Abstract
Objective (s):
Mucoadhesive disc is one of the various routes of drug delivery for curing buccal disease
Materials and Methods:
Every discs containing 70 mg stem bark extract of Ziziphus jujuba were formulated by using Carbopol 934, PVP k30 and gelatin as polymers. Discs were made by granulation and direct compression. Discs were standardized based on the total phenol. Properties such as in vitro and in vivo mucoadhesion, drug release, water uptake, and disintegration were carried out.
Results:
Discs showed excellent mucoadhesion and released high amount of the active ingredients (47%) immediately and completed after approximately the first hour. They had a good adhesion in buccal cavity.
Conclusion:
This study showed that the kinetics of release of the active substance from the mucoadhesive disc obeyed the zero order kinetic and didn’t follow the fick's law. The water uptake and dissolution (DS), increased with the passing of time.
Keywords: Carbopol 934, Mucoadhesive discs, Pharmaceutical tests, PVP(Polyvinylpyrrolidone), Ziziphus jujuba stem bark
Introduction
Amongst the various routes of drug delivery for curing buccal disease, natural or synthetic polymers that bind to biological surfaces are said to be bio-adhesive (bioadhetion). If the mucosa is a biological surface, it is said to be mucoadhesive. The delivery of drugs through the mucosa, increases the breadth and speed of access of drugs to the body. It causes increase in drug concentration at the site of contact, increases drug permeability, promotion of drug delivery and tissue is protected position (1-3). The mucoadhesive properties of these drugs are dependent on polymers. Mucoadhesive polymers are large macromolecules with majority of 100,000 daltons and contain the amine and carboxylic groups that would bind them. Most mucoadhesive polymers, first hydrate and swell and penetrate the mucus by changing the surface tension. However, in this area, there are different theories (4).
Meanwhile, since aphthous stomatitis is one of the problems of oral diseases and can be harassment for the patients, immediate treatment is needed. Based on Iranian traditional documents, buccal inflammatory disease is very important and a separate chapter is devoted to the topic of ulcers and could be searched as “Gholaa” (5-7). It is a kind of ulcer that affects the surface of the mouth and tongue and it can even exceed their inner parts. In the definition of wounds, it is in corrosive lesion groups and deep infectious ulcers (6).
Meantime, based on these topics and on traditional references, Ziziphus jujube was selected for its anti-inflammatory and antimicrobial effects (8). Local practitioners also use powder of stem bark of Jujube to cure Aphthous. Therefore, a decision was made to prepare a mocuadhesive disc from the stem bark extract of jujube. The strategy for designing is principally based on the utilization of mixture of polymers with suitable excipients such as Carbopol and PVP k30.
Z. jujuba (Family: Rhamnaceae) is distributed throughout the Eastern, South-eastern and Central parts of Iran. Its local name in Iran is “Annab” or “Onnab”. The tree is relatively tall and has oval leaves with three parallel leaf veins with thorns (9). Fruits of this plant are widely used in Iranian folk medicine as antitussive, laxative agent and blood pressure reducer (9, 10). Based on Iranian traditional medicine books (11, 12) local traditional healers used powders of stem bark and leaves of Jujube to cure wounds and oral wounds as Aphthous. In this study, mucoadhesive discs were designed containing stem bark extract of Z. jujuba. Another clinical study will be conducted soon to cure the Aphthus (13).
Materials and Methods
Preparation of the extract
The plant parts (stem bark) were cleaned, dried at 40°C, and powdered using electric mills. 100 g of the pulverized stem bark was extracted with water by a Soxhlet apparatus (14).
