Abstract
Objective(s):
Phospholipase C ζ (PLCζ) is considered as a nominee for sperm associated oocyte activating factors and is located back-to-back with CAPZA3, an actin-capping protein controlling actin polymerization during spermiogenesis. They contain a common bidirectional promoter. The objective of this study was to identify individuals with parallel low expression of PLCζ and CAPZA3 mRNA, in hope of detecting genetic defects in this bidirectional promoter.
Materials and Methods:
Semen samples were collected from 24 fertile and 59 infertile individuals with total failed, low and high fertilization rate post intra-cytoplasmic sperm injection (ICSI), as well as globozoospermic individuals. Expression of PLCζ and CAPZA3 were assessed by Real time PCR. In addition, PLCζ was assessed by Western blot.
Results:
Significant correlations between PLCζ with CAPZA3 and also between these two genes with fertilization were observed. Individuals with low fertilization presented significantly lower expression of these two genes. Low expression of PLCζ was also verified by Western analysis. Sequence analysis of bidirectional promoter of these two genes in an individual with parallel low expression of both PLCζ and CAPZA3, revealed a mutation within the CAPZA3 predicted promoter, known as human regulatory factor X4 which is a testis-specific dimeric DNA-binding protein. In the opposite stand, in the same location, the mutation appears to be outside but in the vicinity of PLCζ, in a binding region predicate by Genomatix.
Conclusion:
Parallel assessment of CAPZA3 with PLCζ at mRNA level in individuals with inability to induce oocyte activation may help researcher to identify genetic defects associated with failed fertilization.
Keywords: CAPZA3, Failed fertilization, ICSI, Mutation, PLCζ, Promoter
Introduction
During mammalian fertilization, sperm triggers a remarkable series of intracellular Ca2+ oscillations in oocyte which propagate a chain of biochemical events called ‘oocyte activation’ (1-5). These events are essential for successful fertilization and embryonic development (2, 6-10). Factors involved in induction of oocyte activation are termed sperm-borne oocyte-activating factors (SOAFs). Sperm-specific phospholipase C isoform ζ (PLCζ) (11-15) and post acrosomal WW binding protein (PAWP) are two nominees for SOAFs. PAWP is mainly localized in perinuclear theca of sperm head and can trigger calcium oscillation in oocyte (16-19); however. Wu et al believes that PAWP may act upstream or downstream of Ca2+ signaling pathway (17). Study of literature reveal that PLCζ is a testis specific gene and is introduced as the predominant activator of mammalian oocytes (11-15) and supportive facts are as follows: low expression of PLCζ and mutation in this gene are related with male infertility and may account for low and failed fertilization rate post intra-cytoplasmic sperm injection (ICSI) (20-24). This phenomenon can be overcome by artificial oocyte activation following ICSI (25, 26). Therefore, PLCζ is introduced as a potential marker for assessment of activation capacity of semen samples and its recombinant form can induce intracellular calcium oscillation and oocyte activation in both human and mouse oocyte (22, 27). However, due to variance in distribution and amount of PLCζ assessed by immunofluorescent, this approach has limited application in this filed (28).
In mammals, PLCζ is located back-to-back with another testis-specific gene called CAPZA3 [capping protein (actin filament) muscle Z-line, alpha 3]. These two genes share a common bidirectional promoter with a putative cAMP responsive element modulator of protein recognition site (29, 30). It has been proposed that CAPZA3 might have been a retrogene inserted into the genome, next to PLCζ (31-33).
CAPZA3 mRNA is transcribed in spermatid as an actin-capping protein controlling actin polymerization during acrosomal biogenesis, formation of sperm head morphology (31, 34, 35), capacitation and acrosome reaction (36-41). Therefore, genetic defects disturbing these processes is expected to effect the localization and expression of PLCζ and thereby influencing the activation potential of such a semen samples, like in globozoospermia. Indeed, it has been previously reported the expression of PLCζ is low in globozoospermia (20, 21).
Considering these two genes have a common bidirectional promoter, this study aimed to compare expression of CAPZA3 and PLCζ genes in fertile and infertile individuals and to investigate whether there is a correlation between the expression level of these two genes. The results revealed a good correlation between the mRNA expression of these two genes and individuals with low expression of both genes presented low fertilization rates. Furthermore, we found a mutation in the bidirectional promoter in an individual with previous failed fertilization and low expression of the two genes.
Materials and Methods
Sperm analysis and semen processing
Semen samples were collected from 24 fertile and 59 infertile individuals with male factors referring to the Isfahan Fertility and Infertility Center (IFIC; Isfahan, Iran). All the participants sign consent form. This project was approved by Institutional Review Board (IRB) of Royan Institute.
Samples were acquired by masturbation after 3-4 days of sexual abstinence. One portion of the semen was used for evaluation of sperm parameters according to World Health Organization (42). The remaining portion of the semen sample was washed twice (PBS, pH: 7.4) and used for evaluation of relative expression of PLCζ and CAPZA3 by Real-time PCR. Sperm morphology was assessed by papanicolaou staining.
Experimental design
This study consisted of four groups: 1) Low fertilization (LF-ICSI) group, including 25 individuals with ICSI fertilization rates of 0-25% (20, 43, 44). These couples were contacted and asked to provide semen sample and their cDNA were stored in the Royan cDNA Bank. 2) High fertilization group (HF-ICSI), consisted 14 individuals with ICSI fertilization rates ranging from 50% to 100%. On the day of ovum pickup, the remaining semen samples from these individuals were used to synthesis cDNA. 3) Globozoospermic group, consisted of 20 individuals with stored cDNA in Royan Bank. 4) Fertile group consisted of 24 individuals, participating in embryo donation program. The remaining semen from these individuals was used to synthesis cDNA.
