Abstract
Obesity has been shown to promote renin-angiotensin system (RAS) activity and inflammation in the brain and to be accompanied by increased sympathetic activity and blood pressure (BP). Our previous studies demonstrated that administration of a subpressor dose of angiotensin (Ang) II sensitizes subsequent Ang II-elicited hypertension. The present study tested whether high fat diet (HFD) feeding also sensitizes the Ang II-elicited hypertensive response and whether HFD-induced sensitization is mediated by an increase in RAS activity and inflammatory mechanisms in the brain. HFD did not increase baseline BP, but enhanced the hypertensive response to Ang II compared to a normal fat diet. The sensitization produced by the HFD was abolished by concomitant central infusions of either a tumor necrosis factor α (TNF-α) synthesis inhibitor, pentoxifylline, an Ang II type 1 receptor (AT1-R) blocker, irbesartan or an inhibitor of microglial activation, minocycline. Furthermore, central pretreatment with TNF-α mimicked the sensitizing action of a central subpressor dose of Ang II, whereas central pentoxifylline or minocycline abolished this Ang II-induced sensitization. RT-PCR analysis of lamina terminalis tissue indicated that HFD feeding, central TNF-α or a central subpressor dose of Ang II upregulated mRNA expression of several components of the RAS and proinflammatory cytokines, whereas inhibition of AT1-R and of inflammation reversed these changes. The results suggest that HFD-induced sensitization of Ang II-elicited hypertension is mediated by upregulation of the brain RAS and of central proinflammatory cytokines.
Keywords: High fat diet, Angiotensin II, Sensitization, Blood Pressure, Proinflammatory cytokine
Introduction
Converging lines of evidence indicate that hypertension is characterized by increased activity of the renin-angiotensin system (RAS) and elevated levels of proinflammatory cytokines (PICs). The interactions and synergism between the RAS and PICs have been studied in both the periphery and the central nervous system (CNS). We and others have demonstrated that systemic angiotensin (Ang) II administration elicits an increase in blood pressure (BP) that is accompanied by an upregulation of mRNA expression of PICs in the brain.1,2 Central injections of PICs such as tumor necrosis factor (TNF)-α or interleukin (IL)-1β, significantly elevate BP, renal sympathetic nerve activity (RSNA) and brain angiotensin type 1 receptor (AT1-R) and angiotensin-converting enzyme (ACE1) mRNA.3,4 Conversely, central inhibition of TNF-α synthesis or blockade of AT1-R significantly reduced the hypertensive effect and diminished the upregulation of mRNA for RAS components and PICs produced by systemic Ang II.1,5
Diet-induced obesity (DIO) in both humans and rodents is associated with an increased prevalence of hypertension.6 Head and colleagues recently reported that 3 weeks of high fat diet (HFD) feeding led to increased mean arterial pressure (MAP), heart rate (HR) and RSNA in rabbits. Autonomic ganglion blockade completely abolished the increase in BP, suggesting that this model of obesity-induced hypertension is neurogenic.7–9 Obese animals have been shown to have increased RAS activity and elevated levels of Ang II that upregulates AT1-R in a variety of tissues including the brain.10,11
Recent studies also demonstrate that exposure to a HFD induces hypothalamic as well as peripheral inflammation,12 and one day of exposure to a HFD (60%) has been shown to be sufficient to increase hypothalamic cytokine expression.13 Interestingly, although animals with DIO can restore body weight, insulin levels and leptin sensitivity to normal after being switched to normal fat diet (NFD), BP, RSNA and the activity of central RAS and PICs remain high7,14 These results indicate that obesity itself may induce hypothalamic inflammation and sensitization of the brain to circulating sympathoexcitatory factors such as those from the RAS and these initiate increased BP.
Forebrain structures along with the lamina terminalis (LT) including the subfornical organ (SFO), median preoptic nucleus (MnPO) and organum vasculosum, play important roles in the long-term regulation of BP, body fluid and energy homeostasis.15,16 Our previous work demonstrated that the BP response that manifests as hypertension can be sensitized by treating male rats with mild physiological or dietary challenges at a point earlier in the animal’s lifetime.17–20 Initial studies found that pretreatment with subpressor doses of either Ang II or aldosterone can upregulate the RAS in the LT and sensitize the hypertensive response to a pressor dose of Ang II17,18 and to high dietary salt.19 Given that brain RAS activation and inflammation are common features of both obesity and hypertension, such results prompted us to hypothesize that exposure to HFD may also sensitize the Ang II-elicited hypertensive response, and that the brain RAS and TNF-α signaling may contribute to that response.
