Abstract
Objective(s):
Recently cell therapy is a promising therapeutic modality for many types of disease including acute kidney injury (AKI). Due to the unique biological properties, mesenchymal stem cells (MSCs) are attractive cells in this regard. This study aims to transplant MSCs equipped with nuclear factor E2-related factor 2 (Nrf2) in rat experimental models of acute kidney and evaluate regeneration potential of injured kidney especially expression of injury and repaired biomarkers.
Materials and methods:
Nrf2 was overexpressed in bone marrow-derived MSCs by pcDNA.3.1 plasmid. AKI was induced using glycerol in rat models. The regenerative potential of Nrf2-overexpressed MSCs was evaluated in AKI-Induced animal models using biochemical and histological methods after transplantation. Expression of repaired genes, AQP1 and CK-18, as well as injury markers, Kim-1 and Cystatin C, was also assayed in engrafted kidney sections.
Results:
Our results revealed that transplantation of Nrf2-overexpressed MSCs into AKI-induced rats decreased blood urea nitrogen and creatinine and ameliorated kidney regeneration throughout 14 days. Upregulation of repaired markers and downregulation of injury markers were considerable 14 days after transplantation.
Conclusions:
Overexpression of Nrf2 in MSCs suggests a new strategy to increase efficiency of MSC-based cell therapy in AKI.
Keywords: Acute kidney injury, AQP1, Kim-1, Mesenchymal stem cells, Nrf2
Introduction
Acute kidney injury (AKI) is the state of abrupt decrease in renal filtration function that leads to the accumulation of nitrogenous wastes in blood and loss of fluid, electrolyte and acid-base balance. In developed countries, it is estimated that 15% of adults admitted to hospitals develop AKI (1). AKI-associated mortality is estimated as 50% in hospitalized patients, and 70-80% in patients under intensive care (2).
The causes of acute renal failure are complex. Apoptosis, necrosis, inflammation and oxidative stress are the cellular and molecular events that lead to the end stage renal failure. Many risk factors have also been identified for the development of AKI including hypotension, pulmonary disease, liver failure, sepsis, hypovolemia, increased age, hypertension, pre-existing renal disease, some hyperplasias, hypercalcemia, impaired cardiac output or heart failure, chemotherapy insults and many other medical conditions (3, 4).
Currently, no available therapeutic options exist for decreasing the mortality rate caused by AKI. Kidney transplant is an alternate option but it is limited by organ transplantation problems. It is, therefore, very important to find other therapeutic options to restore renal function (5). In recent years, the use of mesenchymal stem cells (MSCs) is considered as a promising tool for cell therapy of AKI. Several in vitro and animal studies showed that MSCs can restore renal function after induced kidney injury (6-8). The effects of MSCs in tissue regeneration tie with trans-differentiation of MSCs to organ specific cells and secretion of soluble factors that improve cell survival and restore damaged tissue.
Furthermore, clinical studies in phase I and II assess the safety and efficacy of MSCs in patients with AKI (7, 9, 10). This preliminary data on safety and potential role of MSCs in the treatment of acute renal failure together with intrinsic properties of MSCs obtained in large numbers from various sources and their capacity to differentiate into other cell types as well as low immunogenesity make them a reliable candidate for the treatment of acute renal failure (11). However, low viability of MSCs hampers their application in clinical trials. Hence, equipping of MSCs against various stressful conditions might enhance their therapeutic potentialities (12). In this regard, overexpression of the nuclear factor erythroid-2 related factor 2 (Nrf2) in MSCs protects them against cell death and apoptosis which is triggered by hypoxic and oxidative stress conditions (13).
Nrf2 is a transcription factor that binds to the promoter region of several detoxification and antioxidant genes and upregulate the expression of these genes to protect cells against oxidative stress-induced injuries in the cells. It has been shown that Nrf2 protects different cell types and organs from toxic agents and pathogens (14, 15). Also, Nrf2 acts as a major factor to ameliorate oxidative stress and inflammation in renal injuries (16, 17).
In this study, we investigated whether Nrf2 overexpression in MSCs shows higher protective effects especially in terms of expression of molecular injuries/repairs markers in glycerol-induced AKI models of rats than non-recombinant MSCs.
