Abstract
The discovery of a new series of compounds that are potent, selective 5-HT2C receptor agonists is described herein as we continue our efforts to optimize the 2-phenylcyclopropylmethylamine scaffold. Modifications focused on the alkoxyl substituent present on the aromatic ring led to the identification of improved ligands with better potency at the 5-HT2C receptor and excellent selectivity against the 5-HT2A and 5-HT2B receptors. ADMET studies coupled with a behavioral test using the amphetamine-induced hyperactivity model identified four compounds possessing drug-like profiles and having antipsychotic properties. Compound (+)-16b, which displayed an EC50 of 4.2 nM at 5-HT2C, no activity at 5-HT2B, and an 89-fold selectivity against 5-HT2A, is one of the most potent and selective 5-HT2C agonists reported to date. The likely binding mode of this series of compounds to the 5-HT2C receptor was also investigated in a modeling study, using optimized models incorporating the structures of β2-adrenergic receptor and 5-HT2B receptor.
Graphical Abstract
INTRODUCTION
Serotonin or 5-hydroxytryptamine (5-HT) is a major neurotransmitter that is primarily found in the gastrointestinal tract, platelets, and the central nervous system (CNS). It is believed to be involved in the regulation of a variety of physiological functions such as intestinal movements, mood, cognition, and appetite.1 These functions are mediated through serotonin receptors, which belong to the G-protein coupled receptor (GPCR) superfamily and are composed of seven subfamilies (5-HT1–7) and 14 isoforms.2
Recently, the serotonin 2C (5-HT2C) receptor has been shown to be a promising drug target for the treatment of a variety of CNS disorders, including obesity and mental disorders such as schizophrenia, depression, and anxiety.3–7 One of the many advantages of the 5-HT2C receptor as a CNS drug target stems from the fact that it is found almost exclusively in the CNS,8,9 and thus compounds that selectively activate this receptor should have limited impact on peripheral tissues. However, the activation of two other closely related 5-HT2 subtypes, namely the 5-HT2A and 5-HT2B receptors, has been reported to be associated with hallucinations and cardiac valvulopathy, respectively.10 Therefore, the identification of ligands possessing exquisite selectivity against the 5-HT2A and 5-HT2B receptors is a key criterion for the therapeutic advancement of 5-HT2C agonists. The achievement of this goal has been challenging due to the high conservation of molecular determinants involved in ligand recognition within this subfamily of receptors.11
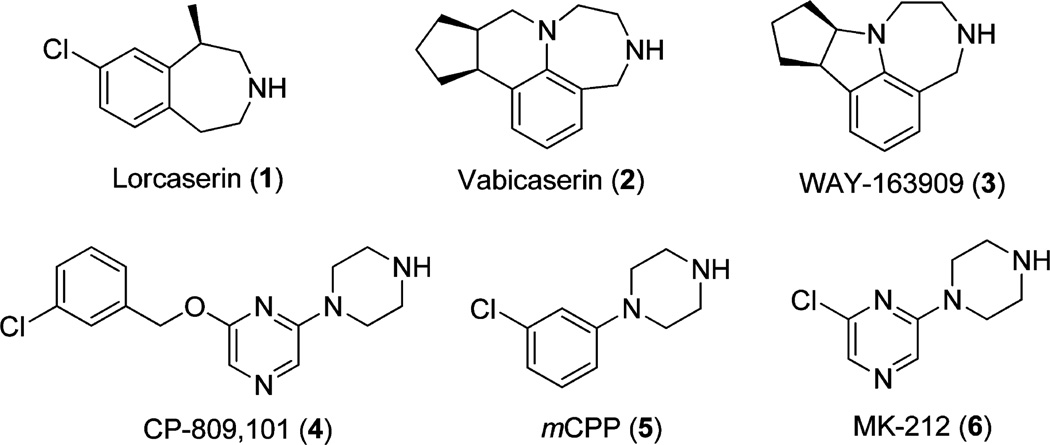
Currently, there are a number of 5-HT2C agonists (Figure 1, 1–6) generated for potential therapeutic uses or as chemical tools for the study of the biological roles of 5-HT2C receptor. Among them, lorcaserin (Belviq, 1) was approved by the FDA in 2012 for the treatment of obesity. Although it was reported to have 100-fold selectivity for 5-HT2C relative to the 5-HT2B subtype, lorcaserin possesses full agonist activity at 5-HT2B (EC50 = 943 ± 90 nM, Emax = 100%).12 Hence, it is not surprising that lorcaserin was found to cause a higher incidence of cardiac valve disorders in clinical trials compared to the placebo group.13
Figure 1.
Representative 5-HT2C agonists.
Vabicaserin (SCA-136, 2), by targeting 5-HT2C receptors (EC50 = 8 nM, Emax = 100%), was tested in clinical trials for the treatment of schizophrenia;14 however, it displayed moderate efficacy on 5-HT2B receptors (Emax = 50%) and good potency (EC50 = 12 or 102 nM depending on receptor densities).15 WAY-163909 (3), an analogue of vabicaserin, was shown to have selectivity toward 5-HT2C (EC50 = 8 nM; Emax = 90%) while possessing no agonist activity at 5-HT2A and weak efficacy at 5-HT2B receptors (Emax = 40%); it has proven to have good preclinical antipsychotic-like activity in several animal models.16 Compound CP-809,101 (4) is one of the most selective and potent 5-HT2C (EC50 = 0.11 nM, Emax = 93%) ligands developed, with about 600-fold 5-HT2C selectivity against 5-HT2B. However, it is still relatively potent at 5-HT2B (EC50 = 65.3 nM, Emax = 57%).17 Because of the genotoxicity of this compound, it could not be advanced to clinical evaluation.18 Nonetheless, CP-809,101 has structural similarities to mCPP (5) and MK-212 (6), two compounds discovered decades ago and used as tools for the pharmacological study of 5-HT2C receptors.19,20
In our own research, we focused on compounds that possessed the 2-phenylcyclopropylmethylamine scaffold (7) to develop selective 5-HT2C agonists. This particular scaffold was derived from an initial high throughput screening (HTS) screening campaign in which tranylcypromine was identified as a hit as described in an earlier publication.21 Rational drug design principles coupled with the evolution of a body of structure–activity relationships (SARs) led to the identification of compounds possessing a 2-cyclopropylmethoxy group at position 2, as illustrated by compounds 8, 9, and 10 (Figure 2). Compound 8, which possesses a fluorine substitution at position 5 of the benzene ring, showed good potency on the 5-HT2C receptor (EC50 = 21 nM), with only moderate selectivity for 5-HT2B (EC50 = 289 nM).22 The replacement of the fluorine atom with a hydroxyl group, as in compound 9, led to the enhancement of both potency and selectivity, but the bioavailability of compound 9 was found to be too low in a later study (F = 3.2% in mice, unpublished data). Compound 10, with no substitution at the same position, showed good potency as a partial agonist (EC50 = 55 nM, Emax = 61%), with great selectivity against both 5-HT2A and 5-HT2B.23 Considering the needs of more selective and potent 5-HT2C agonists for the validation of their therapeutic potential in the treatment of various illnesses associated with 5-HT2C, we conducted a new round of lead optimization on this promising scaffold. The chemical synthesis and SAR results obtained for the new compounds are reported herein, together with a rodent behavioral test and a homology modeling study. These new analogues provide improvements compared to previous compounds.
Figure 2.
Selective 5-HT2C agonists based on 2-phenylcyclopropylme-thylamine scaffold.
RESULTS
Design and Synthesis of 5-Chlorine Substituted Compounds
Prior SAR studies showed that compounds with an alkoxyl group at position 2 provided the most favorable 5-HT2C agonism and selectivity toward 5-HT2A and 5-HT2B, and thus this substitution pattern was therefore retained in further rounds of structural modifications. Through a careful structural analysis of known 5-HT2C agonists as well as other CNS drugs, it is apparent that a chlorine atom represents one of the most frequently used substituents for aromatic rings. This observation is exemplified by mCPP, MK-212, and lorcaserin, all of which contain a chlorine substituent located at a position meta to the nitrogen-containing group. Thus, we introduced the chlorine atom as the substituent at position 5, and further optimization of the alkyl ether group was conducted to improve 5-HT2C potency as well as selectivity over both 5-HT2A and 5-HT2B.
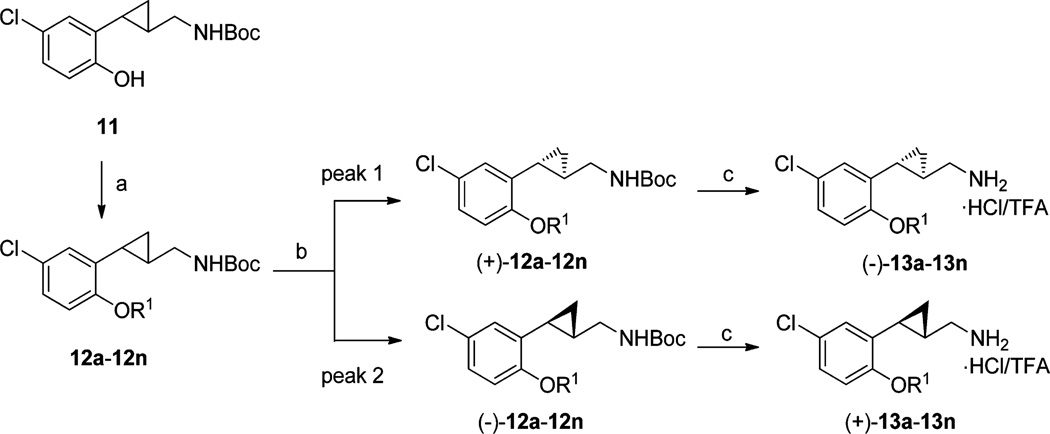
As depicted in Scheme 1, the synthesis of the 5-chlorine containing analogues started from the corresponding phenol 11, which was prepared using methods similar to those reported previously by us for the 5-bromo analogue.23 The cyclopropane ring of 11 was prepared in its trans configuration, as this stereochemistry has proven to be favored in our previous SAR studies. The ether derivatives 12a–12n were prepared either by a standard Williamson ether synthesis with an alkyl halide or by use of the Mitsunobu reaction with the appropriate alcohol (for details of the synthesis of 12a–12n, see Supporting Information, Table S1). Chiral separations of 12a–12n were achieved with preparative chiral HPLC and the corresponding (+)- and (−)-enantiomers were then subjected to Boc deprotection reactions to provide both (−)- and (+)-enantiomers of compounds 13a–13n as their HCl or TFA salts. The absolute configurations of the enantiomers were assigned from the measured optical rotations coupled with the stereochemical correlations reported previously by us.21
Scheme 1.
