Abstract
Objective(s):
Gentamicin is used for the treatment of Gram-negative bacterial infections. However, gentamicin administration is limited because of nephrotoxicity. The aim of the present study was to evaluate the protective effect of crocin against gentamicin-induced nephrotoxicity in rats.
Materials and Methods:
Thirty two male Wistar rats received gentamicin (100 mg/kg, IP), with or without crocin (100 mg/kg, IP) for seven consecutive days. Plasma creatinine and urea-nitrogen concentrations, oxidative stress and histopathological changes of kidney tissues were monitored.
Results:
Administration of gentamicin resulted in significant increases in plasma creatinine and urea-nitrogen concentrations and renal tissue malondialdehyde (MDA) level, and a decrease in the renal tissue ferric reducing/antioxidant power (FRAP) level. Crocin decreased plasma creatinine and urea-nitrogen concentrations and tissue MDA level, but increased the level of tissue FRAP. In addition, gentamicin led to cellular damages including glomerular atrophy, cellular desquamation, tubular necrosis and fibrosis, epithelial oedema of proximal tubules, perivascular edema, vascular congestion and intra-tubular proteinaceous casts, all of which were partially recovered by crocin.
Conclusion:
Crocin has protective effects against functional disturbances, oxidative stress and tissue damages induced by gentamicin.
Keywords: Crocin, Gentamicin, Nephrotoxicity, Oxidative stress tissue-damage
Introduction
Gentamicin, an aminoglycoside antibiotic with a wide spectrum of activities, is vastly used in the treatment of Gram-negative bacterial infections. But its usefulness is limited due to its serious side effects such as nephrotoxicity. It has been shown that up to 30% of people who receive a course of gentamicin treatment develop some symptoms of nephrotoxicity (1, 2). Although the exact mechanism of gentamicin-induced nephrotoxicity is not well understood, numerous studies have found different pathways involved in this process, including production of reactive oxygen species (ROS) and reactive nitrogen species (RNS), reduction in antioxidant defense, activation of inflammatory processes, contraction of mesangial cells, together with a decrease in renal blood flow, which lead to tubular necrosis, leukocyte infiltration, cellular damages, reduced glomerular filtration rate (GFR) and renal dysfunction (2-5). Also, it has been shown that gentamicin- induced nephrotoxicity increases numerous pro-inflammatory cytokines including tumor necrosis factor alpha (TNF-α) and intercellular adhesion molecule-1 (ICAM-1) (6-8). Several studies have demonstrated that compounds with antioxidant and anti-inflammatory properties can reduce gentamicin-induced nephrotoxicity (9-12).
Crocin, a water-soluble carotenoid, is known as the main ingredient of saffron with antioxidant properties that has protective effects against disturbances induced by ischemia/reperfusion in the brain (13), skeletal muscle (14) and kidney (15) as well as cisplatin-induced acute renal failure (16). Also, it has been shown that crocin has anti-inflammatory and analgesic (17, 18), anti-atherosclerotic (19), anti-platelet aggregation (20), and anti-oxidative properties (16, 21). In our previous study, we found that crocin 100 mg/kg reduced mRNA expression of TNF-α and ICAM-1 and decreased oxidative stress and thereby, improved kidney function in rats following ischemia and reperfusion (unpublished data).
As explained, oxidative stress and inflammation are two factors contributing to gentamicin-induced nephrotoxicity and regarding the antioxidant and anti-inflammatory properties of crocin, the present study was designed to evaluate the protective effects of crocin against gentamicin-induced renal disturbances in rats.