ICSI procedure and calculation of fertilization rates were carried out according to our previous study (26). Couples with female factors were excluded in this study. To reduce confounding effect of female factors, patients with fewer than four matured MII oocytes that survived ICSI procedure were excluded from this study. Furthermore, individuals with higher than 10% immature, deformed, and post mature oocytes, or any oocyte with certain types of abnormality, were excluded from this study.
RNA extraction and cDNA synthesis
Total RNA from semen samples were extracted using the ‘RNX-plus method’ according to the manufacturer's protocol (Cinnagen; Iran) and their related concentration and purity were assessed by measuring absorbance at 260 and 260/280 nm ratio, respectively. To eliminate possible contamination of genomic DNA, samples were treated with DNaseI (Fermentas; USA). cDNA synthesis was performed using 2 μg of total RNA with the Revert Aid First Strand cDNA Synthesis kit, utilizing random hexamer primer (Fermentas; USA). Quality of synthesized cDNA was assessed by PCR on GAPDH gene (NM-001101.3, Table 2). PCR products were qualified by electrophoresis on 2% agarose gel. RT-PCR was achieved by the following protocol: one cycle for primary denaturation at 94°C for 4 min, followed by 35 repetitive cycles of denaturation for 45 sec at 94°C, an annealing stage for 45 sec at 60°C and an extension stage for 1 min at 72°C.
Table 1.
Description of semen parameters according to WHO-2010 in four groups involved in this study including fertile, high fertilization, low fertilization and globozoospermia(N=83)
| Group (Number) | Parameter | Median ± SE (min, max) |
|---|---|---|
| Fertile (n=24) | Concentration ×106 | 74.00 ± 9.67 (42, 234) |
| % Sperm motility | 60.00 ± 2.31 (30, 70) | |
| % Abnormal morphology | 96.5 ± 0.52 (90, 97) | |
| Volume(ml) | 3.35 ± 0.3 (1, 6) | |
| High fertilization (HF-ICSI; n=14) | Concentration ×106 | 78.00 ± 16.65 (27, 284) |
| % Sperm motility | 60.00 ± 2.25 (40, 70) | |
| % Abnormal morphology | 99.00 ± 1.14 (87, 100) | |
| Volume(ml) | 2.25 ± 0.39 (1.5, 7) | |
| Low fertilization (LF-ICSI; n=25) | Concentration ×106 | 20.5 ± 17.21 (0.25, 328) |
| % Sperm motility | 40.00 ± 4.13 (2, 65) | |
| % Abnormal morphology | 100.00 ± 0.1 (99, 100) | |
| Volume(ml) | 3.00 ± 0.34 (0.5, 7) | |
| Globozoospermic (n=20) | Concentration ×106 | 76.00 ± 6.8(7, 110) |
| % Sperm motility | 40.00 ± 3.52 (5, 70) | |
| % Abnormal morphology | 100 ± 0.00 (100, 100) | |
| Volume(ml) | 3.00 ± 0.28 (1, 4) |
Table 2.
Name and sequences of primers
| PCR type | Gene symbol | Primer sequences (5´-3´) |
|---|---|---|
| RT-PCR | β-ACTIN | F: CGTGACATTAAGGAGAAGCTGTGC |
| R: CTCAGGAGGAGCAATGATCTTGAT | ||
| hCAPZA3 | F: CAGTGGGTTTGGGCAGCTAC | |
| R: CAGAATATTTTTGGCAGTGTTGG | ||
| Quantitative Real-time PCR | CAPZA3 | F: CTGCCTGCTTTCATGGATAGAC |
| R: TACTCTTTCCTTGTCCTTCCTG | ||
| PLCζ | F: CAGATGCCTTGTTCAGTTATTGTC | |
| R: GCCTTCATTTCCTACGGGTTG | ||
| GAPDH | F: CCACTCCTCCACCTTTGACG | |
| R: CCACCACCCTGTTGCTGTAG |
Quantitative real-time PCR
Real-time PCR was carried out in a thermal cycler Rotor gene 6000 (Corbett). The PCR mixture for each reaction contained 5 μl SYBR premix Ex Taq II (TaKaRa; Japan), 0.5 μl of each primer (5 pmol/μl) and 50ng cDNA adjusted to a final volume of 10 μl using dH2O. All reactions were carried out in triplicate. Real-time specific primer pairs were designed by the Beacon designer 7.5 software (Table 2). The Real-time PCR protocol was comprised of: 4 min at 94 °C followed by 40 repetitive cycles for 10 sec at 94 °C, 30 sec at 60 °C and 61 °C respectively for PLCζ and CAPZA3 and 30 sec at 72 °C. The expression level of PLCζ and CAPZA3 mRNA was normalized to GAPDH expression level as a housekeeping gene. Calculation of relative expression was assessed using the ΔΔCt method (45, 46) and final result was demonstrated as 2-ΔΔCt as previously reported (20).
DNA preparation, cloning and sequencing of putative core promoter
Patient with low expression of both PLCζ and CAPZA3 was asked to donate blood sample. Genomic DNA extraction was carried out using DNeasy Blood & Tissue Kit (Qiagen; Germany) according to manufacturer's instructions. To isolate a part of shared core promoter region, we designed forward and reverse primers for human CAPZA3 promoter (hCAPZA3) (NC_000012.11; 18890723-18891385, Table 2). PCR was performed using 100ng of human genomic DNA and 5pmole of hCAPZA3 primers in a total reaction volume of 25 µl under the following conditions: one cycle of denaturation at 94 °C for 5 min and 35 cycles of successive denaturation at 94 °C for 45 sec, annealing at 61°C for 45 s and extension at 72°C for 1 min followed by a final extension at 72°C for 20 min. PCR products were electrophoresed on 2% agarose gel and then cloned in pTZ57R/T vector according (Fermentas; USA) to InsTAclone™ PCR Cloning Kit protocol (Fermentas; USA). After transformation into Escherchia coli, five positive colonies were selected for colony PCR and plasmid extraction was performed using QIAprep Spin Miniprep Kit (Qiagen; Germany). Plasmids were sent for Dideoxy-chain-termination sequencing with specific hCAPZA3 primers (Table 2). Sequencing data were then analyzed for transcription factor binding sites using Genomatix software through the following site http://www.genomatix.de.
