Abstract
Over the past few years, there is increasing evidence implicating a novel role for Intestinal Alkaline Phosphatase (IAP) in mitigating inflammatory mediated disorders. IAP is an endogenous protein expressed by the intestinal epithelium that is believed to play a vital role in maintaining gut homeostasis. Loss of IAP expression or function is associated with increased intestinal inflammation, dysbiosis, bacterial translocation and subsequently systemic inflammation. As these events are a cornerstone of the pathophysiology of many diseases relevant to surgeons, we sought to review recent research in both animal and humans on IAP’s physiologic function, mechanisms of action and current research in specific surgical diseases.
Keywords: IAP, Sepsis, NEC, IBD, Metabolic Syndrome, Intestinal Microbiome, Bacterial Translocation
Introduction
Intestinal Alkaline Phosphatase (IAP) has an important role in gut mucosal defense. IAP has been shown to be decreased during conditions that commonly effect surgical patients and therefore may contribute to the morbidity experienced by surgical patients. Expression of IAP is known to be affected by prematurity, starvation, and inflammation. Basic research has demonstrated IAP to inactivate bacterial pathogens as well as promote bacterial colonization of the intestine with commensal organisms. Data from several animal and human research trials have demonstrated exogenous IAP may have an effect in mitigating intestinal and systemic inflammation in a variety of diseases commonly treated by surgeons.
Currently, human recombinant for of IAP is undergoing Phase 2 clinical trials and therefore in the near future may become adjunct to other treatment options. The purpose of the review is increase the awareness of IAP for general surgeons and how it may impact their patients. We will review the known mechanisms of action of IAP as well as recent research investigating its role in surgical diseases.
Alkaline Phosphatases
IAP is a member of the Alkaline Phosphatase (AP) family which are ubiquitous enzymes distributed among different tissues throughout the body. In humans, four genes encode AP enzyme isoforms: tissue non-specific AP (TNAP), intestinal AP (IAP). placental AP and germ cell AP [1] (Table 1.) Each of these enzymes shares significant homology. Germ cell AP is predominately expressed by germ cell neoplasms and otherwise is not normally expressed to a significant degree in normal tissue. [2] As one would expect placental AP is expressed by the placenta and normally not expressed by other tissues except for by seminomas and some germ cell neoplasms for which it is used as a tumor marker. [3] TNAP is mainly expressed in liver, bone and kidney but is also found in circulating leukocytes and colon and its expression within the intestine is increased during inflammation. [4, 5] The function of TNAP is not entirely understood but its genetic absence has been linked to hypophosphatemia and therefore it is believed to play a role in bone matrix mineralization. [6] IAP is predominately expressed by the intestinal epithelium whereas the other three isoforms are not. [4, 5] IAP is expressed and secreted by intestinal epithelial cells and remains active within the mucosal membrane as well as the intestinal lumen. IAP is also secreted into the serum, where it remains biologically active. Expression of IAP is found throughout the intestine but is highest in the duodenum while its phosphatase activity is highest in the terminal ileum. [7] The expression of IAP is regulated by developmental stage, nutrition, and inflammation. [4, 5]
Table 1.
Summary of the alkaline phosphatase isoforms and their known clinical significance.
| Isoform | Location | Function |
|---|---|---|
| Tissue non-specific (TNAP) |
|
|
| Intestinal (IAP) |
|
|
| Placental (PLAP) |
|
|
| Germ Cell (GCAP) |
|
|
Mechanisms of Action of IAP and Possible Role in Disease
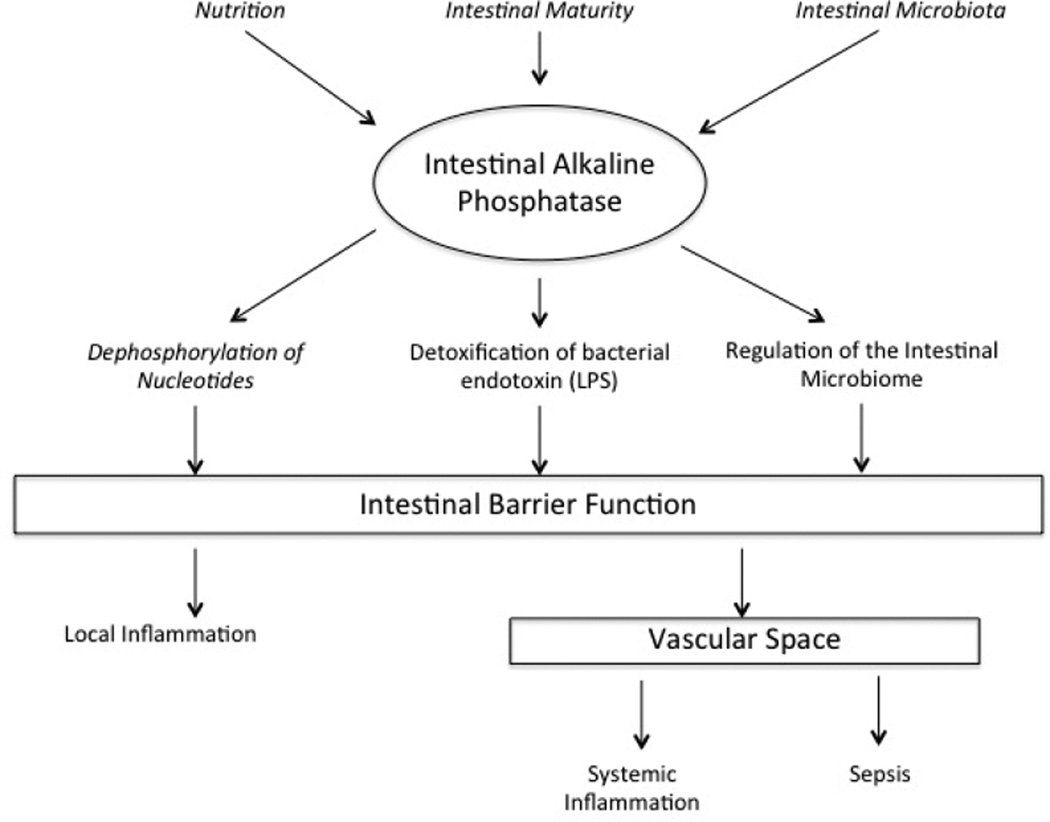
Of these four isoforms, a large amount of focus has been given to IAP and its role in human disease affecting the intestine. The four major functions of IAP in maintaining intestinal homeostasis can broadly be categorized into: regulation of bicarbonate secretion and duodenal surface pH, long chain fatty acid absorption, mitigation of intestinal inflammation through detoxification of pathogen-associated molecular patterns and regulation of the gut microbiome. [4, 5] (Figure 1.) As it’s name suggests IAP functions as a phosphatase and its reported substrates include lipopolysaccharide (LPS), flagellin, CpG DNA, and nucleotide di- and tri-phosphates. [8–10] While all these functions of IAP are important to maintaining intestinal homeostasis, it is the ability of IAP to inactivate LPS, regulate the microbiome and affect metabolism of adenosine tri-phosphate and diphosphate (ATP and ADP, respectively) that warrant specific discussion.
Figure 1.
Adapted from Lalles. [5] Summary of the multiple functions of intestinal alkaline phosphatase.
