Abstract
Objective(s):
Osteoarthritis (OA), as a known degenerative joint disease, is the most common form of arthritis. In this study, we aimed to elucidate unclear pathogenesis of OA.
Materials and Methods:
Rabbit models of OA were established by the transection of the anterior cruciate ligament. Rabbits were randomly divided into three equal groups: the experimental group (OA modeling, treated with estradiol), the control group (OA modeling, treated with normal saline) and the normal group (without OA modeling). The glycosaminoglycan (GAG) and hyaluronan (HA) content of knee joint were collected and assayed. In addition, gene expression of matrix metalloproteinase (MMP)-1, MMP-13 and tissue inhibitor of metalloproteinase (TIMP)-1 were evaluated by real-time PCR and Western blot analysis.
Results:
Animal models were developed successfully. GAG and HA concentrations were significantly increased in the experimental and the normal group compared with the control group (P<0.05 and P<0.01, respectively). Significant increase of GAG level in 6, 9 and 12 week-samples were found in the experimental group compared with the control group (P<0.01). The expression level of MMP-1 and MMP-13 in the experimental group were lower than the control group (P<0.01), but still higher than those of the normal group (P<0.01). TIMP-1 expression level was found to be higher in the experimental group than that of the control and normal group (P<0.01).
Conclusion:
The results suggested the possible role of estradiol in the pathological process of OA via its effect on the MMPs. The results also implied the effect of estradiol intervention on OA.
Keywords: Estradiol, Matrix metalloproteinase, Osteoarthritis, Proteoglycan
Introduction
Osteoarthritis (OA) is a painful, chronic, degenerative joint disease. It is characterized by the articular cartilage damage, osteophytes on the articular surface, synovial cell hyperplasia, synovitis and joint space narrowing (1). Epidemiological studies have shown that OA usually occurs in postmenopausal women, and its incidence is closely linked to estrogen levels (2). Reports revealed that estrogen can delay the degeneration of articular cartilage and reduce the risk of osteoarthritis occurrence (3, 4). However, to understand the efficiency of estrogen intervention, a more comprehensive understanding of the principles is necessary.
The definite mechanisms wherein the estradiol affects OA are not entirely clear. Recent studies suggest that cytokines are important risk factors for OA, and estrogen may protect articular cartilage by regulating expression levels of cytokine (5).
Moreover, it is thought that the cytokine matrix metalloproteinase (MMPs) play a significant role in the pathogenesis of OA by degradation of almost all extracellular matrix of chondrocytes (6). On the other hand, the specific inhibitor of MMPs (tissue inhibitor of metalloproteinases, TIMPs) is endogenic and widespread low molecular weight protein, which can specifically inhibit the activity of MMPs (7). The increased synthesis of MMPs and the decreased synthesis of TIMPs in OA articular cartilage lead to the physiological imbalance between synthesis and degradation of cartilage extracellular matrix (ECM), which may cause cartilage degeneration (8). It is shown that in female patients with OA, the high-dose estrogen inhibits the expression of MMP-1, MMP-13 and TIMP-1 (9). It is also suggested that the balance between MMPs and TIMPs is particularly weighty.
However, findings showed that the expression of MMP-1 can be significantly inhibited by estrogen, but it had no obvious effect on the level of MMP-3, MMP-13 and TIMP-1 (10).
Proteoglycan (PG) is an important part of articular cartilage matrix. Studies of OA pathogenesis have shown that the degradation of PG and collagen fibers is the main physiological and pathological basis of OA occurrence (11, 12). The reduced synthesis and increased decomposition of articular cartilage ECM are one of the important causes of OA cartilage degeneration. Furthermore, PG is an important component of articular cartilage ECM. Glycosaminoglycan (GAG) makes up more than 90% of PG (by weight) (13), and it is the decisive functional group of PG. The PG monomer non-covalently linked to hyaluronan, commonly known as hyaluronic acid (HA) to form an aggregate (14). It is shown that OA reduces the content of GAG and HA, which can be significantly alleviated by estrogen (15). Thereby estrogen can protect OA by inhibiting the degradation of glycoprotein.