Methods
Experimental Protocol
Male Sprague-Dawley rats (10–12 weeks old, Harlan, n=131) were used. Rats were prepared with a lateral ventricular cannula, osmotic minipumps for intracerebroventricular (icv) and subcutaneous drug infusion, and with telemetry probes for continuous blood pressure (BP) monitoring, as previously described.17,18 The icv doses of agents used to induce sensitization or to block AT1-R, TNF-α synthesis or microglial activation were chosen on the basis of published in vivo studies.3,17,21–24
All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by The University of Iowa Animal Care and Use Committee.
Effects of HFD on Ang II-induced hypertension and molecular events in the LT
BP was monitored continuously by telemetry in rats that were fed either NFD (10% calories from lard, 3.85 kcal/g, D12450J, Research Diet, NJ) or HFD (60% calories from lard, 5.24 kcal/g, D12492, Research Diet, NJ) for three weeks (Induction period, I), followed by NFD for two weeks (Expression period, E). During I, rats received icv vehicle (V), the TNF-α synthesis inhibitor pentoxifylline (PTX, 10 µg/h), the AT1-R blocker irbesartan (Irbe, 125 µg/d) or the inhibitor of microglial activation minocycline (Mino, 5 µg/h). These inhibitors were delivered by osmotic pumps (model 2004, 0.25 µl/h for 4 weeks, Alzet) that were disconnected before Ang II infusion. During E, a slow pressor dose of Ang II (120 ng/kg/min) or saline was delivered subcutaneously by osmotic pump (model 2002, 0.5 µl/h for 2 weeks, Alzet). Thus, the primary study groups (n=6/group) were: 1) I-NFD + icv V + E-Ang II, 2) I-HFD + icv V + E-saline, 3) I-HFD + icv V + E-Ang II, 4) I-HFD + icv PTX + E-Ang II, 5) I-HFD + icv Irbe + E-Ang II, and 6) I-HFD + icv Mino + E-Ang II. Three additional HFD groups (n=5/group) received icv PTX, Irbe or Mino without Ang II treatment to determine the effect of each of these inhibitors alone on BP throughout I and E periods. Ganglionic blockade by hexamethonium (30 mg/kg, ip) was conducted during the baseline period, after HFD pretreatment and after 14 days of Ang II infusion. Food consumption, water intake and body weight (BW) were measured once a week.
Five treatment groups (n=5/group) (I-NFD + icv V, I-HFD + icv V, I-HFD + icv PTX, I-HFD + icv Irbe, and I-HFD + icv Mino) were euthanized following I but prior to E for molecular studies to determine the effect of HFD and inhibitors on mRNA expression of RAS and inflammatory elements in the structures lying along the LT (i.e., the SFO, MnPO and organum vasculosum).
Effects of brain RAS and inflammation on Ang II-induced hypertension and molecular events in the LT
Rats were fed normal rat chow (7013 NIH-31 modified rat diet) ad libitum. BP was monitored continuously during an Induction-Delay-Expression (I-D-E) protocol, as described previously.17,18 During I, icv V, a subpressor dose of Ang II (1 ng/kg/min), TNF-α (20 ng/d), Ang II (1 ng/kg/min) plus PTX (10 µg/h) or Ang II (1 ng/kg/min) plus Mino (5 µg/h) was delivered by osmotic minipump (model 2001, 1µl/h, Alzet) for 1 week. To ensure that any exogenous Ang II and antagonists were metabolized, the rats then rested for 1 week (D). After this time, a second pump (model 2002, 0.5µl/h Alzet) was implanted to deliver a slow pressor dose of Ang II (120 ng/kg/min) for 2 weeks (E). Thus, the primary study groups (n=6/group) were: 1) I-icv V + E-Ang II, 2) I-icv Ang II + E-Ang II, 3) I-icv TNF-α + E-Ang II, 4) I-icv Ang II/PTX + E-Ang II, and 5) I-icv Ang II/Mino + E-Ang II.
Five additional groups (n=5/group) underwent identical treatment during I (I-icv V, I-icv TNF-α, I-icv Ang II, I-icv Ang II/PTX and I-icv Ang II/Mino) but were euthanized at the end of the D period to collect LT for analysis of mRNA expression.
