Abstract
Purpose
This study was designed to provide the foundation for combining immunotherapy to induce tumor antigen-specific T cells with proton radiation therapy to exploit the activity of those T cells. We have recently defined “immunogenic modulation,” a mechanism distinct from immunogenic cell death, whereby tumor cells surviving photon radiation therapy nonetheless become more susceptible to cytotoxic T lymphocyte (CTL)-mediated lysis. However, to our knowledge, there are no prior studies examining the role of proton radiation on tumor immune sensitivity.
Materials and Methods
Using cell lines of tumors frequently treated with proton radiation, such as prostate, breast, lung, and chordoma, we examined the effect of proton radiation on the viability and induction of immunogenic modulation in tumor cells by flow cytometric and immunofluorescent analysis of surface phenotype and the functional immune consequences.
Results
These studies show for the first time that a) proton and photon radiation induced comparable upregulation of surface molecules involved in immune recognition (HLA, ICAM-1, and the tumor-associated antigens CEA and MUC-1), b) proton radiation mediated calreticulin cell-surface expression, increasing sensitivity to cytotoxic T-lymphocyte killing of tumor cells, and c) cancer stem cells (CSCs), which are resistant to the direct cytolytic activity of proton radiation, nonetheless upregulated calreticulin after radiation in a manner similar to non-CSCs.
Conclusions
These findings offer a rationale for the use of proton radiation in combination with immunotherapy, including for patients who have failed radiation therapy alone or have limited treatment options.
Keywords: proton radiation, photon radiation, immunogenic modulation, calreticulin, CTL, cancer stem cells
Introduction
Radiation therapy, a standard of care for many tumor types, may not kill all cells in a given tumor due to a) reduced dosing needed to limit damage to healthy tissues and b) intrinsic and extrinsic causes of tumor radioresistance. We have recently defined “immunogenic modulation,” a mechanism distinct from immunogenic cell death (ICD), whereby tumor cells surviving anticancer therapy nonetheless become more susceptible to cytotoxic T lymphocyte (CTL)-mediated lysis. This mechanism of immunogenic modulation has been described with chemotherapy and photon radiation [1,2]. Critical determinants of this novel mechanism are upregulation of surface molecules involved in immune recognition and translocation of calreticulin to the tumor-cell surface [1,3–5].
Photon radiation is highly penetrating, delivering radiation throughout tumor and normal tissue. Thus, dosing may be suboptimal in order to limit damage to healthy tissues. Heavy-particle radiation has been increasingly employed to improve dose conformally to the tumor while sparing normal tissue. The heavy particle enters tissue and deposits a minimal radiation dose on its track to the tumor. The radiation dose increases very gradually with greater depth and lower speed, suddenly rising to a peak, known as the Bragg peak, when the proton is ultimately stopped [6]. However, due to the unique physical properties of this radiation modality, it is unknown if heavy-particle radiation can sensitize tumor cells to immune-mediated killing. We hypothesized that heavy-particle radiation could induce immunogenic modulation of tumor-cell phenotype and upregulate calreticulin on the tumor-cell surface, thereby enhancing productive interactions between CD8+ CTLs and tumor cells.
Materials and Methods
Tumor-cell lines
We focused on cell lines representative of tumors frequently treated with proton radiation; prostate, breast, lung, and chordoma. Cells of human prostate carcinoma (LNCaP clone FGC [ATCC® CRL-1740™], breast carcinoma (MDA-MB-231 [ATCC® HTB-26™]), lung carcinoma (NCI-H1703 [ATCC® CRL-5889™]) were obtained from American Type Culture Collection (Manassas, VA). The chordoma cell line JHC7 was obtained from the Chordoma Foundation (Durham, NC). The negative control cell line representing carcinoma of the pancreas (AsPC-1 [ATCC® CRL-1682™]) was obtained from ATCC. All cell lines were cultured in medium designated by the provider for propagation and maintenance.
Tumor irradiation
Adherent tumor cells in log-growth phase were mock irradiated (0 Gy) or irradiated (8 Gy) with a single dose of photons (137Cs source [Gammacell-40, AECL/Nordion; Kanata, Ontario, Canada]), or 8 Gy/BCGE (cobalt-Gray equivalents) protons, using a passive scattering proton beam (Hitachi ProBeat [Hitachi, Ltd., Tokyo, Japan,]). The energy of the incident proton beam was 200 MeV with a distal 90% range of 19 cm and a spread-out Bragg peak (SOBP) width of 10 cm. The relative biological effectiveness value used to convert the physical dose to Gy was 1.1. The inner walls of the flasks were placed at the center of the SOBP, at a water equivalent depth of 14 cm. An 18×18-cm field size was chosen to encompass the entire flask for homogenous dose delivery. Cells were then washed in fresh medium and incubated at 37°C with 5% CO2. Cells exposed to proton radiation at MD Anderson (TX) were shipped to the NCI (MD) for subsequent analysis. To control for potential shipping stress, each data set is controlled within itself.
CD8+ cytotoxic T lymphocytes (CTLs)
CEA-specific CTLs recognize the CEA epitope YLSGANLNL (CAP-1) [7,8]. Prostate-specific antigen (PSA)-specific CTLs recognize the PSA epitope VLSNDVCAQV [9]. The HLA-A2- and HLA-A24-restricted MUC-1-specific CD8+ CTL lines, designated MUC-1 CTL, recognize the MUC-1 epitopes ALWGQDVTSV and TYHPMSEYPT [9,10]. Brachyury-specific CTLs recognize the brachyury epitope WLLPGTSTL (T-p2) [11]. All T-cell lines were HLA-restricted.
Cytotoxicity assays
CTLs were used as previously described [2]. At 96 h post-irradiation, adherent cells were used as targets in a standard cytotoxicity assay using 111In [4,12]. Here, live tumor cells are metabolically labeled with 111In-labeled oxyquinoline (Medi-Physics Inc., Arlington Heights, IL) and coincubated in 96-well round-bottom plates at 37°C/5% CO2 with HLA restricted T-cells. For indicated experiments, the CTL assay was performed in the presence of calreticulin-blocking peptide (CBP) (MBL International, Woburn, MA, 1.7 µM, 1.43 kDa) or control lymphocytic choriomeningitis virus peptide LCMV NP118–132 (RPQASGVYMGNLTAQ; CPC Scientific, Sunnyvale, CA).