Since numerous factors are involved in OA, it is helpful to perform the multiple-factor conjoint analysis. Previously, the effect of estrogen and progestin had been investigated on the expression of MMPs (16), but the study were only limited to evaluation of mRNA level and no other factors. Therefore, this study presents understanding that the combined action of MMPs and PG is limited. In the current study, the anterior cruciate ligament transaction (ACLT)-based animal model of OA was established and then, the effects of estradiol were analyzed by the changes of PG, MMP1, MMP13 and TIMP-1. The latter three were detected by real-time PCR and Western blot analysis. The focus of this study was to further explore the pathogenesis of OA.
Materials and Methods
Ethic
This research project has been approved by General Hospital of Jinan Military Command, Jinan, China Ethics Committee and was performed in accordance with the ethical standards.
Animal models and treatments
Thirty six adults New Zealand white female rabbits weighing 2 to 3 kg (conventional animals), were provided by the Experimental Animal Center of the Shandong University of Traditional Chinese Medicine. After anesthesia with ketamine (0.1 mg kg-1) intraperitoneally, one knee joint of each rabbit was randomly selected. Based on medial patellar incision, the anterior cruciate ligament was transected under direct vision. The anterior drawer test was performed to confirm the ligament rupture completely. No. 1 silk sutured the joint cavity to establish the OA model (7).
All rabbits were housed individually in each cage with size of 60 cm × 60 cm × 40 cm. Rabbits were randomly divided into three groups: (1) the normal group (n=12) without OA modeling process were feed normally; (2) the experimental group (n=12) were treated with 0. 025 mg mL-1 17β-estradiol (0. 02 mg kg-1, 0.9% normal saline as diluents) (Sigma Chemical Co., St Louis, MO, USA) intramuscularly once a day; (3) the control group was administered by intramuscular injection with normal saline once a day (0.8 ml kg-1).
Content determination of glycosaminoglycan and hyaluronan
Four rabbits were anesthetized and euthanized in 6 weeks, 9 weeks and 12 weeks in each group, respectively. Then the contents of GAG and HA in cartilage were identified. Approximately 10 mg of degenerated articular cartilage of medial femoral condyle were taken. GAG was qualitatively determined by Alcian blue staining according to manufacturer's instruction of rabbit GAG ELISA kit (Shanghai Bioleaf Biotech Co., Ltd., Shanghai, China, M1402-2). Briefly, GAG and Alcian blue can quickly generate soluble GAG-Alcian blue compound. Since light absorption between GAG-Alcian blue compound and Alcian blue is different, the content of GAG could be detected through the measurement of GAG-Alcian blue compound content at 260 nm by colorimetric method. The measurement of HA content was proceeded with specification of the rabbit HA ELISA Kit (Shang hai jiang lai Bio-Technology Co., Ltd., Shanghai, China).
RNA extraction and cDNA obtained
Two samples (degenerated articular cartilage of medial femoral condyle and medial synovial membrane of articular cavity) of each animal group obtained above were homogenized in 1 ml Trizol, and then total RNA was extracted by the RNA extraction kit (Shanghai Sangon Biotech Co., Ltd, Shanghai, China). The integrity of extracted RNA was evaluated by agarose gel electrophoresis. The purity and concentration of total RNA were detected by measurement of the absorbance at 260 nm/280 nm with the use of UV spectrophotometer. Then the RNA was set to the same concentration with 0.1% diethyl pryrocarbonate (DEPC)-treated and autoclaved distilled water.
Reverse transcription-PCR was performed using the provided reagents in Kit (Invitrogen, Carlsbad, CA, USA). The total reaction volume was 20 μl, including 4 μl total RNA, 1μl random oligonucleotide primers (50 μg/ml) and 7.5 μl double distilled water. The mixture was placed on ice for 5 min after denaturation, and the reaction continued by adding RNAs 0.5 μl, 10 mmol dNTP 2 μl, 5× buffer 4 μl, and RTase 1 μl. Then the reaction was achieved at 37°C for 60 min and inactivated at 70°C for 15 min. The cDNA was frozen persevered at -20°C.
Real-time PCR detection
cDNA of 12 weeks euthanized rabbits with normal, control and experimental groups were used. MMP-1, MMP-13, TIMP-1 and β-actin primers were designed. Primer sequences are shown in Table 1, wherein the obtained MMP-1 fragment was 205 bp, MMP-3 fragment was 400 bp, TIMP-1 fragment was 260 bp, and β-actin fragment was 118 bp.
