Abstract
Purpose
This study aims to assess the clinical impact of spot size and the addition of apertures and range compensators on the treatment quality of pencil beam scanning (PBS) proton therapy and to define when PBS could improve upon passive scattering proton therapy (PSPT).
Methods and materials
The patient cohort included fourteen pediatric patients treated with PSPT. Six PBS plans were created and optimized for each patient using three spot sizes (~12, 5.4, and 2.5 mm median sigma at isocenter for 90–230 MeV range), and adding apertures and compensators to plans with the two larger spots. Conformity and homogeneity indices, dose-volume histogram parameters, equivalent uniform dose (EUD), normal tissue complication probability (NTCP) and integral dose were quantified and compared with the respective PSPT plans.
Results
The results clearly indicated that PBS with the largest spots does not necessarily offer a dosimetric or clinical advantage over PSPT. With comparable target coverage, the mean dose (Dmean) to healthy organs was on average 6.3% larger than PSPT when using this spot size. However, adding apertures to plans with large spots improved the treatment quality by decreasing the average Dmean and EUD by up to 8.6 and 3.2% of the prescribed dose, respectively. Decreasing the spot size further improved all plans, lowering the average Dmean and EUD by up to 11.6% and 10.9% compared to PSPT, respectively, and eliminated the need for beam-shaping devices. The NTCP decreased with spot size and addition of apertures, with maximum reduction of 5.4% relative to PSPT.
Conclusions
The added benefit of using PBS strongly depends on the delivery configurations. Facilities limited to large spot sizes (>~8 mm median sigma at isocenter) are recommended to use apertures in order to reduce the treatment-related toxicities, at least for complex and/or small tumors.
Introduction
Proton therapy offers benefits over photon-based radiotherapy for specific patient groups in terms of treatment outcome [1–5]. One of the strongest indications for a proton therapy referral is the curable pediatric solid tumors, since the significantly smaller integral dose deposited by protons contributes to lower adverse side effects compared with photon modalities [6–9]. For these patients, any excessive amount of radiation could negatively impact development and organ function [10–13]. Side effects, besides acute toxicities caused by the high dose exposure, could include neurocognitive/behavioral effects, endocrine abnormalities, vascular effects, and second malignancies [14–20].
Pencil beam scanning (PBS) is a proton therapy delivery technique featuring several dosimetric traits, which depending on the circumstances, could further improve upon what passive scattering proton therapy (PSPT) can achieve in terms of clinical outcome [21–23]. Namely, it provides smaller entrance dose and allows for intensity-modulated treatments enabling dose painting to further spare normal tissue in the vicinity of complex tumor structures. Furthermore, the use of custom fabricated beam-shaping components i.e. apertures (AP) and range compensators (RC) could be avoided, hence reducing scatter neutron dose [20,24], and also saving extra cost, time and effort needed for creating such devices.
These advantages, however, may not be fully realized depending on the specific settings available at each institution for PBS treatments. Suboptimal pencil beams with wide lateral profiles could lead to less steep penumbrae that may not necessarily be more suitable than PSPT, especially to treat smaller sized tumors that are common in pediatric patients. Therefore the question arises whether and when would PBS have additional therapeutic advantages and be a preferred method of treatment over PSPT. More specifically, what spot size should be considered appropriate and if using AP and/or RC could still play a role.
Previous studies have indicated better organ sparing due to reduced PBS spot size for specific patient cases [25,26]. Chanrion et al [27] explored the consequences of unexpected spot size fluctuations on the target coverage in PBS. Dowdell et al [28] investigated the effects of beam-specific apertures on dose characteristics incident on a lucite phantom using Monte-Carlo and measurements. Hyer et al [29] studied the effects of a dynamic collimator system on the lateral dose profile for PBS as a function of spot size and spot spacing in a solid water phantom. Comprehensive patient-based comparison of all possible scenarios (i.e. spot size variations and addition of beam-shaping devices) is currently lacking.
In this study we aim to assess the effect of spot size and the use of beam-specific AP and RC in PBS treatments of pediatric patients. This patient group is more sensitive to dose variations due to their smaller statures and radiosensitive organs, and could highly benefit from any degree of sparing. We compare the dosimetric and clinical endpoints between variable PBS settings and PSPT, by quantifying the plan specific dose-volume indices as well as normal tissue complication probabilities (NTCP) for a relatively large patient cohort.