Materials and Methods
Isolation of MSCs and cell culture
MSCs were prepared from rat bone marrow (4–6 weeks old) as described previously (18). Then, the cells were cultured in culture medium containing dulbecco's modified eagle's medium-low glucose (Gibco, USA) supplemented with 10% fetal bovine serum (Gibco, USA), 1% penicillin and streptomycin (Gibco, USA). Culture flasks were kept at 37°C in a humidified atmosphere containing 5% CO2. Fresh complete medium was replaced every 2 or 3 days. In order to characterize the ex vivo-expanded MSCs, they were analyzed by flowcytometry for the expression of surface markers as described previously (18).
Nrf2 expression plasmid construction and MSCs transfection
To construct the pcDNA 3.1-Nrf2 recombinant vector, the full-length human Nrf2 cDNA was amplified through RT-PCR using specific forward 5’ -CACCATGGGAATGGACTTGGAGCTGCC-3’ and reverse 5’ CTAGTTTTTCTTAACATCTGGCTTCTTAC-3’ primers. Two restriction sites EcoRI and BamHI were added at both end of the gene respectively. The Nrf2 gene was cloned into the EcoRI and BamHI restriction sites of the mammalian expression vector pcDNA3.1 (Invitrogen, USA). DNA sequencing was performed to verify the fidelity of cloning. MSCs were transfected with pcDNA 3.1-Nrf2 plasmid by FuGENE HD (Roche, Germany) transfection reagent following the manufacturer's protocol. The expression level of Nrf2 was evaluated by RT-PCR, and Western blot analysis.
RT-PCR and Western blot analysis
To assess the expression of Nrf2 by reverse transcriptase polymerase chain reaction (RT-PCR), total RNA was isolated from both recombinant pcDNA 3.1-Nrf2 t transfected-MSCs (MSC-Nrf2) and normal MSCs using TriPure reagent (Roche, Germany). Then, the total RNA was used to construct cDNA using first strand cDNA synthesis superscript kit (Invitrogen, USA) according to the manufacturer's protocol. PCR was performed using internal primers forward:
5’ GCGACGGAAAGAGTATGAGCTGG3’ and reverse: 5’ GTTGGCAGATCCACTGGTTTCTG3’.
Western blot analysis was performed to detect the expression of Nrf2 protein. Samples were run on 10% SDS-polyacrylamide gel, and then transblotted onto the PVDF membrane (Roche, Germany) followed by blocking and 3 hr incubation at 4 °C with specific primary antibodies, i.e., anti-β-actin (Sigma, USA) or anti-Nrf2 antibodies (Santa Cruz, USA). Afterwards, the membranes were washed with tris-buffered saline containing 0.1% Tween 20 and incubated with secondary horse raddish peroxidase-conjugated antibody (Sigma, USA). Finally, chemiluminescence solution was used to visualize the expected protein bands on the PVDF membrane.
Induction of experimental models of AKI in rat
Animal experiments were conducted in accordance with the NIH guide for the care and use of laboratory animals. All protocols were conducted in accordance with the ethic committee of Iranian Blood Transfusion Organization (IBTO). Male 6-week-old Rattus norvegicus weighing 160-180 grams (g) were fed with normal chow and also allowed to free excess of water. AKI was induced by intramuscular injection of 5, 8, 10, 13 and 15 ml/Kg glycerol (50%) (Sigma, USA). After 24 hr, water-deprived animals were anesthetized and injected with different doses of glycerol into the left and right hind femoral muscles. Injection of glycerol induced kidney injury was evaluated by analyzing the levels of blood urea nitrogen (BUN) and serum creatinine (SCr) along with histological analysis of the kidney sections for interval times (24, 48 and 72 hr)
MSCs transplantation
The rats were divided into five groups: i) AKI group (n = 10), ii) AKI/PBS group which were AKI-induced rats received PBS (n=10), iii) AKI/MSC group which were AKI-induced rats received normal MSCs (n=10), iv) AKI/MSC-V group which were AKI-induced rats received transfected-MSCs with empty pcDNA3.1 vector (MSC-V) (n = 10) and v) AKI/MSC-Nrf2 group which were AKI-induced rats received transfected-MSCs with recombinant pcDNA3.1-Nrf2 vector (MSC-Nrf2) (n=10). Normal rats which received PBS were considered as control (Cont.).
MSC-Nrf2, MSC-V and MSCs were administered to AKI-induced rats 24 hr after induction of AKI. 1.5×106 of these mentioned cells were suspended in 300 µl of PBS, and then intravenously injected to the rats. Blood samples were collected from rats to determine the level of BUN and SCr on the 3th, 7th and 14st days (19). After 14 days, rats were sacrificed according to the ethical principles for animal research, and the kidney tissues were collected to go through RT-PCR and histological techniques.