Western blot analysis
To perform immunoblotting, fresh semen samples were obtained from 5 individuals: one fertile sample, two infertile samples (previously Q RT- PCR analysis showed low expression of CAPZA3 gene in both individuals but PLCζ expression was within the range of fertile individuals for one individual and low in the other individual) and two globozoospermic samples. Protein was extracted using TRI Reagent (Sigma-Aldrich; USA) and protein concentration of each sample was estimated by Bradford assay (Bio-Rad; USA) to determine total protein load per each lane.
Equal amounts of each sample containing 30 μg of protein was subjected to 12% SDS- polyacrylamide gel electrophoresis (PAGE) and then transferred to PVDF membrane (Bio-Rad; USA). Blocking was achieved with 10% non-fat dry milk overnight incubation followed by incubating 20 µg/ml anti PLCζ antibody polyclonal (1:100 dilution; a gift from Dr Parrington, Department of Pharmacology, University of Oxford, UK.) in 2% Skimmed milk for 90 min at 25°C and washed in TBS–T, three times each for 15 min. Finally, the membranes were incubated with Horseradish peroxidase-conjugated polyclonal Goat Anti-mouse IgG (1:5000; Dako) for 1 hr at room temperature.
The immune reactivity on Western blots was detected by GE Amersham ECL Plus Western Blotting Detection Reagent (Amersham; GE Health care) by exposure to X-ray films.
Statistical analysis
Microsoft Excel and SPSS (version 17) were used to analyze data. Data are expressed as means ± SEM (standard error of mean). For assessment of correlations, Pearson correlation coefficients were used. Differences in genes expression between four groups were assessed using one-way ANOVA. P<0.05 was considered as significant.
Results
The mean ages of fertile and infertile men were 35.09±1.14 and 35.37±0.94 year. The mean ages of their partners were 29.73±0.87 and 29.85±0.89, respectively. Descriptive analysis of the semen parameters including concentration, motility, abnormal morphology and volume in fertile, high fertilization post-ICSI (>50%), failed or low fertilization post-ICSI (<25%), and globozoospermic groups are presented in Table 1.
Comparison of relative expression of PLCζ, CAPZA3 and fertilization rate
Relative expression of PLCζ and CAPZA3 mRNA were assessed with quantitative PCR, and results were compared between fertile, HF-ICSI, LF-ICSI, and globozoospermic groups. The relative expressions of PLCζ were 136.36± 20.09, 135.61± 37.93, 44.83± 9.43, and 102.72± 14.83 in fertile, HF-ICSI, LF-ICSI and globozoospermic groups, respectively (Figure 1). This parameter was significantly higher in the fertile (P<0.05) and HF-ICSI (P<0.05) groups compared to LF-ICSI group. There was no difference in relative expressions of PLCζ between globozoospermic group compared to fertile and HF-ICSI groups, and also with LF-ICSI group.
Figure 1.
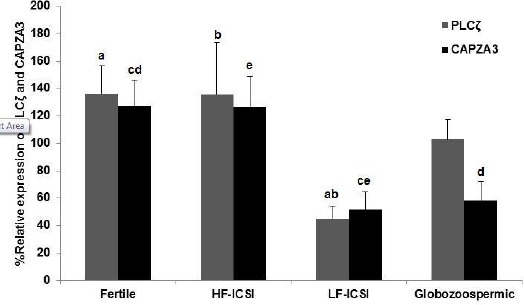
Relative expressions of CAPZA3 and PLCζ mRNA in fertile men, infertile individuals with low or total failed fertilization rate (LF-ICSI), high fertilization rate (HF-ICSI) and globozoospermic cases were quantified and normalized with GAPDH. Common letters indicate significant difference between samples at P<0.05. (N=83)
Relative expressions of CAPZA3 were 127.23±18.95, 126.56±22.40, 51.97±12.44, and 58.28±13.99 in fertile, HF-ICSI, LF-ICSI and globozoospermic groups, respectively (Figure 1). This parameter was significantly higher in the fertile group compared to LF-ICSI (P<0.05) and globozoospermic (P<0.05) groups. In addition, relative expression of CAPZA3 was significantly higher in HF-ICSI group compared to LF-ICSI group (P<0.05).
Relationship between relative expression of PLCζ and CAPZA3 with fertilization rate
Figure 2 shows a significant correlation between percentage fertilization with relative expression of PLCζ (r=0.50; P<0.05) and CAPZA3 (r=0.465; P<0.05).
Figure 2.