Methods and Materials
1. Patient Cohort
Fourteen pediatric patients (7 CNS, 4 head & neck, 2 pelvic and 1 thoracic solid tumors) treated with PSPT were selected for this study. This was a representative patient cohort due to variability in tumor histology, location and size, and patient age. Tumor characteristics (i.e. histology and volume) and some key treatment plan information (i.e. number of beams and the prescribed dose) are listed in table 1. In summary, plans originally included 4–13 beams and the prescribed dose ranged between 36–57.6 Gy(RBE), delivered in 1.8 Gy(RBE) per fraction. In all the cases, the tumor volume and organs at risk (OAR) were identified and delineated by the treating physician or neuro-anatomist. Most targets included clinical target volumes (CTV) as well as boost volumes (GTV), and in some complex cases (e.g. patients 11 and 12), multiple targets, including the nodes, were involved. The most relevant OARs for cranial patients included optic nerves, brainstem, bilateral cochlea, pituitary gland, optic chiasm, hypothalamus, hippocampi, parotids, lacrimal glands, and non-tumor (healthy) brain tissue. For pelvic/thoracic patients, the OARs included rectum, bladder, femoral heads, growth plates, uterus, lungs, spinal cord and esophagus. Not all OARs were studied for all patients. Skin was additionally segmented for all patients.
Table 1.
Patient and treatment-plan specifications.
| Patient # | Histology | Target volume GTV–CTV [cc] | # of fields PBS (all–reduced)/PSPT | Prescribed dose GTV–CTV [Gy] |
|---|---|---|---|---|
| 1 | Ewing sarcoma (pelvic) | 384 | 3/5 | 57.6 |
| 2 | Germinoma | 22–170 | 6–4/6 | 36.0–21.0 |
| 3 | Bladder/Prostate rhabdomyosarcoma | 41–106 | 7/7 | 50.4–30.6 |
| 4 | Ewing sarcoma (thoracic) | 7–94 | 4–3/5 | 55.8–43.2 |
| 5 | Astrocytoma | 90 | 5/5 | 52.2 |
| 6 | Ependymoma | 75–127 | 6/6 | 55.8–54.0 |
| 7 | Craniopharyngioma | 11 | 4–3/4 | 52.2 |
| 8 | Germinoma | 36–203 | 7/7 | 36.0–21.0 |
| 9 | Embryonal rhabdomyosarcoma | 61 | 6/6 | 50.4 |
| 10 | Orbital rhabdomyosarcoma | 5–7 | 4/4 | 45.0–36.0 |
| 11 | Embryonal rhabdomyosarcoma | 149 | 3/10 | 52.2 |
| 12 | Ependymoma | 12–41 | 5–3/5 | 54.0–52.2 |
| 13 | Embryonal rhabdomyosarcoma | 139–222 | 5/13 | 50.4–36.0 |
| 14 | Astrocytoma | 47 | 4–3/4 | 52.2 |
2. Treatment planning
An in-house developed fully validated and clinically commissioned treatment planning system XXX (version XXX) was used to create six new PBS plans for each patient. Three distinct pencil beam spot sizes were defined in XXX. In this context, beams with sigma of 16.8×18.6−8.2×8.9 (median~11×13.6) mm, 9.3–4.6 (median~5.4) mm, and 4.4–2.2 (median~2.5) mm at isocenter for 90–230 MeV range in air, are referred to here as large, medium and small spots, respectively [30]. PBS plans were created and optimized with the following specifications: (1) large spot size (no device) (2) large spot size (with AP), (3) large spot size (with AP and RC), (4) medium spot size (no device), (5) medium spot size (with AP) and (6) small spot size (no device). The devices were identical to those used in PSPT plans. The aperture model in PBS dose calculation engine considers the projection of the aperture at the calculation depth based on the source and aperture positions, and convolves the proton spot component-wise over the aperture edge for a continuous transition. The convolution Gaussian spread is modeled as a virtual source position/size to conform to measurements. Hence, the spot-size contribution in the penumbra is significantly reduced. The general PBS planning parameters were kept the same as PSPT, e.g. the air gap (2–5cm), beam orientation, AP margins (8–10mm), and RC smearing distance (3–10mm). The uncertainties were taken into account for both modalities as per our current clinical practice. RC was only used for the large spots where the spot size at the distal layer was larger than the RC smearing, allowing for the distal dose to be trimmed closer to the tumor shape.
