Abstract
BACKGROUND
Isolated left ventricular apical hypoplasia with several different unrecognized dimensions is a newly discovered congenital anomaly of the heart.
CASE REPORT
In this report, we describe a case of cardiomyopathy of this type occurring in a 13-year-old male with a history of mental retardation and sudden cardiac death (SCD) of second-degree relatives. The patient was referred for an evaluation of cardiac status. An echocardiography analysis demonstrated a spherical left ventricle (LV) appearance with mild mitral regurgitation. Cardiac magnetic resonance imaging (MRI) confirmed a spherical and truncated LV appearance. The right ventricle was found to have elongated and wrapped around the LV, and diverticulum was also seen in the cardiac MRI.
CONCLUSION
To the best of our knowledge, this is to present the first case of LV apical hypoplasia combined with LV diverticulum and a family history of SCD. As more cases featuring this cardiomyopathy type are recognized, it will be easier to elucidate the natural history and management of such cardiac anomalies.
Keywords: Cardiomyopathy, Hypoplasia, Magnetic Resonance Imaging Scan, Sudden Cardiac Death
Introduction
Isolated left ventricular apical hypoplasia with several different unrecognized dimensions is a newly discovered congenital anomaly of the heart, as described for the first time by Fernandez-Valls et al.1 This congenital anomaly was first hypothesized as isolated left ventricular hypoplasia with no specific symptoms such as atypical chest pain, fatigue, or breathlessness, and even as an asymptomatic anomaly. Magnetic resonance imaging (MRI) role in diagnosing cardiac anomalies or masses is strongly recommended.2 This anomaly was first described in detail on the basis of an MRI modality. Initial evidence indicated that the symptoms of affected patients were fully relieved by anti-heart failure medications.3,4 The first case of congenital left ventricular hypoplasia described was reported as an isolated phenomenon. However, it has been indicated in some reports in conjunction with other cardiovascular abnormalities such as cyanotic congenital anomalies, transposition of the cardiac valves, and aortic stenosis.5-7
In the present study, we investigate the other cardiac anomalies accompanying left ventricular apical hypoplasia.
Case Report
A 13-year-old male patient with a history of sudden cardiac death (SCD) in two of his second-degree relatives, referred to our clinic to evaluate a possible cardiac disease. He also had mental retardation from birth. The presenting symptoms were relatively mild and non-specific and included shortness of breath and chest discomfort. The patient’s hemodynamic condition was evaluated through a physical examination and an assessment of his vital signs. The physical examination identified grade II systolic murmurs. Electrocardiography (ECG) analysis showed a normal sinus rate and rhythm, right axis deviation (110°), and a low precordial voltage with poor R-wave progression.
Transthoracic echocardiograms showed moderate to severe left ventricle (LV) systolic dysfunction. In addition, mild tricuspid regurgitation was presented. Besides enlargement of the left atrium and moderate mitral regurgitation, other cardiac valves showed no significant abnormality.
Since the LV apex was not clear in the four-chamber view of the echocardiographic evaluation (Figure 1) and to study the kinetic features of the congenital malformation and its morphological characteristics, we performed contrast-enhanced cardiac MRI, using a 1.5 T whole-body scanner (Avanto, Siemens, Erlangen). For signal reception, an eight-element cardiac phased-array receiver surface coil was used. We performed retrospective ECG-triggered steady-state free precession (SSFP) sequence for the evaluation of LV myocardial thickness, as well as kinetic, parietal segmental, and the global contractility. We oriented the sequences to the short-axis and long-axis (atrium-ventricular and four-chamber axes) using parameters as follows: TR 3.8 ms; TE 1.8 ms; flip angle (FA) 70°; matrix scan 256 × 256; field of view (FOV) 400; thickness 8 mm; gap 2 mm; 25 cardiac phases per cycle; and retrospective synchronization. Cine cardiac MRI with a four-chamber view showed a truncated appearance of the spherical LV with a bulging of the interventricular septum (IVS) toward the right ventricle (white arrow). It also indicated invagination of fatty material and elongation of a normally functioning right ventricle around the deficient LV apex (Figure 2).