Inactivation of LPS
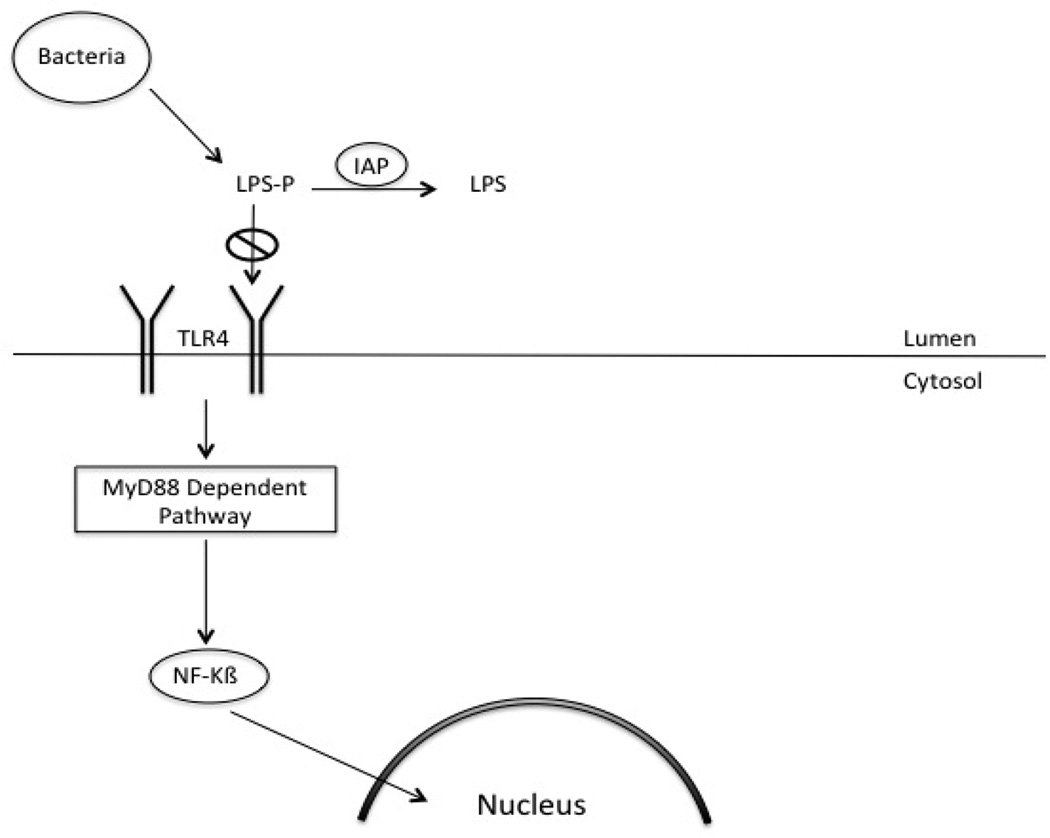
LPS is a constituent of the cell wall of gram-negative bacteria and is abundant in the gastrointestinal tract. It has been implicated in causing systemic inflammation and septic shock. The toxicity of LPS resides in the Lipid-A moiety, which permits it to bind to toll-like receptor-4 (TLR4). Removal of one of the two phosphate groups on the Lipid- A moiety reduces LPS toxicity 100 fold. [11] (Figure 2.) This reduction in the toxicity of LPS inhibits downstream intracellular signaling. LPS acts by binding to TLR4, which acts through two distinct pathways to cause inflammation. These two pathways are either dependent or independent on the adaptor molecule, MyD88. The MyD88 dependent pathway acts mainly through NF-kB to cause release of proinflammatory cytokines. [12] By preventing the activation of TLR4, IAP prevents the activation of NF-kB and its subsequent translocation into the nucleus. Ultimately, this prevents the expression of pro-inflammatory cytokines.
Figure 2.
Diagram depicting the mechanisms by which IAP prevents LPS mediated inflammation. IAP dephosphorylates LPS resulting in a decrease in toxicity. Additionally, IAP blocks activation of NF-kB preventing translocation to the nucleus and expression of pro-inflammatory cytokines.
The role of IAP in inactivating LPS and preventing intestinal inflammation was first examined in vivo in zebrafish. [13] These studies made two interesting observations. The first observation was that the presence of bacteria is necessary to induce the expression of IAP in the intestine. Using conventionally reared zerbafish it was determined that IAP expression is significantly increased 5–8 days post-fertilization. However, in germ-free zebrafish, IAP expression is significantly diminished indicating the presence of bacteria is required for IAP expression. Additionally, when germ-free zebrafish were fed bacteria, IAP expression increased to normal levels. Further, feeding LPS alone to germ free zebrafish was sufficient to induce expression of IAP. The second observation was that IAP could inactive LPS in vivo and prevent intestinal inflammation. In comparison to wild-type, IAP knock-down zebrafish had significantly increased neutrophil recruitment to the small intestine with ingestion of LPS. [14] However, when IAP was ingested prior to LPS, the intestinal inflammation was significantly diminished. These studies indicate that IAP plays a key role in suppressing the inflammatory response to LPS in the intestine. In summary, these experiments demonstrate that the presence of LPS and/or bacteria induce the expression of IAP and in absence of IAP LPS and/or bacteria lead to intestinal inflammation. Therefore one can conclude that IAP is an essential protective mechanism for intestinal inflammation.