Renal function analysis
To analyze the renal function of AKI-induced and transplanted rats, blood samples were collected from the intracardiac punctures of anesthetized rats after induction of AKI and cell therapy. The animals were anesthetized using ketamine (100 mg/kg) and xylazine (10 mg/kg). The levels of BUN and SCr were measured with BUN and SCr assay kits (Biosystem, Spain) using an automatic analyzer (BT 3000 PL/S, Italy).
Histological analysis of kidney tissue
Kidney tissues were fixed in 4% paraformaldehyde, dehydrated in graded concentrations of alcohols, and then embedded in paraffin. Next, the tissues were cut into 5-μm sections and stained with hematoxilin and eosin (Sigma, USA). Luminal hyaline casts and tubular necrosis (cell debris in tubular lumen, denudation of tubular basement membrane and nuclear fragmentation) were assessed and scored according to six levels based on the percentage of tubule affection under a high-power field (HPF) using a light microscope.
RT-PCR and real-time RT-PCR analysis of kidney sections
To evaluate the effects of MSCs transplantation on the expression of renal biomarkers in kidney tissues, RNA was extracted from renal tissue and cDNA was synthesized. RT-PCR was performed to evaluate expression of repaired genes: aquaporin 1 (AQP1) and cytokeratin-18 (CK-18); injury biomarkers: kidney injury molecule-1 (Kim-1) and Cystatin C. Quantitative real-time RT-PCR was also performed using SYBR green master mix (Takara, Japan). Reactions were performed in triplicate with Rotor-gene RG-3000 (Corbett, Germany), the specificity was monitored using melting curve analysis after cycling and the ΔCt was calculated automatically. β-actin was used as normalizer.
Results
Characterization of MSCs
MSCs were isolated from bone marrow through their adhesion to tissue culture surfaces. In order to evaluate the rat MSCs phenotype, cell-surface antigens were analyzed by flow cytometry. These cells revealed the typical cell-surface antigens of MSCs, and were positive for CD44, CD105and CD29 antigens, while they were negative for hematopoietic antigens i.e., CD45 and CD34 (18).
Nrf2 was overexpressed in MSC-Nrf2
Three days post-transfection of the MSCs with the recombinant pCDNA3.1-Nrf2 plasmid, the over expression of Nrf2 gene was analysed by RT-PCR. In contrast to the normal MSCs which revealed no Nrf2 mRNA expression, over expression of Nrf2 was detected in MSC-Nrf2 that transfected with pCDNA3.1-Nrf2 (Figure 1A). The overexpression of the Nrf2 protein was also detected in these cells by western blotting, while no detectable amount of Nrf2 protein was found in normal MSCs (Figure 1B).
Figure 1.
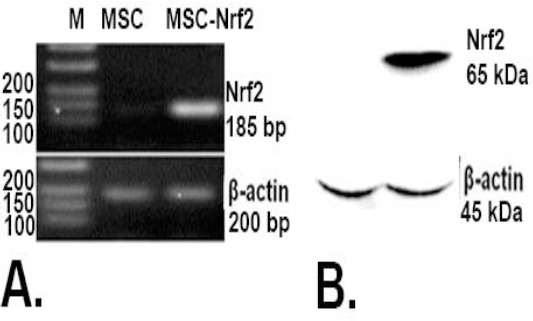
Overexpression of Nrf2 in transfected MSCs with the recombinant vector Nrf2-pcDNA 3.1; A. RT-PCR analysis of MSCs 72 hr after transfection. MSCs transfected with Nrf2-pcDNA 3.1 (MSC-Nrf2) expressed Nrf2, but detectable expression of Nrf2 was not observed in non-manipulated MSCs as the control group. β-actin was used for normalization; B. Western blot analysis of transfected cell. MSC-Nrf2 expressed Nrf2 protein
Glycerol induced AKI in rat experimental models
Intramuscular administration of different doses of glycerol (50%) caused AKI in rats. Elevations in BUN was considerable 24 hr after injection of 8 ml/Kg of glycerol in comparison to control that no received any glycerol (400±10 mg/dl, versus 21±6 mg/dl, p≤0.001) (Figure 2A). SCr level was also significantly increased in 8 ml/Kg glycerol-received rats (4.5±0.2 mg/dl) in compared with control (0.3 ± 0.1 mg/dl, P≤0.001) (Figure 2B).
Figure 2.