Flow cytometry analysis
At 96 h post-irradiation, tumor cells were examined by flow cytometry, as previously described [13]. Cells were examined on FACSCalibur or FACSVerse cytometers, using the monoclonal antibodies targeting HLA-ABC-FITC, HLA-ABC-PE-Cy7, ICAM-1 (CD54)-PE, CEA (CD66)-FITC, MUC-1 (CD227)-FITC, CD24-PerCP-Cy5.5, CD44-FITC, and the appropriate isotype-matched controls (BD Biosciences, San Jose, CA). The monoclonal antibodies targeting CD70-FITC, CD275 (ICOSL)-PE, CD137L (4-1BBL)-PE, CD252 (OX40L)-PE, PD-L1 (CD274)-PE, CTLA-4 (CD152)-PE, and CD227 (MUC-1)-PE were obtained from BioLegend (San Diego, CA). Antibodies targeting CD133-APC (Miltenyi Biotec, San Diego, CA) and calreticulin-PE (R&D Systems, Minneapolis, MN) were also used. Isotype control staining was < 5% for all samples analyzed. Viability was examined using LIVE/DEAD Fixable Violet Dead Stain Kit (Thermo Fisher Scientific, Rockville, MD). Cell surface expression was evaluated on live cells gated by FSC/SSC and LIVE/DEAD staining.
Immunofluorescence and confocal microscopy
At 96 h post-exposure to photon or proton radiation, expression of calreticulin and HLA-ABC was examined in 1×105 cells deposited onto microscope slides using cytocentrifugation (Shandon Cytospin, Thermo Fisher Scientific) and fixed in 3% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA). After permeabilization with 0.05% Triton X-100, cells were incubated with monoclonal antibodies (1:100 dilution) targeting calreticulin (Abcam, San Francisco, CA) or HLA-ABC (BD Biosciences), followed by an Alexa Fluor 488 conjugated secondary antibody (1:250 dilution). Cells were counterstained with DAPI (Thermo Fisher), and examined by fluorescence (Leica Microsystems, Buffalo Grove, IL) or confocal (Zeiss, Dublin, CA) microscopy. Relative calreticulin and HLA-ABC expression levels were calculated using Image J software by normalizing the intensity values to their respective mock-irradiated controls.
Statistical analysis
Significant differences among treatment groups were determined by unpaired Student’s t test with a 2-tailed distribution. The effect of CBP on CTL sensitivity was examined by 1-way ANOVA with Tukey’s multiple comparison test. All statistical analyses were based on a confidence interval of 95% using Prism 6.0f software (GraphPad Software Inc., La Jolla, CA), and reported as P values.
Results
Human tumor cells of diverse origin recovering from photon or proton radiation show similar patterns of immunogenic modulation
We have previously shown that human carcinoma cells recovering from sublethal exposure to photon radiation harbor multiple changes in the expression of proteins involved in immune recognition, including of TAAs and ICAM-1 [14]. Termed ‘immunogenic modulation’, this process has been shown to be distinct from that of immunogenic cell death [14]. Here, we sought to examine if human carcinoma cells recovering from exposure to proton radiation harbor a similar immunogenic modulation signature. Prostate (LNCaP), breast (MDA-MB-231), lung (NCI-H1703), and chordoma (JHC7) tumor cells were mock irradiated (0 Gy) or exposed to proton or photon radiation in a single dose of 8 Gy (Table 1). After recovering for 96 h, tumor cells were examined for cell-surface expression of HLA-ABC, the tumor-associated antigens (TAAs) CEA and MUC-1, as well as ICAM-1. As shown in Table 1, exposure of LNCaP cells to proton or photon radiation significantly increased expression of HLA-ABC, CEA, MUC-1, and ICAM-1. Similar results were observed in breast carcinoma cells. Both modalities of radiation upregulated these proteins to a similar extent in lung and chordoma cell lines. LNCaP cells were also evaluated for changes in expression of positive and negative costimulatory molecules (Supplemental Table 1). Proton radiation upregulated expression of costimulatory molecules CD70 and ICOS-L, while downregulating expression of the inhibitor molecule PD-L1.
Table 1.
Human tumor cells of diverse origin recovering from photon or proton radiation harbor similar patterns of immunogenic modulation on the cell surface.
| Surface Expression: % Positive cells (MFI) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gy | Viability | HLA | CEA | MUC-1 | ICAM-1 | Fraction per cell line |
|||
| Radiation Modality | LNCaP | Photon | 0 | 95 | 92(104) | 2 (27) | 8 (27) | 3 (30) | |
| 8 | 84 | 92 (425) | 15 (181) | 8 (70) | 6 (53) | 4/4 | |||
| Proton | 0 | 96 | 99 (123) | 2 (21) | 9 (32) | 3 (38) | |||
| 8 | 86 | 95 (304) | 9 (102) | 12 (58) | 6 (750) | 4/4 | |||
| MDA-MB-231 | Photon | 0 | 78 | 99 (415) | 4 (38) | 17 (138) | 90 (250) | ||
| 8 | 65 | 99 (886) | 4 (220) | 31 (210) | 95 (1110) | 4/4 | |||
| Proton | 0 | 91 | 99 (390) | 3 (63) | 4 (13) | 69 (129) | |||
| 8 | 76 | 99 (682) | 4 (109) | 14 (81) | 86 (751) | 4/4 | |||
| H1703 | Photon | 0 | 92 | 99 (633) | 2 (39) | 47 (25) | 7 (40) | ||
| 8 | 77 | 99 (930) | 2 (190) | 25 (119) | 25 (119) | 3/4 | |||
| Proton | 0 | 96 | 99 (409) | 2 (31) | 21 (32) | 4 (34) | |||
| 8 | 81 | 99 (501) | 2 (52) | 27 (47) | 16 (45) | 3/4 | |||
| JHC7 | Photon | 0 | 83 | 98 (536) | 5 (239) | 11 (120) | 57 (235) | ||
| 8 | 76 | 98 (580) | 10 (180) | 12 (113) | 63 (243) | 2/4 | |||
| Proton | 0 | 88 | 99 (579) | 13 (133) | 10 (99) | 87 (254) | |||
| 8 | 81 | 99 (667) | 15 (179) | 12 (102) | 90 (395) | 3/4 | |||
| 28/32 (87.5%) | |||||||||
Human prostate (LNCaP), breast (MDA-MB-231), lung (H1703), and chordoma (JHC7) carcinoma cells were mock-irradiated (0 Gy) or exposed to a single dose of 8 Gy photon or proton radiation. After 96 h, cells were analyzed by flow cytometry. Viability was determined for the entire cell populations by live/dead staining as described in materials and methods. For surface expression of HLA-ABC (MHC-I), CEA, MUC-1, and ICAM-1, analysis was conduced on gated viable cells. Numbers indicate percentage of positive cells. Numbers in parentheses denote mean fluorescence intensity (MFI). Bold type indicates marked upregulation (≥ 10% increase in percent of cells or 50% increase in MFI not observed in isotype control vs. untreated cells). Data shown is representative of experiment repeated 3 times with similar results.