Materials and Methods
The test protocol
Male Wistar rats (200–250 g) were housed three per cage at a controlled temperature (23±2 °C) and exposed to artificial 12 hr/12 hr light/dark cycle. Rats were allowed to access to food (pellets) and water, ad libitum. All procedures were done according to international guides for the care and use of laboratory animals (8th Edition), and all efforts were made to minimize the number of animals used and their suffering. Animals were randomly divided into 4 groups of 8 rats. The total test period was 12 days, during which the first group (saline-saline) received normal saline intraperitoneally at the same volume as the drugs received by other groups. The second group (saline-crocin) had a similar protocol; however, in addition to saline, they also received crocin (Sigma, London, UK) 100 mg/kg body weight per day, IP. The third group (saline-gentamicin) received intraperitoneal gentamicin injection (Caspian, Iran) for 7 days from the 6th to 12th day at 100 mg/kg body weight, IP. The fourth group received crocin from the first to the last day of the experiment and gentamicin from the 6th to 12th day (Crocin-gentamicin group).
At the end of experiment, the animals were anesthetized with ether and blood samples were collected from abdominal aorta for determination of creatinine and urea-nitrogen concentrations. Then, the right kidneys were removed and immediately frozen in liquid nitrogen for the evaluation of oxidative stress by malondialdehyde (MDA) and ferric reducing/antioxidant power (FRAP) assay. Also, the left kidneys were removed and preserved in formalin 10% for hematoxylin and eosin staining and histological studies. At the end, the animals were sacrificed by deep anesthesia (22).
Measurement protocol
Plasma creatinine and urea-nitrogen concentrations were measured by autoanalyzer (Technicon, RA-1000, USA). To assess oxidative stress, MDA and FRAP values in renal tissue were measured as previously explained in detail (23-24). The value of MDA, as an index of lipid peroxidation, was determined according the Ohkawa et al method (25). This method is based on the reaction of MDA with thiobarbituric acid (TBA) at acidic pH and high temperature, which results in a pink complex with a maximum light absorption at wavelength of 532nm. Also, Benzie and Strain method was used for the measurement of FRAP (26). This method is based on the ability of plasma or tissue extracts in reducing Fe3+ ions to Fe2+ in the presence of 2,4,6-tris (2-pyridyl) -s-triazine (TPTZ). At low pH, the reduction of Fe3+-TPTZ to ferrous produces a blue complex with maximum light absorption at the wavelength of 593 nm. All used chemicals were purchased from Sigma (Sigma, London, UK).
In order to assess tissue damages and leukocyte infiltration, all the slides stained with H & E were studied by an expert pathologist using light microscope. Bowman's space size and glomerular diameter were measured using light microscope and scaled ocular lens. Tissue damages were graded in terms of glomerular atrophy, cellular desquamation, tubular necrosis and fibrosis, epithelial oedema of proximal tubules, perivascular edema, vascular congestion and intra-tubular proteinaceous casts. To classify tissue damages, the decrease in glomerular diameter in the group with the most severe changes, in comparison to the sham group, was considered as the 100% damage and in other groups the changes were scored accordingly. Other cell damages were calculated as the percentage of the total area observed under the microscope. Damage scoring was as follows: no damage was considered as zero, 1-20 % damage as grade 1; 21-40 % as grade 2; 41- 60 % damage as grade 3, 61-80 % as grade 4 and 81-100 % as grade 5. Finally, the total histopathologic score was calculated which was equal to the sum of all different degrees of damages. In addition, the slides were studied to determine leukocyte infiltration in kidney tissue. For this purpose, the number of leukocytes in 20 microscopic fields (each one 0.14 mm2) was counted, and the average was used to estimate the quantity for each mm2 (27, 28).
Statistical analysis
The data were expressed as mean±SEM. Comparisons of the measured parameters among different groups were make using one way ANOVA followed by Duncan's Post hoc test, and LSD test was used to determine the exact P-value. To compare the total histopathological scores among different groups, non-parametric Kruskal-Wallis multiple comparison and Mann Whitney tests were used. All analyses were done by SPSS-18 software and significance level was accepted at P<0.05.