For PBS plans, spot spacing was set equal to 0.7 times the sigma of the pencil beam's lateral profile and the layer spacing was equal to 0.8 times the width of the most distal Bragg peak at 80% dose level. Furthermore, the spot placement margins were set to 15 mm lateral and 10 mm distal of the target in the beam direction, and the unnecessary spots were automatically eliminated. Intensity-modulated proton therapy (IMPT) optimization was applied. Lucite range shifters were used for beams with shallow (<~6 cm) range.
The prescribed dose and treatment constraints were extracted from the patient charts. Using the multicriteria optimization (MCO) [31] engine in XXX, the maximum dose to patients and minimum target dose were set as plan constraints. The objectives were to minimize the OAR mean dose and maintain the target coverage and total dose uniformity. All plans were created using identical criteria for fair comparisons.
Although not all the beams used in PSPT were always needed to achieve optimized PBS plans for most cases, we kept the default beam configuration constant for a fair comparison during the primary analysis. The field configuration was simplified for 8 cases under the guidance of clinical dosimetrists to yield plans better suited for PBS delivery, including patients 11 and 12 where due to highly complex targets and field-patching in the original plans, keeping all the beams would not be sensible for PBS (see table 1). Comparison between original and reduced number of fields was only performed for medium-spot plans.
3. Data analysis
Optimized PBS plans were compared with the existing PSPT plans. Conformity index (CI) and homogeneity index (HI) were calculated as defined by RTOG [32]. Additionally, CI at 50% prescription dose was calculated to further compare the medium dose exposure of the surrounding tissue. PBS plans were normalized to PSPT (matching dose at 98% volume of the GTVs). Dose volume histograms (DVH) were analyzed for the target and all OARs, by extracting the mean dose (Dmean), dose 2% volume (D2) and volume receiving 90% of the prescribed dose (V90). The equivalent uniform dose (EUD) and normal tissue complication probability (NTCP) were calculated as parameterized by Niemierko et al [33]. The parameters for the gEUD and NTCP models are organ-dependent and taken from the literature [34,35]. The differences between dose and volume indices were assessed as the percentage of the proscribed dose and total volume, respectively. Furthermore, the integral dose (Dint) (defined as the total energy deposited in the patient) was calculated and compared for all plans, as a measure of the extent of patient radiation exposure, and hence the estimated risk of late toxicities.
Results
1. Effect of spot size variation
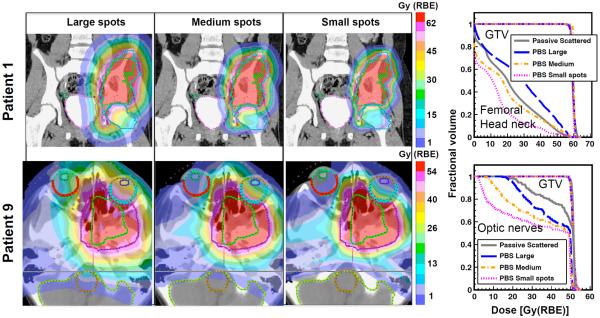
The pencil beam spot size has a significant effect on the PBS plan quality. Figure 1 shows the dose distribution and DVH curves for two representative patients in the cranial and pelvic/thoracic categories. The PBS target coverage was adequate in all cases, with average GTV D98 being 1% smaller than PSPT. After normalization, the average D2 was 2.2% larger than PSPT. The CI considering the GTVs were on average 1.27, 1.14 and 1.10 for large, medium and small spots, respectively, compared to 1.45 for PSPT. The average HI was comparable between all cases (~1.1). For PBS plans with large spots, the dose to most nearby organs was larger than PSPT. This is due to the rather wide lateral penumbrae leading to larger and more spread-out dose distribution adjacent to the primary treatment field. As the spot size was reduced, the field penumbrae were sharpened and the dose to organs located both laterally and distally to the beam decreased. The Dmean for all OARs averaged over all patients was 6.3% and up to 19% larger for PBS with large spot than PSPT. For some organs such as skin, however, the mean dose was smaller than PSPT for most patients even when using large spots due to the better proximal dose conformity. For PBS plans with medium and small spots, the average Dmean over the OARs was 5.2% and 11.6%, and EUD 6.6% and 10.9% smaller than PSPT, respectively. For large, medium and small spots, V90 was on average 6.9%, 12.3% and 14.1% smaller than PSPT, for all patient/OARs. Furthermore, CI50 reduced by 37.3 and 51.5% for medium and small spots relative to large spots, respectively. Hence, the medium and small spot PBS (even with no devices) were clinically superior to PSPT.