Figure 1.
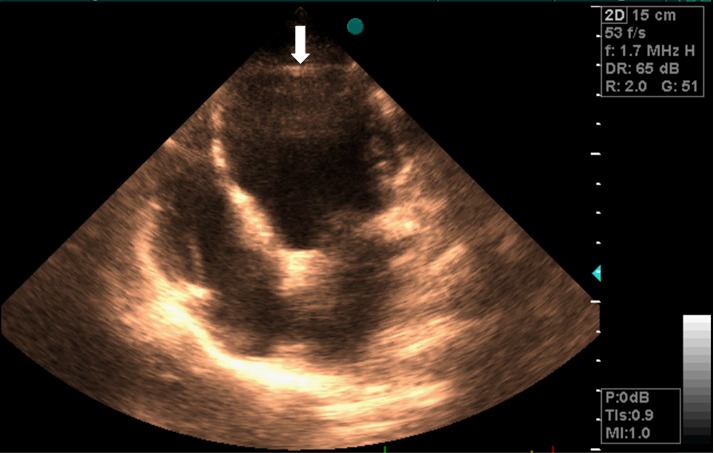
Four-chamber view in a transthoracic echocardiogram showing enlargement of the left ventricle (LV) (The LV apical structure was unclear in this view)
Figure 2.
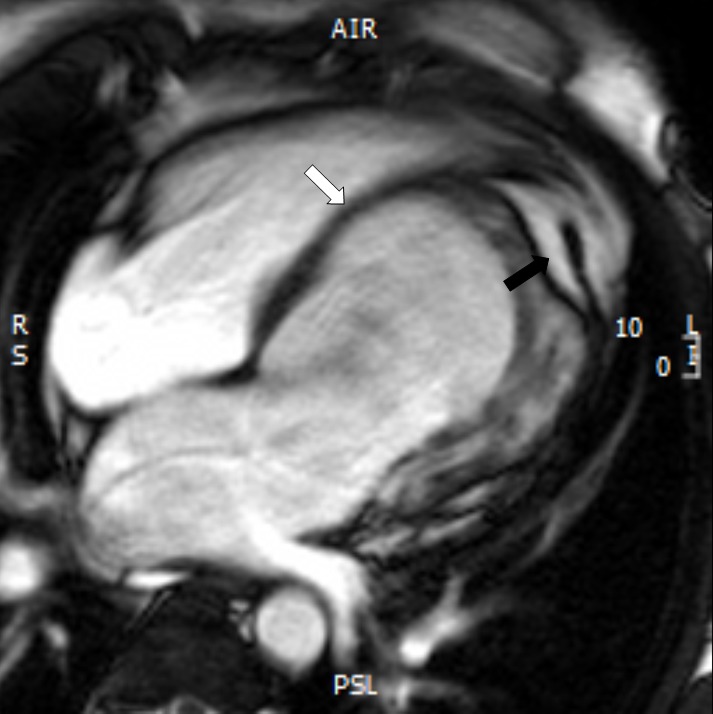
Cine cardiac magnetic resonance imaging (MRI) image in a four-chamber view shows bulging of the interventricular septum (IVS) toward the right ventricle (white arrow) and invagination of fatty material (black arrow)
The stack of the axial view using SSFP sequences showed a small cavity indicating contractile myocardial out pouching located in the mid-posterolateral LV wall and containing all three layers of the ventricular wall, which suggested LV diverticulum. Furthermore, LV volumes were enlarged, and the ejection fraction was decreased.
Black-blood T1-weighted sequences with and without fat saturation on short-axis and long-axis views were performed to assess alterations of the myocardial signals (Figure 3). Finally, late gadolinium enhancement MRI (LGE-MRI) was performed by means of magnitude-reconstructed and phase-sensitive inversion recovery prepared using a fast gradient echo sequence. After 10 minutes from the administration of 0.2 mmol/kg of gadoterate meglumine (Dotarem®, Guerbet, France), LGE-MR images were obtained along the same axis plane and with the same slice thickness as in the cine MRI. The acquisition parameters were as follows: TR = 600 ms; TE = 3.4 ms; FA = 25°; acquisition matrix = 156 × 256; FOV = 320 × 400 mm; slice number = 10 slices; and cardiac phase = mid-diastole. There were no signs of fibrosis or necrosis tissue presenting in the circumferential wall of the diverticulum showing by the delayed enhancement images (Figure 4).