Results
The effect of crocin on gentamicin induced renal functional disturbances
Crocin administration to saline-crocin group could not change plasma creatinine and urea-nitrogen concentrations in comparison with saline-saline group (Figure 1). Gentamicin led to significant increase in creatinine and urea-nitrogen concentrations in saline-gentamicin group as compared to saline-saline group (P<0.001 for both). The administration of crocin could significantly decrease creatinine and urea-nitrogen concentrations as compared to saline-gentamicin group (P<0.001 for both). However, creatinine and urea-nitrogen concentrations were still significantly higher than those in saline-saline group.
Figure 1.
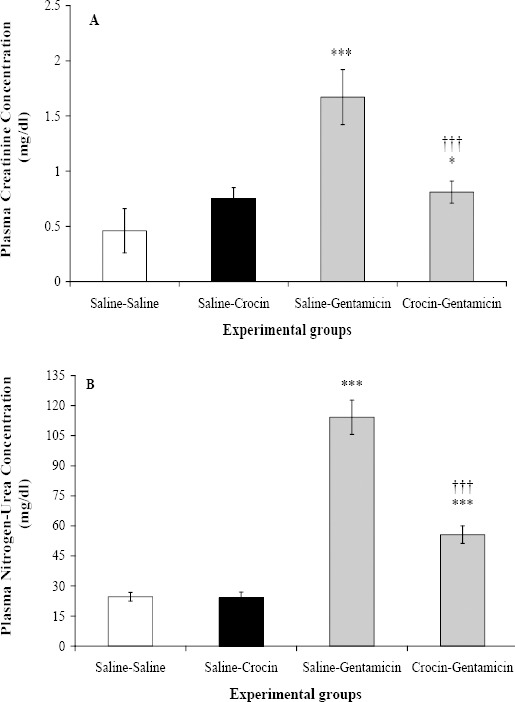
Effect of crocin on plasma creatinine (A) and urea-nitrogen (B) concentrations following gentamicin-induced nephrotoxicity in rats which received normal saline (saline-saline), crocin (saline-crocin), gentamicin (saline-gentamicin) or crocin plus gentamicin, (crocin-gentamicin), n=8. *** P<0.001, as compared to saline-saline group. †††P<0.001, for comparison between crocin- gentamicin and saline-gentamicin groups
The effect of crocin on oxidative stress caused by gentamicin
The MDA and FRAP levels in saline-crocin group were not significantly different from those in saline-saline group (Figure 2). Gentamicin significantly increased tissue MDA level in saline-gentamicin group compared to saline-saline group (P<0.01). Crocin administration caused a significant reduction in MDA level (P<0.05), so that it reached MDA value in saline-saline group. Also, the value of FRAP in kidney tissue of saline-gentamicin group was equal to 5.26±0.25 μmol/g kidney weight which was significantly lower than that in saline-saline group (P<0.001). In crocin-gentamicin group, FRAP level raised to 7.25±0.28 μmol/g kidney weight, which was significantly higher than that in saline-gentamicin group (P<0.001).
Figure 2.