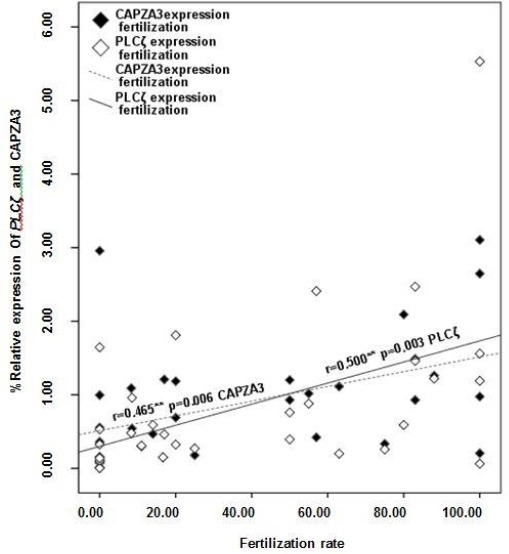
Correlation between relative expression of PLCζ and CAPZA3 with fertilization rate (N=33)
Relationship between relative expression of PLCζ and CAPZA3
Considering PLCζ and CAPZA3 have a common bidirectional promoter; it is questionable, whether there is a correlation between expression of PLCζ and CAPZA3. Therefore, relative expression of PLCζ and CAPZA3 were assessed and Figure 3 shows a strong significant correlation between the relative expressions of these two genes (r=0.502, P<0.05).
Figure 3.
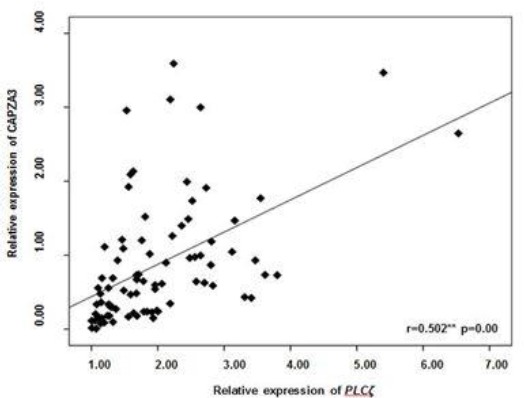
Correlation between PLCζ and CAPZA3 mRNA expression (N=83)
Relationship between relative expression of CAPZA3 and sperm parameters
Figure 4 shows a significant correlation between relative expression of CAPZA3 and percentage of sperm motility (r= 0.268; P<0.05). The relationships between CAPZA3 mRNA level with sperm concentration (r=0.22; P < 0.053) was close to be significant and was insignificant with morphology (r=-0.164; P < 0.24).
Figure 4.
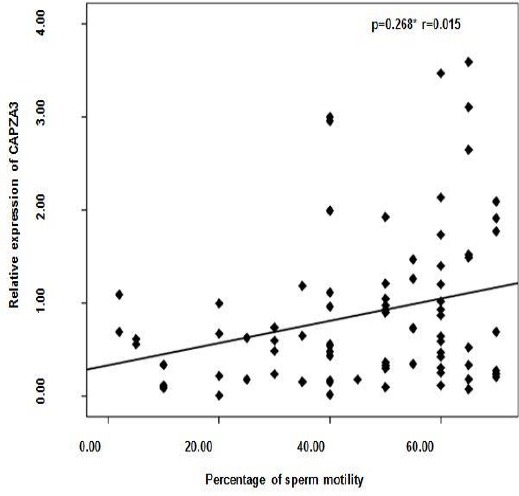
Correlation between percentage of sperm motility and relative expression of CAPZA3 mRNA (N=82)
CAPZA3-PLCζ promoter analysis
To determine whether the low expression of CAPZA3 and PLCζ were linked to changes in the shared promoter region of these two genes, sequence analysis of promoter region (661bp) was performed on genomic DNA of one infertile man with low expression level of these two genes and then was blasted with database sequence in NCBI site (http://www.ncbi.nlm.nih.gov).
A base change at position 18890772 (NC_000012.11) of CAPZA3-PLCζ promoter was identified which would lead to the substitution of cytosine (C) for thymine (T) on DNA sequence of promoter. It is noticeable that polymorphism of this nucleotide has not been reported so far and whether such substitution can be considered as SNP need to be investigated.
This mutation which was homogenous was further confirmed in reverse reading through sequencing. To investigate whether this nucleotide change is within a binding site for transcriptional factors, we analyzed the shared promoter using Genomatics software program. Analyses revealed numerous binding sites for various transcription factors and interestingly aforementioned base change is located in the regulatory factor X, 4 (RFX4) transcription factor binding site belonging to X-box binding factors family (Figure 5).
Figure 5.

Prediction and characterization of putative shared promoter region for CAPZA3 and PLCζ genes. A. Sequence of the shared promoter region for CAPZA3 and PLCζ characterized in this study using genomatix GMBH software. Forward and reverse primers are written with underlined letters. The transcription start sites and translation start sites are indicated by arrowhead. B. Partial sequencing of the sample derived from patient D. Note that a nucleotide change (T) was observed in place of (C) in regulatory X4 binding site
Furthermore, the results of Genomatix software revealed that the mutation is within the CAPZA3 predicted promoter, a predicated region known for RFX4. In the opposite stand, the mutation is located in the vicinity of the PLCζ in a binding region predicate by Genomatics as DR1.
Assessment of PLCζ expression using Western blot analysis
Western blot analysis performed with the PLCζ antibody showed an intense band (70 kDa) in lysates of sperm cells from fertile control (E). A similar band was observed in lysates of sperm cells from infertile men elucidating normal expression of PLCζ mRNA (C). Moreover, no signaling was detected in infertile man, for whom we sequence the promoter region, with low expression of both CAPZA3 and PLCζ mRNA (D).
In samples obtained from globozoospermic men (lane A and B), PLCζ was also not detect. Western blot with β-actin antibody carried out as a control to confirm integrity and equal amount loading of each protein sample (Figure 6). We also aimed to assess protein expression of CAPA3, but we were not able to detect an appropriate band for CAPA3 antibody using commercialized antibody.
Figure 6.