Polyphenolic compounds assay
Determination of total phenolic content of aqueous extracts were evaluated using gallic acid as standard. Total phenolics were determined colorimetrically by the Folin-Ciocalteu method. The prepared extracts (1 ml) were mixed with 5 ml of Folin-Ciocalteu reagent (previously diluted tenfold with distilled water) and allowed to stand at room temperature for 10 min. A 4 ml sodium bicarbonate solution (75 g.l-1) was added to the mixture. After 30 min at room temperature, absorbance was measured at 765 nm using a UV spectrophotometer (Pharmacia Biotech). Total phenolics were determined using a calibration curve obtained from measuring the absorbance of a known concentration of gallic acid (GA) standard (20–200 mg.l-1). The concentrations are expressed as milligrams of GA equivalents per g dry extract (15, 16).
Preparation of mucoadhesive formulation
Carbopol 934 (Lot No: 8835274, BF Goodrich), Zinc oxide (Sepidaj factory, Iran), gelatin (Merck, Germany), PVP K30 (Sigma Aldrich, Germany) and glycerin (Sigma Aldrich, Germany) were used to prepared mucoadhesive formulation.
The mucoadhesive paste was first prepared by mixing Zinc oxide and gel of Carbopol 934, then gelatin and solution of PVP K30 in warm water (about 60°C) which was then added to the mixture. Glycerin was added to prevent drying of paste. This paste was also prepared with other inert powder including: Starch, Bentonite, Avicel, and Talc.
It was appreciated that none of them, lacked good adhesion, except a mixture of Zinc oxide. Finally, the stem bark extract was incorporated in this paste (10% or 70 mg/g) (17).
Due to the incompliance of patients with the paste, the decision was made to make a mucoadhesive disc. The discs were prepared by direct compression, drying the paste at 60°C, crushing, changed to granules and then compressed into a flattened disc with 0.7 g and, 1 cm, 1.5 mm weight, diameter and thickness respectively.
Swelling studies
Water uptake and erosion of discs were evaluated by determining the percentage of hydration, disc erosion and dissolution (DS) according to the following formulas:
% of Hydration = (W2-W1)/W2 × 100, DS = (W1-W3)/W1 × 100
W1: The initial weight of discs before floating in saliva. W2: the weight of discs after floating in saliva. W3: The final weight after drying the discs.
Where W1, W2 and W3 are the weights of each group in artificial saliva for different times (0.5, 1, 1.5, 2, 4 hr) reweighted after removal of excess saliva and the dried discs after being kept in a desiccator for 48 hr respectively (18).
Mechanical mucoadhesion test
This test was performed using a material test machine (Hounsfield) to simulate in vitro condition and in fact was measured in terms of the force needed to pull out a disc from a gelatin gel layer (30% w/w), this layer is similar to the oral mucosa. The discs were fixed to the upper probe of the machine. The gelatin layer was made by pouring gelatin solution on the lower probe. Then, the discs were first fixed with a glass slide that hanged with a thread to the upper probe, wetted with 0.1 ml of water and forced on the gelatin layer that was previously fixed on the lower probe, by means of a 20 g weight for 30 sec. Then, the upper probe was raised at 180 mm/sec until the disc was separated from the gel. The recorded ultimate force was when this point shows the adhesive bond strength between the disc and gel layer. This test was carried out for 5 discs and averaged (19, 20).
In vivo mucoadhesion study
This study was performed by applying discs on 10 healthy volunteer women between the ages of 30-35 years, to assess the residence time of the discs. Each disc was attached to the mucosa by applying a light force with the volunteer's finger for 20 sec who did not consume water or food during the study. The mucoadhesion time was either monitored by the volunteers (themselves) or by the project team. For this part of the study, ethical approval was obtained from the Ethics Committee of Tehran University of Medical Sciences with code 116 (18).
In vitro drug release
This experiment was performed by paddle method, based on USP at 37°C and 100 rpm in tank of artificial saliva (900 ml). Ten discs were placed at the bottom of the tank. When the paddle was rounding (above 5 cm of discs). Ten ml of saliva samples were drawn every 10 min and the total phenol content were measured using UV-Vis spectrophotometer and repeated 5 times (18, 21).