Exposure of tumor cells to sublethal doses of photon or proton radiation significantly increases expression of histocompatibility leukocyte antigens
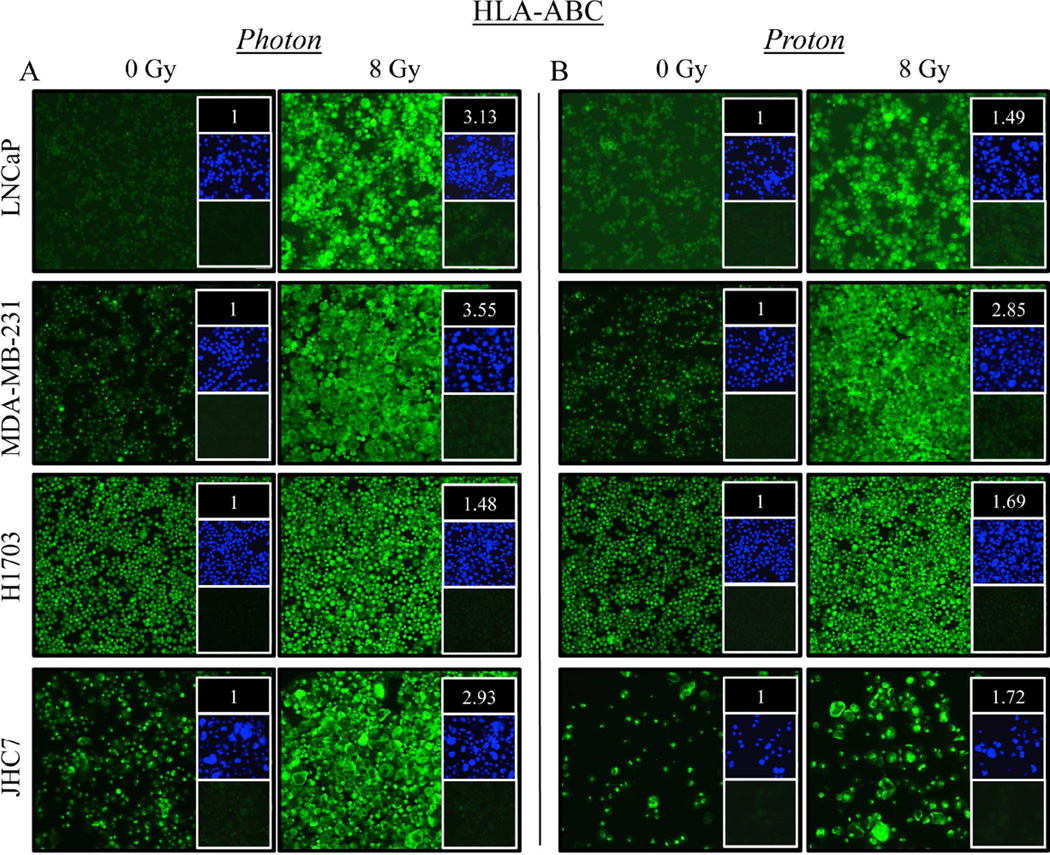
We next examined the cellular expression of histocompatibility leukocyte antigens A, B, and C (HLA-ABC) by immunofluorescence. As shown in Figure 1A, HLA-ABC expression in LNCaP cells exposed to photon irradiation increased 3.13-fold relative to controls. A marked upregulation of HLA-ABC was also observed upon exposure of LNCaP cells to proton radiation (Fig. 1B), albeit to a lesser degree. In contrast, both photon (3.55-fold) and proton (2.85-fold) radiation induced similar upregulation of HLA-ABC in MDA-MB-231 cells relative to mock-irradiated controls. Similar results were seen with H1703 and JHC7 cells.
Figure 1. Exposure of distinct human tumor types to sublethal doses of photon or proton radiation significantly increases expression of histocompatibility leukocyte antigens.
Human prostate (LNCaP), breast (MDA-MB-231), lung (H1703), and chordoma (JHC7) cells were mock irradiated (0 Gy) or exposed to a single dose of 8 Gy (A) photon or (B) proton radiation. After 96 h, HLA-ABC (green) expression was examined by immunofluorescence (10× magnification). Upper inset: HLA-ABC mean fluorescence intensity (MFI) normalized to controls. Middle inset: DAPI nuclear stain (blue). Lower inset: isotype control. Data are representative of 2 independent experiments.