Synthesis of 5-Chloro Compoundsa
aReagents and conditions: (a) Williamson ether synthesis with R1X or Mitsunobu reaction with appropriate alcohols; (b) chiral prep-HPLC separation; (c) 13c, 13d, 13f, 13h, and 13i, TFA/CH2Cl2, rt, 1h; 13a, 13b, 13e, 13g, 13j–13n, 2 M HCl in diethyl ether, rt, 24–48 h.
On the basis of the SAR data from our previous work, we hypothesized that the alkoxyl substituents at position 2 on the benzene ring might be accommodated within a hydrophobic cavity, which could vary in size among the 5-HT2C, 5-HT2A, and 5-HT2B receptors. This cavity might be slightly larger for the 5-HT2C receptors compared to the 5-HT2A and 5-HT2B receptors based on the observation that (1) small groups attached to position 2 displayed good activity at all three subtypes (less selectivity) and (2) increasing the size of the substituent resulted in a decrease in activity at 5-HT2A and 5-HT2B while retaining good activity at 5-HT2C. Accordingly, we believed that by achieving a proper balance of size and lipophilicity, we should be able to identify more potent 5-HT2C agonists with improved selectivity against the 5-HT2A and 5-HT2B subtypes.
To explore this assumption, compounds with varied alkoxyl groups were designed and synthesized. Functional activities at the 5-HT2C, 5-HT2A, and 5-HT2B receptors were determined, and physiochemical properties related to possible CNS penetration such as the CLogP and LogBB values were calculated using the ACD Percepta program. These values are listed in Table 1. Generally, the (+)-isomers of all new compounds were found to be more potent than the (−)-isomers, which is consistent with our previously published SAR data.23 As evidenced by compounds 13a, 13b, and 13c, potency at the 5-HT2C receptor decreases with elongation of the alkyl chain, while selectivity over 5-HT2B and 5-HT2A improves. n-Propyl substitution as in compound (+)-13b was favored with respect to both activity and selectivity. The change from an n-propyl group to an isopropyl group gave compound (+)-13d that has similar 5-HT2C activity but decreased selectivity against 5-HT2B (33-fold selectivity for (+)-13b vs 11-fold selectivity for (+)-13d). The cyclopropylmethoxy group was the preferred ether substituent at position 2 of the benzene ring based on our previous findings;22,23 however, the use of this group as found in compound (+)-13e afforded only a moderate potency at 5-HT2C receptors (EC50 = 251 nM, Emax = 75%). Incorporation of an additional heteroatom such as oxygen or sulfur into the ether appendage, as exemplified by compounds 13f and 13g, led to a large reduction in 5-HT2C activity as well as a predicted decrease in blood-brain barrier (BBB) penetration based upon the calculated LogBB values.
Table 1.
5-HT2C Activity and Selectivity Profiles of Compounds 13a–13n and Their Predicted Physiochemical Propertiesa,b
| R1 | Compound | EC50, nM (Emax) | CLogP | LogBB | ||
|---|---|---|---|---|---|---|
| 5-HT2C | 5-HT2B | 5-HT2A | ||||
| - | 5-HT | 0.21 (100%) | 0.92 (100%) | 1.88(100%) | - | - |
| - | lorcaserin | 3.6 (99%) | 478 (92%) | 302 (68%) | - | - |
| (−)-13a | 646 (72%) | 2307 (26%) | NA | 2.50 | −0.02 | |
| (+)-13a | 13 (91%) | 86 (45%) | 1215 (49%) | |||
| (−)-13b | 1228(31%) | 3004 (21%) | NA | 2.94 | 0.23 | |
| (+)-13b | 103 (72%) | 3436 (25%) | NA | |||
| (−)-13c | 683 (50%) | NA | NA | 3.36 | 0.42 | |
| (+)-13c | 288 (33%) | NA | NA | |||
| (−)-13d | 2481 (37%) | 5660 (19%) | NA | 2.84 | 0.15 | |
| (+)-13d | 91 (86%) | 997 (37%) | 4002 (19%) | |||
| (−)-13e | 1082(51%) | 3034 (32%) | NA | 2.87 | 0.23 | |
| (+)-13e | 251 (75%) | 5012 (20%) | NA | |||
| (−)-13f | 667 (45%) | 5300 (32%) | NA | 2.11 | −0.22 | |
| (+)-13f | 183(8%) | NA | NA | |||
| (−)-13g | 1762(11%) | NA | NA | 2.87 | −0.06 | |
| (+)-13g | 244 (24%) | NA | NA | |||
| (−)-13h | 737 (42%) | 4295 (28%) | NA | 2.69 | 0.07 | |
| (+)-13h | 60 (80%) | 2211 (32%) | NA | |||
| (−)-13i | 365 (71%) | 4411(30%) | NA | 2.17 | −0.43 | |
| (+)-13i | 63 (65%) | 4602 (29%) | 1005 (21%) | |||
| (−)-13j | NA | 6877 (33%) | NA | 2.98 | 0.16 | |
| (+)-13j | 133 (81%) | 1688 (27%) | NA | |||
| (+)-13k | NA | 6094 (39%) | NA | 3.23 | 0.37 | |
| (+)-13k | 212 (90%) | 422 (32%) | 903 (26%) | |||
| (−)-131 | 1127 (59%) | 4521 (31%) | NA | 2.23 | 0.01 | |
| (+)-131 | 32 (86%) | 632(31%) | 1476 (35%) | |||
| (−)-13m | 914 (64%) | 1485 (30%) | 1305 (43%) | 2.43 | 0.11 | |
| (+)-13m | 91 (81%) | 839 (27%) | 1811 (31%) | |||
| (−)-13n | 1258 (24%) | 6166 (22%) | NA | 2.48 | 0.07 | |
| (+)-13n | 206 (60%) | 4911 (22%) | NA | |||
Functional data were acquired with recombinant, stably expressed human serotonin receptors in the HEK-293 cell line, using a fluorescence imaging plate reader (FLIPR) assay; “NA”, no activity at 10 µM.
CLogP and LogBB values were calculated for the free bases using the ACD Percepta program.
Unsaturated alkyl groups, such as allyl and propargyl, were preferred both for their potency and selectivity, as revealed by compounds (+)-13h and (+)-13i. (+)-13h showed a moderately good pharmacological profile, with 60 nM potency at 5-HT2C, 37-fold selectivity against 5-HT2B (2211 nM), and no activity at 5-HT2A. Furthermore, good physiochemical properties were predicted for this compound. Similar potency and selectivity was observed for (+)-13i as well, however, it was predicted to have low brain penetration, as indicated by its LogBB value of −0.43. Allyl ethers bearing small modifications were also explored, as exemplified by compounds 13j and 13k. The presence of a fluorine substituent as in compound (+)-13j resulted in a 2-fold reduction in 5-HT2C activity compared to (+)-13h (133 vs 60 nM) while maintaining the same general selectivity profile against 5-HT2B and 5-HT2A. The presence of the fluorine atom might provide some advantage in terms of pharmacokinetics due to the possibility of reducing oxidative metabolism of the double bond. By adding an additional methyl group to the position 2 of the allyl appendage as in compound (+)-13k, the potency was reduced to 212 nM at the 5-HT2C receptors while its selectivity also decreased in comparison to compounds 13h and 13j.
The 2-fluoroethyl ether analogue (+)-13l displayed 32 nM potency at 5-HT2C and good selectivity against both 5-HT2A and 5-HT2B. Upon introducing one additional fluorine atom into the molecule by way of a 2,2-difluoroethyl appendage as in analogue 13m, a 3-fold decrease in activity at 5-HT2C (EC50 = 91 nM vs 32 nM) was observed. This drop in activity may result from either increased steric hindrance or electronic effects. Additionally, the incorporation of a 3-fluoropropyl ether appendage as in (+)-13n also led to a drop in potency (EC50 = 206 nM) compared to the simple unsubstituted propyl derivative (+)-13b, although (+)-13n possessed a reasonably good selectivity profile.
On the basis of the data from Table 1, compounds (+)-13h and (+)-13l represent the best ligands among this series of compounds, but they are about 10-fold less potent than lorcaserin, which shows an EC50 value of 3.6 nM on 5-HT2C in the functional calcium-based assay. Although lorcaserin displays around 100-fold selectivity over both 5-HT2B and 5-HT2A, it acts as a full or partial agonist at both receptors. As such, lorcaserin has potential side effect issues as discussed previously. Compounds (+)-13h and (+)-13l, along with (+)-13j, which might provide better PK properties due to the presence of the fluorine substitution, were advanced to in vitro ADMET studies.
ADMET Studies
Compounds (+)-13h, (+)-13j, and (+)-13l were evaluated for selected in vitro ADME properties and toxicity profiles (Table 2). In Caco-2 permeability studies, compounds (+)-13h, (+)-13j, and (+)-13l displayed greater than 35 × 10−6 cm/s A–B permeability in pH 7.4 buffer solution, and A–B/B–A ratios of 3.6, 3.5, and 2.0, respectively. When incubated with human and CD-1 mouse liver microsomes, all three compounds retained reasonably good stability, with more than 50% compound remaining after 1 h. Interestingly, although compound (+)-13h and (+)-13j displayed similar stability in the mouse liver microsomes test (60% vs 62%), (+)-13j had much better stability in the human microsomes study compared to (+)-13h (80% vs 52%), which could be due to the presence of the fluorine substitution as expected. Relatively low human plasma protein binding (57–75%) was measured for compounds (+)-13h, (+)-13j, and (+)-13l, which was expected based on their good CLogP values (between 2 and 3 as in Table 1), as Log P is an important indicator that determines protein binding properties.24
Table 2.