Table 1.
Primers of the matrix metalloproteinase (MMP-1), MMP-3, tissue inhibitor of metalloproteinase (TIMP)-1 and β-actin
| Sense strand | Anti-sense strand | |
|---|---|---|
| MMP-1 | 5’-GGTATGATGAATATAAACG-3’ | 5’-CTGCAGTTGAACCAGCT-3’ |
| MMP-13 | 5’-GGCCATCTCTTCCTTCAG-3’ | 5’-GTCACTTTCTTTGCATTTGG-3’ |
| TIMP-1 | 5’-TACACCCCCGCCATGG-3’ | 5’-GTCCACAAGCAATGAGTG-3’ |
| β-actin | 5’-ATGTTTGTGATGGGCGTGAA-3’ | 5’-CGAAGTGGTCGTGGATGA-3’ |
Total 25 μl reaction system including 100 ng cDNA, Real-time Sybr Green PCR regent (Invitrogen, Carlsbad, CA, USA), 5 μl 5× real-time PCR buffer, 0.5 μl MgC12, 0.75 μl dNTP, 0.4 μl up stream primer, 0.4 μl down stream primer, 0.25 μl Taqpolymerase, 2μl template, and water made up to 25 μl. The cycling conditions were as follows: denaturation at 94 °C for 30 sec, annealing (MMP-1 at 60 °C; MMP- 2 at 58 °C, TIMP-1 at 61 °C) 30sec, and extension at 72 °C for 30 sec. Cycles were as follows: MMP-1, 50 cycles; MMP-2, 46 cycles; TIMP-1, 45 cycles. Amplification reaction was conducted in the ABI Prism 7900HT real-time thermal cycler (Applied Biosystems, Carlsbad, CA, USA). The β-actin was used as internal control for the separate groups mRNA expression.
Western blot analysis
Approximately, 10 mg degenerated articular cartilage of medial femoral condyle of the articular cavity was taken from 12 weeks euthanized rabbits in normal, control and experimental groups. After washing twice with phosphate-buffered saline (PBS), samples were grinded and lysed in radio immunoprecipitation assay buffer and collected by centrifugation (14,000 rpm for 20 min, 4 °C). Protein concentrations of the samples were determined, and the desired volume of each sample for equal protein was calculated. About 50 μg protein of each sample was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose (NC). The NC membrane was initially blocked with 5% skim milk (TBS-T) and shaken at room temperature for 1 to 2 hr. Then the blocking solution diluted primary antibody (MMP-1, MMP-13, TIMP-1, 1:1000, Santa Cruz Biotechnology Inc., Santa Cruz, California, USA) was added to NC membrane and incubated at 4°C overnight. Subsequently, the NC membrane was washed with TBS-T for three times (each time for 15 min). In the dark, the enhanced chemiluminescence (ECL) reagent (Millipore Co., Billerica, MA, USA) was used to expose the membrane to film.
Statistical analysis
Statistical analysis was performed and comparisons among the groups were computed on SPSS 17.0. Data were analyzed by ANOVA analysis and pair wise comparison of samples means were examined by least significant difference (LSD) with (x ± s). P-values less than 0.05 was considered statistically significant.
Results
Construction of osteoarthritis animal model
The articular cartilage surface of the normal rabbits was smooth, with normal color, no cartilage surface cracks and dents, and no osteophyte formation (Figure 1 A). In the OA model rabbits, the joints were swollen and deformed, the cartilage color was gray, erosion was found on the rough surface, surface fissures and fibrosis were mainly found in the tackle and the femoral condyle of cartilage, defects were found on the double-femoral condyles and surface cartilage of tibial articular condyle, subchondral bone was exposed, and osteophytosis was obvious (Figure 1 B).
Figure 1.