Exposure of human carcinoma cells to sublethal doses of photon or proton radiation significantly increases sensitivity to antigen-specific CTL lysis
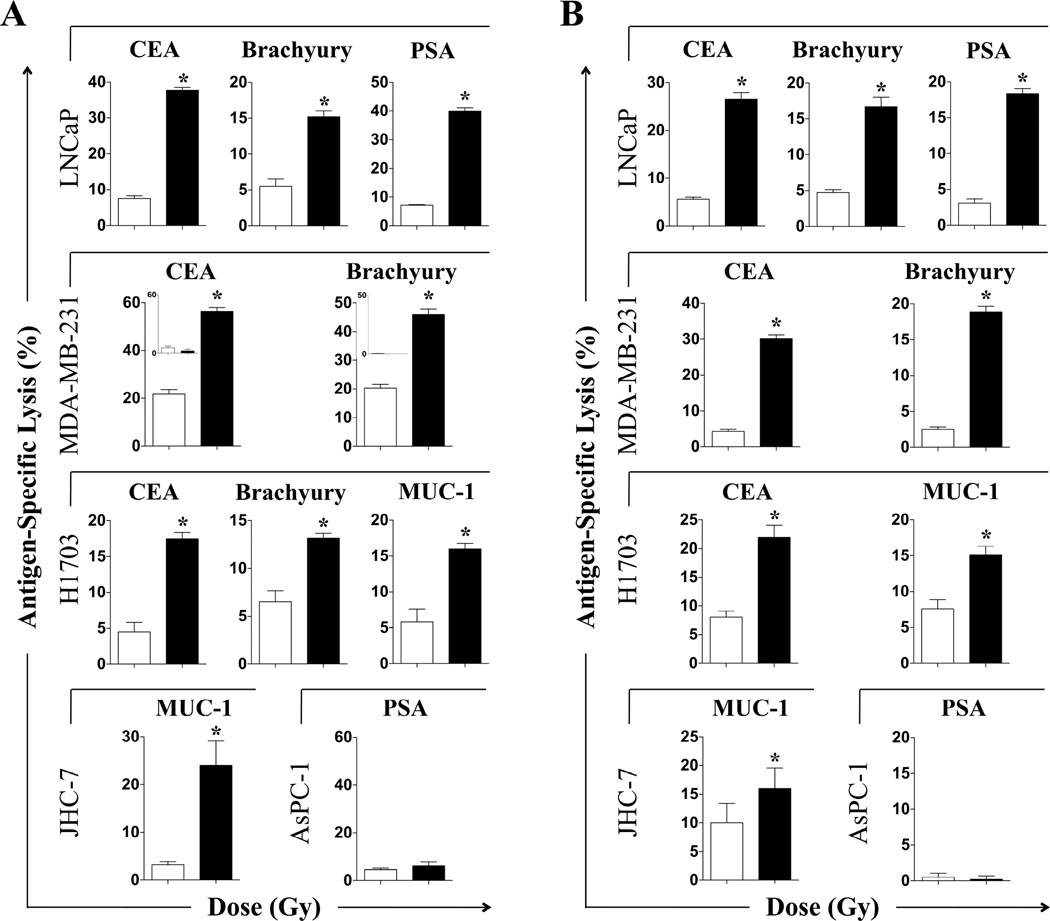
After exposure to photon radiation, LNCaP cells were significantly more sensitive to CEA- and brachyury-specific T-cell lysis (P < 0.0001 for both) (Fig. 2A). Exposure to photon radiation significantly increased the sensitivity of MDA-MB-231 and H1703 cells to CTLs specific for CEA and brachyury (P < 0.0001 for both). JHC7 and LNCaP cells were also more sensitive to MUC-1- or PSA-specific lysis relative to controls after photon radiation (P < 0.0001 for both). CTL killing was MHC I-restricted as determined by absence of significant lysis of HLA-A2/-A24 negative AsPC-1 carcinoma cells, after 8 Gy or mock irradiation (Fig. 2A, lower right panel and insets). Similar results were observed with LNCaP, MDA-MB-231, H1703, and JHC7 cells 96 h post-proton irradiation (Fig. 2B).
Figure 2. Exposure of human carcinoma cells to sublethal doses of photon or proton radiation significantly increases sensitivity to antigen-specific CTL lysis.
Human prostate (LNCaP), breast (MDA-MB-231), lung (H1703), and chordoma (JHC7) tumor cells were mock-irradiated (0 Gy; open bars) or exposed to a single dose of 8 Gy (closed bars) (A) photon or (B) proton radiation. After 96 h, cells were used as targets in a CTL-lysis assay using CEA-, MUC-1-, brachyury-, or PSA-specific CD8+ T cells as effectors. To verify that effector T cells were HLA-restricted, CTLs were incubated with CEA+, HLA-A2/A24− AsPC-1 carcinoma cells (MDA-MB-231 inset, panel A). To verify antigen specificity, PSA-specific CTLs were incubated with PSA− AsPC-1 cells exposed to 0 or 8 Gy (panel A, lower right). Results are presented as mean ± S.E.M. from 3–6 replicate wells. This experiment was repeated 1–3 times with similar results. *, statistical significance relative to controls.
Carcinoma cells recovering from sublethal exposure to photon or proton radiation have increased cell-surface expression of calreticulin
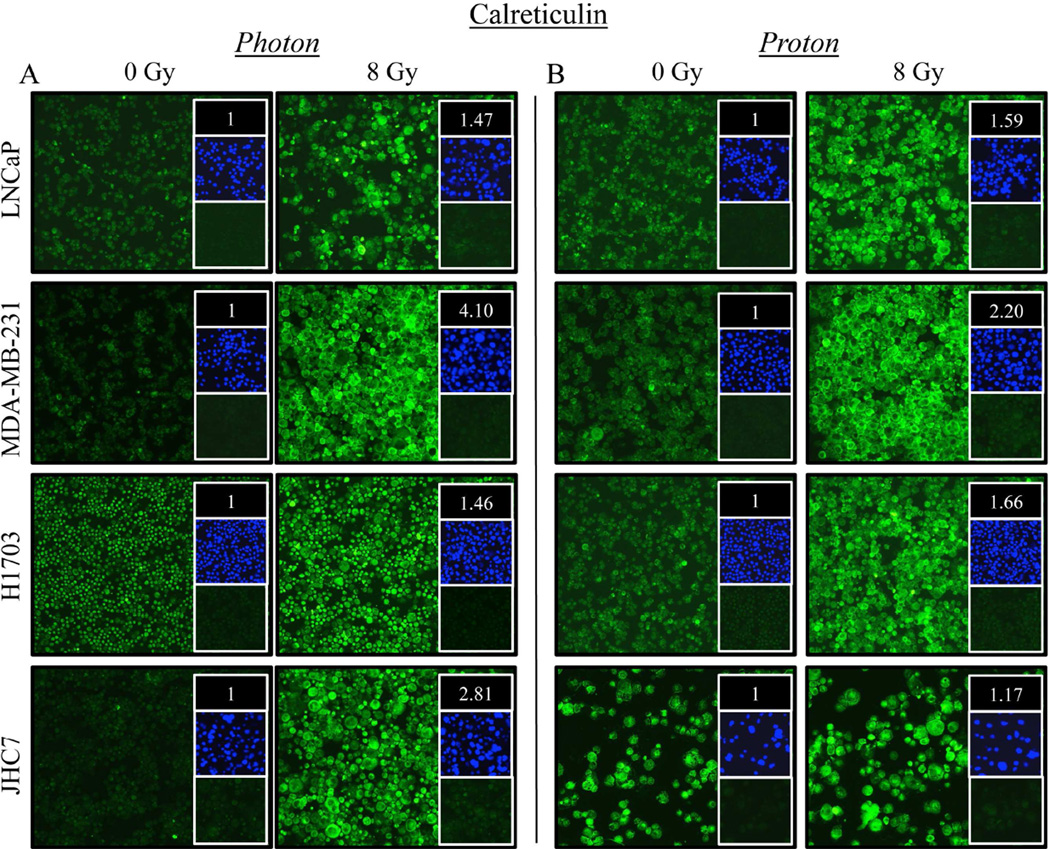
Prostate carcinoma cells exposed to photon radiation had a 47% increase in surface and intracellular expression of calreticulin (Fig. 3A). A similar increase in calreticulin expression (59%) was observed in cells exposed to proton radiation relative to mock-irradiated control cells (Fig. 3B). A comparable magnitude of calreticulin upregulation was also observed in H1703 cells, where calreticulin expression increased 46% and 66% after exposure to photon (Fig. 3A) or proton (Fig. 3B) radiation, respectively. Significant increases in calreticulin expression were also observed in breast carcinoma and chordoma cells after exposure to either type of radiation, albeit to different extents.