In Vitro ADMET Data for Compounds (+)-13h, (+)-13j, and (+)-13la
| compd | (+)-13h | (+)-13j | (+)-13l | |
|---|---|---|---|---|
| Caco-2 permeability (pH 7.4, × 10−6 cm/s) |
A–B | 35.6 | 38.3 | 35.5 |
| B–A | 9.8 | 10.8 | 17.5 | |
| microsomal stability (%) | human | 52 | 80 | 95 |
| mouse | 60 | 62 | 74 | |
| human PPB at 10 µM (%) | 75 | 68 | 57 | |
| CYP inhibition at 10 µM (%) |
3A | 27 | 27 | 22 |
| 2D6 | 91 | 80 | 79 | |
| 2C9 | 56 | 51 | 33 | |
| 2C19 | 32 | 33 | 21 | |
| 2B6 | 86 | 77 | 58 | |
| hERG IC50 (µM) | 0.27 | 3.98 | 2.00 | |
| Ames fluctuation test | negative | negative | negative |
This study was conducted at Cerep, Inc. (Redmond, USA); microsomal stability is presented as % compound remaining after 1 h incubation with human/mouse liver microsomes; the CYP inhibition assays were performed using human liver microsomes; midazolam, dextromethorphan, diclofenac, omeprazole, and bupropion were used as test substrates for the 3A, 2D6, 2C9, 2C19, and 2B6 isoforms, respectively; hERG inhibition was tested on CHO cells using the automated patch-clamp method; the Ames fluctuation test was performed with the TA98, TA100, TA1535, and TA1537 strains, with/without S9, at concentrations of 10, 50, and 100 µM.
Human cytochrome P450 (CYP) enzyme inhibition studies were conducted using the five most important isoforms, 3A, 2D6, 2C9, 2C19, and 2B6, at compound concentrations of 10 µM. Similar profiles were observed for all three compounds, with low inhibition of CYP3A and CYP2C19 and relatively higher inhibition of the other three isoforms (>50%). Compound (+)-13j shows a slightly better profile compared to (+)-13h, which indicates another benefit of the fluorine substituent. In the hERG inhibition test with the automated patch-clamp assay, moderate inhibition was observed with compounds (+)-13j and (+)-13l, while (+)-13h was found to have an IC50 of 0.27 µM. The Ames test was also conducted to preclude any mutagenic potential of these compounds; all three compounds showed negative results. Because of the high hERG activity of (+)-13h, this compound was not further evaluated. Thus, only compounds (+)-13j and (+)-13l were submitted to the in vivo brain penetration studies.
Brain penetration studies of (+)-13j and (+)-13l were conducted with CD1 mice, and the results are listed in Table 3. Excellent brain penetration was observed for both compounds, with brain concentrations of greater than 50 µM achieved at a dose of 10 mg/kg (ip) and with brain/plasma ratios over 10 at both time-points (0.5 and 2 h). The excellent brain penetration for these two compounds coupled with low plasma protein binding should ensure optimal CNS distribution of both compounds. The lower plasma concentrations would also help to mitigate possible cardiac toxicity related to hERG inhibition observed in the previous ADMET studies.
Table 3.
Brain Penetration Study of Compounds (+)-13j and (+)-13l in CD1 Micea
| Compd | time-point (h) |
brain concentration (ng/g) |
plasma concentration (ng/mL) |
ratio (brain/plasma)b |
|---|---|---|---|---|
| (+)-13j | 0.5 | 17460 | 1445 | 12.1 |
| 2 | 14670 | 903 | 16.2 | |
| (+)-13l | 0.5 | 12795 | 1200 | 10.7 |
| 2 | 13350 | 874 | 15.3 |
CD1 mice were administrated a single dose of both compounds (10 mg/kg, ip, formulation in pure water).
Density of mouse brain tissue was calculated as 1 g/mL.
While both compounds (+)-13j and (+)-13l showed good pharmacological profiles, acceptable ADMET properties, and excellent brain penetration, their moderate hERG inhibition could be a concern for further development. A similarity analysis of their structures with known hERG inhibitors revealed that the scaffold embodied by this series of compounds matched one of the common structural templates known to cause hERG inhibition, which is comprised of a three-point pharmacophore containing an aromatic ring, an adjacent hydrogen-bond donor, and an ionizable N atom.25 However, the 5-fluoro containing compound 8 displayed no hERG inhibition in our previous studies.22 Thus, replacement of the chlorine atom by the less lipophilic fluorine atom at position 5 might lead to a decrease in hERG inhibition. Moreover, from the SAR data gleaned from the study of the 5-chloro bearing compounds, we concluded that some of the alkoxyl substitutions at position 2 on the benzene ring may provide advantages over the cyclopropylmethoxy group found in compounds 13e and 8 in terms of potency and selectivity. Therefore, the introduction of these substituents into the 5-fluoro containing scaffold might produce compounds with better potency and, more importantly, reduced hERG inhibitory activity. Four different substituents from Table 1 were chosen for this purpose, and the synthesis and evaluation of these compounds are described below.
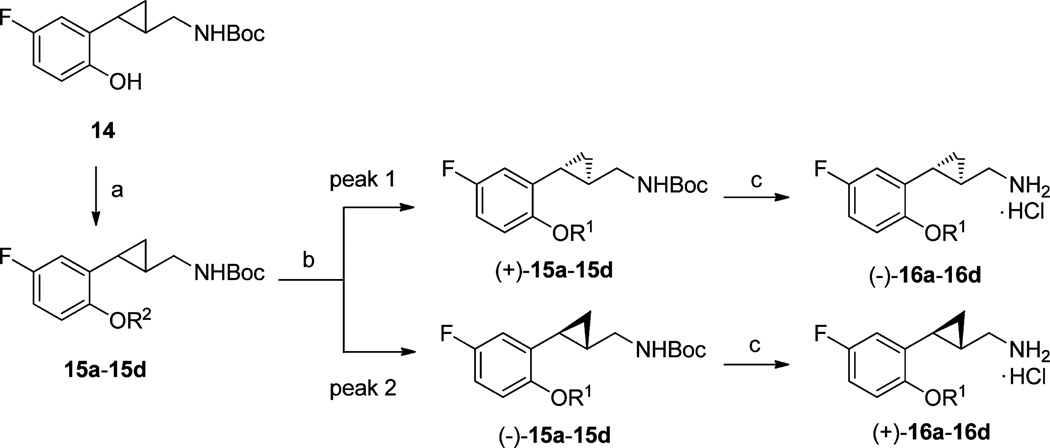
Routes to the 5-fluoro compounds are depicted in Scheme 2. 5-Fluorophenol 14 was prepared and used as the starting material, the synthesis of which has been reported previously.22 Alkylation of 14 with 1-iodopropane, allyl bromide, or 3-chloro-2-fluoropropene and a Mitsunobu reaction with 2-fluoroethanol provided the corresponding ethers 15a–15d in excellent yields (for details of the synthesis of 15a–15d, see Supporting Information, Table S2). These intermediates were separated using chiral preparative HPLC and then deprotected with HCl in diethyl ether to give both (−)- and (+)-enantiomers of compound 16a–16d. Similarly, absolute configurations of the enantiomers were assigned based on the measured optical rotations as described above.
Scheme 2.
Synthesis of 5-Fluoro Compoundsa
aReagents and conditions: (a) Williamson ether synthesis with R1X or Mitsunobu reaction with appropriate alcohols; (b) chiral prep-HPLC separation; (c) 2 M HCl in diethyl ether, rt, 24 h, 64–80%.
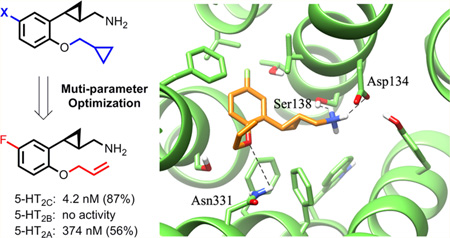
Pharmacological profiles of the 5-fluoro compounds are summarized in Table 4. All (+)-enantiomers had better efficacy than their (−)-enantiomers. However, a significant increase in potency at the 5-HT2C receptor was observed for these compounds compared to their 5-chloro analogues, while excellent selectivity was maintained. Compound (+)-16a, bearing an n-propyl group, displayed an EC50 value of 11 nM at 5-HT2C and was 9-fold more potent than its 5-chloro analogue (+)-13b (EC50 = 103 nM). It showed a high degree of selectivity over 5-HT2B (EC50 = 1994 nM) and 5-HT2A (no activity). The allyl ether bearing derivative (+)-16b (EC50 = 4.2 nM) has a potency equal to that of lorcaserin. It showed much better selectivity over 5-HT2B (no activity observed), with an 89-fold selectivity against 5-HT2A (EC50 = 374 nM). The attachment of a fluorine atom to the allyl group, with the intent to enhance metabolic stability, led to (+)-16c that was slightly less potent at 5-HT2C (22 nM) but had an improved selectivity over 5-HT2A. The 2-fluoroethoxy bearing compound (+)-16d showed enhanced potency at 5-HT2C (EC50 = 3.4 nM), along with excellent functional selectivity over both 5-HT2B (no activity observed) and 5-HT2A (>100-fold selectivity).
Table 4.
| R2 | Compound | EC50, nM(Emax) | CLogP | LogBB | ||
|---|---|---|---|---|---|---|
| 5-HT2C | 5-HT2B | 5-HT2A | ||||
| - | 5-HT | 0.21 (100%) | 0.92 (100%) | 1.88(100%) | - | - |
| - | lorcaserin | 3.6 (99%) | 478 (92%) | 302 (68%) | - | - |
| (−)-16a | 296 (80%) | 735(11%) | NA | 2.35 | 0.24 | |
| (+)-16a | 11 (88%) | 1994(33%) | 1025 (15%) | |||
| (−)-16b | 157(87%) | NA | 697 (56%) | 2.50 | −0.02 | |
| (+)-16b | 4.2 (87%) | NA | 374 (56%) | |||
| (−)-16c | 985 (62%) | NA | NA | 2.48 | 0.23 | |
| (+)-16c | 22 (91%) | NA | 1666(17%) | |||
| (−)-16d | 514(77%) | NA | 2994 (20%) | 2.94 | 0.23 | |
| (+)-16d | 3.4 (89%) | NA | 359 (76%) | |||
Functional data were acquired with recombinant, stably expressed human 5-HT receptors in the HEK-293 cell line, using a fluorescence imaging plate reader (FLIPR) assay; “NA”, no activity at 10 µM.