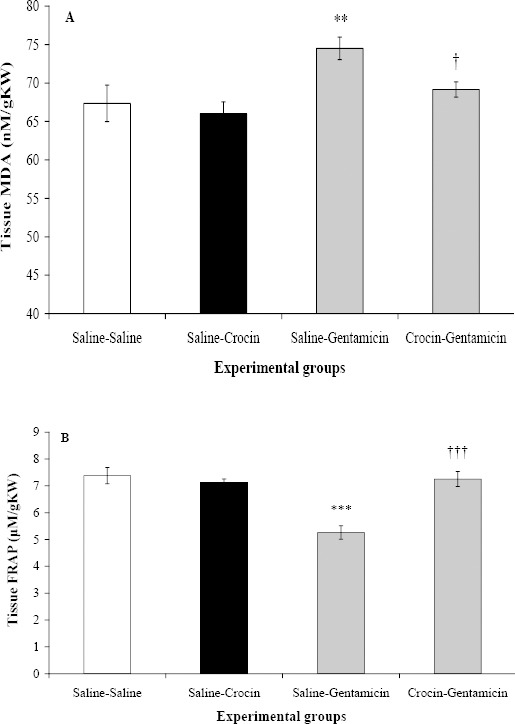
Effect of crocin on (A) renal tissue malondialdehyde (MDA) and (B) ferric reducing/antioxidant power (FRAP) levels following gentamicin-induced nephrotoxicity in rats which received normal saline (saline-saline), crocin (saline-crocin), gentamicin (saline-gentamicin) or crocin plus gentamicin (crocin-gentamicin), n=8. *** P<0.001, as compared to saline-saline group. †††P<0.001, for comparison between crocin- gentamicin and saline-gentamicin groups
The effect of crocin on gentamicin-induced renal tissue damages
Histopathological study revealed that gentamicin reduced the glomerular diameter in saline-gentamicin group compared to saline-saline group, which represented glomerular atrophy (Figure 3 and Table 1). Moreover, gentamicin caused a marked increase in cellular desquamation, tubular necrosis and fibrosis, epithelial oedema of proximal tubules, perivascular edema, vascular congestion and intra-tubular proteinaceous casts. Pretreatment with crocin could alleviate this damages in such a way that the total histopathologic score which had significantly increased by gentamicin (P<0.05), was markedly decreased by crocin to its level in saline-saline group (Table 1). As shown in Figure 4, gentamicin led to a mild infiltration of leukocytes into the renal interstitium which was markedly reduced after crocin administration.
Figure 3.
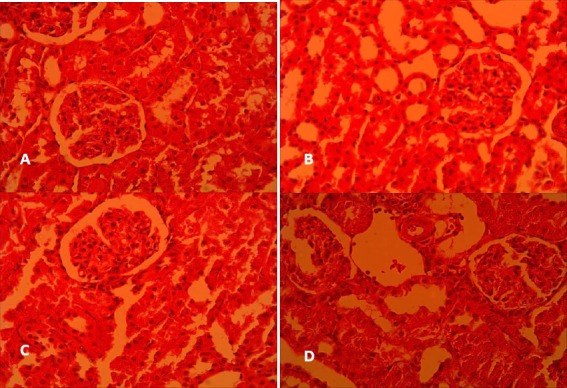
Histopathologic alterations in cortical area of rat kidneys following gentamicin administration and the effect of crocin on them in (A) saline-saline, (B) saline-crocin, (C) saline-gentamicin and (D) crocin-gentamicin groups. (Haematoxylin and eosin, 400x)
Table 1.
Effects of crocin administration on renal histopathologic scores induced by gentamicin
| Histopathology | Experimental groups | |||
|---|---|---|---|---|
| Saline-saline | Saline-crocin | Saline-gentamicine | Crocin-gentamicine | |
| Glomerular atrophy | 0 | 0.25 | 5 | 0.25 |
| Cellular desquamation | 0.75 | 0.25 | 3.75 | 1.75 |
| Tubular necrosis | 0 | 0 | 2.5 | 0.5 |
| Tubular fibrosis | 0 | 0 | 1.5 | 0.25 |
| Epithelial oedema of proximal tubules | 0 | 0.5 | 2.25 | 0.5 |
| Perivascular edema | 0.5 | 0.75 | 3.5 | 1.5 |
| Vascular congestion | 0 | 1 | 2.75 | 0.9 |
| Intra-tubular proteinaceous casts | 0 | 0 | 2.9 | 0.85 |
| Total histopathologic score | 1.25 | 2.75 | 24.15* | 6.5† |
Histopathological scores in rats which received normal saline (saline- saline), crocin (saline-crocin), gentamicin (saline -gentamicin) or crocin plus gentamicin (crocin- gentamicin)
P<0.05, as compared to saline-saline group
P<0.05, for comparison between crocin- gentamicin and saline-gentamicin groups
Figure 4.