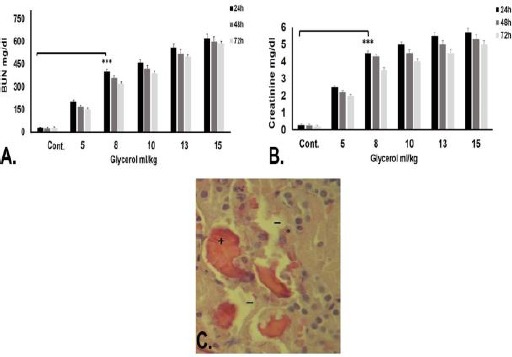
Confirmation of acute kidney injury (AKI) induction in rat models. A. Biochemical analysis of blood urea nitrogen (BUN) 24, 48 and 72 hr after intramuscular injection of different glycerol doses. B. Biochemical analysis of serum creatinine (SCr) 24, 48 and 72 hr after intramuscular injection of different glycerol doses. BUN and SCr were significantly higher in 8 ml/Kg glycerol-received rats for 24 hr in comparison with control (those without any injection) (*** P≤0. 001). C. Histological analysis of kidney tissue 24 hr after intramuscular injection of 8 ml/kg glycerol. Tubular necrosis (-), casts (+), and loss of brush border (*) indicated kidney injury in glycerol-received rates. Considering the fact that Injection of high glycerol doses (10, 13, and 15 ml/Kg) led to more death in rat models, 8 ml/Kg of glycerol was selected as the optimized AKI-inducing dose
Histological analysis of the kidney sections verified the tubular necrosis, casts, and loss of brush border 24 hr after injection of 8 ml/kg glycerol (Figure 2C).
Administration of 10, 13 and 15 ml/kg glycerol led to death of the rats after 72 hr. Therefore, 8 ml/kg of glycerol after 24 hr was considered the optimized dose and time for induction of AKI in rats.
MSC-Nrf2 transplantation improves renal function in glycerol-induced AKI rats
To investigate the efficacy of MSC transplantation in improving renal function, the BUN and SCr concentrations was evaluated in MSC and MSC-Nrf2-received groups (AKI/MSC and AKI/MSC-Nrf2) as well as AKI/PBS group which received PBS (sham group) after 3, 7 and 14 days along with controls.
On days 7th and 14th after cell therapy, the BUN and SCr levels decreased in cell therapy groups. The BUN levels of AKI/MSC-Nrf2 which transplanted with Nrf2 overexpressed-MSCs were decreased significantly compared to that of AKI/MSC which transplanted with normal MSCs during 14 days after transplantation (P≤0.01 and p≤0.001) (Figure 3A). Interestingly, over-expression of Nrf2 in MSCs increased the protective effect of MSCs, and the levels of SCr dropped off more in rats treated by MSC-Nrf2 in comparison to those of transplanted with MSCs (p≤0.05 and p≤0.01) (Figure 3B). These results verify the role of Nrf2 over-expression to improve efficacy of MSC cell therapy for improvement of renal function in glycerol-induced renal injury.
Figure 3.
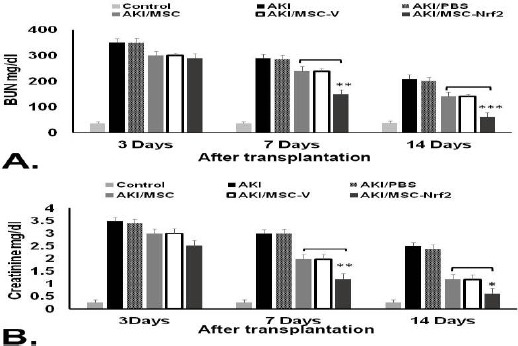
Evaluation of MSCs therapeutic potentialities in AKI-induced rats using biochemical analysis. Serum samples were collected 3, 7, and 14 days after transplantation of different MSC groups in AKI-induced rats. A. BUN serum level significantly dropped off in AKI/MSC-Nrf2 group transplanted with MSC-Nrf2 in comparison to those transplanted with MSCs (AKI/MSC). B. SCr serum level was also much lower in AKI/MSC-Nrf2 compared to other groups. AKI: AKI-induced rats without any injection, AKI/MSC-V: AKI-induced rats transplanted with empty vector transfected-MSCs, AKI/PBS: AKI-induced rats injected with PBS, Control: normal rat with no treatment (*** P≤0. 001, ** P≤0. 01 and * P≤0.05)
Nrf2 increase the re-construction potential of MSCs in animal models of AKI
Kidney tissue was collected on days 14th after cell therapy. Histological analysis of the kidney sections verified that MSC and MSC-Nrf2-treated groups were recovering in comparison with the group receiving PBS. The degree of micro aggregate-like degeneration and necrosis of renal tubular epithelial cells were lower in AKI/MSC and AKI/MSC-Nrf2 rats compared with the AKI group (Figure 4 A, B and C). Next, to evaluate the effects of MSCs on the proliferation of tubular cells in AKI models, the number of casts and tubular necrosis were estimated per field. In AKI/MSC and AKI/MSC-Nrf2 groups, the number of both the cast and tubular necrosis was lower than those of AKI group (Figure 4D).