Results
Result of stem bark of plant and extract assay
The aqueous extract had the highest content of total phenol (123.729±0.07 mg gallic acid equivalent/g). pH of the extract was 5.59. The percentage yield of the dry aqueous extract of the plant was 11.46% w/w. This part of the study was done, just for plant evaluation.
Swelling-hydration and DS studies
Hydration was usually very fast in all discs, due to the porosity and capillary tube within their mass. After 2 hr, hydration was 34% and after 4 hr it was 32% and after 4 hr, destruction of the discs began and after 24 hr they were completely destroyed.
Figure 1 indicates the percent of hydration against of time as could be seen as expected. There is a direct relationship between the time and water uptake (hydration) up to the limitation of discs capacity. It means that the hydration becomes 34% and 32% after 2 and 4 hr respectively. After that, the discs were begun to destroyed and 24 hr they were destroyed completely.
Figure 1.
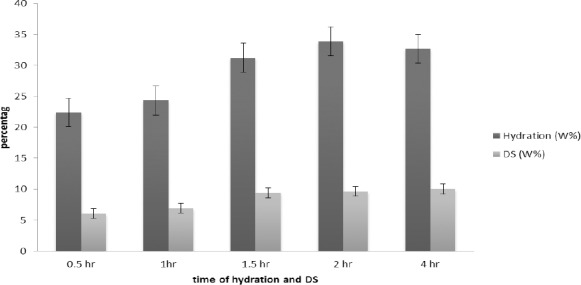
Comparison between hydration and dissolution of discs in period of time. hydration and dissolution within two hours, both are on the rise
Figure 1, also shows the trend and comparison of swelling due to hydration with dissolution. As could be seen, the DS confirms the good hydration of the mucoadhesive discs.
Figure 2 shows rate of hydration and describes kinetic of this rate. Since R= 0.9538, the rate of hydration followed semi zero –order kinetic.
Figure 2.
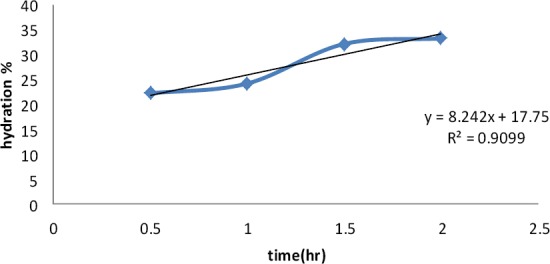
The rate of hydration of mocuahesive discs in saliva solution
Table 2 shows the average adhesion strength for 120 min, before the commencement of disintegration.
Table 1.
Bond strength of discs / simulated mucosa systems
| Samples | Sample 1 (Pa) | Sample 2 (Pa) | Sample 3 (Pa) | Sample 4 (Pa) | Sample 5 (Pa) |
|---|---|---|---|---|---|
| Disc contain extract+ Carbopol+PVP k30+ Gelatin (Group A) | 6.42 | 6.71 | 5.69 | 4.24 | 4.88 |
| Disc contain extract+ Carbopol+ Gelatin (Group B) | 3.94 | 2.88 | 3.605 | 2.42 | 2.503 |
| Disc contain extract+ Carbopol (Group C) | 1.57 | 1.33 | 1.24 | 0.975 | 1.17 |
extract: Water extract of Ziziphus jujuba
Table 2.
Duration of the remaining discs on the mouth mucosa of healthy volunteer woman between the ages of 30-35 years
| Person 1 | Person 2 | Person 3 | Person 4 | Person 5 | Person 6 | Person 7 | Person 8 | Person 9 | Person 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Time of adhesion | 125 min | 90 min | 120 min | 125min | 110 min | 120 min | 90 min | 115 min | 100 min | 120 min |
Mucoadhesion studies
Mixtures of polymers were compared in this study. The formulation contains Carbopol 934, PVP K30 and gelatin had the highest mucoadhesion properties compared to other formulation containing Carbopol and gelatin or that contains only Carbopol. Hence, the Carbopol 934P: PVP K30: gelatin mixture was selected for further studies. Based on Table 1, the ultimate force for separating the disc from gel (simulated mucosa) of the groups was according to the trend A> B> C.