Figure 3.
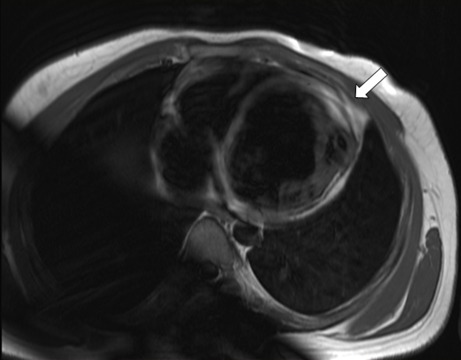
A T1-weighted image shows bright tissue replaced in the left ventricle (LV) apical position (white arrow), which could suggest the presence of fat replacement
Figure 4.
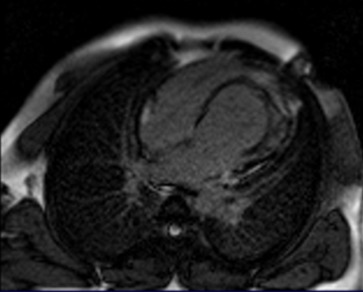
A late gadolinium enhancement (LGE) image performed 10 minutes after the contrast media injection showed no intramyocardial hyperenhancement area but indicated the presence of myocardial tissue in the out pouching; this confirmed the congenital nature of the diverticulum
Discussion
Previous studies reported a limited number of cases relating to LV apical hypoplasia accompanied by other cardiac diseases.5-7
In our case report, the patient had LV diverticulum, mental retardation, and a family history of SCD. To our knowledge, this is the first case of LV apical hypoplasia combined with LV diverticulum. In our patient, although not documented, the family history of SCD may also have raised suspicions of a familial pattern of disease at least in a subset of patients, which necessitated screening of other family members.
Although the exact mechanism is unclear, it is believed that deficient partitioning of both ventricles during embryonic life may lead to a spherical LV with an elongated right ventricular covering around its truncated apex. Furthermore, the finding of LV diverticula may be a physiological consequence of the severity of the dysplasia, which makes the LV wall weak and leads to the formation of diverticula. The main manifestation relates to an indication of four definitive components of isolated left ventricular apical hypoplasia on MRI, including: (1) left ventricular truncation with systolic dysfunction; (2) substitution of the left ventricular apex with fat tissue; (3) papillary muscles originating from the anteroapical region; and (4) the wrapping of the right ventricle around the LV.3,4,8,9-13 We also demonstrate the novel finding of LV diverticulum.
In spite of scarce knowledge, the presented cases depict a spectrum from no symptoms or non-specific symptoms, mainly during childhood, to systolic or diastolic dysfunction of the congestive heart failure, or even malignant tachyarrhythmia, usually in adulthood. The hemodynamic condition of the anomaly described in our report looked like restrictive cardiomyopathy manifested by left ventricular dysfunction7 or by a reduced left ventricular ejection fraction.3,5,13,14 Although there is little documentation on this phenomenon, a close follow-up of patients is recommended if only for the purpose of evaluating the signs and symptoms of pulmonary hypertension, heart failure, and potentially malignant tachyarrhythmia.11,13 Furthermore, screening of other family members if there is a suspicious family history may be valuable.
Conclusion
In this case report, we presented a teenage patient with combined LV apical hypoplasia, LV diverticulum, and a suspicious family history. A study of other living family members using echocardiography was negative. Our patient is still undergoing intensive treatment with standard drugs for systolic heart failure and is also under close observation for the occurrence of an arrhythmia and ventricular dysfunction.
Acknowledgments
None.
Footnotes
Conflicts of Interest
Authors have no conflict of interests.