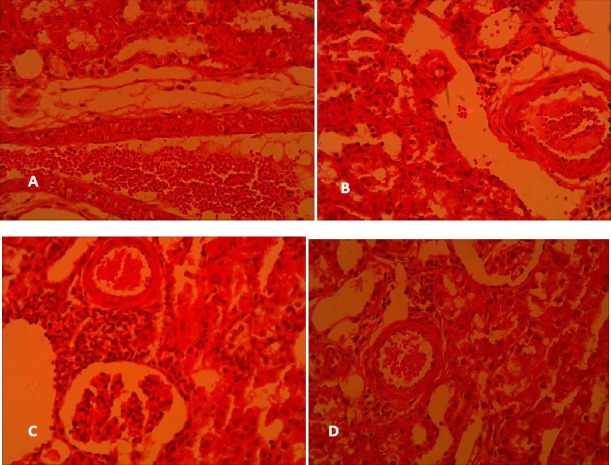
Leukocytes infiltration in cortical area of rat kidneys following gentamicin administration and the effect of crocin on it in (A) saline-saline, (B) saline-crocin, (C) saline-gentamicin and (D) crocin-gentamicin groups. (Haematoxylin and eosin, 400x)
Discussion
In this study, for the first time, we investigated the protective effects of crocin against gentamicin-induced renal functional disturbances, oxidative stress, and tissue damages in rats. The results showed that intraperitoneal administration of crocin could improve gentamicin-induced nephrotoxicity in rats. In the present study, gentamicin administration caused multiple histological damages and leukocytes infiltration (Table 1). Several experimental evidence have suggested that gentamicin causes cell damage in the kidney by stimulating ROS production (29-32). Moreover, when exposed to ROS, the kidneys of the rats that received gentamicin, suffered more because of reduced antioxidant defense system enzymes (33). Cellular damage and necrosis, in turn, stimulate the inflammatory processes and ICAM-1 recruitment, which exacerbates the leukocytes migration to the injured site (4). In this study, gentamicin increased renal tissue MDA and decreased the FRAP values, which shows increased ROS production and decreased antioxidant defense power. Gentamicin also caused a mild leukocytes infiltration into interstitium. Crocin administration could relieve all cell damages induced by gentamicin. It seemed that crocin, due to its known antioxidant properties, inhibited the production of ROS and thereby, prevented the fragmentation of DNA and ultimately reduced cell necrosis (21, 34). On the other hand, reduced cell necrosis and exfoliation into the tubular lumen, in addition to alleviating the inflammatory processes and leukocyte recruitment, opened the tubular flow which in turn improved the glomerular filtration rate, resulting in improved renal functional parameters. In line with this hypothesis, Hosseinzadeh and colleagues in their study on mice, demonstrated that the administration of crocin could decrease methyl methane sulfonate-induced DNA damage (34). It has also been shown that crocin has anti-inflammatory properties. In the study conducted by Nam et al, crocin by reducing the release of TNF-α, IL-1β, and reactive oxygen species and reducing the activation of NF-κB, exerted its anti-inflammatory effects in cultured glial cells (17). Therefore, it seemed that crocin, due to its antioxidant properties, reduced cellular damages in kidneys which together with its anti-inflammatory properties, limited leukocytes infiltration.
In the present study, gentamicin administration resulted in a marked increase in plasma creatinine and urea-nitrogen concentrations (Figure 1), which was in line with the results of previous studies (35-36). Since plasma creatinine concentration is inversely related to GFR, it seems that increased plasma creatinine concentration was due to a decrease in GFR (37). It has been shown that gentamicin by increasing renal vascular resistance and tubular necrosis, and decreasing glomerular ultrafiltration coefficient (Kf), reduces GFR (2). Crocin could reduce plasma creatinine and urea-nitrogen concentrations. By reducing oxidative stress and cellular damages, crocin may have weakened the GFR reducing parameters and consequently improved plasma creatinine and urea-nitrogen concentrations. In a study conducted by Xuan et al, they also found that crocin increased choroids and retinal blood flow (38). Similarly, crocin probably had vasodilator effects in the kidneys and by increasing renal blood flow and correcting GFR, decreased plasma creatinine and urea-nitrogen concentrations.