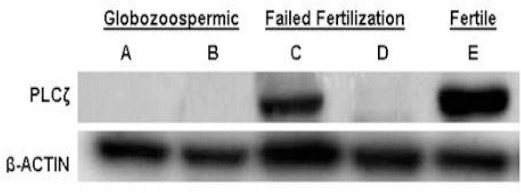
Western blot analysis for PLCζ compared with β-ACTIN. Upper panel shows PLCζ western blotting of five semen samples from two globozoospermic (A and B), two non-globozoospermic patients (C,D) and one fertile individual (E) respectively. Lower panel is B-actin staining of the same individual to confirm integrity of protein content related to immunoblotting of the respective samples with an antibody against β-ACTIN. For simplicity of presentation PLCζ the extra non-specific band were crop out and only 70kDa was presented
Discussion
Bioinformatics studies show that PLCζ is located back-to-back with CAPZA3, another testis-specific gene. These two genes appear to have a common bidirectional promoter (29). Quantitative analysis of CAPZA3 and PLCζ mRNA revealed that the expressions of these transcripts were significantly lower in individuals with low or failed fertilization compared to fertile or individuals with high fertilization rates or fertile individual. These results are also consistent with western blot data. Total absence of PLCζ protein in globozoospermia with low expression of this transcript in these patients are consistent with the inability of sperm form these individuals to induce oocyte activation post ICSI. However, since the transcript of PLCζ is present in the sperm of these individuals, one may expect to see certain degree of translation. Two explanations can be provided for this observation: 1) RNA is not translated, 2) RNA is translated but to a reduce extent and the proteins are not located in their proper position and are lost during cytoplasmic engulfment or removal or residual bodies during final stages of spermiogenesis. This was the situation in the western blot analysis of the two globozoospermic samples in this study. However, further study is required to make sure this is true for all the globozoospermia samples. Indeed, the latter explanation is more likely, since in globozoospermia acrosomal biogenesis is affected and one of the defective mechanisms in these patients is defects in protein transportation. Therefore, the sperm of these individuals lack perinuclear theca, likely to contain PLCζ protein. It is also important to note one may not expect to see lower expression of PLCζ at mRNA and protein levels in all the failed fertilization cases as failed fertilization is a multifactorial phenomenon. This is the reason for presence of adequate amount of PLCζ protein in some of these individuals. We also observed a significant correlation between the relative expressions of the two transcripts, verifying the bioinformatics data and suggesting important role of these two genes in maintaining fertilization and fertility. In concordance with our results, several studies have shown that reduced expression PLCζ is linked with male infertility and provided evidence for importance of PLCζ in mammalian fertilization (20, 21, 24). Furthermore, Yoon and colleagues showed that repetitive fertilization failure after ICSI is associated with oocyte activation failure linked to the inability of sperm to initiate Ca2+ oscillations and this deficiency can be functionally associated with reduced or absence of expression of PLCζ in sperm of these patients (27). However, it is important to note, a paper by Kashir, et al (2013) showed varied expression of PLCζ in fertile individuals and some of these fertile men showed low expression of this protein (28). A varied localization of PLCζ independent of sperm morphology has been also reported (47).
In accordance with our data showing lower expression of CAPZA3 in individuals with low fertilization rate or complete failed fertilization, Geyer et al also showed that missense mutation in this gene leads to infertility in mice (48).
Unlike the expression level of PLCζ, the expression of CAPZA3 in globozoospermic group was significantly lower than the fertile group. This observation is consistent with role of the CAPZA3 in shaping the sperm head and especially in acromogenesis (36). In contrary to our previous report (20), the expression of PLCζ was lower in globozoospermic group compared to fertile individuals but this difference was not significant. This difference between the two studies is very likely related to the number of globozoospermic individuals between the two studies (8 vs. 20 individuals). It is important to note that globozoo-spermia have heterogenic etiology with different genes involved in this phenomenon. There are reports of pregnancies with round sperm in absence of artificial oocyte activation following ICSI. Therefore, heterogenic etiology of globozoospermia may account for this difference (49-51).
Furthermore, the mean relative expression of CAPZA3 compared to PLCζ is significantly lower in globozoospermic individuals while such a difference was not observed in the other groups. We have no explanation regarding this difference, despite existence of a common bidirectional promoter with the two genes. In addition to above explanation, a hypothetical explanation is differential translation of the two genes during spermiogenesis or anomalies associated with promoter region of these genes or within the genes.
Considering the fact that these two genes have common bidirectional promoter, therefore we hypothesized that individual with low or absence of expression of these two genes may have some sort of mutation or deletion associated with this promoter region. Therefore, we obtained blood from one of the individuals which showed low levels of expression and translation of these two genes and DNA sequencing revealed a mutation located in the core sequence of binding site of a transcription factor, RFX4 from X-box binding factor family in CAPZA3 promoter. The expression of this factor is restricted to testis (52). Interestingly, this mutation on the opposite strand was predicted in a binding site for DR1 according to Genomatix. This site is outside but in the vicinity of the Genomatics predicated PLCζ promoter and we observed down regulation of both transcriptions in this individual, therefore, further studies are required to see whether this region is also part of the PLCζ promoter or if it has any effect on expression of PLCζ. However the exact role of this factor has not been unraveled yet. These results suggest that through simultaneous analysis of CAPZA3 and PLCζ, one might be able to select infertile individuals, the etiology of infertility of whom may be related to mutations or anomalies related to promoter region of these two genes. However, further studies at cellular level are required to clear the direct relation of these phenomena to anomalies related to common promoter region of these genes. To our knowledge no such a mutation has been reported before, but mutation in PLCζ gene has been reported previously and has been associated with failed fertilization (23).