Further evidence in support of the attenuation of the LPS load from the intestinal microbiome is IAP location within intestinal epithelial cells. IAP is anchored on the apical membrane of intestinal epithelial cells. [15] IAP can also be cleaved off the apical membrane and secreted within the lumen. [16–18] The intestinal epithelial microvilli secrete vesicles that are highly enriched with functional proteins, which include IAP. [19] What is most important about these vesicles is that while they were shown to be able to dephosphorylate LPS, they prevented the adhesion of pathogens and commensal bacteria to intestinal epithelial cells in vitro. Additionally, the presence of pathogens stimulated the secretion of these vesicles. [20]
The Intestinal Microbiome
In addition to providing a defense against microbes, recent evidence supports that IAP plays a role in determining which bacteria colonize the gut. [21] Bacterial 16S small subunit ribosomal RNA genes were used to examine the microbiome in the feces of IAP knock-out mice and compared to wild-type mice. The IAP knock-out mice feces contained fewer and less diverse bacteria than the wild-type mice. Phylogenetic analysis showed that IAP-KO mice have more Clostridia class of bacteria belonging to the Firmicutes phylum than WT mice. This difference was reversed by feeding the IAP knock-out mice supplemental IAP. In the same study, the effect of antibiotics on the intestinal microbiome and subsequent infection from Salmonella typhimurium was examined. Streptomycin was fed to both IAP knockout and wild-type mice and then the feces were examined for the presence of commensal bacteria for several days. In comparison to the IAP knockout mice, the wild-type mice recovered commensal bacteria in the feces much more quickly and had a lower risk of mortality when fed Salmonella typhimurium. [21]
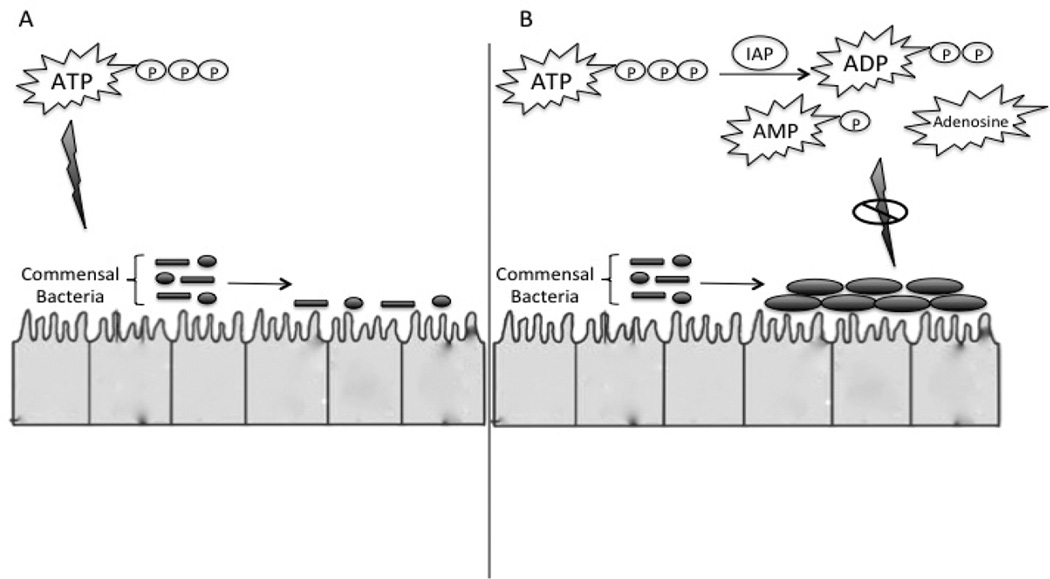
Dephosphorylation of Tri- and Di-Phosphorylated Nucleotides
In addition to detoxification of bacterial pathogens, IAP may also help regulate the intestinal microbiome. One mechanism recently identified by which IAP may positively regulate the intestinal microbiome is through dephosphorylation of phosphorylated nucleotides in the intestinal lumen. [22] Both in vitro and in vivo, increasing quantities of Adenosine Triphosphate (ATP) promoted less bacterial diversity and inhibited the growth of commensal bacteria. In a series of experiments it was identified that IAP promoted the growth of aerobic and anaerobic bacteria in stool and did so by conversion of ATP to adenosine within the intestinal lumen. (Figure 3.) Adult IAP knock-out (KO) mice were shown to have fewer and also different types of aerobic and anaerobic microbes in their stools compared with wild type (WT) mice. This abnormality was reversed by providing supplemental enteral IAP to the IAP KO mice.
Figure 3.
Adapted from Malo et al. [22] IAP helps to regulate the intestinal microbiome through dephosphorylation of phosphorylated nucleotides (ATP) in the intestinal lumen. Increasing quantities of ATP promote less bacterial diversity and inhibited the growth of commensal bacteria. This effect is ameliorated by dephoshorylation of nucleotides.
Similarly, conversion of ATP and ADP to adenosine may have a protective role in sepsis induced acute kidney injury. Adenosine is a known scavenger of oxygen free radicals. Adenosine also may confer protective effects in the kidney by vasodilation of the arterioles of the renal medulla. This is associated with an increase in medullary blood flow and thus medullary oxygenation. [23]
Specific Surgical Disease Where IAP May Play a Role in Disease and Treatment
Inflammatory Bowel Disease
The etiology of inflammatory bowel disease (IBD), which includes Crohn’s disease and ulcerative colitis, remains relatively unknown. IBD is characterized by chronic inflammation of the intestine. The exaggerated inflammatory response is thought to be multifactorial and a combination of genetic, immunological and bacterial factors. [24] It is hypothesized that inappropriate and ongoing activation of the mucosal immune system against luminal contents are the cause of the intestinal inflammation. Activation of the innate immune system within the intestine is dependent upon recognition of microbes and their ligands by pattern recognition receptors. Bacterial products like LPS and flagellin are known to illicit necessary mucosal defense immune responses that prevent invasion of opportunistic bacteria. However, dysregulation of the host immune response or impaired mucosal defense against gut flora may lead to excessive intestinal inflammation. [25–27]
It remains uncertain whether the excessive inflammation is due to dysregulation of the mucosal immune response or impaired protective factors. However, it may be a combination of both. In both children and adults who have IBD, the colonic mucosa has been found to have a significantly higher TLR4 expression when compared to healthy controls. [28, 29] Furthermore, this dysregulated immune response occurs in response to the intestinal flora since germ free mice fail to develop intestinal inflammation in a mouse model of colitis. [30] There is evidence that the intestinal flora in IBD patients is altered. The biggest alteration most studies examining the microbiome in IBD patients is a reduction in alpha diversity, representing the total numbers of species present. [31] In addition to increased inflammatory regulators, there appears to be impaired protective factors in patients with IBD. As previously discussed, IAP plays a central role in the regulation of intestinal inflammation and decreased expression of IAP has been demonstrated to be associated with IBD. [26] IAP was found to be significantly decreased in the terminal ileum of patients with inflamed tissue obtained from intestinal biopsies via colonoscopy from adult patients with both Ulcerative Colitis and Crohn’s disease. Additionally, the IAP expression in non-inflamed tissue of those with IBD was decreased compared to healthy controls. Decreased expression of IAP has also been reported in pediatric patients with IBD. [32]
These finding suggested that decreased expression of IAP may play a role in IBD. Several studies have examined whether exogenous IAP could prevent colitis in experimental animal models. In particular, enteral administration of dextran sulfate sodium to rats has been used as a validated model to initiate intestinal injury similar to that observed in IBD. Using this model, supplementation of enteral IAP was shown to be protective against intestinal injury. Rats given IAP demonstrated decreased inflammatory changes as observed histologically, as well as reduced inflammatory cytokine expression in the terminal ileum and colon. [33] Further, gut barrier dysfunction is known to occur from a variety of insults. In mice with gut barrier dysfunction induced by intestinal ischemia either by temporary SMA occlusion or remote ischemia/reperfusion, the absence of IAP in IAP-knockout mice resulted in increased severity of intestinal inflammation and increased bacterial translocation when compared to wild-type mice. [34] Similarly it has been demonstrated that IAP knockout mice had a more severe histologic injury as well as increased inflammatory cytokine expression when compared to wild-type mice in a murine model of chronic colitis. [35] More importantly, exogenous administration of IAP significantly attenuated inflammation in both IAP knockout and wild-type mice in the chronic colitis model. These data suggest that the absence of IAP is associated with an increased severity of intestinal injury in colitis models and supplemental, enteral replacement could be protective.
Based on these animal experiments, the potential use of enteral IAP to treat IBD was further explored in patients who had steroid and/or immunosuppressant moderate to severe ulcerative colitis. [36] Twenty-one patients were given a 7-day course of calf IAP and were reevaluated at day 21. At 21 days these patients had a significant decrease in their disease severity as measured by the Mayo score and Modified Truelove-Witts Severity index. The improvement in clinical symptoms as associated with a reduction in serum CRP and stool calprotectin suggesting decreased intestinal inflammation. IAP was well tolerated without signs of toxicity in this study.
While the etiology of IBD is complex and multifactorial, current evidence indicates IAP may have a therapeutic role without the risk of harmful effects associated with current immunotherapies. (Table 2.) To further examine the therapeutic effectiveness of IAP in the treatment of IBD, a phase II clinical trial is expected to begin enrollment in the near future using a human recombinant form of IAP.
Table 2.