Figure 4.
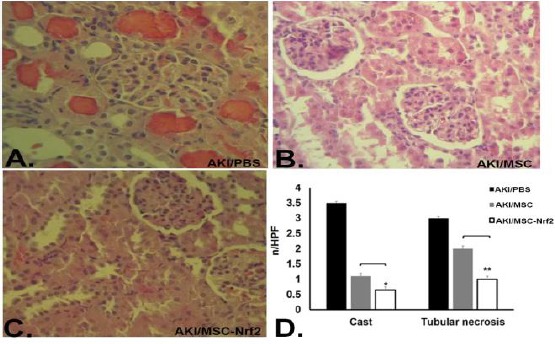
Evaluation of MSCs therapeutic potentialities in AKI-induced rats using histological analysis. Kidney sections were prepared to assess kidney regeneration 14 days after transplantation of different MSCs groups in AKI-induced rats. A. AKI/PBS group transplanted with PBS showed more casts and tubular necrosis. B. AKI/MSC group transplanted with normal MSCs indicated partial kidney regeneration. C. Rare casts and tubular necrosis were observed in AKI/MSC-Nrf2 group that was transplanted with Nrf2 overexpressed MSCs. D. Quantification of histological analysis results. Casts and tubular necrosis significantly decreased in AKI/MSC-Nrf2 in comparison to AKI/MSC group (** P≤0. 01 and * P≤0.05)
However, in AKI/MSC-Nrf2 group, the tubular cells show higher proliferation rate, and the number of casts decreased significantly over 14 days (P≤0.05 and P≤0.01) (Figure 4D). These results indicate the successful engraftment of MSCs in injured kidneys that decrease the severity of injuries and repair the damaged tissue, and also more improvement in AKI/MSC-Nrf2 group confirmed the successful function and resistance of Nrf2-MSCs.
MSC-Nrf2-based cell therapy increases repaired genes and decreases injury biomarkers in AKI
The expression of AQP1, CK-18 as repaired genes and Kim-1 and Cystatin C as injury biomarkers was assayed by RT-PCR. Two weeks after cell therapy, the results of RT-PCR revealed low expression of AQP1 and CK-18 in AKI, AKI/MSCs and AKI/MSC-V groups. However, rats engrafted with MSC-Nrf2 indicated a high level of both AQP1 and CK-18 expressions. Conversely, the expression of Kim-1 and Cystatin C enhanced in AKI group (Figure 5A). This finding was further confirmed by real-time RT-PCR analysis. Expression of AQP1, CK-18 was significantly up-regulated in AKI/MSC-Nrf2 (P≤0.05) (Figure 5B). Engraftment of MSC-Nrf2 into AKI-induced rat models down-regulated the expression levels of injury biomarkers in AKI/MSC-Nrf2 in comparison to AKI/MSC groups (P≤0.001) (Figure 5C). Taken together, the increase in repaired genes and parallel decrease in injury biomarkers of AKI/MSC-Nrf2 groups indicate the beneficial role of Nrf2 in the regenerative process.
Figure 5.