Figure 1.
Effect of spot size on the dose distribution and corresponding DVH curves for patients 1 and 9.
2. Effect of using beam-shaping devices
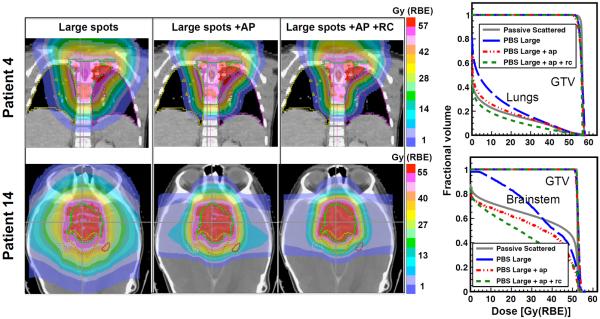
Beam-specific APs effectively sharpened the lateral dose penumbrae. In most cases when only AP was used in plans with large spots, the OAR dose became smaller or at least equivalent to PSPT plans. The exception was for organs distal to specific fields, where RC was also necessary to achieve superior sparing than PSPT. Adding RC to plans with large spots always led to dosimetrically superior plans to PSPT. Plans with medium spots also improved further with AP, although the degree of improvement was smaller than the large spots. Figure 2 illustrates the effect of adding AP alone as well as both devices on plans with large spots for two representative cases. In case of patient 4, for example, the lung mean dose decreased by 4.4% after applying AP only but still remained 2.3% larger than PSPT, and further decreased to 3.2% less than PSPT, when adding RC. In case of patient 14, the mean dose to the brainstem already dropped to 6.3% below PSPT by adding AP only, due to the reduced penumbrae width for the laterally oriented fields. Adding RC further reduced the brainstem dose by another 9.3%, mainly due to the close proximity of the structure to the distal edge of the anterior oblique field. Adding AP to medium spot plans reduced the brainstem mean dose by 6.3% compared with 15.2% for large spots (not shown).
Figure 2.
Effect of using AP and RC on dose distributions and DVH curves for targets and example OARs for patients 4 and 14.
When using beam-shaping devices, the CI was reduced on average by 0.08 and 0.12 below the case with no devices for large spots with AP only and with both devices, respectively, and by 0.03 for medium spots with AP. CI50 was reduced by 30.9, 41 and 9.4% for the above scenarios, respectively. Uniform target dose was more easily achieved when adding devices, as D2 was reduced. Average OAR Dmean/EUD were 2.3/2.4%, 7.1/5.7% and 9.3/8.1% smaller than PSPT for large spots with AP, large spots with both devices, and medium spots with AP, respectively. For large spots, adding APs on average reduced the OAR Dmean/EUD by 8.6/3.1%, whereas adding both devices reduced these values by 13.4/6.5% compared with no devices, respectively. For medium spots, these reductions were 4.1/1.6%. Therefore, beam-shaping devices were deemed necessary for large spots to achieve plans equivalent or better than PSPT.
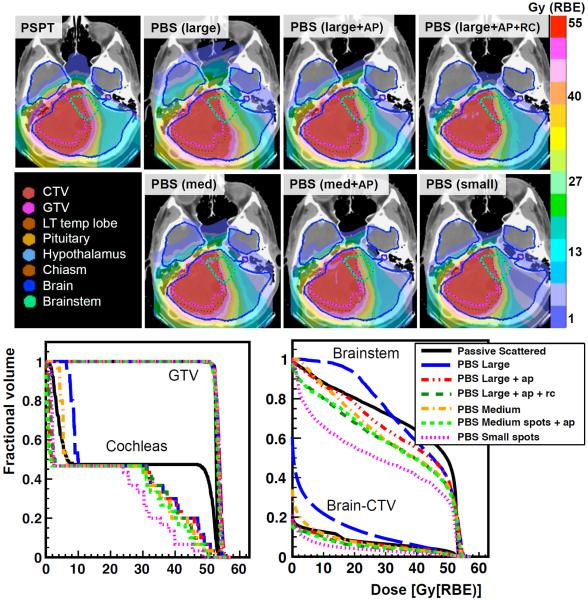
3. Overall comparison
Figure 3 shows a comparison of the dose distributions and DVH curves between all PBS plans and the PSPT plan for patient 5. Although the OAR dose decreases with decreasing the spot size and adding both devices, the dosimetric advantage over PSPT when adding AP alone to plans with large spots was organ-dependent. Also the benefits of adding AP sometimes exceeded that of reducing the spot size. For example, in figure 3 the mean dose to cochleas when using large spots with AP was 2.5% smaller than using medium spots with no AP. When adding AP to medium spots, the mean dose was then reduced relative to large spots with both devices. The CI/CI50 decreased as devices were added and further as the spot size decrease but always remained above the lower limit of 1.0.