In vitro release studies
The release rate of the active ingredients, based on polyphenolic compounds, was the average of 47% in one hour (Figure 3). This high rate of release is due to high solubility of the phenolic compounds in aqueous media, as well as mucosa cavity. An assay was performed just in 1 hr because the fact that the duration of the treatment and patient compliance is not more than 1 hr. On the other hand, it could be seen that the local concentration of the active ingredients is high enough to treat the lesion of that site. Although the sticking time is high enough (2 hr) but is not an indication of treatment within this time.
Figure 3.
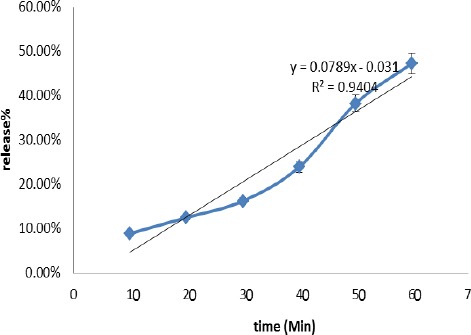
Rate of release the aqueous extract from the discs containing zinc oxide and carbopol and other polymers against time. Based of “R” the releasing kinetic of the polyphenolic compounds from the discs is nearly Zero order
Figure 4.
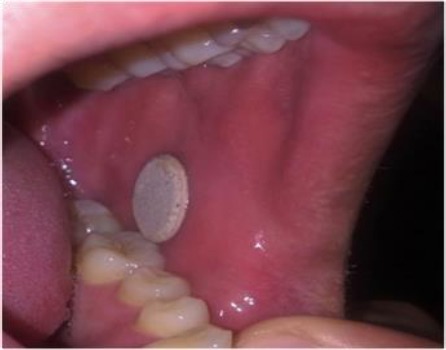
A photograph of the applied mucoadhesive discs on the mucosa of the volunteer's mouth
In vivo mucoadhesion study
As indicated in the previous section, all discs remain attached to the mucosa for 2 hr. The high adhesion time may be the result of a large number of hydrogen bonding of the mucoadhesive, Carbopol included in the formulation. Meanwhile, none of the discs had bad taste, irritation and/or pain in the buccal cavity.
Discussion
To provide a novel approach to survey the traditional medicine hypotheses, this study aimed at applying Iranian natural herbs as new formulation.
Since each herbal drug should be standardized based on one of the active components (secondary metabolites) (21), total phenols were chosen. The total phenols include flavonoids and tannins and these metabolites identify the Rhamnaceae family and that they have anti-inflammatory and wound healing properties and they can help to improve irritant disorders (17, 22) such as Aphthus.
In this study, suitable polymers were chosen because adhesion properties are important. These polymers should have good adhesion in mouth pH, water soluble and compatible with plant extracts as well as its pH. Hence, Carbopol 934 and PVP k30 were chosen. Carbopol, in addition to being water-soluble, is active in slightly acidic to alkaline range and carboxylic groups with the formation of hydrogen bonds with the surface of the mucosa, create room for suitable adhesion (4, 23). PVP k30 is water soluble and compatible with Jujube extract. It makes the film layer very good and is used in the structure of tablets as a binder. On the other hand, it is a polar polymer with ability to form hydrogen bonds (24, 25). In the presence of saliva, these bonds are formed with mucosa and adhesion will be more. Role of gelatin is increasing the pH in the final product in addition to improving the adhesion.
According to this, results show that hydration happened fast in all the tablets, due to the capillary and porosity into the mass of tablets that is due to the hydrophilic and mucoadhesive polymers. Based on the results shown in Figure 1, disc hydration capacity increased till 2 hr and then it was completed in the next 2 hr, after that, it was reduced. This result is a good indication of disc durability, when used for the mucosa of the human mouth.