Table of studies providing evidence to the multifactorial nature of Inflammatory Bowel Disease and how the use of IAP may mitigate some of the factors.
| Author | Study Type | Findings |
|---|---|---|
| Szebeni B et al. (2008) |
|
|
| Cario et al. (2000) |
|
|
| Madsen, KL (1999) |
|
|
| Madsen, KL (1999) |
|
|
| Molnar et al. (2012) |
|
|
| Ramasamy et al. (2011) |
|
|
| Tuin et al. (2008) |
|
|
| Goldberg et al. (2008) |
|
|
| Lukas et al. (2010) |
|
|
Necrotizing Enterocolitis
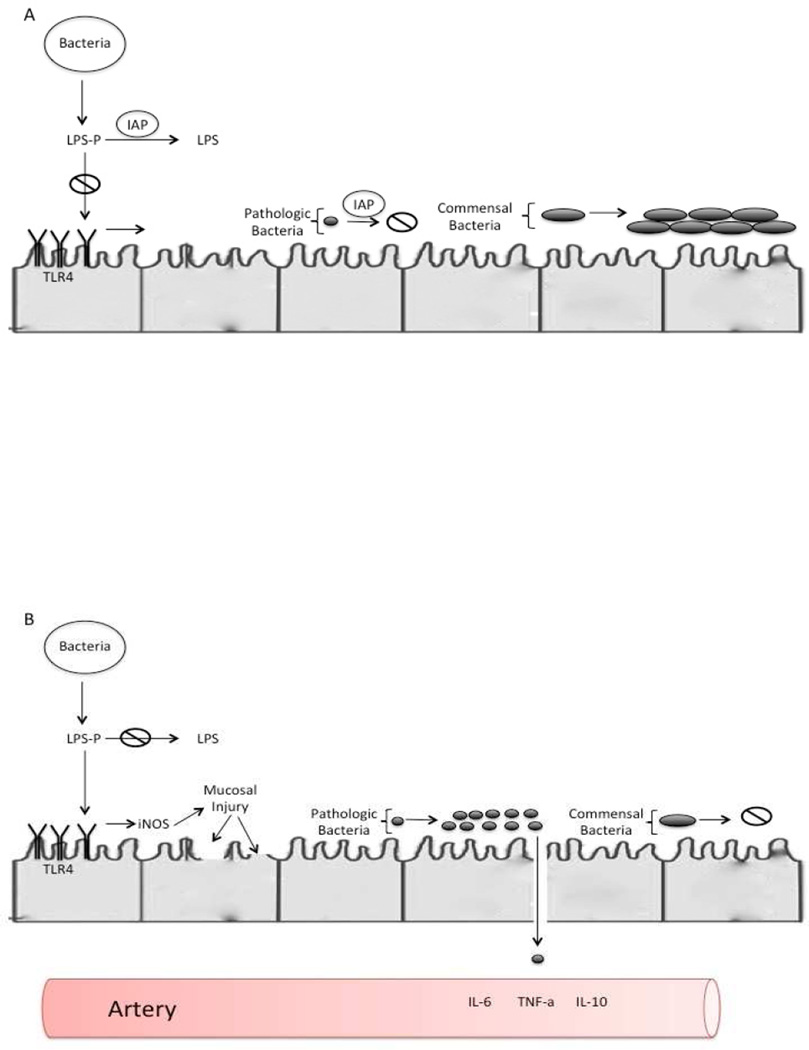
Necrotizing enterocolitis (NEC) is a disease of unknown etiology that affects the gastrointestinal tract of premature infants. It is the most common life-threatening gastrointestinal surgical emergency, occurring in about 7% of infants with a birth weight of 500 – 1500g. [37] The mortality rate of NEC ranges between 20 – 30%, and is the highest among those who require surgery. [38] However, NEC is rare in the full term infant. There are numerous risk factors associated with development of NEC, however, prematurity and formula feeding has been the only consistent observation in those who develop NEC. Despite many risk factors being recognized, the etiology of NEC remains elusive. The pathogenesis of NEC is incompletely understood, but generally considered to be a multifactorial disease process. A combination of genetic predisposition, immature intestine that is highly immunoreactive and abnormal bacterial colonization are all believed to be causative factors. [39, 40] (Figure 4.)
Figure 3.
The premature intestine is associated with increased TLR4 expression. Shortly before the onset of necrotizing enterocolitis, there is an alteration in the microbiome with increased numbers of pathogenic bacteria. This leads to an increase LPS leading to mucosal injury and decreased mucosal restitution. However, as shown in figure A IAP deactivates intraluminal LPS preventing activation of the TLR4 cascade. Additionally, it is hypothesized that IAP prevents the alteration in the microbiome.
There are many differences between the mature and immature intestine that increase it susceptibility to inflammation. Amniotic fluid contains multiple proteins, which are important for maturation of enterocytes. [41] In the intrauterine environment, the intestinal epithelial cells are continuously exposed to these proteins. However, preterm enterocytes have not been provided the opportunity to mature and therefore, are hyper-responsive to stimulation. [41, 42] Consequentially, when exposed to microbes and luminal contents, the immature enterocyte may have an exaggerated and robust inflammatory response. [41, 43]
The hyper-responsive mucosa of the neonatal intestine maybe due to exaggerated stimulation of the immune response or impaired regulation of the inflammatory response. It is likely a combination of the two that lead to the development of NEC. TLR4 receptor expression has been shown to be increased shortly after birth. [44, 45] Mice deficient in TLR4 are protected from the development of NEC. [46] Additionally, stressors such as early enteral feeds, hypoxia and LPS have been demonstrated to initiate translocation of the NF-kB to the nucleus with increased expression of cytokines. [42] Growing evidence suggests that abnormal colonization, or dysbiosis may occur in the newborn intestine just prior to development of NEC. [47, 48] One of the larger studies to date suggested that dysbiosis in the first two weeks of life correlated with subsequent risk of development of NEC. [49] Using 16S RNA gene sequencing from stool samples it was demonstrated that newborns who later developed NEC had lower alpha-diversity, indicated a lower diversity of the types of bacterial species present. In addition to the loss of diversity, the intestinal microbiome of newborns with NEC had less Propionibacterium and increased amounts of Firmicutes and/or Proteobacteria compared to those without NEC. The increased abundance of gram-negative bacteria as demonstrated by increased Proteobacteria may result in excessive TLR4 activation which is believed to be an important early event in the pathogenesis of NEC. Propionibacterium is a commensal bacterium and thought to help mitigate the risk of developing NEC. However, none of those that developed NEC had detectable levels of Propionibacterium. This finding is consistent with the concept that the early presence of commensal bacteria helps induce intestinal homeostasis. Another study examined the intestinal microbiome and identified that the predominant organism found in the feces of preterm newborns with sepsis was also found in the blood cultures. [50]
One important question is whether IAP is decreased in NEC. [51] A recent study in fact does demonstrate when compared to control rats, IAP protein expression and activity were significantly decreased in animals with experimental NEC. However, when animals subjected to the NEC protocol received exogenous enteral IAP, they were found to have comparable histology and IAP expression and activity to that of controls. [51] Subsequent follow-up experiments using the same NEC model have shown enteral IAP decreased both intestinal and systemic inflammatory mediator production as well as improved gut barrier function. [42, 52, 53] While the enteral formulation helped reduce the severity of the intestinal injury, systemic administration did not. However, despite having no effect on intestinal inflammation, systemic IAP was shown to significantly decrease the systemic inflammatory response suggesting that systemic administration of IAP may be useful clinically to treat NEC related sepsis. [52]
Another important question is whether the deficiency of IAP leads to an increased risk of NEC or if NEC leads to decreased IAP expression. This question has not been answered, however, it was observed that preterm rats do have decreased expression of IAP. The preterm rats also exhibited increased expression of pro-inflammatory cytokines in the intestine and had increased intestinal permeability compared to full-term rats. It was also shown that the intestine of the preterm rat compared to the term rat was more sensitive to LPS stimulation. Importantly, simply feeding supplemental IAP decreased both the intestinal inflammation and permeability. Based on these findings the use of prophylactic enteral IAP may be a novel therapeutic strategy to prevent NEC.