Figure 3.
Dose distributions comparing PSPT and all PBS plans, and DVH curves for GTV, cochleas, brainstem and brain for patient 5.
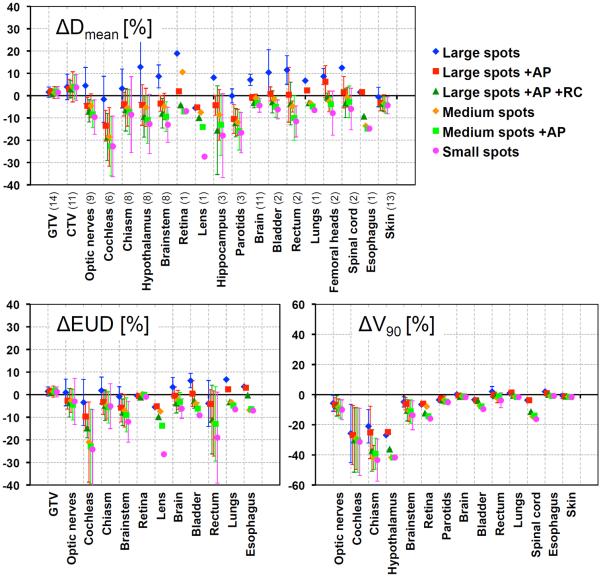
Figure 4 summarizes the mean and standard deviation of percent differences of organ Dmean, EUD and V90 between each PBS and the PSPT plan averaged over all patients. Positive values indicate larger PBS doses than PSPT. It clearly illustrates the trend of decreasing OAR dose as the spot sizes decrease and beam-shaping hardware are added. For all cases, the NTCP with acute toxicity endpoints for PBS was either equivalent or slightly smaller than PSPT. Absolute NTCP of up to 8.4% was found, and the largest NTCP reduction was 5.4% for patient 10 lens, when using small spots. Other examples were cochleas for patients 5 and 11 where NTCP decrease by 4.0% and 3.0% for small spots relative to PSPT, respectively. Also for patient 14 (figure 2), the brainstem NTCP was decreased by up to 1.5%. In all cases, NTCP decreased when reducing the spot size and using devices.
Figure 4.
Percent difference of Dmean, EUD and V90 between PSPT and all PBS plans for all OARs (averaged over number of patients shown in parenthesis). Error bars represent standard deviations.
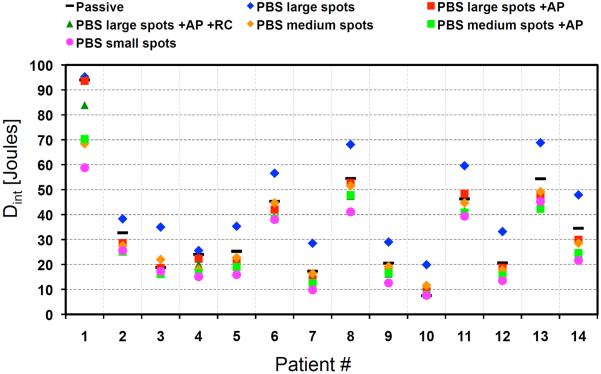
The integral doses for all plans are quantified in figure 5. As expected, plans with large spots had the largest Dint, which was on average 10.4 J and up to 16.2 J larger than PSPT. Dint decreased below PSPT after adding beam-shaping devices and decreasing the spot size. On average, Dint decreased by 6.3, 16.2, 4.9, 19.7 and 26.2% for PBS plans 2 through 6, respectively.
Figure 5.
Integral dose for PSPT and PBS plans for all patients.
The findings were similar for cranial and thoracic/pelvic patient groups and no significant site dependence was observed, despite differences in tumor depth and size.