Figure 3. Carcinoma cells recovering from exposure to photon or proton radiation have increased surface and intracellular expression of calreticulin.
Calreticulin (green) expression was examined by immunofluorescence (10× magnification) 96 h after a single 8-Gy dose of (A) photon or (B) proton radiation of human prostate (LNCaP), breast (MDA-MB-231), lung (H1703), or chordoma (JHC7) cells. Upper inset: MFI normalized to that of mock-irradiated controls. Middle inset: DAPI nuclear stain (blue). Lower inset: isotype control. Data are representative of 2 independent experiments. This experiment was repeated 2 times with similar results.
Carcinoma cells recovering from exposure to photon or proton radiation show increased calreticulin expression on the cell surface, resulting in heightened sensitivity to CTL-mediated killing
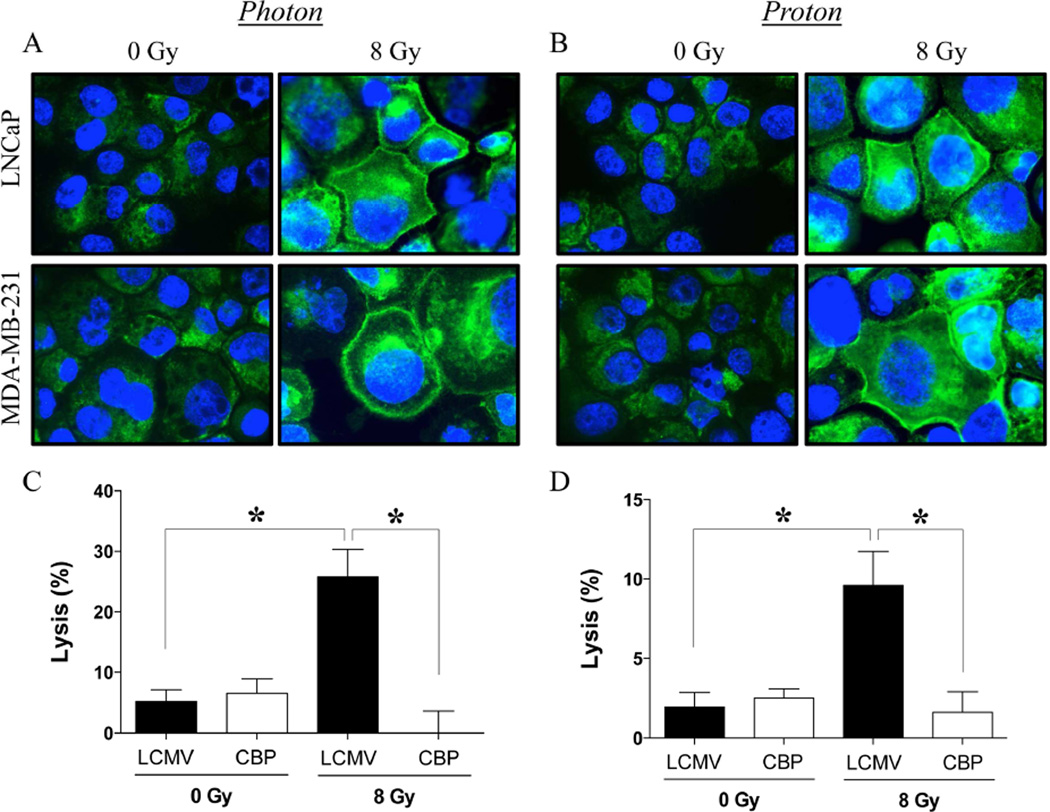
Increased calreticulin cell-surface expression was observed in prostate and breast carcinoma cells after exposure to both photon (Fig. 4A) and proton (Fig. 4B) radiation. MDA-MB-231 cells were used as targets for CEA-specific CTL lysis after radiation exposure, in the presence of an LCMV peptide control or a calreticulin-blocking peptide (CBP), previously shown to prevent interaction between surface calreticulin and CTLs [14,15]. As shown in Figure 4C, MDA-MB-231 cells recovering from exposure to photon radiation and co-incubated with control peptide were lysed by CEA-specific T cells to a significantly greater extent than mock-irradiated controls (P = 0.0003). However, in the presence of a CBP, the photon-induced increase in CTL lysis of MDA-MB-231 carcinoma cells was completely abrogated (P = 0.0003). Further, in the presence of LCMV peptide, proton-irradiated MDA-MB-231 cells were also significantly more sensitive to CEA-specific CTL killing (Fig. 4D, P = 0.002). However, in the presence of a CBP, the level of CTL lysis of proton-irradiated MDA-MB-231 cells was significantly reduced (P = 0.002).
Figure 4. Carcinoma cells recovering from exposure to photon or proton radiation show increased calreticulin expression on the cell surface, resulting in heightened sensitivity to CTL-mediated killing.