While enteral IAP supplementation was found to decrease the severity of intestinal injury in an NEC model, the use of systemic IAP did not decrease intestinal injury. [52] However, when given by intra-peritoneal injection, IAP decreased the expression of serum cytokines in rat pups with experimental NEC compared to placebo. This suggests that systemic IAP may not prevent NEC but may be useful in combating the systemic sepsis associated with NEC.
Sepsis
One of the most common causes of mortality in the surgical patient is sepsis. This is a complex disease process characterized by a massive inflammatory response leading to release of large quantities of inflammatory cytokines, including tumor necrosis factor, interferon-γ, and interleukin-2. This results in hemodynamic changes leading to hypotension, poor tissue perfusion and multi-organ failure. Despite increases in medical management of the septic patient, there is still a mortality rate of approximately 30%. [54] While the mainstay of treatment of the septic patient is source control, few novel treatments have proven to be effective in mitigating end-organ injury due to sepsis.
Given that LPS is a potent instigator of sepsis and the ability of IAP to inactivate LPS, several investigators have explored the systemic use of IAP to treat sepsis. Bentala et al. treated mice with the placental AP after injection of a lethal dose of LPS. [55, 56] The survival rate at six days after injection of LPS improved from 57% to100% with placental AP treatment. Due to the fact that calf IAP is a rich source of alkaline phosphatase, further studies were conducted to determine if IAP has the same physiologic effects in vivo as placental AP. [57] Mice who received IAP after a lethal dose of E. coli had a survival rate of 80%, compared to 20% when saline was given. In addition, those who received IAP had no change in the white blood cell count and the Tumor Necrosis Factor-α response was suppressed by 98%. [57] Su et al. were able to provide additional support by inducing septic shock secondary to peritonitis in sheep by intra-peritoneal injection of feces. Sheep who received IAP demonstrated improved gas exchanged as measured by the PaO2/FiO2 ratio, decreased concentrations of interleukin-6 and prolonged survival. [58]
In addition to increasing the survival, IAP has been demonstrated to improve acute kidney injury (AKI). Animal experiments indicate that following an ischemic injury alkaline phosphatase activity is depleted in the kidney. [59] Besides the effect of IAP to inactivate LPS, IAP may also play an important part in host defense by dephosphorylating extracellular ATP to adenosine. Adenosine exerts potent anti-inflammatory and renal tissue protective effects. [23]
Based on these and other animal studies, phase 1 and 2 clinical trials using IAP in septic patients to prevent AKI have been conducted using an intravenous formulation of bovine derived IAP. Systemic IAP was well tolerated and had little to no observed toxicity or side effects. [60] Septic patients who received IAP had significant improvement in kidney function, as demonstrated by decreases in median plasma creatinine levels and creatinine clearance. [61, 62] This was associated with the IAP treated patients having a trend towards reduction in dialysis requirement and duration. [62] Additionally, septic patients without AKI were less likely to develop AKI after the infusion of IAP. [61] IAP has demonstrated a benefit not only in levels of inflammation, but reducing mortality rates and end-organ damage.
A randomized clinical trial evaluating a human-recombinant form of IAP is currently enrolling patients in a Phase 2 randomized clinical trial. IAP may prove to be an interesting adjunct in the management of the septic patient.
Antibiotic Associated Diarrhea
The use of antibiotics has increased over the years to treat bacterial infections. However, their use is not completely benign. While antibiotics do eradicate bacterial infections, they have also been implicated in destroying the normal commensal flora. This allows for opportunistic bacteria to colonize and infect the host. [63] For example, mice treated with streptomycin require a smaller inoculation of Salmonella enterica serovar Typhimurium (<10 colony forming units, CFU), compared to 1 million CFU required to infect control mice. [64] This reduction in intestinal commensal flora has been demonstrated to be dose dependent. [65] The replacement of commensal bacteria with pathogenic bacteria has clinical consequences. Mice pretreated with antibiotics followed by oral gavage of 105 CFU of C. difficile died within 3 days of treatment, whereas mice not exposed to antibiotics failed to demonstrate evidence of disease. [66] The cause of antibiotic associated diarrhea has been the focus of many epidemiologic studies. The implicating cause has been identified as replacement of the commensal flora with pathogenic bacteria, with C. difficile being a common pathogen. [67–69]
The role of IAP in maintaining the microbiota has been described above. IAP deficient mice and found fewer and different types of bacterial species compared to wild type mice. [21] Mice treated with antibiotics had fewer and different types of bacteria. When the antibiotic treated mice also received IAP throughout treatment, they experienced rapid restoration of the gut flora that would have otherwise have been lost. [21] However, maintenance of the gut flora does not directly provide evidence of a clinical difference in the IAP treated mice. To investigate this point, mice were given antibiotics as well as gavage fed C. difficile and S. Typhimurium with and without IAP. Mice who received IAP were able to maintain their weight, had reduced clinical severity score and gut inflammation, as well as demonstrating improved survival compared to mice that were not given IAP. [64]
From the evidence provided it is likely that IAP provides protection from antibiotic associated diarrhea through the maintenance of the intestinal microbiota. Although further work in humans is needed, IAP may prove to be an excellent adjunct to antibiotic therapy to prevent not only associated diarrhea but colitis as well, especially in the critically ill.
Metabolic Syndrome
Metabolic syndrome is composed of a cluster of disorders that include central obesity (abdominal fat distribution), insulin resistance, abnormal lipid profile (dyslipidemia), fatty liver, and hypertension. [70, 71] This syndrome has long-term consequences and ultimately leads to type II diabetes, nonalcoholic fatty liver disease and atherosclerosis. Those who suffer from this syndrome have a significantly higher mortality rate largely due to coronary heart disease and other cardiovascular disease. [72] This is concerning as the number of adults in the United States suffering from this syndrome has been estimated to be approaching almost 40%. [73]
Recent research has suggested chronic endotoxemia is an underlying factor in the pathogenesis of metabolic syndrome. One factor associated with chronic endotoxemia is a high-fat diet. [74] Mice that were given a 4-week high-fat diet had similar levels of circulating endotoxin to mice that underwent 4 weeks of continuous subcutaneous infusion of LPS. [74] Importantly, mice that were fed a high-fat diet had a change in the intestinal microbiome to a high proportion of LPS-containing species. [74] This persistent endotoxemia leads to a chronic low-grade inflammatory state with increased levels of inflammatory cytokines. [74] Additionally, the chronic inflammation is associated with damage to pancreatic beta cells, hepatocytes and vascular endothelium. [75–77]
One proposed mechanism for this chronic systemic inflammation is due to intestinal LPS being complexed with chylomicrons. [78] A high fat diet leads to excess chylomicron formation (with complexed LPS) which enter systemic circulation through mesenteric lymph nodes. High fat diets not only cause systemic inflammation, but local intestinal inflammation as well. [79] The increased expression of inflammatory cytokines in the intestine increases gut permeability, promotes bacterial or endotoxin translocation, and further worsens systemic inflammation.