On the other hand, disc hydration capacity and water uptake play an important role in drug release. Water activates the polymer swelling process, improves the adhesive step and finally facilitates penetration of the mucosa. On the opposite side, polymer swelling is important for mucoadhesion. In this process, saliva is absorbed in the mass of tablets and causes the swelling of polymers such as Carbopol and PVP k30. These polymers form gel, in and around the discs matrix and substances are released from them by passive diffusion. The larger surface area which is provided by disc structure could accelerate the diffusion process and reduce the total release time to lower than 2 hr (26). Therefore, the produced structure could predominantly govern the diffusion and particularly, phenolic compound release.
The swelling phenomena can increase solubility as a result of a correlation between parameters of combination and structural properties (27, 28) and desired solubility is associated with ample release of phenolic compounds. Furthermore, increasing the released value of phenolic compound could intensify the release of residual phenolic compounds, due to increased porosity and swelling (29).
The swelling may cause disintegration of discs ultimately. Therefore, the time of loses the integrity and disc dissolution should be known. For comparison and further study, this was appreciated by measuring the initial disc weight and final weight after immersion in the saliva solution and drying.
The figure also shows that the capacity of water uptake is limited and became full after 2 hr. It should be noted that the swelling alone is not the ultimate goal for mucoadhesion, rather the other factors particularly the sticking time and its bond strength should be taken into account for the quality of mucoadhesive during which the drug release and thereby the treatment would be completed. Therefore, the mucoadhesive discs were tested in a real manner, the in vivo tests were performed on the mucosa of the volunteers’ mouth.
As a result of Fig 1, the water uptake and DS increase with increasing time. It shows that if the swelling increases, DS will also increase until 2 hr. It means that increasing the hydration property may result in higher release of the active ingredients and finally, increase in disintegration also.
On the other hand, based on Figure 2, the rate of hydration followed semi zero –order kinetic (R= 0.9538), meaning that time is the only factor affecting the hydration (up to 2 hr).
In the mucoadhesion study, different polymers may produce tougher complexes and help to link on mucus. If the number of polymers present in the same media were lower, the number of hydrogen bonding with water and mucus will be reduced, thereby making the links weaker. This study investigated that the hydrophilic properties of these polymers could be the reason for the linking with the mucosa and when the number of polymers were more, the linking was more, due to the high possibility of forming more hydrogen bonds (4, 20, 30). It is possible to modulate the drug release, which depends on the interaction between water, polymer, and drug. Since the quick release was needed to improve Aphthous, long attachment was not necessary, therefore it should not be used for polymers that form ionic and covalent bonding or have high viscosity in order to release slowly (sustain release) and more systemic effects)4(. These discs are kind of matrix with short acting and local effects. It is necessary that hydrophilic polymers are used in preparation of these discs.
Based on the release test, as could be postulated the releasing kinetic of the polyphenolic compounds from the discs is nearly Zero order (r=0.9697). This means that compounds release was constant within the time and the mechanism of diffusion was considered non-Fickian type (due to swelling and water uptake). The release of effective polyphenolic compound followed the diffusion and was potentially excellent for the treatment. There is a little bias from the straight line (r=0.9999) as already seen in all heterogeneous systems including all discs and tablets (19, 24, 31).
And finally based on in vitro mucoadhesive test is, due to water uptake from the mucosa during which the PVP dissolves and facilitates the penetration of water throughout the pores and capillary canals within the discs. Based on the results of release tests conducted after 1hr, 47% of the active materials of the extract were released and after 2 hr, almost all of them may be released. For treatment of Aphthous, we need a rapid therapeutic effect. Therefore, 120 min adhesion is suitable for use as cure.