CLogP and LogBB values were calculated for the free bases using the ACD Percepta program.
To further investigate their drug-like character, compounds (+)-16b and (+)-16d were tested for both CYP and hERG inhibition. As shown in Table 5, improved CYP inhibition profiles were measured for (+)-16b and (+)-16d relative to compounds (+)-13h, (+)-13j, and (+)-13l. Less than 50% inhibition was observed at CYPs 3A4, 2D6, 2C9, and 2C19 for both compounds, while higher inhibition was observed at 2B6. As the 2B6 isoform is present in relatively low abundance in comparison to the other CYPs,26 these results are encouraging. In the hERG inhibition assay, although concentration-dependent inhibition was observed for (+)-16b and (+)-16d, both compounds afforded less than 50% inhibition at the highest concentration tested, which was 30 µM. These results suggest that the compounds may present a reasonable safety margin, as both compounds are active at the 5-HT2C receptor in the low nanomolar range.
Table 5.
CYP and hERG Inhibition Data for Compounds (+)-16b and (+)-16d
| assay | (+)-16b | (+)-16d | |
|---|---|---|---|
| CYP inhibition at 10 µMa (%) | 3A4 | 15 | 10 |
| 2D6 | 43 | 24 | |
| 2C9 | 35 | 16 | |
| 2C19 | 22 | 26 | |
| 2B6 | 80 | 6 | |
| hERG inhibitionb (%) | 0.37 µM | 6.4 | 6.0 |
| 1.1 µM | 8.7 | 11 | |
| 3.3 µM | 18 | 15 | |
| 10 µM | 29 | 25 | |
| 30 µM | 45 | 35 |
CYP inhibition test was performed using human liver microsomes; midazolam, dextromethorphan, tolbutamide, (S)-mephenytoin, and bupropion were used as test substrates for the 3A4, 2D6, 2C9, 2C19, and 2B6 isoforms, respectively.
hERG inhibition was tested on CHO cells using the automated patch-clamp method.
Further pharmacological profiling was conducted for compounds (+)-16b and (+)-16d to investigate potential off-target activity. Binding studies were performed at 45 targets, including GPCRs, ion channels, neurotransmitter transporters, and sigma receptors, by the Psychoactive Drug Screening Program (PDSP). (+)-16b and (+)-16d were found to display high affinity for the 5-HT2B and 5-HT2C receptors, with Ki values in the range of 30–50 nM. However, as already described, both compounds have been shown to be “functionally” selective for the 5-HT2C receptor. (+)-16b showed <1 µM Ki values at the 5-HT1A, 5-HT6, 5-HT7, α2A, α2B, α2C, and dopamine D3 receptor, while (+)-16d showed binding at this same level at only α2A. None of the other targets analyzed were found to show any significant off-target affinity for these two compounds (Supporting Information, Table S3). Compared to the other known 5-HT2C agonists reported to date, compounds (+)-16b and (+)-16d thus represent attractive molecules in terms of both their 5-HT2C potency and selectivity.
In Vivo Test in a Schizophrenia-like Animal Model
The pharmacological profiles and ADMET properties of the best compounds from both the 5-chloro and 5-fluoro series, (+)-13j, (+)-13l, (+)-16b, and (+)-16d, prompted their assessment in an animal model to explore their potential antipsychotic effects. One of the advantages of developing 5-HT2C agonists as antipsychotics is that they decrease levels of dopamine in limbic brain regions,27 which have been proven to mediate the antipsychotic effects of current antischizophrenia drugs. Meanwhile, agonism of the 5-HT2c receptor does not affect dopamine levels in the striatum, the region associated with extrapyramidal side effect of the “typical” antipsychotics.28 Moreover, the antiobesity effect of 5-HT2C agonists is well-known, exemplified by the approval of lorcaserin as an antiobesity drug. Thus, these agonists do not have the potential side effect of weight gain, which is observed with many antipsychotics which have properties of 5-HT2C antagonists or inverse agonists.29
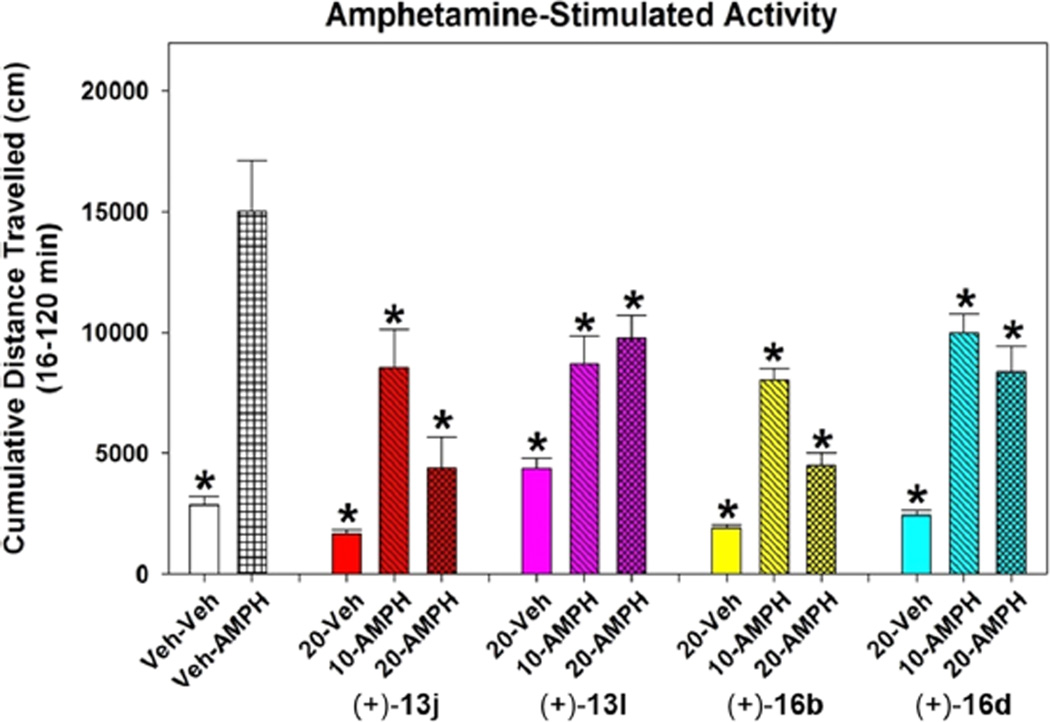
Amphetamine-induced hyperactivity is a well-recognized rodent test used to assess the antipsychotic potential of a compound. To examine this possibility, compounds (+)-13j, (+)-13l, (+)-16b, and (+)-16d were tested in this model (details of the behavioral test are provided in the Experimental Section). Adult male C57BL/6J mice were given the vehicle (Veh) or one of the test compounds and were placed into the open field for 15 min. They were removed and administered the Veh or d-amphetamine (AMPH) and returned immediately to the open field for 105 min. As shown in Figure 3, d-amphetamine significantly increased locomotor activity from 16 to 120 min in the Veh–AMPH compared to the Veh–Veh group. Administration of each of the four compounds at the 10 or 20 mg/kg doses significantly reduced the AMPH-stimulated hyperlocomotion relative to the Veh–AMPH group (p-values <0.035). Although responses to 10 and 20 mg/kg (+)-13l and (+)-16d were not differentiated by dose, compounds (+)-13j and (+)-16b dose dependently suppressed the hyperactivity such that locomotion at the 20 mg/kg doses was similar to that of the Veh–Veh group. Importantly, the 20 mg/kg dose of each the four compounds alone (20-Veh groups) had little influence on the spontaneous activity of the injected animals. The reduction in AMPH-stimulated hyperlocomotion, especially by compounds (+)-13j and (+)-16b, indicate their potential as antipsychotic agents and encourage further studies of other behaviors.
Figure 3.
Cumulative locomotor activity following injection of the vehicle (Veh) or 3 mg/kg d-amphetamine (AMPH). AMPH-stimulated locomotor activity in the Veh–AMPH group was higher than that in all other groups tested. Locomotion in the Veh–Veh group was similar to that in the (+)-13j–Veh, (+)-13l–Veh, (+)-16b–Veh, and (+)-16d–Veh groups (i.e., 20-Veh groups). Both 10 and 20 mg/kg of each of the four compounds decreased the AMPH-stimulated hyperlocomotion, with 20 mg/kg (+)-13j and (+)-16b being the most efficacious. N = 8–16 mice/group; *, p < 0.035, from the Veh–AMPH group.
Homology Modeling to Elucidate the Binding Mode of Key Compounds to the 5-HT2C Receptor
Following our SAR studies, we wanted to obtain some idea as to how (+)-16b might interact with the 5-HT2C receptor. Thus, the 5-HT2C primary sequence was retrieved from UniProt (P28335, 5HT2C_HUMAN), and two 5-HT2C homology models were generated. The first model (5-HT2C_inact) was obtained using the β2-adrenergic receptor (β2-AR) as a template in its inactive state (PDB: 2RH1), while the second model (5-HT2C_act) was generated by combining two templates, namely the resolved structure of 5-HT2B in a complex with ergotamine (PDB: 4IB4) and the β2-AR in its fully active state (PDB: 3SN6). This 5-HT2B-ergotamine model was selected due to the fact that ergotamine has functional selectivity for 5-HT2B, and thus the resolved structure does not represent a fully active state.30 By combining the two templates, the generated active model should benefit from the high homology in the orthosteric site between 5-HT2B and 5-HT2C while conserving features responsible for the fully activated state as taken from the β2-AR. The homology models were built by using Modeller9.12,31 and the best structures were selected for docking studies. Docking simulations were performed using PLANTS, which finds plausible ligand poses through ant colony optimization algorithms (ACO).32 (For details of the modeling study, see Supporting Information.)