Given the pathogenesis of metabolic syndrome, IAP represents a novel therapeutic strategy based on its ability to attenuate LPS mediated inflammation. Mice who were deficient in IAP were found to have increased intestinal permeability and circulating levels of LPS. [80] Additionally, they demonstrated features of metabolic syndrome, such as central obesity and insulin resistance. [80] When control mice were given a high-fat diet supplemented with IAP, not only did IAP supplementation prevent the development of metabolic syndrome, but was also able to reverse the features of metabolic syndrome as well. [80] While this work is still in the preclinical stage, it none-the-less provides encouraging data about a novel treatment strategy to prevent the highly morbid and expensive complications related to metabolic syndrome.
Conclusion
Research examining the role of IAP in multiple disease processes, as well as its role a therapeutic option is a relatively young field of study. IAP has been demonstrated to inactivate LPS as well as promote commensal bacterial colonization of the intestine. Absence of or a deficiency of IAP appear to increase the risk of disease through changes in the microbiome, increased intestinal inflammation and permeability thereby leading to systemic inflammation and potentially sepsis. Preclinical data thus far strongly suggest IAP can mitigate this pathologic process. Recent development of a human recombinant form of IAP has led to an increase potential for commercialization of IAP as a therapeutic strategy. Ongoing clinical studies of the human recombinant IAP will be essential to determine if IAP will be a therapeutic strategy to prevent dysbiosis, intestinal inflammation, and systemic inflammation that occur in a number of disease process that affect surgical patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- Jason Fawley, MD – Literature review and manuscript
- David Gourlay, MD – Literature review and manuscript editing
- Jason Fawley, MD – Author has no financial or personal relationships to disclose
- David Gourlay, MD – Author has no financial or personal relationships to disclose
Bibliography
- 1.Millan JL. Alkaline Phosphatases : Structure, substrate specificity and functional relatedness to other members of a large superfamily of enzymes. Purinergic Signal. 2006;2(2):335–341. doi: 10.1007/s11302-005-5435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Broe ME, Pollet DE. Multicenter evaluation of human placental alkaline phosphatase as a possible tumor-associated antigen in serum. Clin Chem. 1988;34(10):1995–1999. [PubMed] [Google Scholar]
- 3.Neumann A, Keller T, Jocham D, Doehn C. Human placental alkaline phosphatase (hPLAP) is the most frequently elevated serum marker in testicular cancer. Aktuelle Urol. 2011;42(5):311–315. doi: 10.1055/s-0031-1271545. [DOI] [PubMed] [Google Scholar]
- 4.Lalles JP. Intestinal alkaline phosphatase: novel functions and protective effects. Nutr Rev. 2014;72(2):82–94. doi: 10.1111/nure.12082. [DOI] [PubMed] [Google Scholar]
- 5.Lalles JP. Intestinal alkaline phosphatase: multiple biological roles in maintenance of intestinal homeostasis and modulation by diet. Nutr Rev. 2010;68(6):323–332. doi: 10.1111/j.1753-4887.2010.00292.x. [DOI] [PubMed] [Google Scholar]
- 6.MP W. Hypophosphatasia. In: B.A. Scriver CR, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; 1995. [Google Scholar]
- 7.Van Dongen JM, Kooyman J, Visser WJ, Holt SJ, Galjaard H. The effect of increased crypt cell proliferation on the activity and subcellular localization of esterases and alkaline phosphatase in the rat small intestine. Histochem J. 1977;9(1):61–75. doi: 10.1007/BF01007009. [DOI] [PubMed] [Google Scholar]
- 8.Wilkes JM, Garner A, Peters TJ. Studies on the localization and properties of rat duodenal HCO3--ATPase with special relation to alkaline phosphatase. Biochim Biophys Acta. 1987;924(1):159–166. doi: 10.1016/0304-4165(87)90083-3. [DOI] [PubMed] [Google Scholar]
- 9.Moss AK, Hamarneh SR, Mohamed MM, Ramasamy S, Yammine H, Patel P, et al. Intestinal alkaline phosphatase inhibits the proinflammatory nucleotide uridine diphosphate. Am J Physiol Gastrointest Liver Physiol. 2013;304(6):G597–G604. doi: 10.1152/ajpgi.00455.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen KT, Malo MS, Moss AK, Zeller S, Johnson P, Ebrahimi F, et al. Identification of specific targets for the gut mucosal defense factor intestinal alkaline phosphatase. Am J Physiol Gastrointest Liver Physiol. 2010;299(2):G467–G475. doi: 10.1152/ajpgi.00364.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schromm AB, Brandenburg K, Loppnow H, Zahringer U, Rietschel ET, Carroll SF, et al. The charge of endotoxin molecules influences their conformation and IL-6-inducing capacity. J Immunol. 1998;161(10):5464–5471. [PubMed] [Google Scholar]
- 12.Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42(2):145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Bates JM, Mittge E, Kuhlman J, Baden KN, Cheesman SE, Guillemin K. Distinct signals from the microbiota promote different aspects of zebrafish gut differentiation. Dev Biol. 2006;297(2):374–386. doi: 10.1016/j.ydbio.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Bates JM, Akerlund J, Mittge E, Guillemin K. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe. 2007;2(6):371–382. doi: 10.1016/j.chom.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engle MJ, Mahmood A, Alpers DH. Two rat intestinal alkaline phosphatase isoforms with different carboxyl-terminal peptides are both membrane-bound by a glycan phosphatidylinositol linkage. J Biol Chem. 1995;270(20):11935–11940. doi: 10.1074/jbc.270.20.11935. [DOI] [PubMed] [Google Scholar]
- 16.Nakano T, Inoue I, Alpers DH, Akiba Y, Katayama S, Shinozaki R, et al. Role of lysophosphatidylcholine in brush-border intestinal alkaline phosphatase release and restoration. Am J Physiol Gastrointest Liver Physiol. 2009;297(1):G207–G214. doi: 10.1152/ajpgi.90590.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Persson EM, Nilsson RG, Hansson GI, Lofgren LJ, Liback F, Knutson L, et al. A clinical single-pass perfusion investigation of the dynamic in vivo secretory response to a dietary meal in human proximal small intestine. Pharm Res. 2006;23(4):742–751. doi: 10.1007/s11095-006-9607-z. [DOI] [PubMed] [Google Scholar]
- 18.Eliakim R, Mahmood A, Alpers DH. Rat intestinal alkaline phosphatase secretion into lumen and serum is coordinately regulated. Biochim Biophys Acta. 1991;1091(1):1–8. doi: 10.1016/0167-4889(91)90213-h. [DOI] [PubMed] [Google Scholar]