Additional Methods
Please see the online-only Data Supplement.
Results
HFD-induced Sensitization of Ang II hypertension
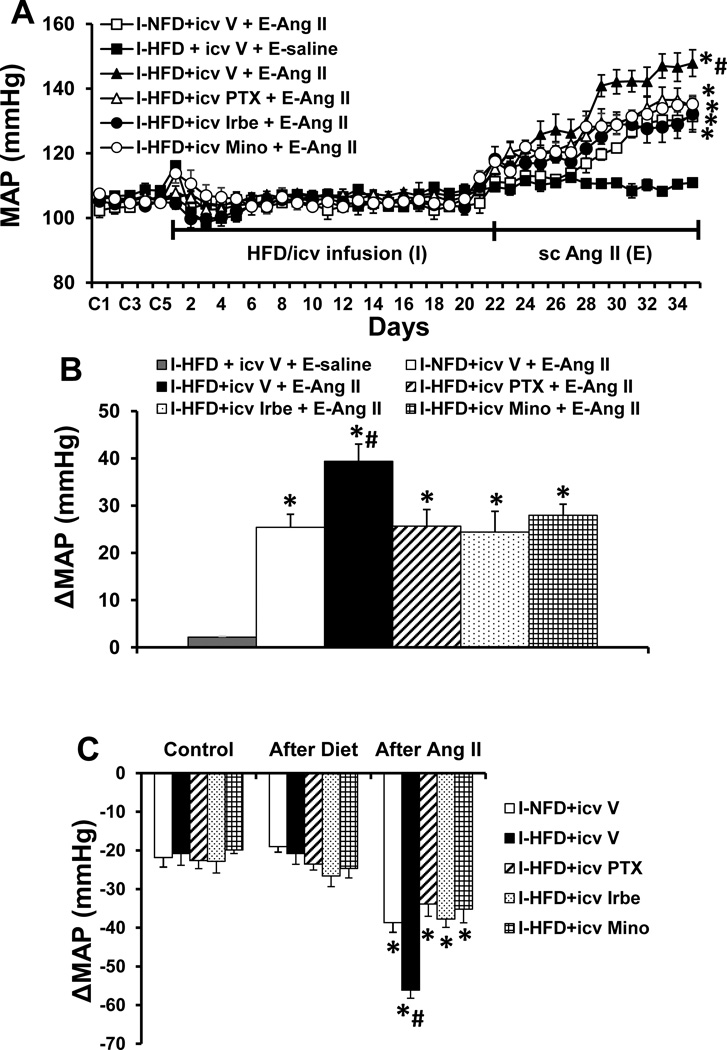
Baseline values for MAP (105.6±2.3 mmHg) and HR (346.9±5.5 beats/min) were comparable prior to and following application of HFD or icv infusion of PTX, Irbe or Mino alone in all groups of rats (Fig. S1). However, over the course of E, Ang II induced a greater increase in MAP in the rats that received the HFD [Δ39.4 ± 3.7 mmHg, two-way ANOVA: effect of HFD, F (1, 30) =8.69, p=0.0061; effect of Ang II, F(2, 30)=93.81, p<0.0001, Fig. 1A, 1B] as compared to the rats pretreated with NFD (Δ25.4 ± 2.8 mmHg). This augmentation of the pressor effect induced by Ang II was blocked by concurrent icv infusions of either PTX [Δ25.6 ± 3.5 mmHg, two-way ANOVA: effect of icv PTX, F(1, 30)=6.53, p=0.0159; effect of Ang II, F(2, 30)=247.6, p<0.0001], Irbe [Δ24.4 ± 4.4 mmHg, two-way ANOVA: effect of icv Irbe, F(1, 30)=9.31, p=0.0047; effect of Ang II, F(2, 30)=117.6, p<0.0001] or Mino [Δ27.9 ± 2.4 mmHg, two-way ANOVA: effect of icv Mino, F(1, 30)=7.31, p=0.0112; effect of Ang II, F(2, 30)=204.7, p<0.0001] along with the HFD pretreatment (p<0.05). Systemic Ang II infusions produced slight, comparable decreases in HR in all groups (Fig. S2).
Figure 1.