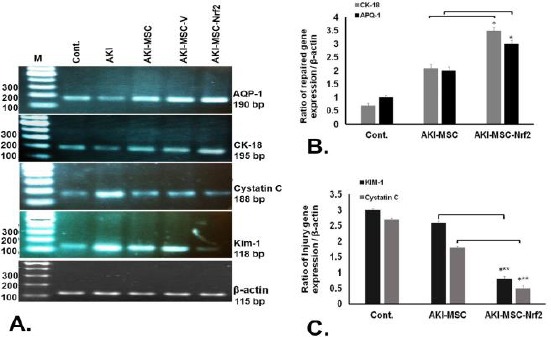
Evaluation of biomarkers of kidney injury in different rats 14 days after cell therapy. Expression of AQP1 and CK-18 (as repaired genes) and Kim-1 and Cystatin C (as injury biomarkers) was assayed in transplanted rats along with control group. A. RT-PCR analysis results indicated that AKI/MSC-Nrf2 up-regulated AQP1, CK-18 and down- regulated Kim-1, Cystatin C in comparison with other groups. B. Quantitative real time RT-PCR analysis of the repaired genes in different transplanted rats. Expression of AQP1 and CK-18 was significantly higher in AKI/MSC-Nrf2 than that of in AKI/MSC. C. Quantitative real time RT-PCR analysis of injury biomarkers in different transplanted rats. Expression of Kim-1 and Cystatin C was down regulated in AKI/MSC-Nrf2 (*** P≤0. 001 and * P≤0.05)
Discussion
Ischemia, nephrotoxic insults and hypoxia are main underlying causes contributing to AKI, a condition in which renal cells are damaged, and finally results in rapid decrease in glomerular filtration rate. AKI is associated with unacceptably high mortality rate (2-4). No effective therapeutic option has been identified for AKI, and supportive measures had no chance in decreasing the mortality rates. Therefore, finding new therapeutic options to manage AKI is of critical importance. In recent years, mesenchymal stem cells (MSC) are considered as a promising therapeutic option for AKI. Many studies indicate the potential role of MSCs in inducing regeneration responses that result in tissue repair and restoring renal function after AKI (6-8).
In current study, we up-regulated Nrf2, one of the main transcription factors of cell antioxidant defense, in MSCs at transcriptional as well as translational level using transfection reagent that decrease the concerns about using of viral vector in preclinical and clinical studies. Nuclear factor-erythroid-2-related factor 2 (Nrf2) plays a central role in natural antioxidant defense system that controls the induction of several antioxidant and detoxifying proteins such as catalase, SOD, NAD(P) H quinone oxidoreductase-1 (NQO1), heme oxygenase-1(HO-1), glutamate cysteine ligase, glutathione S-transferase, glutathione peroxidase and thioredoxin (20). Therefore, Nrf2 plays a central role in the regulation of cellular antioxidant and anti-inflammatory responses and defend against oxidative stress (21).
We induced AKI in rat after injection of 8 ml/kg of for 24 hr. Then, the therapeutic efficacy of these Nrf2-overexpressed-MSCs was assessed by measuring BUN and creatinine concentration and histological analysis in compared with proper controls. To further verify the results, we assessed the expression of other molecular biomarkers. Our result revealed that transplantation of Nrf2 overexpressed-MSCs increase the expression of repaired genes such as AQP-1 and CK-18, and simultaneously down-regulates the expression of injury biomarkers i.e., Kim-1 and Cystatin C. These mentioned genes are known as new biomarkers for determination of renal function or injury (22-24)
The therapeutic effects of MSCs is mainly through paracrine anti-apoptotic, mitogenic, anti-inflammatory and immune-modulating influences leading to promote epithelial proliferation, modulate inflammation, or enhance angiogenesis (25, 26). It has been shown that after MSC infusion, pro-inflammatory cytokines such as IL-6 and TNF-α decreased, and in parallel, anti-inflammatory cytokines such as IL-10 increased (27, 28). Furthermore, MSCs release a variety of growth factors such as vascular endothelial growth factor (VEGF), insulin-like growth factor-1 (IGF-1), hepatocyte growth factor (HGF) that help to tubular regeneration and renal recovery after AKI (27, 29).
Several studies investigated the mechanisms to make MSCs more resistant against the environment which they are supposed to be transplanted (12).
Mohammadzadeh et al (2015) reported that Nrf2-overexpressed MSCs ameliorated kidney regeneration in Cisplatin-AKI-induced rats. They used Gat way technique and transducted MSCs with adenovirus viral vector to over express Nrf2 in them. They evaluated therapeutic potentialities of manipulated-MSCs 11 days after transplantation using biochemical and pathological methods. They did not assay the expression of repaired genes such as AQP-1 and CK-18, and down-regulation of injury biomarkers, Kim-1 and Cystatin C (30).
Liu and colleagues administrated MSCs in combination with erythropoietin (EPO) to improve the repair effects of these cells in AKI-induced mice. This strategy resulted in fast reduction in serum BUN and creatinine along with pathological changes in tubular cells. They showed that the expression of CK-18 was significantly up regulated in the renal cell after transplantation of MSCs in combination with EPO (31).