Conclusion
As hydrophilic polymers used can easily absorb water and swell, the drug release would affect by these properties. This depends on the interaction between water, polymer and drug. By comparing hydration rate and release rate, we concluded that good water uptake could bring about satisfactory drug release. Based on this study, the rate of release, water uptake, and integrity of the discs are in the same kinetic fashion (zero order kinetic), and a good hydration is associated with good drug release. On the other hand, by increasing the number of polymers, adhesion increased with the possibility of forming higher amounts of hydrogen bonds. Also, hydration and dissolution rate diagrams have similar configuration against time. It is concluded that they have similar configuration and fashion of the quality for the product, which helps to anticipate the duration of the treatment.
Acknowledgment
This project was helped by Dr Hadi Afrasiabi, Professor in industrial pharmacy. We thank him and his colleagues from School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Shojaei AH. Buccal mucosa as a route for systemic drug delivery: a review. J Pharm Pharm Sci. 1998;1:15–30. [PubMed] [Google Scholar]
- 2.Squier CA, Wertz PW. Permeability and the pathophysiology of oral mucosa. Adv Drug Deliv Rev. 1993;12:13–24. [Google Scholar]
- 3.Hearndena V, Sankar V, Hull K. New developments and opportunities in oral mucosal drug delivery for local and systemic disease. Adv Drug Deliv Rev. 2012;64:16–28. doi: 10.1016/j.addr.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Andrews GP, Laverty TP, Jones DS. Mucoadhesive polymeric platforms for controlled drug delivery. Eur J Pharm Biopharm. 2009;71:505–518. doi: 10.1016/j.ejpb.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 5.Aghili MH. Aghili treatments book. Tehran: Iran Institute of Medicine Studies and Islamic medicine press; 2004. [Google Scholar]
- 6.Azam Khan M. Aksir Azam. Tehran: Institute of Medicine Studies and Islamic medicine press; 2004. [Google Scholar]
- 7.Arzani MA. Akbari Medicine. Tehran: Jalal Publisher; 2008. p. 385. [Google Scholar]
- 8.Mahajan RT, Chopda MZ. Phyto-pharmacology of Ziziphus jujuba Mill- A plant review. Pharmacognosy Review. 2009;3:320–329. [Google Scholar]
- 9.Amin GR. Popular Medicinal Plants of Iran. Tehran: Tehran University of Medical Sciences publisher; 2005. p. 198. [Google Scholar]
- 10.Salehi Soormaghi MH. Medicinal Plants and Herbal Therapy. Tehran: Tehran University of Medical Sciences publisher; 2002. [Google Scholar]
- 11.Avicenna . Canon of Medicine. Tehran: Soroosh Publisher; 1986. p. 314. [Google Scholar]
- 12.Ibn al-Nafis . Al-Shamil fi al-Tibb. Beirut: cultural foundation publication; 1963. [Google Scholar]
- 13.Hamedi Sh, Shams-Ardekani MR, Sadeghpour O, Amin GR, Hajighasemali D, Feyzabadi Z. The most common herbs to cure the most common oral disease: stomatitis recurrent aphthus ulcer (RAU) Iran Red Crescent Med J. 2015 doi: 10.5812/ircmj.21694. new accepts, impress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Handa SS, Rakesh DD. Extraction Technologies for Medicinal and Aromatic Plants. Trieste: international center and high technology; 2008. pp. 156–158. [Google Scholar]
- 15.Fazli R, Nazar Nejad N, Ebrahim zadeh MA. Assess the level of total phenolics and flavonoids and antioxidant activity of bark Beech, Hornbeam and Spruce. Iran J Natl Resources?? 2013;66:339–349. [Google Scholar]