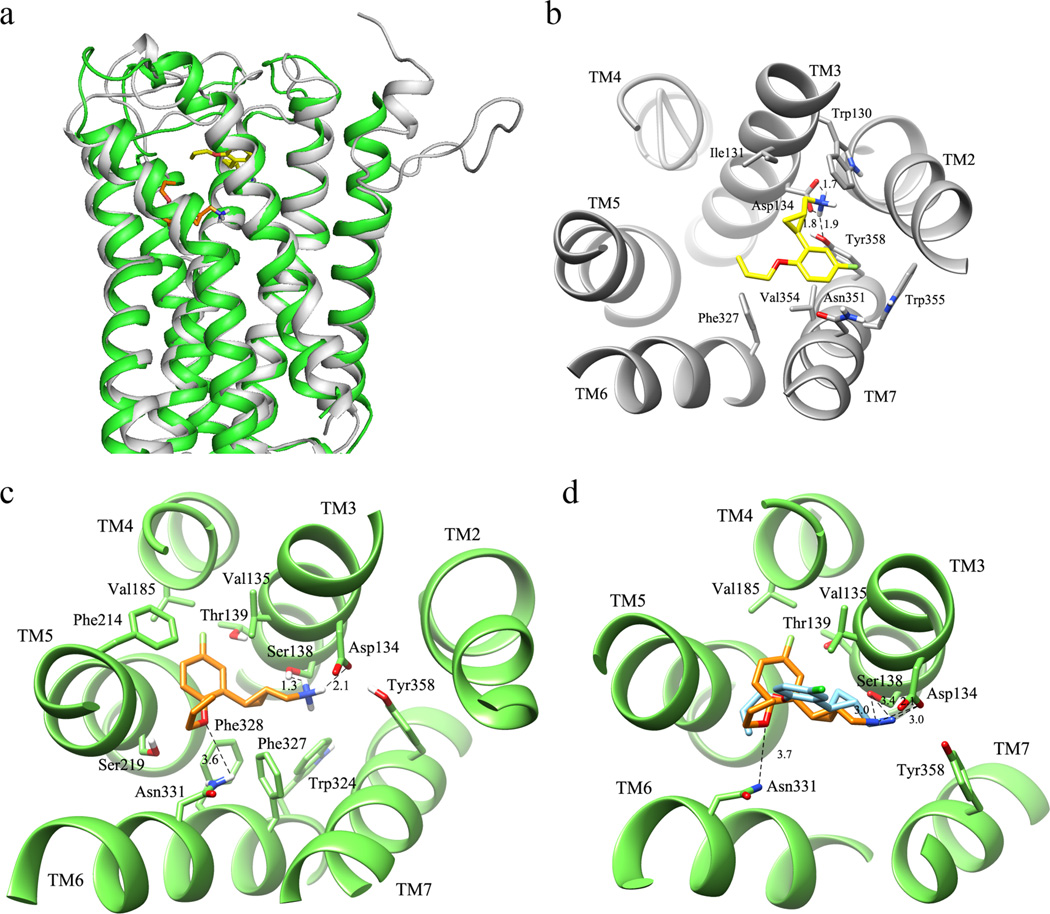
The macroscopic view of compound (+)-16b binding to both the inactive and active conformations of the 5-HT2C and the magnified views of the binding sites are shown in Figure 4. As exemplified in Figure 4a, a significant difference was observed between the binding poses of compound (+)-16b with 5-HT2C_inactive and 5-HT2C_active, as the compound is inserted much deeper into a tighter niche when docking to 5-HT2C_active. As shown with the magnified view in Figure 4b, the computed complexes for the 5-HT2C_inact model are vastly stabilized by the ion pair between the ligand ammonium head and Asp134, a key contact reinforced by clear H-bonds with Ser138 and Tyr358 plus a charge transfer interaction with Trp130. By contrast, the remaining part of the ligand is seen to elicit only weak apolar contacts: (a) the cyclopropane ring approaches Ile131, (b) the phenyl ring is engaged in π-π stacking with Phe327, Trp355, and Tyr358, with the halogen atom approaching the indole nitrogen atom of Trp355, and (c) the alkenyl chain is stabilized by a possible π-π stacking interaction with Phe327.
Figure 4.
Predicted binding poses of compound (+)-16b in 5-HT2C. (a) Docking poses of compound (+)-16b in the 7-transmembrane domain (7TMD) of the 5-HT2C_inactive (compound shown in light yellow, protein shown in gray) and 5-HT2C_active (compound shown in orange, protein shown in green) conformations. (b) Magnified view of binding sites of compound (+)-16b in 5-HT2C_inactive. (c) Magnified view of binding sites of compound (+)-16b in 5-HT2C_active. (d) Overlay of compound (+)-16b (orange) with (+)-13h (sky blue) in the binding pocket.
A significantly different interaction pattern is observed when analyzing the computed complexes for the 5-HT2C_act model. As shown in Figure 4c, the interactions involving the ligand ammonium group are almost superimposable on those seen using the inactive model and involve Asp134, Ser138, and Tyr358, while the cyclopropane ring elicits apolar contacts with Val135 and Val185. The largest differences between the two 5-HT2C models are observed for the contacts that stabilize the remaining portion of the ligands because the phenyl ring is inserted into a tight niche lined by aromatic residues (i.e., Phe214, Trp324, Phe327, Phe328, and Tyr358) with which it can interact via extended π-π stacking interactions, while the ether bridge engages in two possible H-bonds with Ser219 and Asn331. Finally, the alkenyl tail also engages in a possible π-π stacking interaction with Phe214.
Some differences were also observed when the binding pose of (+)-16b was compared to that found for (+)-13h, as is apparent from the overlay shown in Figure 4d. The interactions displayed by the ammonium group of (+)-13h are super-imposable to those of (+)-16b, but the positions of the benzene ring and the direction of the ether side chain are significantly different. The increase of steric hindrance upon changing the fluorine atom to a chlorine atom probably prevents the benzene ring from going deeper into the pocket, thus the ether side chain moves inside while the benzene ring is outside. The π-π stacking interactions within the tight niche lined by aromatic residues would therefore be anticipated to be weaker than those experienced by (+)-16b. This property might explain the fact that the potency of (+)-16b is about 15-fold higher than that of (+)-13h (4.2 vs 60 nM).
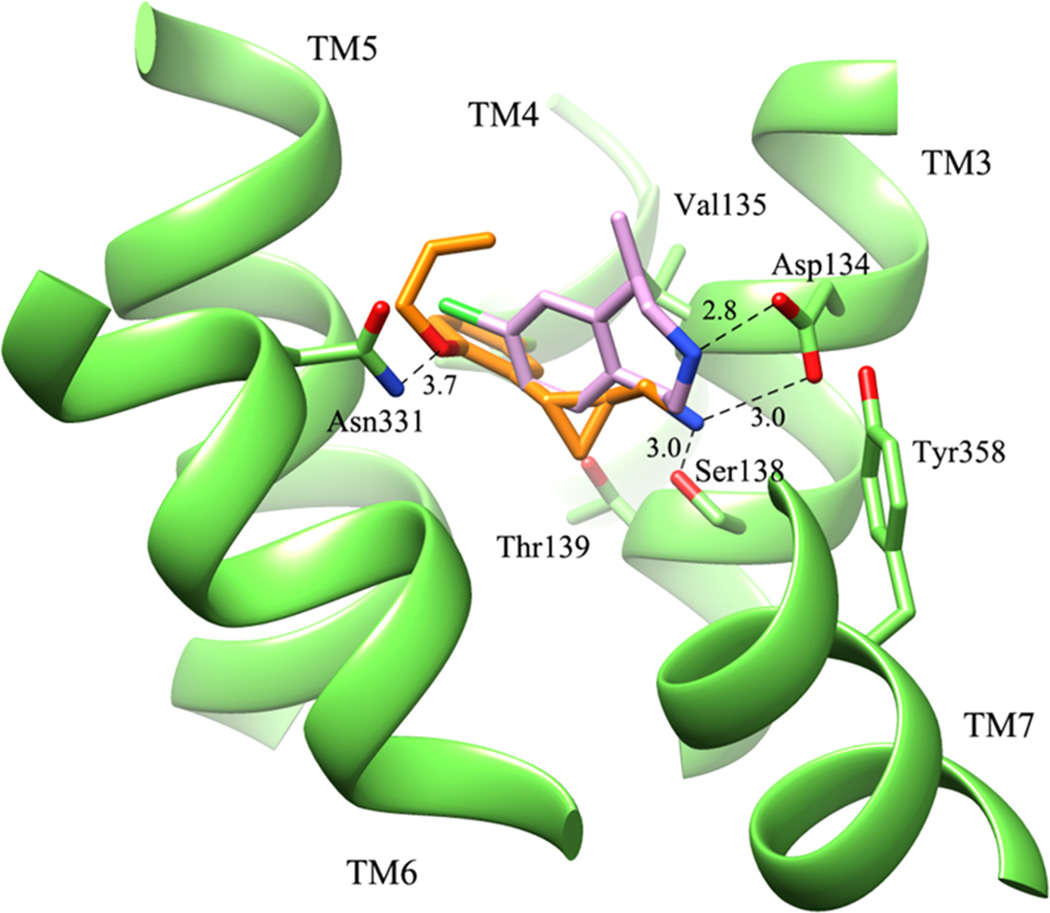
When the binding mode of compound (+)-16b is compared to that of lorcaserin, as shown in Figure 5, (+)-16b shares with lorcaserin the same key interactions that stabilize the ammonium group as well as the extended set of hydrophobic and stacking contacts, with the cyclopropane ring suitably replacing the carbon skeleton of the benzazepine ring. The major differences in the 5-HT2C_act model is that the polar contact, namely the likely H-bond with Asn331, is seen only with compound (+)-16b because of its ether oxygen atom, while the chlorine atom of lorcaserin engages Asn331 in possible hydrophobic contacts.
Figure 5.
Overlay of compound (+)-16b (orange) with lorcaserin (pink) in the binding pocket of 5-HT2C_active.
CONCLUSION
As our continuing efforts to optimize the 2-phenylcyclopro-pylmethylamine scaffold, new compounds bearing chlorine or fluorine substitutions at position 5 on the benzene ring were synthesized, and these modifications together with optimization of the ether substituent at position 2 led to compounds with good potency at the 5-HT2C receptor as well as improved selectivity against the 5-HT2B and 5-HT2A receptors. In turn, ADMET studies coupled with behavioral analysis in the amphetamine-stimulated hyperlocomotion model identified four compounds with good profiles as antipsychotic agents. Compound (+)-16b displayed an EC50 value of 4.2 nM at 5-HT2C, was 89-fold selective against 5-HT2A, and had no significant activity at 5-HT2B. It is one of the most potent and selective 5-HT2C agonists reported to date. At a dose of 20 mg/ kg, (+)-16b completely reversed amphetamine-induced hyperactivity in mice while showing no significant influence on the spontaneous activity of the tested animals. Molecular modeling studies were also performed to elucidate the possible binding poses of compound (+)-16b to the 5-HT2C receptor, using a homology model incorporating both the well-known β2-adrenergic receptor template and recently reported 5-HT2B receptor.