In another study, Masoud et al enhanced in vitro proliferation and survival of MSCs by 6 hours-preconditioning with S-nitroso N-acetyl penicillamin. They transplanted these preconditioned-MSCs into ischemia–reperfusion-induced AKI rats. According to their results, the creatinine clearance and in vivo engraftment was improved and fibrosis was decreased due to enhanced homing of transplanted preconditioned-MSCs (5). Lee et al compared the effect of weekly (up to 5 weeks) administration of bone marrow-MSCs with the effect of once injection of these unique cells in restoration of injured kidney tissue. They indicated that urinary protein excretion, systolic blood pressure, and serum creatinine level obviously improved in remnant kidney injury after repeated MSCs injection (32). There are several studies that conducted to employ MSCs with or without any manipulation for treatment of kidney diseases. Reported results might be various because of different methodology.
Conclusion
In summary, we showed that MSC-Nrf2 cell therapy improves renal function and regeneration following glycerol-induced renal injury. Moreover, Nrf2 overexpression potentiates MSCs against stress-related environment in vivo.
Acknowledgment
We thank Mozhgan Dehghan Harati and Amaneh Mohammadi Roushandeh for their assistance. This study was supported by Islamic Azad University, Arak Branch, grant entitled
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Ftouh S, Thomas M. Acute Kidney Injury Guideline Development G. Acute kidney injury: summary of NICE guidance. BMJ. 2013;347:4930–4939. doi: 10.1136/bmj.f4930. [DOI] [PubMed] [Google Scholar]
- 2.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. JASN. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 3.Basile DP, Anderson MD, Sutton TA. Patho-physiology of acute kidney injury. Comprehensive Physiology. 2012;2:1303–1353. doi: 10.1002/cphy.c110041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silva GB, Junior, Daher Ede F, Mota RM, Menezes FA. Risk factors for death among critically ill patients with acute renal failure. Sao Paulo Med J. 2006;124:257–263. doi: 10.1590/S1516-31802006000500004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masoud MS, Anwar SS, Afzal MZ, Mehmood A, Khan SN, Riazuddin S. Pre-conditioned mesenchymal stem cells ameliorate renal ischemic injury in rats by augmented survival and engraftment. J Transl Med. 2012;10:243–254. doi: 10.1186/1479-5876-10-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Humphreys BD, Bonventre JV. Mesenchymal stem cells in acute kidney injury. Ann Rev Med. 2008;59:311–325. doi: 10.1146/annurev.med.59.061506.154239. [DOI] [PubMed] [Google Scholar]
- 7.Bianchi F, Sala E, Donadei C, Capelli I, La Manna G. Potential advantages of acute kidney injury management by mesenchymal stem cells. World J Stem Cell. 2014;6:644–650. doi: 10.4252/wjsc.v6.i5.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morigi M, Introna M, Imberti B, Corna D, Abbate M, Rota C, et al. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cell. 2008;26:2075–2082. doi: 10.1634/stemcells.2007-0795. [DOI] [PubMed] [Google Scholar]
- 9.Doty J, Affleck D, Horne B, Muhlestein J, Psioda M, Paragamian V, et al. A phase 1 trial of human allogeneic mesenchymal stem cells for the prevention of acute kidney injury in cardiac surgery subjects. Am Soc Neph Kidney Week. 2011:8–13. [Google Scholar]
- 10.Westenfelder C, Togel FE. Protective actions of administered mesenchymal stem cells in acute kidney injury: relevance to clinical trials. Kidney Int Suppl. 2011;1:103–106. doi: 10.1038/kisup.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Togel F, Cohen A, Zhang P, Yang Y, Hu Z, Westenfelder C. Autologous and allogeneic marrow stromal cells are safe and effective for the treatment of acute kidney injury. Stem Cell Dev. 2009;18:475–485. doi: 10.1089/scd.2008.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amiri F, Jahanian-Najafabadi A, Roudkenar MH. In vitro augmentation of mesenchymal stem cells viability in stressful microenvironments. Cell Stress Chaperon. 2015;20:237–251. doi: 10.1007/s12192-014-0560-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohammadzadeh M, Halabian R, Gharehbaghian A, Amirizadeh N, Jahanian-Najafabadi A, Roushandeh AM, et al. Nrf-2 overexpression in mesenchymal stem cells reduces oxidative stress-induced apoptosis and cytotoxicity. Cell Stress Chaperone. 2012;17:553–565. doi: 10.1007/s12192-012-0331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JM, Li J, Johnson DA, Stein TD, Kraft AD, Calkins MJ, et al. Nrf2, a multi-organ protector? FASEB. 2005;19:1061–1066. doi: 10.1096/fj.04-2591hyp. [DOI] [PubMed] [Google Scholar]