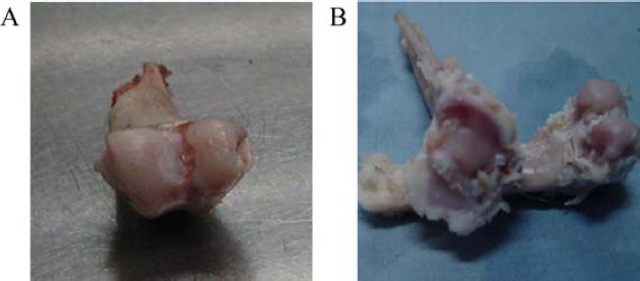
Osteoarthritis model of rabbits
A. The articular cartilage surface of the normal rabbits. B. The articular cartilage surface of the OA model rabbits.
Content of glycosaminoglycan and hyaluronan
In 6, 9 and 12 week-samples, GAG level of the experimental group were all similar with the normal group, but significantly higher than those in the control group (P<0.01). There was a negative correlation between sampling time and GAG content in the control group (P<0.05), while, no significant changes were found in the control and the experimental group. HA contents of the control group were significantly lower than those in the experimental and the normal group (P<0.01). With the extension of sampling time, HA content was significantly reduced (P<0.05 vs. P<0.01) in the control group, while it was considerably increased in the control and the experimental group (P<0.01) (Table 2).
Table 2.
Glycosaminoglycan (GAG) and hyaluronan (HA) in cartilage of normal, control and experimental groups at different sampling times (mg·g-1)
| 6 weeks | 9 weeks | 12 weeks | ||||
|---|---|---|---|---|---|---|
| Groups | GAG | HA | GAG | HA | GAG | HA |
| Normal | 44±1 | 1.05±0.03 | 43±1 | 1.36±0.03* | 44±2 | 1.65±0.02* |
| Control | 33±2 | 0.77±0.01* | 27±1# | 0.55±0.01** | 23±1# | 0.43±0.02*# |
| Experimental | 41±1* | 1.09±0.03 | 42±2* | 1.35±0.02* | 42±2* | 1.66±0.02* |
P< 0.01,
P< 0.05.
Real-time PCR analysis of MMP-1, MMP-13 and TIMP-1
Relative expression levels of MMP-1, MMP-13, TIMP-1 and β-actin were shown in Table 3. The expression levels of MMP-1 and MMP-13 in the control group were significantly higher than that in the normal group (P<0.01). The expression level of TIMP-1 was also decreased compared with the other two groups (P<0.01). Also, the expression level of MMP-1 and MMP-13 in the experimental group were lower than that in the control group (P<0.01), but still higher than that of the normal group (P<0.01), while the TIMP-1 expression level of the experimental group was higher than that in the control and the normal group (P<0.01).
Western blot analysis of MMP-1, MMP-13 and TIMP-1
The results (Figure 2) showed that the protein expression of MMP-1 and MMP-13 of the control group in articular cartilage of rabbit 's knee joint were higher than that of the normal group (P<0.01), whereas the expression of TIMP-1 was lower than that of the normal group (P<0.01). The protein expression levels of MMP-1 and MMP-13 in experimental groups were lower than the control group (P<0.01), while the expression of TIMP-1 was higher than that of the control group (P <0.01).
Figure 2.
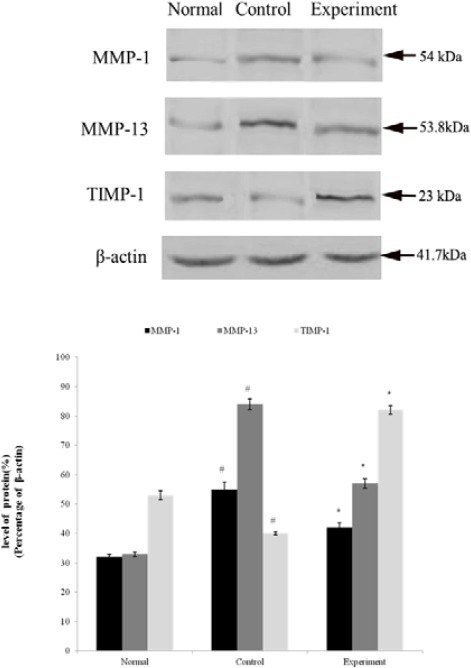
Western blot results of matrix metalloproteinase (MMP-1), MMP-13 and tissue inhibitor of metalloproteinase (TIMP)-1 (at 12 weeks)
#: compared with normal group, P < 0.01. *: compared with control group, P< 0.01
Discussion
OA is the most common form of arthritis, which affects more than 20 million people in the United States, and the number of newly diagnosed cases is increased with years (17). The exact pathogenesis of OA is unclear and needs to be elucidated. In this study, the impact of estradiol on the changes of PG in the OA model of rabbits (established by ACLT) was discussed. In the control group, GAG and HA were considerably lower with the extension of sampling time in comparison with that in the experimental and control group. The followed real-time PCR and Western blot analysis were used to study MMP1, MMP13 and TIMP-1 expression in the model. In the control group, the mRNA and protein level of MMP-1 and MMP-13 was improved compared to that in the normal group, whereas the level of TIMP-1 was decreased.