Cell-surface expression of calreticulin (green) was examined by confocal immunofluorescence (10× magnification) in human prostate (LNCaP) and breast (MDA-MB-231) cells 96 h after a single dose of 8 Gy (A) photon or (B) proton radiation. Blue denotes DAPI nuclear stain. (C–D) Functional role of cell-surface calreticulin on CTL-mediated lysis. MDA-MB-231 cells were mock irradiated (0 Gy) or exposed to a single dose of 8 Gy (C) photon or (D) proton radiation. After 96 h, cells were used as targets in a CEA-specific CTL-lysis assay in the presence of calreticulin-blocking peptide (CBP, open bars) or LCMV control peptide (closed bars). Results are presented as mean ± S.E.M. from 6 replicate wells. *, statistical significance relative to controls.
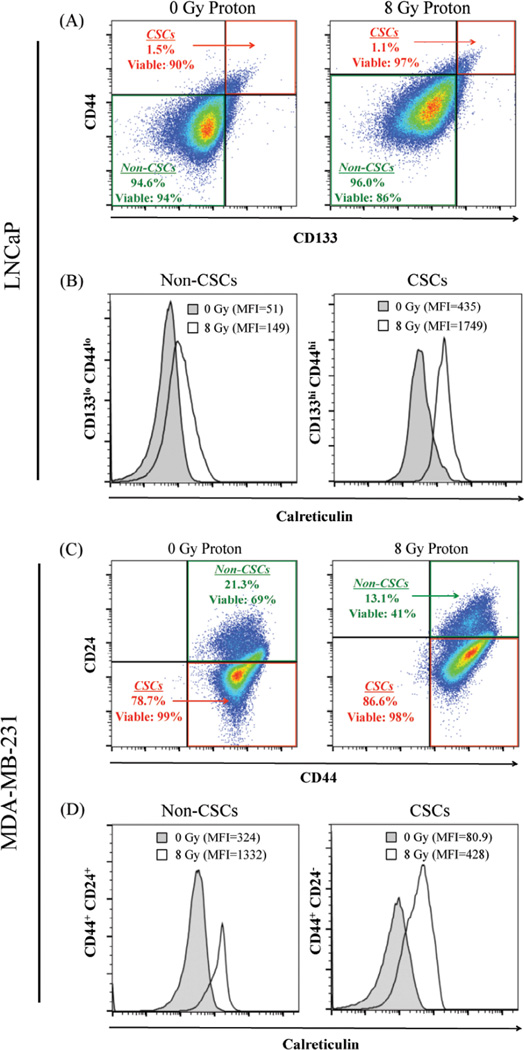
Residential stem-like carcinoma cells exposed to sublethal photon or proton radiation show increased calreticulin expression on the cell surface
As shown in Figure 5A, exposure of prostate carcinoma cells to proton radiation had no significant effect on the viability of stem-like LNCaP populations (CD133hi CD44hi), but some effect on the viability of non-stem-like cells (CD133lo CD44lo). However, exposure to proton radiation significantly increased cell-surface expression of calreticulin in both non-stem-like (2.92-fold) and stem-like (4-fold) cell populations (Fig. 5B). Proton irradiation of MDA-MB-231 cells decreased the non-stem-like population (CD24hi CD44hi), from 21.3% to 13.1%, a 62.6% decrease relative to mock-irradiated breast carcinoma cells, parallel with a 68.3% decrease in cellular viability (Fig. 5C). In contrast, proton irradiation had no significant effect on the viability of stem-like breast carcinoma cells (CD24− CD44hi). However, proton irradiation induced significant translocation of calreticulin to the cell surface of both non-stem-like and stem-like populations of breast carcinoma cells, reflected by a 4.1-fold and 5.3-fold increase relative to mock-irradiated control cells, respectively (Fig. 5D).
Figure 5. Residential CSCs exposed to proton radiation harbor increased calreticulin expression on the cell surface.
(A) Flow cytometry was utilized to identify the stem-like (CD133hi CD44hi, designated CSC) and non-stem-like (CD133lo CD44lo, designated non-CSC) cells in LNCaP populations 96 h after mock (0 Gy) or proton radiation (8 Gy). Insets: percentage and viability of each population. (B) Calreticulin cell-surface expression in non-CSC and CSC populations after mock (closed histograms) or proton (open histograms) radiation. (C) MDA-MB-231 stem-like (CD44+CD24−) and non-stem-like (CD44+CD24+) cell populations. Insets: percentage and viability of each population. (D) Cell-surface expression of calreticulin in non-CSC and CSC populations after mock (closed histograms) or proton (open histograms) radiation. Viability was examined using LIVE/DEAD Fixable Violet Dead Stain. Cell surface expression of markers was evaluated on live cells gated by FSC/SSC and LIVE/DEAD staining.
Discussion
Here, using cell lines representative of tumors frequently treated with proton radiation; prostate, breast, lung, and chordoma, we report that proton radiation induces cardinal signs of immunogenic modulation (upregulation of HLA, ICAM-1, TAAs, and calreticulin) in (Table 1, Figs. 1 and 3). Moreover, the degree of upregulation of these molecules critical to T-cell recognition was similar to that observed following equivalent exposure to photon radiation. Proton radiation exposure enhanced antigen-specific CTL killing across all tumor types, and targeting different TAAs (Fig. 2). CTL-mediated attack requires the recognition of specific HLA/epitope complexes on the surface of tumor cells, which relies on the proper function of multiple antigen-processing machinery proteins, including the chaperone calreticulin. Calreticulin has also been reported to act directly in CTL:tumor cell-surface interactions [14]. Given the increased expression of calreticulin across distinct tumor types exposed to proton radiation (Fig. 3) and, more importantly, its translocation to the cell surface (Fig. 4A and B), we analyzed the functional role of calreticulin in CTL-mediated lysis by using a CBP (Fig. 4C and D). Blocking surface calreticulin abrogated the enhanced CTL killing induced by proton or photon radiation. These data confirm and extend those of Gameiro et al. [14] and Hodge et al. [2], where photon radiation and docetaxel chemotherapy, respectively, were demonstrated to increase tumor sensitivity to CTL attack. In both instances, the enhanced CTL killing mediated by photon radiation or chemotherapy was abolished by blocking calreticulin:CTL interactions or through genetic silencing of calreticulin. It should be noted that these studies, evaluated a single model dose of radiation (8 Gy). Radiation therapy is typically delivered either over a fractionated schedule or in combination with chemotherapeutic agents. We have previously shown the induction of immunogenic modulation by 10 Gy photon radiation delivered either as a single dose or fractionated 2 Gy/day for 5 days [14]. There, the level of immunogenic modulation and subsequent increased sensitivity to T-cell killing was equivalent between the single dose and fractionated dose. The role of hypofractionated vs. hyperfractionated radiation and combination of radiation and chemotherapy on the induction of immunogenic modulation in in-vivo preclinical murine models is currently being evaluated.