The high potency and excellent selectivity profile of compound (+)-16b, together with its significant effects in an animal behavior study, strongly support the further evaluation of this compound as a chemical tool that can be used to elucidate the importance of the 5-HT2C receptors in normal human behaviors as well as in pathophysiological states. Further animal studies are being conducted to explore the use of this compound in the possible treatment of schizophrenia-like behaviors, and these studies will be reported separately.
EXPERIMENTAL SECTION
General
All chemicals and solvents were purchased from Sigma-Aldrich or Fisher Scientific and used without further purification. Microwave reactions were run in Biotage Initiator microwave synthesizer. Synthetic intermediates were purified by CombiFlash flash chromatography on 230–400 mesh silica gel. 1H and 13C NMR spectra were recorded on Bruker DPX-400 or AVANCE-400 spectrometer at 400 and 100 MHz, respectively. NMR chemical shifts were reported in δ (ppm) using residual solvent peaks as standard (CDCl3, 7.26 ppm (1H), 77.23 ppm (13C); CD3OD, 3.31 ppm (1H), 49.15 ppm (13C); DMSO-d6, 2.50 ppm (1H), 39.52 ppm (13C)). Mass spectra were measured in the ESI mode at an ionization potential of 70 eV with an LC-MS MSD (Hewlett-Packard). Purity of all final compounds (greater than 95%) was determined by analytical HPLC (ACE 3AQ C18 column (150 mm × 4.6 mm, particle size 3 µM), 0.05% TFA in H2O/0.05% TFA in MeOH gradient eluting system). Optical rotation values were recorded on Autopol IV automatic polarimeter.
General Method A: Preparation of N-Boc-amines 12a–12n and 15a–15d
Intermediate 11 and 14 was treated with the Williamson ether synthesis or Mitsunobu reaction conditions as described in Scheme 1 and Scheme 2 (for detailed conditions, see Supporting Information, Tables S1 and S2). The reactions were monitored with TLC, and the workup was done with ethyl acetate and water. Crude product was purified with flash chromatography.
General Method B: Chiral Separation of N-Boc-amines 12a–12n and 15a–15d
The racemic intermediates were separated by chiral HPLC. Analytical conditions: RegisCell chiral column (25 cm × 4.6 mm, 10 µM), 1.5–15% EtOH in n-hexane as the fluent phase. Preparative conditions: RegisPack chiral column (25 cm × 21.1 mm, 10 µM), 3–7.5% EtOH in n-hexane as the eluting system (isocratic eluent, stacked injections, flow rate =18 mL/min, λ = 254 and 280 nm). (+)-12a–12n and (+)-15a–15d were isolated as the first-eluting peaks, with (−)-12a–12n and (−)-15a–15d as the second-eluting peaks, both after evaporation appeared as colorless oil or white solids. Optical purity of both enantiomers was determined on analysis HPLC after the separation, and a second separation was done when necessary to guarantee >90% ee optical purity.
General Method C: Deprotection of N-Boc-Amines to Afford HCl Salts (13a, 13b, 13e–13g, 13i–13n, and 16a–16d)
N-Boc-Amines was dissolved in 2 M HCl (g) in diethyl ether (10 mL/mmol substrate) and stirred at room temperature for 24–48 h. The white solids formed were collected by filtration, washed with diethyl ether, and dried over vacuum to give the HCl salts as white solids.
General Method D: Deprotection of N-Boc-Amines to Afford TFA Salts (13c, 13d, and 13h)
To a solution of the N-Boc protected precursor (1 mmol) in CH2Cl2 (10 mL) was added to TFA (1 mL) at 0 °C under an argon atmosphere. The mixture was stirred at room temperature for 1 h. The reaction mixture was concentrated and the residue was dissolved in water and methanol (ratio 4:1). The solution was filtered and then purified by Shidmadzu preparative LC using the following conditions: ACE 5AQ column (150 mm × 21.2 mm, particle size 5 µM). Method: 8–100% 0.05% TFA in MeOH/ 0.05% TFA in H2O, 30 min. Flow rate = 17 mL/min with monitoring at 254 and 280 nm wavelengths. After the solvent was evaporated, the residue was dissolved in distilled water (2–3 mL) and lyophilized to obtain the TFA salt.
Detailed synthetic procedures were described for compounds (−)-16b, (+)-16b, (−)-16d, and (+)-16d. Other compounds were prepared similarly with the general methods described above and the characterization data are provided in the Supporting Information.
tert-Butyl ((2-(2-(Allyloxy)-5-fluorophenyl)cyclopropyl)-methyl)carbamate (15b)
To a solution of compound 14 (282 mg, 1.0 mmol) in anhydrous DMF (2 mL) was added Cs2CO3 (489 mg, 1.5 mmol) and allyl bromide (242 mg, 2.0 mmol), and the mixture was heated in a microwave at 80 °C for 30 min. Water was added, and the mixture was extracted with ethyl acetate, the combined extracts were dried over Na2SO4, concentrated, and purified with flash chromatography (0–30% ethyl acetate in hexanes) to render the title compound as a colorless oil (250 mg, 78%). 1H NMR (400 MHz, CDCl3) δ 6.80 (dt, J = 8.8, 3.2 Hz, 1H), 6.75 (dd, J = 8.8, 4.8 Hz, 1H), 6.61 (dd, J = 9.2, 2.8 Hz), 6.18–6.08 (m, 1H), 5.42 (dd, J = 17.2, 1.6 Hz, 1H), 5.31 (dd, J = 10.4, 1.2 Hz, 1H), 5.08 (br, 1H), 4.61−4.57 (m, 2H), 3.54−3.51 (m, 1H), 2.79−2.76 (m, 1H), 1.93−1.88 (m, 1H), 1.46 (s, 9H), 1.07−1.98 (m, 2H), 0.88−0.82 (m, 1H). 13C NMR (100 MHz, CDCl3) δ 157.3 (d, JCF = 236.9 Hz), 155.9, 153.5, 133.3, 132.6 (d, JCF = 7.4 Hz), 118.1, 113.4 (d, JCF = 23.3 Hz), 112.5 (d, JCF = 22.5 Hz), 112.4 (d, JCF = 8.4 Hz), 79.0, 69.8, 45.2, 28.5, 21.8, 17.0, 11.4. The racemate was separated with RegisPack chiral column (25 cm × 21.1 mm, 10 µM), 3.25% EtOH in n-hexane as the eluting system (isocratic eluent, stacked injections, flow rate =18 mL/min, λ = 254 and 280 nm). (+)-15b (92 mg, 97% ee) was separated as the first peaks and (−)-15b (78 mg, 96% ee) as the second peaks. (+)-15b, [α]D20 +26.4 (c 0.5, CHCl3); (−)-15b, [α]D20 −26.8 (c 0.5, CHCl3).
(−)-((1R,2R)-2-(2-(Allyloxy)-5-fluorophenyl)cyclopropyl)-methanamine Hydrochloride ((−)-16b)
(+)-15b (80 mg, 0.25 mmol) was dissolved in 2 M HCl in diethyl ether (10 mL) and stirred at room temperature for 24 h. The precipitation was collected by filtration, washed with diethyl ether, and dried on vacuum to render the title compound as a white solid (48 mg, 75%). 1H NMR (400 MHz, CD3OD) δ 6.93−6.86 (m, 2H), 6.73 (dd, J = 9.4, 3.2 Hz, 1H), 6.17−6.10 (m, 1H), 5.45 (dd, J = 17.2, 1.6 Hz, 1H), 5.30 (dd, J = 10.4, 1.6 Hz, 1H), 4.60 (d, J = 5.6 Hz, 2H), 3.06 (dd, J = 13.2, 7.6 Hz, 1H), 2.98 (dd, J = 13.2, 4.2 Hz, 1H), 2.21−2.15 (m, 1H), 1.34−1.30 (m, 1H), 1.14−1.02 (m, 2H). 13C NMR (100 MHz, CD3OD) δ 158.8 (d, JCF = 225.7 Hz), 154.9, 135.0, 133.0(d, JCF = 7.4 Hz), 118.2, 114.3 (d, JCF = 17.8 Hz), 114.1, 114.0 (d, JCF = 37.3 Hz), 71.0, 45.1, 19.7, 18.3, 13.7. HRMS (ESI) calculated for C13H17FNO ([M + H]+), 222.1294; found, 222.1273. [α]D20 −16.2 (c 0.4, MeOH).