Besides, in the experimental group, the mRNA and protein level of MMP-1 and MMP-13 was lower and the TIMP-1 was higher than that of the control group. However, the expression levels of both MMP-1 and MMP-13 were higher than that in the normal group. These results implied the effect of estradiol on PG and MMPs/TIMPs in OA model of rabbits.
The degradation of PG and collagen fibers is the main physiological and pathological basis of the occurrence of OA (18, 19). MMPs are thought to be involved in the process of the ECM degradation of articular cartilage (20). It is now widely acknowledged that, OA is caused by the significant imbalance of synthesis and degradation of cartilaginous ECM (21). In addition, PG is made by a core protein, wherein the GAG side chains are covalently bound to this protein and its monomers can further link to the HA non-covalently to form larger aggregates (22). GAG is the decisive functional group and the main part of PG (90% by weight).
Studies suggest that GAG and HA can be referenced for the conversion of PG, since that cartilage GAG content may indirectly reflect the levels of PG (23). MMPs or TIMPs act directly or indirectly on synovial joints and a variety of components of adjacent cells and then result in an abnormal increase of PG metabolism, more decomposition than synthesis of PG, the considerable loss of PG and the destruction of cartilage and subchondral bone (24). Early studies have shown that the action of MMPs was the strongest in the initial stage of cartilage degeneration, which gradually weaken in the middle and late stage. The reason that MMPs expression does not enhance with the increased cartilage degeneration is unclear, which may be related to the distinct regulatory pathways. MMPs and TIMPs can be clustered by the binding of a 1:1 ratio to block the degradation of the former, which is the most important regulation of the local activities of MMPs in tissues (25). Epidemiological studies show that estrogen can slow down the degeneration of articular cartilage (26) and decrease the risk of OA (27). It is reported that MMPs mRNA expression and cytokines mentioned above are significantly inhibited by estrogen. Thereby, PG degradation is indirectly restrained (28). Therefore, estradiol may abnormally increase PG metabolism with the more decomposition than synthesis by increasing or reducing the expression of TIMPs or MMPs, which eventually results in the considerable loss of PG and the cartilage destruction. The experimental results showed that GAG content in different periods of the control group was significantly decreased compared with that in the normal and the experimental group, while the GAG content in other two groups were close to each other. With the progress of OA, the GAG content of the control group was gradually decreased; meanwhile, there were no significant changes in the experimental and the control group. These results were in accordance with other reported results (9, 10, 29). Though the reason that why the level of TIMP-1 in experimental group was much higher than the normal group was not clear, and all these still indicated that exogenous estrogen may have a protective effect by inhibiting the degradation of GAG, delaying the process of OA, and reducing the pathological damage of OA to protect the cartilage and bone.
Conclusion
Since it is difficult to obtain primary degeneration specimens of human cartilage, animal models were established to study the pathological process of cartilage degeneration. In our study, the MMP-1 and MMP-13 expression of OA rabbit models was reduced by estradiol to a certain extent. It implied the effect of intervention on the occurrence of OA. Additionally, exogenesis estradiol may also suppress the occurrence of OA through inhibiting the decomposition of PG. These results implied the probable role of estradiol in the pathological process of OA, which led to a more clear understanding of OA and may help to identify the direction of OA treatment. However, some limitations including the lack of ovariectomized rabbit model of OA, comparison with the OA extent, and the only one dose of estrogen were existed. Therefore, additional studies and clinical researches are needed to clarify these results.
Conflict of interest
All authors declare that they have no conflict of interest to state.