Importantly, our studies demonstrate that tumor cells typically resistant to the cytolytic/cytostatic effects of radiation and other therapies, such as chordoma and cancer stem cells (CSCs), respond to radiation by modulating phenotype. Chordoma is a rare, slow-growing neoplasm of notochord origin that can arise from bone in the skull base and along the spine, and has been reported to be radioresistant, and thus can only be controlled with very high doses of radiation that would damage normal tissue [17]. Because of chordoma’s proximity to the brain and spinal cord, proton therapy is often used due to its radiobiologic and physical properties. In the studies presented here, chordoma cells exposed to proton or photon radiation upregulated HLA, TAAs, ICAM-1, and calreticulin (Table 1, Figs. 1 and 3), and were killed to a significantly greater degree by MUC-1-specific T cells (Fig. 2). We have previously described that photon radiation induced calreticulin translocation on the whole population of tumor cells, and that this translocation is critical for increased sensitivity to T-cell killing [14]. Here, we extended that observation to show calreticulin translocation was induced by either photon or proton radiation (Fig. 3, Fig. 4) and focused on proton radiation to interrogate the expression of calreticulin on the non-CSC and CSC populations. CSCs have been recognized in recent years as important players in the development of solid tumors. CSCs are largely resistant to treatments, including radiation and chemotherapy [18]. It has been shown that radiation can transiently increase the proportion of CSCs, given their radioresistance and the eradication of the non-CSC population [19,20], consistent with our findings (Fig. 5A and C). However, radiation was also capable of significantly increasing calreticulin expression on resident CSC populations (Fig. 5B and D), suggesting a modulatory effect of proton radiation in facilitating immune attack of CSCs. These data differ from those of Zhang et al., who investigated the differential effects of proton and photon radiation on the stem-like population of a paclitaxel-resistant non-small-cell lung cancer cell line [21]. The difference in our observations could be in the parental cell population used (natural CSCs vs. chemotherapy-enriched CSCs). Nonetheless, both studies suggest that proton radiation may preferentially target resistant cells.
Our results might have been predicted based on the relative biologic effectiveness (RBE) of proton (1.1) and photon (1) radiation. However, there are considerable uncertainties in the determination of proton RBE values [22,23]. The data presented here suggest that RBE equivalencies may predict immune sensitivity. However, future studies examining other heavy-particle radiation treatment options, including alpha particle [24] and carbon ion therapy [25] (RBE values of 20), will determine if these other types of radiation can be successfully coupled with immunotherapy.
Conclusions
Radiation-induced immunogenic modulation can be exploited to promote clinical benefit when the treatment is followed by or given concurrent with an immunotherapy regimen. This combination has been described clinically with vaccines [16,26–28]. For reasons noted above, chordoma is a particularly attractive tumor for combination radiotherapy/immunotherapy. Chordoma aberrantly overexpresses the transcription factor brachyury [17], which can be targeted with vaccine [29] followed by radiation therapy, including proton therapy. A phase II trial is now examining this concept [30]. Radiation has also been evaluated clinically in combination with the immune checkpoint inhibitors anti-CTLA-4 and PD-1 [31,32]. [33,34]. It has been reported that single-dose photon radiation therapy downregulated expression of PD-L1 on human prostate cancer cell lines in vitro [35]. Our data confirm and extend those observations: proton radiation of LNCaP prostate tumor cells induced a significant downregulation of PD-L1 (Supplemental Table 1), which could lead to greater T-cell activity in irradiated tumors. In patients, radiation has been reported to upregulate PD-L1 which would support the combination of PD1/PD-L1 monoclonal antibody therapy with radiation [33,34]. These data, taken together, provide a rationale for the use of proton and photon radiation in combination with T-cell mediated immunotherapy (such as vaccines) including for patients who have failed radiation therapy or who have limited treatment options.
Supplementary Material
Summary.
We have shown that sublethal proton radiation mediates tumor changes that can be exploited for immune-mediated attack, including upregulation of surface molecules involved in immune recognition and translocation of calreticulin, which is critical in immune killing. These observations were extended to cells resistant to multiple therapies, such as chordoma and cancer stem cells. Our findings support proton radiation in combination with immunotherapy for patients who have failed radiation therapy alone or have limited treatment options.
Acknowledgments
Grant Support
This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Potential Conflicts of Interest
The authors have no potential conflicts of interest.