REFERENCES
- 1.Fernandez-Valls M, Srichai MB, Stillman A, White RD. Isolated left ventricular apical hypoplasia: a new congenital anomaly described with cardiac tomography. Heart. 2004;90(5):552–5. doi: 10.1136/hrt.2003.010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nezafati MH, Nezafati P. A 25-cm angiomyxoma of the right atrium extending toward the right ventricle and pulmonary artery terminated to the right pulmonary hilum. Ann Thorac Surg. 2014;98(5):1846. doi: 10.1016/j.athoracsur.2014.07.048. [DOI] [PubMed] [Google Scholar]
- 3.Flett A, Elliott PM, Moon J. Cardiovascular magnetic resonance of isolated left ventricular apical hypoplasia. Circulation. 2008;117:e504–e505. doi: 10.1161/CIRCULATIONAHA.107.746503. [DOI] [PubMed] [Google Scholar]
- 4.Haffajee JA, Finley JJ, Brooks EL, Kuvin JT, Patel AR. Echocardiographic characterization of left ventricular apical hypoplasia accompanied by a patent ductus arteriosus. Eur J Echocardiogr. 2011;12(3):E17. doi: 10.1093/ejechocard/jeq170. [DOI] [PubMed] [Google Scholar]
- 5.Chaowu Y, Xin S, Shihua Z, Jianrong L, Hao W. Complete transposition of the atrioventricular valves associated with left ventricular apical hypoplasia. Circulation. 2011;124(21):e538–e539. doi: 10.1161/CIRCULATIONAHA.111.043620. [DOI] [PubMed] [Google Scholar]
- 6.Moon JI, Jeong YJ, Lee G, Choi JH, Lee JW. Isolated left ventricular apical hypoplasia with infundibular pulmonary and aortic stenosis: a rare combination. Korean J Radiol. 2013;14(6):874–7. doi: 10.3348/kjr.2013.14.6.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ong CC, Hia CP, Lim TC, Teo LL. Isolated left-ventricular apical hypoplasia presenting as a left-ventricular mass on echocardiography. Pediatr Cardiol. 2012;33(8):1456–7. doi: 10.1007/s00246-012-0349-x. [DOI] [PubMed] [Google Scholar]
- 8.Motwani M, Witte KK, Plein S, Greenwood JP. Isolated Left ventricular apical hypoplasia evaluated by cardiovascular magnetic resonance and gadolinium enhancement techniques. Journal of the American College of Cardiology. 2011;58(22):2355. doi: 10.1016/j.jacc.2011.02.082. [DOI] [PubMed] [Google Scholar]
- 9.Marin C, Sanchez ML, Maroto E, Ossaba S, Ruiz Y, Zabala JI. MR imaging of isolated left ventricular apical hypoplasia. Pediatr Radiol. 2007;37(7):703–5. doi: 10.1007/s00247-007-0459-4. [DOI] [PubMed] [Google Scholar]
- 10.Melendez G, Munoz L, Meave A. Isolated left ventricular apical hypoplasia. Rev Esp Cardiol. 2010;63(8):984. doi: 10.1016/s1885-5857(10)70191-2. [DOI] [PubMed] [Google Scholar]
- 11.Freedom RM, Black MD, Benson LN. Hypoplastic left heart syndrome. In: Allen HD, Driscoll D, Shaddy R, Feltes TF, editors. Moss & Adams' Heart disease in infants, children, and adolescents: including the fetus and young adult. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. pp. 1011–26. [Google Scholar]
- 12.Irving CA, Chaudhari MP. Fatal presentation of congenital isolated left ventricular apical hypoplasia. Eur J Cardiothorac Surg. 2009;35(2):368–9. doi: 10.1016/j.ejcts.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 13.Vanhecke TE, Decker J, Leonowicz N, Chinnaiyan KM. Isolated left ventricular apical hypoplasia. Congenit Heart Dis. 2011;6(6):646–9. doi: 10.1111/j.1747-0803.2011.00489.x. [DOI] [PubMed] [Google Scholar]
- 14.Starmer G, Younger JF, Stewart P. Multimodality imaging of isolated left ventricular apical hypoplasia. Eur Heart J. 2012;33(5):675. doi: 10.1093/eurheartj/ehr252. [DOI] [PubMed] [Google Scholar]