References
- 1.Attur M, Samuels J, Krasnokutsky S, Abramson SB. Targeting the synovial tissue for treating osteoarthritis (OA): where is the evidence? Best Pract Res Clin Rheumatol. 2010;24:71–79. doi: 10.1016/j.berh.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Spector T, Nandra D, Hart D, Doyle D. Is hormone replacement therapy protective for hand and knee osteoarthritis in women?: The Chingford Study. Ann Rheum Dis. 1997;56:432–434. doi: 10.1136/ard.56.7.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldring MB, Berenbaum F. The regulation of chondrocyte function by proinflammatory mediators: prostaglandins and nitric oxide. Clic Orthop Relat R. 2004;427:S37–46. doi: 10.1097/01.blo.0000144484.69656.e4. [DOI] [PubMed] [Google Scholar]
- 4.Kitazawa R, Kimble R, Vannice J, Kung V, Pacifici R. Interleukin-1 receptor antagonist and tumor necrosis factor binding protein decrease osteoclast formation and bone resorption in ovariectomized mice. J Clin Invest. 1994;94:2397–2406. doi: 10.1172/JCI117606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanko L, Søndergaard B-C, Oestergaard S, Karsdal M, Christiansen C. An update review of cellular mechanisms conferring the indirect and direct effects of estrogen on articular cartilage. Climacteric. 2008;11:4–16. doi: 10.1080/13697130701857639. [DOI] [PubMed] [Google Scholar]
- 6.Felson DT, Kim YJ. The futility of current approaches to chondroprotection. Arthritis Rheum. 2007;56:1378–1383. doi: 10.1002/art.22526. [DOI] [PubMed] [Google Scholar]
- 7.Hayami T, Pickarski M, Wesolowski GA, Mclane J, Bone A, Destefano J, et al. The role of subchondral bone remodeling in osteoarthritis: reduction of cartilage degeneration and prevention of osteophyte formation by alendronate in the rat anterior cruciate ligament transection model. Arthritis Rheum. 2004;50:1193–1206. doi: 10.1002/art.20124. [DOI] [PubMed] [Google Scholar]
- 8.Felson DT. Epidemiology of hip and knee osteoarthritis. Epidemiol Rev. 1988;10:1–28. doi: 10.1093/oxfordjournals.epirev.a036019. [DOI] [PubMed] [Google Scholar]
- 9.Claassen H, Steffen R, Hassenpflug J, Varoga D, Wruck CJ, Brandenburg LO, et al. 17β-estradiol reduces expression of MMP-1,-3, and-13 in human primary articular chondrocytes from female patients cultured in a three dimensional alginate system. Cell Tissue Res. 2010;342:283–293. doi: 10.1007/s00441-010-1062-9. [DOI] [PubMed] [Google Scholar]
- 10.Lee YJ, Lee EB, Kwon YE, Lee JJ, Cho WS, Kim HA, et al. Effect of estrogen on the expression of matrix metalloproteinase (MMP)-1, MMP-3, and MMP-13 and tissue inhibitor of metalloproternase-1 in osteoarthritis chondrocytes. Rheumatol Int. 2003;23:282–288. doi: 10.1007/s00296-003-0312-5. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Lang W, Ye H, Yu F, Li H, Chen J, et al. Tougu Xiaotong capsule inhibits the tidemark replication and cartilage degradation of papain-induced osteoarthritis by the regulation of chondrocyte autophagy. Int J Mol Med. 2013;31:1349–1356. doi: 10.3892/ijmm.2013.1341. [DOI] [PubMed] [Google Scholar]
- 12.Adatia A, Rainsford K, Kean WF. Osteoarthritis of the knee and hip. Part I: aetiology and pathogenesis as a basis for pharmacotherapy. J Pharm Pharmacol. 2012;64:617–625. doi: 10.1111/j.2042-7158.2012.01458.x. [DOI] [PubMed] [Google Scholar]
- 13.Hurst R. Structure, function, and pathology of proteoglycans and glycosaminoglycans in the urinary tract. World J Urol. 1994;12:3–10. doi: 10.1007/BF00182044. [DOI] [PubMed] [Google Scholar]