Our results also suggest that CAPZA3 might be considered as a complementary marker to evaluate the ability of a semen sample to induce oocyte activation. This conclusion is further reiterated by the correlations observed between CAPZA3 and PLCζ mRNA with fertilization rate. In addition, a significant correlation was observed between the relative expressions of CAPZA3 with percentage sperm motility. This observation might be related to role of actin in cellular morphology and motility. This possibility was previously proposed by Howes and colleagues (41). These authors showed that redistribution of actin and their regulatory proteins, along with changing levels of actin polymerization, may play a continuing role for actin in both post testicular sperm maturation and acrosomal exocytosis. One of the actin protein studies by this group was the testis-specific actin capping protein and these authors proposed that these actins may play a role both in the determination of cell shape during spermiogenesis and mis-shapen spermatozoa fails to fertilize because of impaired motility (41).
Conclusion
Analysis mRNA of CAPZA3 along with PLCζ may assist researcher to identify individuals with lack of ability to induce oocyte activation and make them candidate for artificial oocyte activation and through selecting individuals with low expression of the both genes, one might be able to define genetic infertility related to promoter anomalies related with these two testis specific genes.
Acknowledgment
The authors have no financial or commercial conflicts with this project. This study was supported by Royan Institute and we would like to express our gratitude to staff of Isfahan Fertility and Infertility for their full support. The results described in this paper were part of student thesis.
References
- 1.Lawrence Y, Whitaker M, Swann K. Sperm-egg fusion is the prelude to the initial Ca2+ increase at fertilization in the mouse. Development. 1997;124:233–241. doi: 10.1242/dev.124.1.233. [DOI] [PubMed] [Google Scholar]
- 2.Malcuit C, Knott JG, He C, Wainwright T, Parys JB, Robl JM, et al. Fertilization and inositol 1,4,5-trisphosphate (IP3)-induced calcium release in type-1 inositol 1,4,5-trisphosphate receptor down-regulated bovine eggs. Biol Reprod. 2005;73:2–13. doi: 10.1095/biolreprod.104.037333. [DOI] [PubMed] [Google Scholar]
- 3.Ozil JP, Swann K. Stimulation of repetitive calcium transients in mouse eggs. J Physiol. 1995;483:331–346. doi: 10.1113/jphysiol.1995.sp020589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swann K, Yu Y. The dynamics of calcium oscillations that activate mammalian eggs. Int J Dev Biol. 2008;52:585–594. doi: 10.1387/ijdb.072530ks. [DOI] [PubMed] [Google Scholar]
- 5.Yu Y, Nomikos M, Theodoridou M, Nounesis G, Lai FA, Swann K. PLCζ causes Ca2+oscillations in mouse eggs by targeting intracellular and not plasma membrane PI(4,5)P(2) Mol Biol Cell. 2012;23:371–380. doi: 10.1091/mbc.E11-08-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malcuit C, Kurokawa M, Fissore RA. Calcium oscillations and mammalian egg activation. J Cell Physiol. 2006;206:565–573. doi: 10.1002/jcp.20471. [DOI] [PubMed] [Google Scholar]
- 7.Nomikos M, Elgmati K, Theodoridou M, Calver BL, Cumbes B, Nounesis G, et al. Male infertility-linked point mutation disrupts the Ca2+ oscillation-inducing and PIP(2) hydrolysis activity of sperm PLCζ. Biochem J. 2011;434:211–217. doi: 10.1042/BJ20101772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parrington J, Lai FA, Swann K. The soluble mammalian sperm factor protein that triggers Ca2+ oscillations in eggs: Evidence for expression of mRNA(s) coding for sperm factor protein(s) in spermatogenic cells. Biol Cell. 2000;92:267–275. doi: 10.1016/s0248-4900(00)01064-9. [DOI] [PubMed] [Google Scholar]
- 9.Runft LL, Jaffe LA, Mehlmann LM. Egg activation at fertilization: where it all begins. Dev Biol. 2002;245:237–254. doi: 10.1006/dbio.2002.0600. [DOI] [PubMed] [Google Scholar]
- 10.Kashir J, Deguchi R, Jones C, Coward K, Stricker SA. Comparative biology of sperm factors and fertilization-induced calcium signals across the animal kingdom. Mol Reprod Dev. 2013;80:787–815. doi: 10.1002/mrd.22222. [DOI] [PubMed] [Google Scholar]
- 11.Cox LJ, Larman MG, Saunders CM, Hashimoto K, Swann K, Lai FA. Sperm phospholipase Cζfrom humans and cynomolgus monkeys triggers Ca2+ oscillations, activation and development of mouse oocytes. Reproduction. 2002;124:611–623. doi: 10.1530/rep.0.1240611. [DOI] [PubMed] [Google Scholar]
- 12.Knott JG, Kurokawa M, Fissore RA, Schultz RM, Williams CJ. Transgenic RNA interference reveals role for mouse sperm phospholipase Cζin triggering Ca2+ oscillations during fertilization. Biol Reprod. 2005;72:992–996. doi: 10.1095/biolreprod.104.036244. [DOI] [PubMed] [Google Scholar]
- 13.Kouchi Z, Fukami K, Shikano T, Oda S, Nakamura Y, Takenawa T, et al. Recombinant phospholipase Cζhas high Ca2+ sensitivity and induces Ca2+ oscillations in mouse eggs. J Biol Chem. 2004;279:10408–10412. doi: 10.1074/jbc.M313801200. [DOI] [PubMed] [Google Scholar]