4. Effect of reducing the number of beams
Simplifying the beam configuration for a representative subgroup yielded overall comparable plans to the original ones. Omitting some beams resulted in slight rise in mean dose in some organs while sparing others, due to the redistribution of the dose. For example, in case of patient 7, for equivalent target coverage, eliminating the posterior field decreased the brainstem Dmean by 6.6% at the cost of increasing the optic nerves Dmean by 4.6%.
Discussion
This study was designed to not only guide on the possible benefits and limitations of PBS versus PSPT, which is currently the primary clinical practice for pediatric patients, but mainly to assess the dependence of PBS plan quality upon delivery specifications. We included a cohort of patients with cranial/head & neck and thoracic/pelvic solid tumors. The PBS beam spot size was varied to simulate the beam characteristics available at different institutions. Furthermore, the impact of applying AP and RC to plans with larger spots was examined. We found that if the spot size is large, as could be the case in facilities that have implemented a PBS system within their operating double scattering nozzle, the plans could be dosimetrically inferior to PSPT. As the spot size is reduced the penumbrae will shrink and so will the dose to the healthy organs, making the plans more clinically desirable. However, if the possibility of upgrading to smaller spot sizes is not available, beam-specific AP and RC similar to ones used in PSPT could be applied to achieve clinically superior plans to PSPT. We also found that simplifying the beam configuration could yield dosimetrically equivalent plans to ones with beams optimized to PSPT, hence not affecting the general conclusions.
Our results agreed with the findings of Hyer et al [29] who saw a 40% reduction of mean normal tissue dose when using collimators for 9 mm spot size in air. Since their definition of normal tissue included the entire non-tumor region in a water tank, it was comparable to our results on integral dose that was decreased by on average 32% when adding AP to large spots.
Although NTCP for most organs studied were not significantly different between plans due to relatively extreme endpoints (e.g. necrosis, blindness, etc), considering smaller thresholds made large differences in terms of late toxicities. For example, according to previous study by Fuss et al [36], doses of 32 and 39 Gy to the whole brain could cause IQ drop in children to below 90 and 80 points, respectively. Using these numbers, we found up to 38.0% and 6.4% smaller chance of IQ reduction to below 90 and 80 points, respectively, for PBS with small spots compared to PSPT. We also found considerable reductions in NTCP for hypothalamus when considering growth hormone deficiency as an endpoint [37].
While including AP could largely improve PBS plans, these devices are known to be the primary contributors to the scatter neutron dose in organs away from the treatment field in PSPT. For PBS, however, this dose has been found to be much smaller than PSPT [24,38] due to the fact that in PBS apertures only trim the penumbrae rather than blocking a portion of the fields as done in PSPT. Furthermore, although large spots with no devices were inferior to PSPT for pediatric cases studied, it might still be beneficial for treatment of very large tumors due to considerable simplification of treatment planning and delivery [21].
It should be noted that PSPT and PBS plans were created using different planning systems and optimization algorithms. However, both dose calculation engines were based on Hong's pencil beam algorithm [39]. Therefore, differences are expected to be negligible and not impact our general conclusions.
When considering the comparison between PBS and PSPT, the issue of robustness was not taken into account in this work. Plan robustness to setup/range uncertainty as a function of spot size for IMPT should be thoroughly investigated. This is especially worth exploring for facilities with smaller spot size and for patient sites with highly mobile and variable tumors. Furthermore, adding AP and RC could make plans more susceptible to loss of robustness, due to increased conformity and sharper dose falloffs.
Conclusion
For the patient cohort studied, we can conclude that PBS plan quality improves as the spot size decreases and/or beam-shaping devices are included. We recommend that institutions whose available spot size exceeds ~8 mm median sigma at isocenter for 90-230 MeV range to consider using AP and even RC depending on the patient case. Hence, PBS is the favorable proton therapy modality to treat pediatric patients due to the apparent clinical and dosimetric advantages.