(+)-((1S,2S)-2-(2-(Allyloxy)-5-fluorophenyl)cyclopropyl)-methanamine Hydrochloride ((+)-16b)
(−)-15b (60 mg, 0.19 mmol) was dissolved in 2 M HCl in diethyl ether (8 mL) and stirred at room temperature for 24 h. The precipitation was collected by filtration, washed with diethyl ether, and dried on vacuum to render the title compound as a white solid (32 mg, 66%). 1H NMR (400 MHz, CD3OD) δ 6.93−6.86 (m, 2H), 6.73 (dd, J = 9.6, 3.2 Hz, 1H), 6.17−6.10 (m, 1H), 5.45 (dd, J = 17.2, 1.6 Hz, 1H), 5.30 (dd, J = 10.4, 1.6 Hz, 1H), 4.60 (d, J = 4.2 Hz, 2H), 3.07 (dd, J = 12.8, 7.2 Hz, 1H), 2.98 (dd, J = 13.2, 5.6 Hz, 1H), 2.21−2.17 (m, 1H), 1.35−1.31 (m, 1H), 1.14−1.04 (m, 2H). 13C NMR (100 MHz, CD3OD) δ 158.8 (d, JCF = 235.7 Hz), 155.0, 135.0, 133.0(d, JCF = 7.4 Hz), 118.2, 114.3 (d, JCF = 17.0 Hz), 114.1, 114.0 (d, JCF = 36.1 Hz), 71.0, 45.1, 19.7, 18.3, 13.7. HRMS (ESI) calculated for C13H17FNO ([M + H]+), 222.1294; found, 222.1273. [ α]D20 +17.5 (c 0.2, MeOH).
tert-Butyl ((2-(5-Fluoro-2-(2-fluoroethoxy)phenyl)-cyclopropyl)methyl)carbamate (15d)
To a solution of compound 14 (282 mg, 1.0 mmol), triphenylphosphine (787 mg, 3.0 mmol) and 2-fluoroethanol (192 mg, 3.0 mmol) in anhydrous THF (3 mL) was cooled at 0 °C and was added diethylazodicarboxylate (522 mg, 3.0 mmol) dropwise. The solution was then heated in microwave reactor at 60 °C for 40 min. The mixture was purified with flash chromatography (0–30% ethyl acetate in hexanes) after concentration to render 15d as a colorless oil (320 mg, 98%). 1H NMR (400 MHz, CDCl3) δ 6.82 (dt, J = 8.8, 2.8 Hz, 1H), 6.77 (dd, J = 8.0, 4.8 Hz, 1H), 6.62 (dd, J = 9.2, 2.8 Hz, 1H), 5.02 (br, 1H), 4.88−4.73 (m, 2H), 4.31−4.20 (m, 2H), 3.53−3.48 (m, 1H), 2.84−2.78 (m, 1H), 1.96−1.92 (m, 1H), 1.46 (s, 9H), 1.10−1.05 (m, 1H), 1.02−0.98 (m, 1H), 0.88−0.82 (m, 1H). 13C NMR (100 MHz, CDCl3) δ 157.7 (d, JCF = 237.5 Hz), 155.9, 153.4, 133.2 (d, JCF = 7.4 Hz), 113.5 (d, JCF = 23.4 Hz), 112.9 (d, JCF = 8.5 Hz), 112.6 (d, JCF = 22.7 Hz), 81.9 (d, JCF = 169.7 Hz), 79.1, 68.5 (d, JCF = 20.2 Hz), 45.1, 28.5, 20.0, 16.8, 11.5. The racemate was separated with RegisPack chiral column (25 cm × 21.1 mm, 10 µM), 7.5% EtOH in n-hexane as the eluting system (isocratic eluent, stacked injections, flow rate = 18 mL/min, λ = 254 and 280 nm). (+)-15d (120 mg, > 99% ee) was separated as the first peaks and (−)-15d (110 mg, > 99% ee) as the second peaks. (+)-15d, [α]D20 +20.2 (c 0.3, CHCl3); (−)-15d, [α]D20 −18.7 (c 0.3, CHCl3).
(−)-((1R,2R)-2-(5-Fluoro-2-(2-fluoroethoxy)phenyl)-cyclopropyl)methanamine Hydrochloride ((−)-16d)
Compound (+)-15d (110 mg, 0.34 mmol) was dissolved in 2 M HCl in diethyl ether (8 mL) and stirred at room temperature for 24 h. The precipitation was collected by filtration, washed with diethyl ether, and dried on vacuum to render the title compound as a white solid (61 mg, 69%). 1H NMR (400 MHz, CD3OD) δ 6.97−6.86 (m, 2H), 6.76 (dd, J = 9.2, 2.8 Hz, 1H), 4.87−4.75 (m, 2H), 4.32−4.20 (m, 2H), 3.04−3.00 (m, 2H), 2.19−2.15 (m, 1H), 1.28−1.17 (m, 2H), 1.06−1.02 (m, 1H). 13C NMR (100 MHz, CD3OD) δ 159.0 (d, JCF = 236.0 Hz), 155.0, 133.0 (d, JCF = 7.4 Hz), 114.6 (d, JCF = 24.0 Hz), 114.2 (d, JCF = 22.8 Hz), 114.0 (d, JCF = 8.1 Hz), 83.6 (d, JCF = 166.6 Hz), 69.7 (d, JCF = 18.9 Hz), 45.0, 20.0, 18.3, 13.0. HRMS (ESI) calculated for C12H16F2NO ([M + H]+), 228.1200; found, 228.1179. [α]D20 −3.3 (c 0.3, MeOH).
(+)-((1S,2S)-2-(5-Fluoro-2-(2-fluoroethoxy)phenyl)-cyclopropyl)methanamine Hydrochloride ((+)-16d)
This compound was prepared from (−)-15d as described for (−)-16d as a white solid. 1H NMR (400 MHz, CD3OD) δ 6.97−6.88 (m, 2H), 6.76 (dd, J = 9.6, 3.2 Hz, 1H), 4.87−4.74 (m, 2H), 4.32−4.22 (m, 2H), 3.03 (d, J = 7.6 Hz, 2H), 2.20−2.15 (m, 1H), 1.28−1.25 (m, 1H), 1.22−1.17 (m, 1H), 1.07−1.02 (m, 1H). 13C NMR (100 MHz, CD3OD) δ 159.0 (d, JCF = 236.2 Hz), 155.0, 133.1 (d, JCF = 7.3 Hz), 114.6 (d, JCF = 24.0 Hz), 114.2 (d, JCF = 17.5 Hz), 114.1 (d, JCF = 3.1 Hz), 83.6 (d, JCF = 166.6 Hz), 69.7 (d, JCF = 19.8 Hz), 45.0, 20.0, 18.3, 13.1. HRMS (ESI) calculated for C12H16F2NO ([M + H]+), 228.1200; found, 228.1173. [α]D20 +3.6 (c 0.3, MeOH).
Calcium Flux Assay
Flp-In-293 cells stably expressing the human 5-HT2A, 5-HT2B, or 5-HT2C-INI were grown for 24–48 h in DMEM containing 10% dialyzed FBS before seeding. Cells were plated into Poly-l-Lys-coated 384-well black clear bottom cell culture plates in DMEM with 1% dialyzed FBS at a density of 12000 cells per 50 µL per well for 24 h. Preceding the experiment, culture medium was removed and 20 µL of assay buffer (20 mM Hepes, pH 7.40, Hanks’ balanced salt solution, 2.5 mM probenecid, 1× FLIPR calcium dye) was added and cells were incubated at 37 °C for 1 h. Serial dilutions of each tested drug were prepared at 3× final concentration and transferred to 384-well plates. Each drug plate contained 5-HT and lorcaserin in serial dilutions for internal reference. Cell and drug plates were placed in a FLIPRTETRA fluorescence imaging plate reader (Molecular Dynamics). The FLIPRTETRA was programmed to read baseline for 10 s (1 read/s) and then add 10 µL of drug/well and read for an additional 120 s. Fluorescence was normalized to the average of the baseline (first 10 reads), and the maximum fold increase peak was determined for each drug and controls (5-HT and lorcaserin). Data were plotted as a function of drug concentration and were normalized compared to the internal 5-HT reference for each plate recorded. Normalized data were regressed using a sigmoidal dose–response function. Data of two independent experiments (n = 2) conducted in quadruplicate are presented. Analyses were performed using the software from GraphPad Prism 6.0. 5-HT2C EC50 confidence intervals (5-HT, 0.16–0.26 nM; lorcaserin, 3.1–3.9 nM), Emax Std Error (5-HT, 100% ± 1.04; lorcaserin, 99% ± 0.75); 5-HT2B EC50 confidence intervals (5-HT, 0.66–1.17 nM; lorcaserin, 429–527 nM), Emax Std Error (5-HT, 100% ± 1.69; lorcaserin, 92% ± 3.01); 5-HT2A EC50 confidence intervals (5-HT, 1.60–2.16 nM; lorcaserin, 275–329 nM), Emax Std Error (5-HT, 100% ± 1.03; lorcaserin, 68% ± 0.83).
Open Field Activity
Adult male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were injected (ip) with the vehicle (Veh, 0.9% saline from Butler Schein Animal Health, Dublin, Ohio) or different doses of compounds (+)-13j, (+)-13l, (+)-16b, or (+)-16d (compounds were dissolved in 0.9% saline and injected intraperitoneally at 5 mL/kg) and placed into the open field for 15 min. The mice were removed and administered (ip) the Veh or 3 mg/kg amphetamine (AMPH; Sigma-Aldrich, St. Louis, MO) and returned to the open field for 105 min. Locomotor activity was monitored as distance traveled in an automated Omnitech Digiscan apparatus using VersaMax software (AccuScan Instruments, Columbus, OH). The results are presented as means and standard errors of the mean using SPSS software (IBM, Armonk, NY). The data from the 0–15 min interval were analyzed by three-way ANOVA for condition (vehicle and AMPH), treatment (compounds (+)-13j, (+)-13l, (+)-16b, and (+)-16d), dose (10 or 20 mg/kg), and dose nested in treatment (to reflect the different doses tested per compound). Because activities among the groups at the 0- 15 min interval were significantly different, a RMANOVA was run with a sequential sum of squares (to control for the group differences at 0–15 min) for test interval (0–15 and 16–120 min), condition, treatment, dose, and dose (treatment) to control for these group differences. All posthoc analyses were by Bonferroni corrected pairwise comparisons where a p < 0.05 was considered significant.
Supplementary Material
Acknowledgments
Financial support from the National Institute of Health (NIH) (no. R01MH99993) and NIMH Psychoactive Drug Screening Program is gratefully acknowledged.