- 14.Parrington J, Jones ML, Tunwell R, Devader C, Katan M, Swann K. Phospholipase C isoforms in mammalian spermatozoa: potential components of the sperm factor that causes Ca2+ release in eggs. Reproduction. 2002;123:31–39. doi: 10.1530/rep.0.1230031. [DOI] [PubMed] [Google Scholar]
- 15.Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, et al. PLC zeta: a sperm-specific trigger of Ca2+ oscillations in eggs and embryo development. Development. 2002;129:3533–3544. doi: 10.1242/dev.129.15.3533. [DOI] [PubMed] [Google Scholar]
- 16.Aarabi M, Sutovsky P, Oko R. Re: Is PAWP the ‘real’ sperm factor? Asian J Androl. 2015;17:446–9. doi: 10.4103/1008-682X.145071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu AT, Sutovsky P, Manandhar G, Xu W, Katayama M, Day BN, et al. PAWP, a sperm-specific WW domain-binding protein, promotes meiotic resumption and pronuclear development during fertilization. J Biol Chem. 2007;282:12164–12175. doi: 10.1074/jbc.M609132200. [DOI] [PubMed] [Google Scholar]
- 18.Aarabi M, Balakier H, Bashar S, Moskovtsev SI, Sutovsky P, Librach CL, Oko R. Sperm-derived WW domain-binding protein, PAWP, elicits calcium oscillations and oocyte activation in humans and mice. FASEB J. 2014;28:4434–40. doi: 10.1096/fj.14-256495. [DOI] [PubMed] [Google Scholar]
- 19.Aarabi M, Yu Y, Xu W, Tse MY, Pang SC, Yi YJ, et al. The testicular and epididymal expression profile of PLCζin mouse and human does not support its role as a sperm-borne oocyte activating factor. PLoS One. 2012;7:1–12. doi: 10.1371/journal.pone.0033496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aghajanpour S, Ghaedi K, Salamian A, Deemeh MR, Tavalaee M, Moshtaghian J, et al. Quantitative expression of phospholipase C zeta, as an index to assess fertilization potential of a semen sample. Hum Reprod. 2011;26:2950–2956. doi: 10.1093/humrep/der285. [DOI] [PubMed] [Google Scholar]
- 21.Heytens E, Parrington J, Coward K, Young C, Lambrecht S, Yoon SY, et al. Reduced amounts and abnormal forms of phospholipase C zeta (PLCzeta) in spermatozoa from infertile men. Hum Reprod. 2009;24:2417–2428. doi: 10.1093/humrep/dep207. [DOI] [PubMed] [Google Scholar]
- 22.Kashir J, Jones C, Lee HC, Rietdorf K, Nikiforaki D, Durrans C, et al. Loss of activity mutations in phospholipase C zeta (PLCζ) abolishes calcium oscillatory ability of human recombinant protein in mouse oocytes. Hum Reprod. 2011;26:3372–3387. doi: 10.1093/humrep/der336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kashir J, Konstantinidis M, Jones C, Lemmon B, Lee HC, Hamer R, et al. A maternally inherited autosomal point mutation in human phospholipase C zeta (PLCζ) leads to male infertility. Hum Reprod. 2012;27:222–231. doi: 10.1093/humrep/der384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoon SY, Jellerette T, Salicioni AM, Lee HC, Yoo MS, Coward K, et al. Human sperm devoid of PLC zeta 1 fail to induce Ca2+ release and are unable to initiate the first step of embryo development. J Clin Invest. 2008;118:3671–3681. doi: 10.1172/JCI36942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nasr-Esfahani MH, Deemeh MR, Tavalaee M. Artificial oocyte activation and intracytoplasmic sperm injection. Fertil Steril. 2009;94:520–526. doi: 10.1016/j.fertnstert.2009.03.061. [DOI] [PubMed] [Google Scholar]
- 26.Nasr-Esfahani MH, Tavalaee M, Deemeh MR, Arbabian M, Parrington J. Can assessment of total acrosin activity help predict failed or low fertilization rate ICSI for implementation of artificial oocyte activation? The Open Andrology Journal. 2010;2:19–26. [Google Scholar]
- 27.Yoon SY, Eum JH, Lee JE, Lee HC, Kim YS, Han JE, et al. Recombinant human phospholipase C zeta 1 induces intracellular calcium oscillations and oocyte activation in mouse and human oocytes. Hum Reprod. 2012;27:1768–1780. doi: 10.1093/humrep/des092. [DOI] [PubMed] [Google Scholar]
- 28.Kashir J, Jones C, Mounce G, Ramadan WM, Lemmon B, Heindryckx B, et al. Variance in total levels of phospholipase C zeta (PLC-ζ) in human sperm may limit the applicability of quantitative immunofluorescent analysis as a diagnostic indicator of oocyte activation capability. Fertil Steril. 2013;99:107–117. doi: 10.1016/j.fertnstert.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Coward K, Ponting CP, Chang HY, Hibbitt O, Savolainen P, Jones KT, et al. Phospholipase C zeta, the trigger of egg activation in mammals, is present in a non-mammalian species. Reproduction. 2005;130:157–163. doi: 10.1530/rep.1.00707. [DOI] [PubMed] [Google Scholar]
- 30.Hurst S, Howes EA, Coadwell J, Jones R. Expression of a testis-specific putative actin-capping protein associated with the developing acrosome during rat spermiogenesis. Mol Reprod Dev. 1998;49:81–91. doi: 10.1002/(SICI)1098-2795(199801)49:1<81::AID-MRD9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 31.Miyagawa Y, Tanaka H, Iguchi N, Kitamura K, Nakamura Y, Takahashi T, et al. Molecular cloning and characterization of the human orthologue of male germ cell-specific actin capping protein alpha3 (cpalpha3) Mol Hum Reprod. 2002;8:531–539. doi: 10.1093/molehr/8.6.531. [DOI] [PubMed] [Google Scholar]