Acknowledgements
This project was supported by the NCI Federal Share of Program income earned by Massachusetts General Hospital on C06 CA059267. The authors would like to give special thanks to Judy Adams for her support and suggestions on alternative PBS beam configurations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: none
References
- 1.Goitein M. Magical protons? Int J Radiat Oncol Biol Phys. 2008;70:654–6. doi: 10.1016/j.ijrobp.2007.10.057. [DOI] [PubMed] [Google Scholar]
- 2.Steneker M, Lomax AJ, Schneider U. Intensity modulated photon and proton therapy for the treatment of head and neck tumors. Radiother Oncol. 2006;80:263–267. doi: 10.1016/j.radonc.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 3.Efstathiou JA, et al. Adjuvant radiation therapy for early stage seminoma: proton versus photon planning comparison and modeling of second cancer risk. Radiother Oncol. 2012;103:12–7. doi: 10.1016/j.radonc.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 4.Olsen DR, et al. Proton therapy - a systematic review of clinical effectiveness. Radiother Oncol. 2007;83:123–32. doi: 10.1016/j.radonc.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Kandula S, Zhu X, Garden AS, et al. Spot scanning beam proton therapy vs intensity-modulated radiation therapy for ipsilateral head and neck malignancies: a treatment planning comparison. Med Dosim. 2013;38:390–4. doi: 10.1016/j.meddos.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Yock TI, Tarbell NJ. Technology insight: Proton beam radiotherapy for treatment in pediatric brain tumors. Nat Clin Pract Oncol. 2004;1:97–103. doi: 10.1038/ncponc0090. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman KE, Yock TI. Radiation therapy for pediatric central nervous system tumors. J Child Neurol. 2009;24:1387–96. doi: 10.1177/0883073809342275. [DOI] [PubMed] [Google Scholar]
- 8.Yock TI, et al. Proton Radiotherapy for Ependymoma: Initial Clinical Outcomes and Dose Comparisons for Intensity Modulated Radiation With Photons, Proton Radiation, and Intensity Modulated Proton Therapy. Int J Radiat Oncol Biol Phys. 2007;69:S575–S576. [Google Scholar]
- 9.Yock TI, et al. Results from a Prospective Trial of Proton Radiotherapy for Medulloblastoma: Clinical Outcomes including Hearing and Neurocognitive. Int J Radiat Oncol Biol Phys. 2011;81:S113. [Google Scholar]
- 10.Armstrong GT, Stovall M, Robison LL. Long-term effects of radiation exposure among adult survivors of childhood cancer: results from the childhood cancer survivor study. Radiat Res. 2010;174:840–50. doi: 10.1667/RR1903.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hudson MM, et al. High-risk populations identified in Childhood Cancer Survivor Study investigations: implications for risk-based surveillance. J Clin Oncol. 2009;27:2405–14. doi: 10.1200/JCO.2008.21.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meadows AT, et al. Second neoplasms in survivors of childhood cancer: findings from the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27:2356–62. doi: 10.1200/JCO.2008.21.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neglia JP, et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2006;98:1528–37. doi: 10.1093/jnci/djj411. [DOI] [PubMed] [Google Scholar]
- 14.Oeffinger KC, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–82. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 15.Meeske KA, et al. Factors associated with health-related quality of life in pediatric cancer survivors. Pediatr Blood Cancer. 2007;49:298–305. doi: 10.1002/pbc.20923. [DOI] [PubMed] [Google Scholar]
- 16.Diller L, et al. Chronic disease in the Childhood Cancer Survivor Study cohort: a review of published findings. J Clin Oncol. 2009;27:2339–55. doi: 10.1200/JCO.2008.21.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duffner PK. Risk factors for cognitive decline in children treated for brain tumors. European J Pedi Neurology. 2010;14:106–115. doi: 10.1016/j.ejpn.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Zacharatou Jarlskog C, Paganetti H. Risk of developing second cancer from neutron dose in proton therapy as function of field characteristics, organ, and patient age. Int J Radiat Oncol Biol Phys. 2008;72:228–35. doi: 10.1016/j.ijrobp.2008.04.069. [DOI] [PubMed] [Google Scholar]
- 19.Paganetti H, et al. Assessment of radiation-induced second cancer risks in proton therapy and IMRT for organs inside the primary radiation field. Phys Med Biol. 2012;57:6047–61. doi: 10.1088/0031-9155/57/19/6047. [DOI] [PubMed] [Google Scholar]