- 19.McConnell RE, Higginbotham JN, Shifrin DA, Jr, Tabb DL, Coffey RJ, Tyska MJ. The enterocyte microvillus is a vesicle-generating organelle. J Cell Biol. 2009;185(7):1285–1298. doi: 10.1083/jcb.200902147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shifrin DA, Jr, McConnell RE, Nambiar R, Higginbotham JN, Coffey RJ, Tyska MJ. Enterocyte microvillus-derived vesicles detoxify bacterial products and regulate epithelial-microbial interactions. Curr Biol. 2012;22(7):627–631. doi: 10.1016/j.cub.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malo MS, Alam SN, Mostafa G, Zeller SJ, Johnson PV, Mohammad N, et al. Intestinal alkaline phosphatase preserves the normal homeostasis of gut microbiota. Gut. 2010;59(11):1476–1484. doi: 10.1136/gut.2010.211706. [DOI] [PubMed] [Google Scholar]
- 22.Malo MS, Moaven O, Muhammad N, Biswas B, Alam SN, Economopoulos KP, et al. Intestinal alkaline phosphatase promotes gut bacterial growth by reducing the concentration of luminal nucleotide triphosphates. Am J Physiol Gastrointest Liver Physiol. 2014;306(10):G826–G838. doi: 10.1152/ajpgi.00357.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vallon V, Muhlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev. 2006;86(3):901–940. doi: 10.1152/physrev.00031.2005. [DOI] [PubMed] [Google Scholar]
- 24.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3(7):521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 25.Garrett WS, Gordon JI, Glimcher LH. Homeostasis and inflammation in the intestine. Cell. 2010;140(6):859–870. doi: 10.1016/j.cell.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448(7152):427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 27.Mizoguchi A, Mizoguchi E, Bhan AK. Immune networks in animal models of inflammatory bowel disease. Inflamm Bowel Dis. 2003;9(4):246–259. doi: 10.1097/00054725-200307000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Szebeni B, Veres G, Dezsofi A, Rusai K, Vannay A, Mraz M, et al. Increased expression of Toll-like receptor (TLR) 2 and TLR4 in the colonic mucosa of children with inflammatory bowel disease. Clin Exp Immunol. 2008;151(1):34–41. doi: 10.1111/j.1365-2249.2007.03531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68(12):7010–7017. doi: 10.1128/iai.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madsen KL, Malfair D, Gray D, Doyle JS, Jewell LD, Fedorak RN. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm Bowel Dis. 1999;5(4):262–270. doi: 10.1097/00054725-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, et al. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut. 2006;55(2):205–211. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molnar K, Vannay A, Szebeni B, Banki NF, Sziksz E, Cseh A, et al. Intestinal alkaline phosphatase in the colonic mucosa of children with inflammatory bowel disease. World J Gastroenterol. 2012;18(25):3254–3259. doi: 10.3748/wjg.v18.i25.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tuin A, Poelstra K, de Jager-Krikken A, Bok L, Raaben W, Velders MP, et al. Role of alkaline phosphatase in colitis in man and rats. Gut. 2009;58(3):379–387. doi: 10.1136/gut.2007.128868. [DOI] [PubMed] [Google Scholar]
- 34.Goldberg RF, Austen WG, Jr, Zhang X, Munene G, Mostafa G, Biswas S, et al. Intestinal alkaline phosphatase is a gut mucosal defense factor maintained by enteral nutrition. Proc Natl Acad Sci U S A. 2008;105(9):3551–3556. doi: 10.1073/pnas.0712140105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramasamy S, Nguyen DD, Eston MA, Alam SN, Moss AK, Ebrahimi F, et al. Intestinal alkaline phosphatase has beneficial effects in mouse models of chronic colitis. Inflamm Bowel Dis. 2011;17(2):532–542. doi: 10.1002/ibd.21377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lukas M, Drastich P, Konecny M, Gionchetti P, Urban O, Cantoni F, et al. Exogenous alkaline phosphatase for the treatment of patients with moderate to severe ulcerative colitis. Inflamm Bowel Dis. 2010;16(7):1180–1186. doi: 10.1002/ibd.21161. [DOI] [PubMed] [Google Scholar]
- 37.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364(3):255–264. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fitzgibbons SC, Ching Y, Yu D, Carpenter J, Kenny M, Weldon C, et al. Mortality of necrotizing enterocolitis expressed by birth weight categories. J Pediatr Surg. 2009;44(6):1072–1075. doi: 10.1016/j.jpedsurg.2009.02.013. discussion 1075–6. [DOI] [PubMed] [Google Scholar]
- 39.Sharma R, Tepas JJ, 3rd, Hudak ML, Mollitt DL, Wludyka PS, Teng RJ, et al. Neonatal gut barrier and multiple organ failure: role of endotoxin and proinflammatory cytokines in sepsis and necrotizing enterocolitis. J Pediatr Surg. 2007;42(3):454–461. doi: 10.1016/j.jpedsurg.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 40.Markel TA, Crisostomo PR, Wairiuko GM, Pitcher J, Tsai BM, Meldrum DR. Cytokines in necrotizing enterocolitis. Shock. 2006;25(4):329–337. doi: 10.1097/01.shk.0000192126.33823.87. [DOI] [PubMed] [Google Scholar]
- 41.Lebenthal A, Lebenthal E. The ontogeny of the small intestinal epithelium. JPEN J Parenter Enteral Nutr. 1999;23(5 Suppl):S3–S6. doi: 10.1177/014860719902300502. [DOI] [PubMed] [Google Scholar]
- 42.Rentea RM, Welak SR, Fredrich K, Donohoe D, Pritchard KA, Oldham KT, et al. Early enteral stressors in newborns increase inflammatory cytokine expression in a neonatal necrotizing enterocolitis rat model. Eur J Pediatr Surg. 2013;23(1):39–47. doi: 10.1055/s-0032-1329704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. 2006;368(9543):1271–1283. doi: 10.1016/S0140-6736(06)69525-1. [DOI] [PubMed] [Google Scholar]
- 44.Gribar SC, Sodhi CP, Richardson WM, Anand RJ, Gittes GK, Branca MF, et al. Reciprocal expression and signaling of TLR4 and TLR9 in the pathogenesis and treatment of necrotizing enterocolitis. J Immunol. 2009;182(1):636–646. doi: 10.4049/jimmunol.182.1.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hackam DJ, Afrazi A, Good M, Sodhi CP. Innate immune signaling in the pathogenesis of necrotizing enterocolitis. Clin Dev Immunol. 2013;2013:475415. doi: 10.1155/2013/475415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sodhi CP, Neal MD, Siggers R, Sho S, Ma C, Branca MF, et al. Intestinal epithelial Toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology. 2012;143(3):708–718. e1–e5. doi: 10.1053/j.gastro.2012.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morowitz MJ, Poroyko V, Caplan M, Alverdy J, Liu DC. Redefining the role of intestinal microbes in the pathogenesis of necrotizing enterocolitis. Pediatrics. 2010;125(4):777–785. doi: 10.1542/peds.2009-3149. [DOI] [PubMed] [Google Scholar]