Conclusion
Our findings have provided strong evidence that crocin has protective effects against gentamicin-induced nephrotoxicity in rats. This protective effect might be due to its antioxidant and anti-inflammatory properties or other pathways which require further studies.
Acknowledgment
This article is derived from a research project (No. 92428) approved by the Research Deputy of Kermanshah University of Medical Sciences, Kermanshah, Iran. We hereby wish to appreciate their sincere collaboration.
Conflict of interest
The authors declare no competing financial interest.
References
- 1.Ali BH, Al Zaabi M, Blunden G, Nemmar Al. Experimental Gentamicin Nephrotoxicity and Agents that Modify it: A Mini-Review of Recent Research. Basic Clin Pharmacol Toxicol. 2011;109:225–232. doi: 10.1111/j.1742-7843.2011.00728.x. [DOI] [PubMed] [Google Scholar]
- 2.Martinez-Salgado C, López-Hernández FJ, Lopez-Novoa JM. Glomerular nephrotoxicity of aminoglycosides. Toxicol Appl Pharmacol. 2007;223:86–98. doi: 10.1016/j.taap.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Ali BH. Agents ameliorating or augmenting experimental gentamicin nephrotoxicity: some recent research. Food Chem Toxicol. 2003;41:1447–1452. doi: 10.1016/s0278-6915(03)00186-8. [DOI] [PubMed] [Google Scholar]
- 4.Balakumar P, Rohilla A, Thangathirupathi A. Gentamicin-induced nephrotoxicity: do we have a promising therapeutic approach to blunt it? Pharmacol Res. 2010;62:179–186. doi: 10.1016/j.phrs.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int. 2011;79:33–45. doi: 10.1038/ki.2010.337. [DOI] [PubMed] [Google Scholar]
- 6.Geleilete TJ, Melo GC, Costa RS, Volpini RA, Soares TJ, Coimbra TM. Role of myofibroblasts, macrophages, transforming growth factor-beta endothelin, angiotensin-II, and fibronectin in the progression of tubulointerstitial nephritis induced by gentamicin. J Nephrol. 2001;15:633–642. [PubMed] [Google Scholar]
- 7.Tang WW, Feng L, Mathison JC, Wilson CB. Cytokine expression, upregulation of intercellular adhesion molecule-1, and leukocyte infiltration in experimental tubulointerstitial nephritis. Lab Invest. 1994;70:631–638. [PubMed] [Google Scholar]
- 8.Wahl SM, Hunt DA, Wakefield LM, McCartney-Francis N, Wahl LM, Roberts AB, et al. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci USA. 1987;84:5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anandan R, Subramanian P. Renal protective effect of hesperidin on gentamicin-induced acute nephrotoxicity in male Wistar albino rats. Redox Rep. 2012;17:219–226. doi: 10.1179/1351000212Y.0000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feyissa T, Asres K, Engidawork E. Renoprotective effects of the crude extract and solvent fractions of the leaves of Euclea divinorum Hierns against gentamicin-induced nephrotoxicity in rats. J Ethnopharmacol. 2013;145:758–766. doi: 10.1016/j.jep.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Stojiljkovic N, Stoiljkovic M, Randjelovic P, Veljkovic S, Mihailovic D. Cytoprotective effect of vitamin C against gentamicin-induced acute kidney injury in rats. Exp Toxicol Pathol. 