References
- 1.Gameiro SR, et al. Radiation-induced survival responses promote immunogenic modulation to enhance immunotherapy in combinatorial regimens. Oncoimmunology. 2014;3:e28643. doi: 10.4161/onci.28643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodge JW, et al. Chemotherapy-induced immunogenic modulation of tumor cells enhances killing by cytotoxic t lymphocytes and is distinct from immunogenic cell death. Int J Cancer. 2013;133:624–636. doi: 10.1002/ijc.28070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakraborty M, et al. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated t-cell killing. Cancer Res. 2004;64:4328–4337. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 4.Garnett CT, et al. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic t lymphocytes. Cancer Res. 2004;64:7985–7994. doi: 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 5.Kudo-Saito C, et al. The requirement of multimodal therapy (vaccine, local tumor radiation, and reduction of suppressor cells) to eliminate established tumors. Clinical Cancer Research. 2005;11:4533–4544. doi: 10.1158/1078-0432.CCR-04-2237. [DOI] [PubMed] [Google Scholar]
- 6.Lawrence JH, et al. Alpha and proton heavy particles and the bragg peak in therapy. Trans Am Clin Climatol Assoc. 1964;75:111–116. [PMC free article] [PubMed] [Google Scholar]
- 7.Tsang KY, et al. Generation of human cytotoxic t cells specific for human carcinoembryonic antigen epitopes from patients immunized with recombinant vacciniacea vaccine. J Natl Cancer Inst. 1995;87:982–990. doi: 10.1093/jnci/87.13.982. [DOI] [PubMed] [Google Scholar]
- 8.Tsang KY, et al. Phenotypic stability of a cytotoxic t-cell line directed against an immunodominant epitope of human carcinoembryonic antigen. Clin Cancer Res. 1997;3:2439–2449. [PubMed] [Google Scholar]
- 9.Tsang KY, et al. A human cytotoxic t-lymphocyte epitope and its agonist epitope from the nonvariable number of tandem repeat sequence of muc-1. Clin Cancer Res. 2004;10:2139–2149. doi: 10.1158/1078-0432.ccr-1011-03. [DOI] [PubMed] [Google Scholar]
- 10.Jochems C, et al. Identification and characterization of agonist epitopes of the muc1-c oncoprotein. Cancer immunology, immunotherapy : CII. 2014;63:161–174. doi: 10.1007/s00262-013-1494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tucker JA, et al. Identification and characterization of a cytotoxic t-lymphocyte agonist epitope of brachyury, a transcription factor involved in epithelial to mesenchymal transition and metastasis. Cancer immunology, immunotherapy : CII. 2014;63:1307–1317. doi: 10.1007/s00262-014-1603-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelbard A, et al. Combination chemotherapy and radiation of human squamous cell carcinoma of the head and neck augments ctl-mediated lysis. Clin Cancer Res. 2006;12:1897–1905. doi: 10.1158/1078-0432.CCR-05-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogino T, Wang X, Ferrone S. Modified flow cytometry and cell-elisa methodology to detect hla class i antigen processing machinery components in cytoplasm and endoplasmic reticulum. J Immunol Methods. 2003;278:33–44. doi: 10.1016/s0022-1759(03)00224-2. [DOI] [PubMed] [Google Scholar]
- 14.Gameiro SR, et al. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced t-cell killing. Oncotarget. 2014;5:403–416. doi: 10.18632/oncotarget.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chao MP, et al. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by cd47. Science translational medicine. 2010;2:63ra94. doi: 10.1126/scitranslmed.3001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwilas AR, et al. In the field: Exploiting the untapped potential of immunogenic modulation by radiation in combination with immunotherapy for the treatment of cancer. Front Oncol. 2012;2:104. doi: 10.3389/fonc.2012.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Maio S, et al. Novel targeted therapies in chordoma: An update. Ther Clin Risk Manag. 2015;11:873–883. doi: 10.2147/TCRM.S50526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Islam F, et al. Translational potential of cancer stem cells: A review of the detection of cancer stem cells and their roles in cancer recurrence and cancer treatment. Exp Cell Res. 2015;335:135–147. doi: 10.1016/j.yexcr.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Lagadec C, et al. Survival and self-renewing capacity of breast cancer initiating cells during fractionated radiation treatment. Breast Cancer Res. 2010;12:R13. doi: 10.1186/bcr2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pajonk F, Vlashi E, McBride WH. Radiation resistance of cancer stem cells: The 4 r's of radiobiology revisited. Stem Cells. 2010;28:639–648. doi: 10.1002/stem.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, et al. Therapy-resistant cancer stem cells have differing sensitivity to photon versus proton beam radiation. J Thorac Oncol. 2013;8:1484–1491. doi: 10.1097/JTO.0b013e3182a5fdcb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tommasino F, Durante M. Proton radiobiology. Cancers (Basel) 2015;7:353–381. doi: 10.3390/cancers7010353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paganetti H. Relative biological effectiveness (rbe) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys Med Biol. 2014;59:R419–R472. doi: 10.1088/0031-9155/59/22/R419. [DOI] [PubMed] [Google Scholar]
- 24.Sartor O, et al. Targeted use of alpha partcles: Current status in cancwer therapeutics. Nuclear Medicine and Radiation Therapy. 2102;3 [Google Scholar]
- 25.Schlaff CD, et al. Bringing the heavy: Carbon ion therapy in the radiobiological and clinical context. Radiat Oncol. 2014;9:88. doi: 10.1186/1748-717X-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gulley JL, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11:3353–3362. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 27.Gulley JL, et al. A pilot safety trial investigating a vector-based vaccine targeting carcinoembryonic antigen in combination with radiotherapy in patients with gastrointestinal malignancies metastatic to the liver. Expert Opin Biol Ther. 2011;11:1409–1418. doi: 10.1517/14712598.2011.615741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heery C, et al. Interim analysis of a phase ii randomized clinical trial of samrium-153 (sm-153) with or without psa-tricom vaccine in metastatic castration-resistant prostate cancer after docetaxel. J Clin Oncol. 2012;30 abstr 2526. [Google Scholar]
- 29.Palena C, Hamilton DH. Immune targeting of tumor epithelial-mesenchymal transition via brachyury-based vaccines. Adv Cancer Res. 2015;128:69–93. doi: 10.1016/bs.acr.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.A randomized, double-blind, phase 2 trial of gi-6301 (yeast-brachyury vaccine) versus placebo in combination with standard of care definitive radiotherapy in locally advanced, unresectable, chordoma. 2015 doi: 10.1002/onco.13720. Clinicaltrials.gov; 15-C-0118. [DOI] [PMC free article] [PubMed]
- 31.Hiniker SM, Chen DS, Knox SJ. Abscopal effect in a patient with melanoma. The New England journal of medicine. 2012;366:2035. doi: 10.1056/NEJMc1203984. author reply 2035–2036. [DOI] [PubMed] [Google Scholar]
- 32.Twyman-Saint Victor C, et al. Radiation and dual checkpoint blockade activate nonredundant immune mechanisms in cancer. Nature. 2015;520:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Derer A, et al. Radio-immunotherapy-induced immunogenic cancer cells as basis for induction of systemic anti-tumor immune responses - pre-clinical evidence and ongoing clinical applications. Front Immunol. 2015;6:505. doi: 10.3389/fimmu.2015.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Derer A, et al. Immune-modulating properties of ionizing radiation: Rationale for the treatment of cancer by combination radiotherapy and immune checkpoint inhibitors. Cancer immunology, immunotherapy : CII. 2015 doi: 10.1007/s00262-015-1771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bernstein MB, et al. Radiation-induced modulation of costimulatory and coinhibitory t-cell signaling molecules on human prostate carcinoma cells promotes productive antitumor immune interactions. Cancer Biother Radiopharm. 2014;29:153–161. doi: 10.1089/cbr.2013.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.