- 16.Yousefbeyk F, Gohari AR, Hashemighahderijan Z, Ostad SN, Salehi Sourmaghi MH, Amini M, et al. Bioactive terpenoids and flavonoids from Daucus littoralis Smith subsp, hyrcanicus Rech.f, an endemic species of Iran. Daru. 2014;22:12. doi: 10.1186/2008-2231-22-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basanta Kumar J, Bhabagrahi R, Subrat K. Wound Healing Potential of Ziiphus xyloprus wild. (Rhamnaceae) Stem bark ethanol extract using in vitro and in vivo model. J Drug Deliv Ther. 2012;2:41–46. [Google Scholar]
- 18.Perioli L, Ambrogi V, Giovagnoli S, Ricci M, Blasi P, Rossi C. Mucoadhesive Bilayerd Tablets For Buccal Sustained Release of Flurbiprofen. AAPS PharmSciTech. 2007;8:E20–E27. doi: 10.1208/pt0802034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Llabot JM, Manzo RH, Allemandi DA. Double-layered mucoadhesive tablets containing nystatin. AAPS PharmSciTech. 2002;3:47–52. doi: 10.1007/BF02830620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kate VK, Payghan SA. Effect of bioadhesion and permeability on dissolution behaviour of piroxicam mucoadhesive fast disintegrating tablet. inventi rapid. Pharm Tech. 2013;2:2–7. [Google Scholar]
- 21.United States Pharmacopeial Convention, US pharmacopeia 35, National Formulary. United States Pharmacopeial Publisher; 2012. [Google Scholar]
- 22.Phillipson JD. Phytochemistry and medicinal plants. Phytochem. 2001;56:237–243. doi: 10.1016/s0031-9422(00)00456-8. [DOI] [PubMed] [Google Scholar]
- 23.Anlar S, Capan Y, Hincal AA. Physico-chemical and bioadhesive properties of polyacrylic acid polymers. Pharmazie. 1993;48:285–287. [PubMed] [Google Scholar]
- 24.Aulton ME. Pharmaceutics ?The Science of Dosage From Design. 2nd ed. Mashhad: Mashhad University of Medical Sciences Press; 2001. pp. 168–191. [Google Scholar]
- 25.Afshari Taromi F, Valadoost Tabrizi V. Review of polymerization polyvinylpyrrolidone (PVP) by solution method and Synthetic studies. J Polym Sci Technol. 1993;6:38–47. [Google Scholar]
- 26.Nguyen TT, Ghosh C, Hwang SG, Chanunpanich N, Park JS. Porous core/sheath composite nanofibers fabricated by coaxial electrospinning as a potential mat for drug release system. Int J Pharm. 2012;439:296–306. doi: 10.1016/j.ijpharm.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 27.Wang FP, Mu PH, Zhang JY, Li WX, Wang QZ, Du XZ. Study on preparation and swelling kinetics of P (AA-co-C8PhEO10Mac) pH-sensitive hydrogel in vitro drug release study. J Appl Polym Sci. 2013;130:1981–1989. [Google Scholar]
- 28.Malacarne J, Carvalho RM, de Goes MF, Svizero N, Pashley DH, Tay FR, et al. Water sorption/solubility of dental adhesive resins. Dent Mater. 2006;22:973–980. doi: 10.1016/j.dental.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 29.Nishitani Y, Yoshiyama M, Hosaka K, Tagami J, Donnelly A, Carrilho M, et al. Use of Hoy's solubility parameters to predict water sorption/solubility of experimental primers and adhesives. Eur J Oral Sci. 2007;115:81–86. doi: 10.1111/j.1600-0722.2007.00430.x. [DOI] [PubMed] [Google Scholar]
- 30.Salamat-Miller N, Chittchang M, Johnston TP. The use of mucoadhesive polymers in buccal drug delivery. Adv Drug Deliv Rev. 2005;57:1666–1691. doi: 10.1016/j.addr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Singh B, Chakkal SK, Ahuja N. Formulation and optimization of controlled release mucoadhesive tablets of atenolol using response surface methodology. AAPS PharmSciTech. 2006;7:E1–E10. doi: 10.1208/pt070103. [DOI] [PubMed] [Google Scholar]