ABBREVIATIONS USED
- 5-HT
serotonin
- β2-AR
β2-adrenergic receptor
- ADMET
absorption, distribution, metabolism, excretion and toxicity
- AMPH
d-amphetamine
- Boc
tert-butyloxycarbonyl
- BBB
blood–brain barrier
- CHO
Chinese hamster ovary cells
- CNS
central nervous system
- CYP
cytochrome P450
- DMF
dimethylformamide
- FDA
US Food and Drug Administration
- FLIPR
fluorescence imaging plate reader
- GPCR
G-protein coupled receptor
- HEK-293
human embryonic kidney 293 cells
- hERG
human ether-a-go-go-related gene
- HPLC
high-performance liquid chromatography
- PDB
Protein Data Bank
- PPB
plasma protein binding
- SAR
structure–activity relationship
- TFA
trifluoroacetic acid
- TM
transmembrane
- TMD
transmembrane domain
- Veh
vehicle
Footnotes
ASSOCIATED CONTENT
Reaction conditions for the preparation of 12a–12n and 15a–15d intermediates, characterization data for all other compounds, pharmacological profiling data of compounds (+)-16b and (+)-16d, and details of the modeling study. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
REFERENCES
- 1.Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu. Rev. Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nichols DE, Nichols CD. Serotonin receptors. Chem. Rev. 2008;108:1614–1641. doi: 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- 3.Meltzer HY, Roth BL. Lorcaserin and pimavanserin: emerging selectivity of serotonin receptor subtype-targeted drugs. J. Clin. Invest. 2013;123:4986–4991. doi: 10.1172/JCI70678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith BM, Thomsen WJ, Grottick AJ. The potential use of selective 5-HT2C agonists in treating obesity. Expert Opin. Invest. Drugs. 2006;15:257–266. doi: 10.1517/13543784.15.3.257. [DOI] [PubMed] [Google Scholar]
- 5.Sargent BJ, Henderson AJ. Targeting 5-HT receptors for the treatment of obesity. Curr. Opin. Pharmacol. 2011;11:52–58. doi: 10.1016/j.coph.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Rosenzweig-Lipson S, Comery TA, Marquis KL, Gross J, Dunlop J. 5-HT(2C) agonists as therapeutics for the treatment of schizophrenia. Handb. Exp. Pharmacol. 2012:147–165. doi: 10.1007/978-3-642-25758-2_6. [DOI] [PubMed] [Google Scholar]
- 7.Mombereau C, Kawahara Y, Gundersen BB, Nishikura K, Blendy JA. Functional relevance of serotonin 2C receptor mRNA editing in antidepressant- and anxiety-like behaviors. Neuropharmacology. 2010;59:468–473. doi: 10.1016/j.neuropharm.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pazos A, Hoyer D, Palacios JM. The binding of serotonergic ligands to the porcine choroid plexus: characterization of a new type of serotonin recognition site. Eur. J. Pharmacol. 1984;106:539–546. doi: 10.1016/0014-2999(84)90057-8. [DOI] [PubMed] [Google Scholar]
- 9.Berg KA, Clarke WP, Cunningham KA, Spampinato U. Fine-tuning serotonin 2c receptor function in the brain: molecular and functional implications. Neuropharmacology. 2008;55:969–976. doi: 10.1016/j.neuropharm.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nichols DE. Hallucinogens. Pharmacol. Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Lee J, Jung ME, Lee J. 5-HT2C receptor modulators: a patent survey. Expert Opin. Ther. Pat. 2010;20:1429–1455. doi: 10.1517/13543776.2010.518956. [DOI] [PubMed] [Google Scholar]
- 12.Thomsen WJ, Grottick AJ, Menzaghi F, Reyes-Saldana H, Espitia S, Yuskin D, Whelan K, Martin M, Morgan M, Chen W, Al-Shamma H, Smith B, Chalmers D, Behan D. Lorcaserin, a novel selective human 5-hydroxytryptamine2C agonist: in vitro and in vivo pharmacological characterization. J. Pharmacol. Exp. Ther. 2008;325:577–587. doi: 10.1124/jpet.107.133348. [DOI] [PubMed] [Google Scholar]
- 13.Lorcaserin In obesity: unacceptable risks. Prescrire Int. 2014;23:117–120. [PubMed] [Google Scholar]
- 14.Liu J, Ogden A, Comery TA, Spiros A, Roberts P, Geerts H. Prediction of Efficacy of Vabicaserin, a 5-HT2C Agonist, for the Treatment of Schizophrenia Using a Quantitative Systems Pharmacology Model. CPT Pharmacometrics Syst. Pharmacol. 2014;3:e111. doi: 10.1038/psp.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunlop J, Watts SW, Barrett JE, Coupet J, Harrison B, Mazandarani H, Nawoschik S, Pangalos MN, Ramamoorthy S, Schechter L, Smith D, Stack G, Zhang J, Zhang G, Rosenzweig-Lipson S. Characterization of vabicaserin (SCA-136), a selective 5-hydroxytryptamine 2C receptor agonist. J. Pharmacol. Exp. Ther. 2011;337:673–680. doi: 10.1124/jpet.111.179572. [DOI] [PubMed] [Google Scholar]
- 16.Marquis KL, Sabb AL, Logue SF, Brennan JA, Piesla MJ, Comery TA, Grauer SM, Ashby CR, Jr, Nguyen HQ, Dawson LA, Barrett JE, Stack G, Meltzer HY, Harrison BL, Rosenzweig-Lipson S. WAY-163909 [(7bR,10aR)-1,2,3,4,8,9,10,10a–octahydro-7bH-cyclopenta-[b][1,4]diazepino[6,7,1-hi]indole]: A novel 5-hydroxytryptamine 2C receptor-selective agonist with preclinical antipsychotic-like activity. J. Pharmacol. Exp. Ther. 2007;320:486–496. doi: 10.1124/jpet.106.106989. [DOI] [PubMed] [Google Scholar]
- 17.Siuciak JA, Chapin DS, McCarthy SA, Guanowsky V, Brown J, Chiang P, Marala R, Patterson T, Seymour PA, Swick A, Iredale PA. CP-809,101, a selective 5-HT2C agonist, shows activity in animal models of antipsychotic activity. Neuropharmacology. 2007;52:279–290. doi: 10.1016/j.neuropharm.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 18.Kalgutkar AS, Dalvie DK, Aubrecht J, Smith EB, Coffing SL, Cheung JR, Vage C, Lame ME, Chiang P, McClure KF, Maurer TS, Coelho RV, Jr, Soliman VF, Schildknegt K. Genotoxicity of 2-(3-chlorobenzyloxy)-6-(piperazinyl)-pyrazine, a novel 5-hydroxytryptamine2c receptor agonist for the treatment of obesity: role of metabolic activation. Drug Metab. Dispos. 2007;35:848–858. doi: 10.1124/dmd.106.013649. [DOI] [PubMed] [Google Scholar]
- 19.Callahan PM, Cunningham KA. Involvement of 5-HT2C receptors in mediating the discriminative stimulus properties of m-chlorophenylpiperazine (mCPP) Eur. J. Pharmacol. 1994;257:27–38. doi: 10.1016/0014-2999(94)90690-4. [DOI] [PubMed] [Google Scholar]
- 20.Fiorella D, Helsley S, Rabin RA, Winter JC. 5-HT2C receptor-mediated phosphoinositide turnover and the stimulus effects of m-chlorophenylpiperazine. Psychopharmacology (Berlin, Ger.) 1995;122:237–243. doi: 10.1007/BF02246545. [DOI] [PubMed] [Google Scholar]
- 21.Cho SJ, Jensen NH, Kurome T, Kadari S, Manzano ML, Malberg JE, Caldarone B, Roth BL, Kozikowski AP. Selective 5-hydroxytryptamine 2C receptor agonists derived from the lead compound tranylcypromine: identification of drugs with antidepressant-like action. J. Med. Chem. 2009;52:1885–1902. doi: 10.1021/jm801354e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozikowski AP, Cho SJ, Jensen NH, Allen JA, Svennebring AM, Roth BL. HTS and rational drug design to generate a class of 5-HT(2C)-selective ligands for possible use in schizophrenia. ChemMedChem. 2010;5:1221–1225. doi: 10.1002/cmdc.201000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen G, Cho SJ, Huang XP, Jensen NH, Svennebring A, Sassano MF, Roth BL, Kozikowski AP. Rational Drug Design Leading to the Identification of a Potent 5-HT(2C) Agonist Lacking 5-HT(2B) Activity. ACS Med. Chem. Lett. 2011;2:929–932. doi: 10.1021/ml200206z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamazaki K, Kanaoka M. Computational prediction of the plasma protein-binding percent of diverse pharmaceutical compounds. J. Pharm. Sci. 2004;93:1480–1494. doi: 10.1002/jps.20059. [DOI] [PubMed] [Google Scholar]
- 25.Aronov AM. Predictive in silico modeling for hERG channel blockers. Drug Discovery Today. 2005;10:149–155. doi: 10.1016/S1359-6446(04)03278-7. [DOI] [PubMed] [Google Scholar]
- 26.Turpeinen M, Zanger UM. Cytochrome P450 2B6: function, genetics, and clinical relevance. Drug Metab. Drug Interact. 2012;27:185–197. doi: 10.1515/dmdi-2012-0027. [DOI] [PubMed] [Google Scholar]
- 27.Millan MJ, Dekeyne A, Gobert A. Serotonin (5-HT)2C receptors tonically inhibit dopamine (DA) and noradrenaline (NA), but not 5-HT, release in the frontal cortex in vivo. Neuropharmacology. 1998;37:953–955. doi: 10.1016/s0028-3908(98)00078-1. [DOI] [PubMed] [Google Scholar]
- 28.Di Giovanni G, Di Matteo V, Di Mascio M, Esposito E. Preferential modulation of mesolimbic vs nigrostriatal dopaminergic function by serotonin (2C/2B) receptor agonists: a combined in vivo electrophysiological and microdialysis study. Synapse. 2000;35:53–61. doi: 10.1002/(SICI)1098-2396(200001)35:1<53::AID-SYN7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 29.Leysen JE. 5-HT2 receptors. Curr. Drug Targets: CNS Neurol. Disord. 2004;3:11–26. doi: 10.2174/1568007043482598. [DOI] [PubMed] [Google Scholar]
- 30.Wacker D, Wang C, Katritch V, Han GW, Huang XP, Vardy E, McCorvy JD, Jiang Y, Chu M, Siu FY, Liu W, Xu HE, Cherezov V, Roth BL, Stevens RC. Structural features for functional selectivity at serotonin receptors. Science. 2013;340:615–619. doi: 10.1126/science.1232808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marti-Renom MA, Stuart AC, Fiser A, Sanchez R, Melo F, Sali A. Comparative protein structure modeling of genes and genomes. Annu. Rev. Biophys. Biomol. Struct. 2000;29:291–325. doi: 10.1146/annurev.biophys.29.1.291. [DOI] [PubMed] [Google Scholar]
- 32.Korb O, Stutzle T, Exner TE. Empirical scoring functions for advanced protein-ligand docking with PLANTS. J. Chem. Inf. Model. 2009;49:84–96. doi: 10.1021/ci800298z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.