- 32.Valentin M, Balvers M, Pusch W, Weinbauer GF, Knudsen J, Ivell R. Structure and expression of the mouse gene encoding the endozepine-like peptide from haploid male germ cells. Eur J Biochem. 2000;267:5438–5449. doi: 10.1046/j.1432-1327.2000.01603.x. [DOI] [PubMed] [Google Scholar]
- 33.Vanin EF. Processed pseudogenes: characteristics and evolution. Ann Rev Genet. 1985;19:253–272. doi: 10.1146/annurev.ge.19.120185.001345. [DOI] [PubMed] [Google Scholar]
- 34.Kierszenbaum AL, Tres LL. Structural and transcriptional features of the mouse spermatid genome. J Cell Biol. 1975;65:258–270. doi: 10.1083/jcb.65.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monesi V. Synthetic activities during spermatogenesis in the mouse RNA and protein. Exp Cell Res. 1965;39:197–224. doi: 10.1016/0014-4827(65)90023-6. [DOI] [PubMed] [Google Scholar]
- 36.Sosnik J, Buffone MG, Visconti PE. Analysis of CAPZA3 localization reveals temporally discrete events during the acrosome reaction. J Cell Physiol. 2010;224:575–580. doi: 10.1002/jcp.22211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tokuhiro K, Miyagawa Y, Tanaka H. Characterizing mouse male germ cell-specific actin capping protein alpha3 (CPalpha3): dynamic patterns of expression in testicular and epididymal sperm. Asian J Androl. 2008;10:711–718. doi: 10.1111/j.1745-7262.2008.00426.x. [DOI] [PubMed] [Google Scholar]
- 38.Brener E, Rubinstein S, Cohen G, Shternall K, Rivlin J, Breitbart H. Remodeling of the actin cytoskeleton during mammalian sperm capacitation and acrosome reaction. Biol Reprod. 2003;68:837–845. doi: 10.1095/biolreprod.102.009233. [DOI] [PubMed] [Google Scholar]
- 39.Breitbart H, Cohen G, Rubinstein S. Role of actin cytoskeleton in mammalian sperm capacitation and the acrosome reaction. Reprod. 2005;129:263–268. doi: 10.1530/rep.1.00269. [DOI] [PubMed] [Google Scholar]
- 40.Dvoráková K, Moore HD, Sebková N, Palecek J. Cytoskeleton localization in the sperm head prior to fertilization. Reproduction. 2005;130:61–69. doi: 10.1530/rep.1.00549. [DOI] [PubMed] [Google Scholar]
- 41.Howes EA, Hurst SM, Jones R. Actin and actin-binding proteins in bovine spermatozoa: potential role in membrane remodeling and intracellular signaling during epididymal maturation and the acrosome reaction. J Androl. 2001;22:62–72. [PubMed] [Google Scholar]
- 42.WHO. WHO laboratory manual for the examination and processing of human semen. Fifth edition. Geneva, Switzerland: World Health Organization, WHO Press; 2010. [Google Scholar]
- 43.Montag M, Köster M, van der Ven K, Bohlen U, van der Ven H. The benefit of artificial oocyte activation is dependent on the fertilization rate in a previous treatment cycle. Reprod Biomed Online. 2012;24:521–526. doi: 10.1016/j.rbmo.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Ebner T, Montag M, Van der Ven K, Shebl O, Oppelt P. Live birth after artificial oocyte activation using a ready-to-use ionophore: a prospective multicentre study. Reprod Biomed Online. 2015;30:359–365. doi: 10.1016/j.rbmo.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 45.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 46.Vaerman JL, Saussoy P, Ingargiola I. Evaluation of real-time PCR data. J Biol Regul Homeost Agents. 2004;18:212–214. [PubMed] [Google Scholar]
- 47.Kashir J, Nomikos M, Swann K, Lai FA. PLCζor PAWP: revisiting the putative mammalian sperm factor that triggers egg activation and embryogenesis. Mol Hum Reprod. 2015;21:383–388. doi: 10.1093/molehr/gav009. [DOI] [PubMed] [Google Scholar]
- 48.Geyer CB, Inselman AL, Sunman JA, Bornstein S, Handel MA, Eddy EM. A Missense Mutation in the Capza3 Gene and Disruption of F-actin Organization in Spermatids of repro32 Infertile Male Mice. Dev Biol. 2009;330:142–152. doi: 10.1016/j.ydbio.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuentz P, Vanden Meerschaut F, Elinati E, Nasr-Esfahani MH, Gurgan T, Iqbal N, et al. Assisted oocyte activation overcomes fertilization failure in globozoospermic patients regardless of the DPY19L2 status. Hum Reprod. 2013;28:1054–1061. doi: 10.1093/humrep/det005. [DOI] [PubMed] [Google Scholar]
- 50.Dirican EK, Isik A, Vicdan K, Sozen E, Suludere Z. Clinical pregnancies and livebirths achieved by intracytoplasmic injection of round headed acrosomeless spermatozoa with and without oocyte activation in familial globozoospermia: case report. Asian J Androl. 2008;10:332–336. doi: 10.1111/j.1745-7262.2008.00248.x. [DOI] [PubMed] [Google Scholar]
- 51.Stone S, Mahony FO, Khalaf Y, Taylor A, Braude P. A normal livebirth after intracytoplasmic sperm injection for globozoospermia without assisted oocyte activation. Hum Reprod. 2000;15:139–141. doi: 10.1093/humrep/15.1.139. [DOI] [PubMed] [Google Scholar]
- 52.Morotomi-Yano K, Yano K, Saito H, lwama A, Miki Y. Human regulatory factor X 4 (RFX4) is a testis-specific dimeric DNA-binding protein that cooperates with other human RFX members. J Biol Chem. 2002;277:836–842. doi: 10.1074/jbc.M108638200. [DOI] [PubMed] [Google Scholar]