2012;64:69–74. doi: 10.1016/j.etp.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Broushaki MT, Asadpour E, Sadeghnia HR, Dolati K. Effect of pomegranate seed oil against gentamicin-induced nephrotoxicity in rat. J Food Sci Technol. 2012:3510–3514. doi: 10.1007/s13197-012-0881-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng YQ, Liu JX, Wang JN, Xu L. Effects of crocin on reperfusion-induced oxidative/nitrative injury to cerebral microvessels after global cerebral ischemia. Brain Res. 2007;1138:86–94. doi: 10.1016/j.brainres.2006.12.064. [DOI] [PubMed] [Google Scholar]
- 14.Hosseinzadeh H, Modaghegh MH, Safari Z. Crocus sativus L.(Saffron) extract and its active constituents (crocin and safranal) on ischemia-reperfusion in rat skeletal muscle. Evid Based Complement Alternat Med. 2009;6:343–350. doi: 10.1093/ecam/nem125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hosseinzadeh H, Sadeghnia HR, Ziaee T, Danaee A. Protective effect of aqueous saffron extract (Crocus sativus L.) and crocin, its active constituent, on renal ischemia-reperfusion-induced oxidative damage in rats. J Pharm Pharm Sci. 2005;8:387–393. [PubMed] [Google Scholar]
- 16.Naghizadeh B, Boroushaki MT, Vahdati Mashhadian N, Mansouri SMT. Protective effects of crocin against cisplatin-induced acute renal failure and oxidative stress in rats. Iran Biomed J. 2008;12:93–100. [PubMed] [Google Scholar]
- 17.Nam KN, Park YM, Jung HJ, Lee JY, Min BD, Park SU, et al. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur J Pharmacol. 2010;648:110–116. doi: 10.1016/j.ejphar.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Tamaddonfard E, Farshid AA, Eghdami K, Samadi F, Erfanparast A. Comparison of the effects of crocin, safranal and diclofenac on local inflammation and inflammatory pain responses induced by carrageenan in rats. Pharmacological Rep. 2013;65:1272–1280. doi: 10.1016/s1734-1140(13)71485-3. [DOI] [PubMed] [Google Scholar]
- 19.Lee IA, Lee JH, Baek NI, Kim DH. Antihyperlipidemic effect of crocin isolated from the fructus of Gardenia jasminoides and its metabolite crocetin. Biol Pharm Bull. 2005;28:2106–2110. doi: 10.1248/bpb.28.2106. [DOI] [PubMed] [Google Scholar]
- 20.Liakopoulou-Kyriakides M, Skubas AI. Characterization of the platelet aggregation inducer and inhibitor isolated from Crocus sativus. Biochem. Int. 1990;22:103–110. [PubMed] [Google Scholar]
- 21.Asdaq SMB, Inamdar MN. Potential of Crocus sativus (saffron) and its constituent, crocin, as hypolipidemic and antioxidant in rats. Appl biochem biotechnol. 2010;162:358–372. doi: 10.1007/s12010-009-8740-7. [DOI] [PubMed] [Google Scholar]
- 22.Najafi H, Firouzifar MR, Shafaat O, Changizi Ashtiyani S, Hosseini N. Protective effects of tribulus terrestris L extract against acute kidney injury induced by reperfusion injury in rats. Iran J Kidney Dis. 2014;8:292–298. [PubMed] [Google Scholar]
- 23.Changizi Ashtiyani S, Najafi H, Jalalvandi S, Hosseinei F. Protective effects of Rosa canina L fruit extracts on renal disturbances induced by reperfusion injury in rats. Iran J kidney dis. 2013;7:290–298. [PubMed] [Google Scholar]