- 14.Chan F, Choi H, Underhill C. Hyaluronan and chondroitin sulfate proteoglycans are colocalized to the ciliary zonule of the rat eye: a histochemical and immunocytochemical study. Histochem Cell Biol. 1997;107:289–301. doi: 10.1007/s004180050114. [DOI] [PubMed] [Google Scholar]
- 15.Yang J, Lü J, Shen X. Effects of estrogen on the changes of proteoglycan in articular cartilage matrix of rabbits with osteoarthritis [J] J Xi’an Jiaotong Univ. 2004;25:584–586. [Google Scholar]
- 16.Song Y, Wu Z, Lin S, Weng X, Qiu G. The effect of estrogen and progestin on the expression of matrix metalloproteinases, tissue inhibitor of metalloproteinase and interleukin-1beta mRNA in synovia of OA rabbit model. Natl Med J China. 2003;83:498–503. [PubMed] [Google Scholar]
- 17.Ashford S, Williard J. Osteoarthritis: A review. Nurse Pract. 2014;39:1–8. doi: 10.1097/01.NPR.0000445886.71205.c4. [DOI] [PubMed] [Google Scholar]
- 18.Grenier S, Bhargava MM, Torzilli PA. An in vitro model for the pathological degradation of articular cartilage in osteoarthritis. J Biomech. 2014;47:645–652. doi: 10.1016/j.jbiomech.2013.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heinegård D, Saxne T. The role of the cartilage matrix in osteoarthritis. Nat Rev Rheumatol. 2011;7:50–56. doi: 10.1038/nrrheum.2010.198. [DOI] [PubMed] [Google Scholar]
- 20.Giacalone PL, Daurés JP, Faure JM, Boulot P, Hedon B, Laffargue F. The effects of mifepristone on uterine sensitivity to oxytocin and on fetal heart rate patterns. Eur J Obstet Gynecol Reprod Biol. 2001;97:30–34. doi: 10.1016/s0301-2115(00)00506-6. [DOI] [PubMed] [Google Scholar]
- 21.Wu H, Du J, Hu Y, Liu W, Fu G, Zhang J. Expression of MMP-1 MMP-13 and TlMP-1 mRNA in cartilage and synovium of experimentally induced rabbit ACLT osteoarthritis [J] Chin J Rheumatol. 2002;6:169–173. [Google Scholar]
- 22.Taskiran D, Stefanovicracic M, Georgescu H, Evans C. Nitric-oxide mediates suppression of cartilage proteoglycan synthesis by interleukin-1. Biochem Biophys Res Commun. 1994;200:142–148. doi: 10.1006/bbrc.1994.1426. [DOI] [PubMed] [Google Scholar]
- 23.Pottenger LA, Webb JE, Lyon NB. Kinetics of extraction of proteoglycans from human cartilage. Arthritis Rheum. 1985;28:323–330. doi: 10.1002/art.1780280313. [DOI] [PubMed] [Google Scholar]
- 24.Loeser RF. Chondrocyte integrin expression and function. Biorheology. 2000;37:109–116. [PubMed] [Google Scholar]
- 25.Rhee JS, Diaz R, Korets L, Hodgson JG, Coussens LM. TIMP-1 alters susceptibility to carcinogenesis. Cancer Res. 2004;64:952–961. doi: 10.1158/0008-5472.can-03-2445. [DOI] [PubMed] [Google Scholar]
- 26.Sniekers Y, Weinans H, Bierma-Zeinstra S, Van Leeuwen J, Van Osch G. Animal models for osteoarthritis: the effect of ovariectomy and estrogen treatment–a systematic approach. Osteoarthritis Cartilage. 2008;16:533–541. doi: 10.1016/j.joca.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Imgenberg J, Rolauffs B, Grodzinsky A, Schünke M, Kurz B. Estrogen reduces mechanical injury-related cell death and proteoglycan degradation in mature articular cartilage independent of the presence of the superficial zone tissue. Osteoarthritis Cartilage. 2013;21:1738–1745. doi: 10.1016/j.joca.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Hannum CH, Wilcox CJ, Arend WP, Joslin FG, Dripps DJ, Heimdal PL, et al. Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor. Nature. 1990;343:336–340. doi: 10.1038/343336a0. [DOI] [PubMed] [Google Scholar]
- 29.Parker DA, Beatty KT, Giuffre B, Scholes CJ, Coolican MR. Articular cartilage changes in patients with osteoarthritis after osteotomy. Am J Sport Med. 2011;39:1039–1045. doi: 10.1177/0363546510392702. [DOI] [PubMed] [Google Scholar]