- 15.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Ann Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 16.Liu M, Grigoryev DN, Crow MT, Haas M, Yamamoto M, Reddy SP, et al. Transcription factor Nrf2 is protective during ischemic and nephrotoxic acute kidney injury in mice. Kidney Int. 2009;76:277–285. doi: 10.1038/ki.2009.157. [DOI] [PubMed] [Google Scholar]
- 17.Ruiz S, Pergola PE, Zager RA, Vaziri ND. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013;83:1029–1041. doi: 10.1038/ki.2012.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halabian R, Tehrani HA, Jahanian-Najafabadi A, Habibi Roudkenar M. Lipocalin-2-mediated upregulation of various antioxidants and growth factors protects bone marrow-derived mesenchymal stem cells against unfavorable microenvironments. Cell Stress Chaperone. 2013;18:785–800. doi: 10.1007/s12192-013-0430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edelstein CL. Biomarkers of acute kidney injury. Adv Chronic Kidney Dis. 2008;15:222–234. doi: 10.1053/j.ackd.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li W, Khor TO, Xu C, Shen G, Jeong WS, Yu S, et al. Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem Pharmacol. 2008;76:1485–1489. doi: 10.1016/j.bcp.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernhardt WM, Campean V, Kany S, Jurgensen JS, Weidemann A, Warnecke C, et al. Preconditional activation of hypoxia-inducible factors ameliorates ischemic acute renal failure. JASN. 2006;17:1970–1978. doi: 10.1681/ASN.2005121302. [DOI] [PubMed] [Google Scholar]
- 22.Sohn SJ, Kim SY, Kim HS, Chun YJ, Han SY, Kim SH, et al. In vitro evaluation of biomarkers for cisplatin-induced nephrotoxicity using HK-2 human kidney epithelial cells. Toxicol Lett. 2013;217:235–242. doi: 10.1016/j.toxlet.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 23.Bonventre JV. Kidney injury molecule-1 (KIM-1): a urinary biomarker and much more. Nephrol Dial Transplant. 2009;24:3265–3268. doi: 10.1093/ndt/gfp010. [DOI] [PubMed] [Google Scholar]
- 24.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Ame J Kidney Dis. 2002;40:221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 25.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Ame J Physiol Renal Physiol. 2005;289:31–42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 26.Togel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Ame J Physiol Renal Physiol. 2007;292:1626–1635. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- 27.Abdel Aziz M, Wassef M, Rashed L, Mhfouz S, Omar N, Elsebaie MM. Mesenchymal stem cells therapy in acute renal failure: possible role of hepatocyte growth factor. J Stem Cell Res Ther. 2011;1:109–120. [Google Scholar]
- 28.Deng J, Kohda Y, Chiao H, Wang Y, Hu X, Hewitt SM, et al. Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney Int. 2001;60:2118–2128. doi: 10.1046/j.1523-1755.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- 29.Khan M, Akhtar S, Mohsin S, NK S, Riazuddin S. Growth factor preconditioning increases the function of diabetes-impaired mesenchymal stem cells. Stem Cell Dev. 2011;20:67–75. doi: 10.1089/scd.2009.0397. [DOI] [PubMed] [Google Scholar]
- 30.Mohammadzadeh-Vardin M, Habibi Roudkenar M, Jahanian-Najafabadi A. Adenovirus-mediated over-expression of Nrf2 within mesenchymal stem cells (MSCs) protected rats against acute kidney injury. Adv Pharm Bull. 2015;5:201–208. doi: 10.15171/apb.2015.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu N, Han G, Cheng J, Huang J, Tian J. Erythropoietin promotes the repair effect of acute kidney injury by bone-marrow mesenchymal stem cells transplantation. Exp Biol Med. 2013;238:678–686. doi: 10.1177/1535370213489486. [DOI] [PubMed] [Google Scholar]
- 32.Lee SR, Lee SH, Moon JY, Park JY, Lee D, Lim SJ, et al. Repeated administration of bone marrow-derived mesenchymal stem cells improved the protective effects on a remnant kidney model. Ren Fail. 2010;32:840–848. doi: 10.3109/0886022X.2010.494803. [DOI] [PubMed] [Google Scholar]