- 24.Jafarey M, Changizi Ashtiyani S, Najafi H. Calcium dobesilate for prevention of gentamicin-induced nephrotoxicity in rats. Iran J Kidney Dis. 2014;8:46–52. [PubMed] [Google Scholar]
- 25.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 26.Benzie IF, Strain JJ. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999;299:15–27. doi: 10.1016/s0076-6879(99)99005-5. [DOI] [PubMed] [Google Scholar]
- 27.Sternberg SS. Diagnostic Surgical Pathology. 3rd ed. Philadelphia: Lippincott: Williams & Wilkins; 1996. pp. 1701–1785. [Google Scholar]
- 28.Ysebaert DK, De Greef KE, Vercautern SR, Ghielli M, Verpooten GA, Eyskens EJ, et al. Identification and kinetics of leukocytes after severe ischaemia/reperfusion renal injury. Nephrol Dial Transplant. 2000;15:1562–1574. doi: 10.1093/ndt/15.10.1562. [DOI] [PubMed] [Google Scholar]
- 29.Balakumar P, Chakkarwar VA, Kumar V, Jain A, Reddy J, Singh M. Experimental models for nephropathy. J Renin Angiotensin Aldosterone Syst. 2008;9:189–195. doi: 10.1177/1470320308098343. [DOI] [PubMed] [Google Scholar]
- 30.Maldonado PD, Barrera D, Rivero I, Mata R, Medina-Campos ON, Hernandez-Pando R, et al. Antioxidant sallylcysteine prevents gentamicin-induced oxidative stress and renal damage. Free Radic Biol Med. 2003;35:317–324. doi: 10.1016/s0891-5849(03)00312-5. [DOI] [PubMed] [Google Scholar]
- 31.Walker PD, Barri Y, Shah SV. Oxidant mechanisms in gentamicin nephrotoxicity. Ren Fail. 1999;21:433–442. doi: 10.3109/08860229909085109. [DOI] [PubMed] [Google Scholar]
- 32.Zorov DB. Amelioration of aminoglycoside nephrotoxicity requires protection of renal mitochondria. Kidney Int. 2010;77:841–843. doi: 10.1038/ki.2010.20. [DOI] [PubMed] [Google Scholar]
- 33.Pedraza-Chaverrı J, Maldonado PD, Medina Campos ON, Olivares-Corichi IM, Granados-Silvestre MA, Hernandez-Pando R, et al. Garlic ameliorates gentamicin nephrotoxicity: relation to antioxidant enzymes. Free Radic Biol Med. 2000;29:602–611. doi: 10.1016/s0891-5849(00)00354-3. [DOI] [PubMed] [Google Scholar]
- 34.Hosseinzadeh H, Abootorabi A, Sadeghnia HR. Protective effect of crocus sativus stigma extract and crocin (trans-crocin 4) on Methyl Methanesulfonate–induced DNA damage in mice organs. DNA Cell Biol. 2008;27:657–664. doi: 10.1089/dna.2008.0767. [DOI] [PubMed] [Google Scholar]
- 35.Abdel-Raheem IT, Abdel-Ghany AA, Mohamed GA. Protective effect of quercetin against gentamicin-induced nephrotoxicity in rats. Biol Pharm Bull. 2009;32:61–67. doi: 10.1248/bpb.32.61. [DOI] [PubMed] [Google Scholar]
- 36.Sardana A, Kalra S, Khanna D, Balakumar P. Nephroprotective effect of catechin on gentamicin-induced experimental nephrotoxicity. Clin Exp Nephrol. 2014;19:178–184. doi: 10.1007/s10157-014-0980-3. [DOI] [PubMed] [Google Scholar]
- 37.Star RA. Treatment of acute renal failure. Kidney Int. 1998;54:1817–1831. doi: 10.1046/j.1523-1755.1998.00210.x. [DOI] [PubMed] [Google Scholar]
- 38.Xuan B, Zhou YH, Li N, Min ZD, Chiou GC. Effects of crocin analogs on ocular blood flow and retinal function. J Ocul Pharmacol Ther. 1999;15:143–152. doi: 10.1089/jop.1999.15.143. [DOI] [PubMed] [Google Scholar]