- 48.Weng M, Walker WA. The role of gut microbiota in programming the immune phenotype. J Dev Orig Health Dis. 2013;4(3):203–214. doi: 10.1017/S2040174412000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morrow AL, Lagomarcino AJ, Schibler KR, Taft DH, Yu Z, Wang B, et al. Early microbial and metabolomic signatures predict later onset of necrotizing enterocolitis in preterm infants. Microbiome. 2013;1(1):13. doi: 10.1186/2049-2618-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Madan JC, Salari RC, Saxena D, Davidson L, O'Toole GA, Moore JH, et al. Gut microbial colonisation in premature neonates predicts neonatal sepsis. Arch Dis Child Fetal Neonatal Ed. 2012;97(6):F456–F462. doi: 10.1136/fetalneonatal-2011-301373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whitehouse JS, Riggle KM, Purpi DP, Mayer AN, Pritchard KA, Jr, Oldham KT, et al. The protective role of intestinal alkaline phosphatase in necrotizing enterocolitis. J Surg Res. 2010;163(1):79–85. doi: 10.1016/j.jss.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 52.Riggle KM, Rentea RM, Welak SR, Pritchard KA, Jr, Oldham KT, Gourlay DM. Intestinal alkaline phosphatase prevents the systemic inflammatory response associated with necrotizing enterocolitis. J Surg Res. 2013;180(1):21–26. doi: 10.1016/j.jss.2012.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rentea RM, Liedel JL, Welak SR, Cassidy LD, Mayer AN, Pritchard KA, Jr, et al. Intestinal alkaline phosphatase administration in newborns is protective of gut barrier function in a neonatal necrotizing enterocolitis rat model. J Pediatr Surg. 2012;47(6):1135–1142. doi: 10.1016/j.jpedsurg.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 54.Zimmerman JE, Kramer AA, Knaus WA. Changes in hospital mortality for United States intensive care unit admissions from 1988 to 2012. Crit Care. 2013;17(2):R81. doi: 10.1186/cc12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bentala H, Verweij WR, Huizinga-Van der Vlag A, van Loenen-Weemaes AM, Meijer DK, Poelstra K. Removal of phosphate from lipid A as a strategy to detoxify lipopolysaccharide. Shock. 2002;18(6):561–566. doi: 10.1097/00024382-200212000-00013. [DOI] [PubMed] [Google Scholar]
- 56.Verweij WR, Bentala H, Huizinga-van der Vlag A, Miek van Loenen-Weemaes A, Kooi K, Meijer DK, et al. Protection against an Escherichia coli-induced sepsis by alkaline phosphatase in mice. Shock. 2004;22(2):174–179. doi: 10.1097/01.shk.0000132485.05049.8a. [DOI] [PubMed] [Google Scholar]
- 57.Beumer C, Wulferink M, Raaben W, Fiechter D, Brands R, Seinen W. Calf intestinal alkaline phosphatase, a novel therapeutic drug for lipopolysaccharide (LPS)-mediated diseases, attenuates LPS toxicity in mice and piglets. J Pharmacol Exp Ther. 2003;307(2):737–744. doi: 10.1124/jpet.103.056606. [DOI] [PubMed] [Google Scholar]
- 58.Su F, Brands R, Wang Z, Verdant C, Bruhn A, Cai Y, et al. Beneficial effects of alkaline phosphatase in septic shock. Crit Care Med. 2006;34(8):2182–2187. doi: 10.1097/01.CCM.0000229887.70579.29. [DOI] [PubMed] [Google Scholar]
- 59.Khundmiri SJ, Asghar M, Khan F, Salim S, Yusufi AN. Effect of reversible and irreversible ischemia on marker enzymes of BBM from renal cortical PT subpopulations. Am J Physiol. 1997;273(6 Pt 2):F849–F856. doi: 10.1152/ajprenal.1997.273.6.F849. [DOI] [PubMed] [Google Scholar]
- 60.Heemskerk S, Masereeuw R, Moesker O, Bouw MP, van der Hoeven JG, Peters WH, et al. Alkaline phosphatase treatment improves renal function in severe sepsis or septic shock patients. Crit Care Med. 2009;37(2):417–423. e1. doi: 10.1097/CCM.0b013e31819598af. [DOI] [PubMed] [Google Scholar]
- 61.Heemskerk S, Pickkers P, Bouw MP, Draisma A, van der Hoeven JG, Peters WH, et al. Upregulation of renal inducible nitric oxide synthase during human endotoxemia and sepsis is associated with proximal tubule injury. Clin J Am Soc Nephrol. 2006;1(4):853–862. doi: 10.2215/CJN.00490206. [DOI] [PubMed] [Google Scholar]
- 62.Pickkers P, Heemskerk S, Schouten J, Laterre PF, Vincent JL, Beishuizen A, et al. Alkaline phosphatase for treatment of sepsis-induced acute kidney injury: a prospective randomized double-blind placebo-controlled trial. Crit Care. 2012;16(1):R14. doi: 10.1186/cc11159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van der Waaij D, Berghuis-de Vries JM, Lekkerkerk L-v. Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. J Hyg (Lond) 1971;69(3):405–411. doi: 10.1017/s0022172400021653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alam SN, Yammine H, Moaven O, Ahmed R, Moss AK, Biswas B, et al. Intestinal alkaline phosphatase prevents antibiotic-induced susceptibility to enteric pathogens. Ann Surg. 2014;259(4):715–722. doi: 10.1097/SLA.0b013e31828fae14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sekirov I, Tam NM, Jogova M, Robertson ML, Li Y, Lupp C, et al. Antibiotic-induced perturbations of the intestinal microbiota alter host susceptibility to enteric infection. Infect Immun. 2008;76(10):4726–4736. doi: 10.1128/IAI.00319-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen X, Katchar K, Goldsmith JD, Nanthakumar N, Cheknis A, Gerding DN, et al. A mouse model of Clostridium difficile-associated disease. Gastroenterology. 2008;135(6):1984–1992. doi: 10.1053/j.gastro.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 67.Kachrimanidou M, Malisiovas N. Clostridium difficile infection: a comprehensive review. Crit Rev Microbiol. 2011;37(3):178–187. doi: 10.3109/1040841X.2011.556598. [DOI] [PubMed] [Google Scholar]
- 68.Vaishnavi C. Clinical spectrum & pathogenesis of Clostridium difficile associated diseases. Indian J Med Res. 2010;131:487–499. [PubMed] [Google Scholar]
- 69.Hookman P, Barkin JS. Clostridium difficile associated infection, diarrhea and colitis. World J Gastroenterol. 2009;15(13):1554–1580. doi: 10.3748/wjg.15.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C, American Heart A et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 71.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 72.Malik S, Wong ND, Franklin SS, Kamath TV, L'Italien GJ, Pio JR, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110(10):1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 73.Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care. 2005;28(11):2745–2749. doi: 10.2337/diacare.28.11.2745. [DOI] [PubMed] [Google Scholar]
- 74.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 75.Hohmeier HE, Tran VV, Chen G, Gasa R, Newgard CB. Inflammatory mechanisms in diabetes: lessons from the beta-cell. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S12–S16. doi: 10.1038/sj.ijo.0802493. [DOI] [PubMed] [Google Scholar]
- 76.Gieling RG, Wallace K, Han YP. Interleukin-1 participates in the progression from liver injury to fibrosis. Am J Physiol Gastrointest Liver Physiol. 2009;296(6):G1324–G1331. doi: 10.1152/ajpgi.90564.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 78.Ghoshal S, Witta J, Zhong J, de Villiers W, Eckhardt E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res. 2009;50(1):90–97. doi: 10.1194/jlr.M800156-JLR200. [DOI] [PubMed] [Google Scholar]
- 79.Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NM, Magness S, et al. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One. 2010;5(8):e12191. doi: 10.1371/journal.pone.0012191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kaliannan K, Hamarneh SR, Economopoulos KP, Nasrin Alam S, Moaven O, Patel P, et al. Intestinal alkaline phosphatase prevents metabolic syndrome in mice. Proc Natl Acad Sci U S A. 2013;110(17):7003–7008. doi: 10.1073/pnas.1220180110. [DOI] [PMC free article] [PubMed] [Google Scholar]