- 20.Athar BS, Bednarz B, Seco J, Hancox C, Paganetti H. Comparison of out-of-field photon doses in 6 MV IMRT and neutron doses in proton therapy for adult and pediatric patients. Phys Med Biol. 2010;55:2879–91. doi: 10.1088/0031-9155/55/10/006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kooy HM, et al. A case study in proton pencil beam scanning delivery. Int J Radiat Oncol Biol Phys. 2010;76:624–30. doi: 10.1016/j.ijrobp.2009.06.065. [DOI] [PubMed] [Google Scholar]
- 22.Clasie B, Depauw N, Fransen M, et al. Golden beam data for proton pencil-beam scanning. Phys Med Biol. 2012;57:1147–58. doi: 10.1088/0031-9155/57/5/1147. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lomax AJ, et al. Treatment planning and verification of proton therapy using spot scanning: initial experiences. Med Phys. 2004;31:3150–7. doi: 10.1118/1.1779371. [DOI] [PubMed] [Google Scholar]
- 24.Clasie B, Wroe A, Kooy H, et al. Assessment of out-of-field absorbed dose and equivalent dose in proton fields. Med Phys. 2010;37:311–321. doi: 10.1118/1.3271390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D, Dirksen B, Hyer D, et al. Impact of spot size on plan quality of spot scanning proton radiosurgery for peripheral brain lesions. Med Phys. 2014;41:121705. doi: 10.1118/1.4901260. [DOI] [PubMed] [Google Scholar]
- 26.van de Water TA, Lomax AJ, Bijl HP, Schilstra C, Hug EB, Langendijk JA. Using a reduced spot size for intensity-modulated proton therapy potentially improves salivary gland-sparing in oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2012;82:e313–9. doi: 10.1016/j.ijrobp.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Chanrion MA, Ammazzalorso F, Wittig A, Engenhart-Cabillic R, Jelen U. Dosimetric consequences of pencil beam width variations in scanned beam particle therapy. Phys Med Biol. 2013;58:3979–93. doi: 10.1088/0031-9155/58/12/3979. [DOI] [PubMed] [Google Scholar]
- 28.Dowdell SJ, Clasie B, Depauw N, et al. Monte Carlo study of the potential reduction in out-of-field dose using a patient-specific aperture in pencil beam scanning proton therapy. Phys Med Biol. 2012;57:2829. doi: 10.1088/0031-9155/57/10/2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hyer DE, Hill PM, Wang D, Smith BR, Flynn RT. Effects of spot size and spot spacing on lateral penumbra reduction when using a dynamic collimation system for spot scanning proton therapy. Phys Med Biol. 2014;59:N187–N196. doi: 10.1088/0031-9155/59/22/N187. [DOI] [PubMed] [Google Scholar]
- 30. XXX.
- 31.Craft DL, Hong TS, Shih HA, Bortfeld TR. Imroved planning time and plan quality through multicriteria optimization for intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82:e83–90. doi: 10.1016/j.ijrobp.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feuvret L, Noël G, Mazeron J-J, Bey P. Conformity index: A review. Int J Radiat Oncol Biol Phys. 2006;64:333–342. doi: 10.1016/j.ijrobp.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 33.Niemierko A. A generalized concept of equivalent uniform dose (EUD) (Abstract) Med phys. 1999;26:1100. [Google Scholar]
- 34.Gay HA, Niemierko A. A free program for calculating EUD-based NTCP and TCP in external beam radiotherapy. Physica Medica. 2007;23:115–125. doi: 10.1016/j.ejmp.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Oinam AS, Singh L, Shukla A, Ghoshal S, Kapoor R, Sharma SC. Dose volume histogram analysis and comparison of different radiobiological models using in-house developed software. J Med Phys. 2011;36:220–229. doi: 10.4103/0971-6203.89971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuss M, Poljanc K, Miller DW, Archambeau JO, Slater JM, Slater JD, Hug EB. Normal tissue complication probability (NTCP) calculations as a means to compare proton and photon plans and evaluation of clinical appropriateness of calculated values. Int J Cancer. 2000;90:351–358. doi: 10.1002/1097-0215(20001220)90:6<351::aid-ijc7>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 37.Merchant TE, Rose SR, Bosley C, Wu S, Xiong X, Lustig RH. Growth hormone secretion after conformal radiation therapy in pediatric patients with localized brain tumors. J Clinical Oncol. 2011;29:4776–80. doi: 10.1200/JCO.2011.37.9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phys Med Biol. XXX. In press.
- 39.Hong L, et al. A pencil beam algorithm for proton dose calculations. Phys Med Biol. 1996;41(8):1305–1330. doi: 10.1088/0031-9155/41/8/005. [DOI] [PubMed] [Google Scholar]