Augmented pressor effects induced by Ang II during the expression (E) period in rats after pretreatment with HFD during the induction (I) period. This effect was attenuated by central inhibition of TNF-α synthesis, AT1-R or microglia activation (Fig. 1A). Figure 1B shows the changes in MAP after infusion of Ang II during E in all groups. Figure 1C shows the decreases in MAP in response to ganglionic blockade with hexamethonium at baseline, after 3 weeks of diet treatment and on day 14 after infusion of Ang II in all groups. I-NFD = pretreatment with normal fat diet during I; I-HFD = pretreatment with high fat diet during I; icv V = central treatment with vehicle during I; I-HFD+icv PTX = pretreatment with HFD plus central treatment with TNF-α synthesis inhibitor pentoxifylline during I; I-HFD+icv Irbe = pretreatment with HFD plus central treatment with AT1-R blocker irbesartan during I; I-HFD+icv Mino = pretreatment with HFD plus central treatment with inhibitor of microglial activation minocycline during I; E-saline = peripheral treatment with saline during E; E-Ang II = peripheral treatment with a pressor dose of Ang II during E. (* significant difference vs. baseline or after diet treatment; # significant difference vs. I-NFD+icv V + E-Ang II and other groups fed with HFD plus central blocker treatment during I)
The hexamethonium-induced decreases in MAP were comparable in all groups before HFD treatment (averaged −21.6 ± 0.6 mmHg, Fig. 1C). HFD alone or HFD combined with central inhibition of TNF-α synthesis, AT1-R or microglial activation also had no effect on the decrease in MAP produced by acute ganglionic blockade (averaged −23.8 ± 1.4 mmHg, Fig. 1C). However, following 14 days of Ang II infusion, acute hexamethonium injection resulted in significant decreases in MAP in both NFD- and HFD-treated rats (p<0.05 vs before infusion of Ang II). Notably, HFD pretreated rats exhibited an enhanced decrease in MAP (HFD −56.1± 2.3 mmHg vs NFD −38.7± 2.5, p<0.05, Fig. 1C), which was attenuated by central inhibition of TNF-α synthesis (−33.8 ± 3.2 mmHg, p<0.05), AT1-R (−37.8 ± 4.4 mmHg, p<0.05) or microglial activation (−35.2 ±3.5 mmHg, p<0.05).
Caloric Intake, Feed Efficiency and Body Weight during HFD and Systemic Ang II
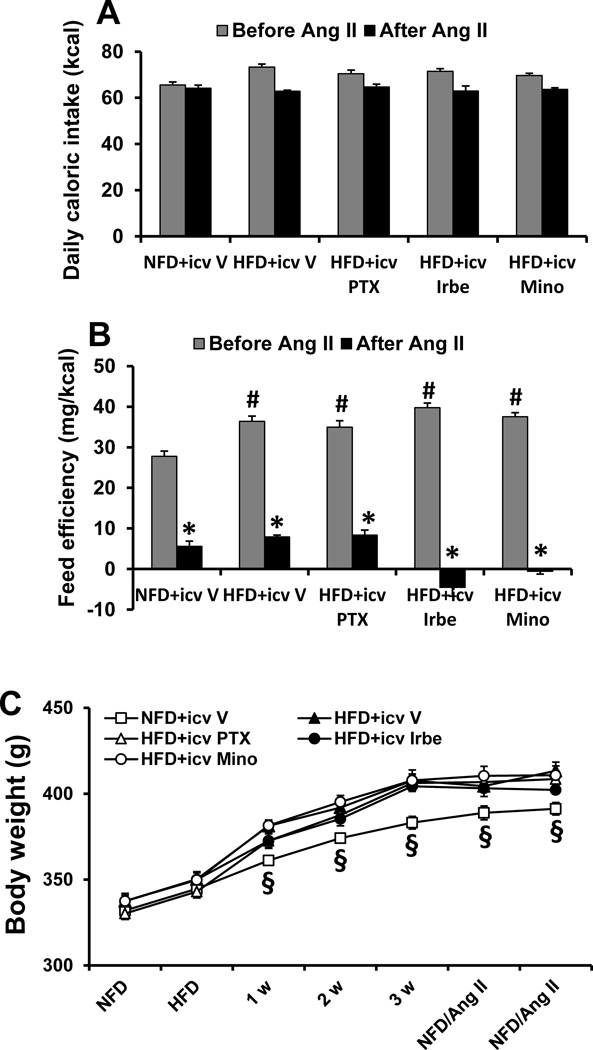
During I, all groups of rats fed the HFD had slight increases in caloric intake (71.2±3.5 Kcal/day, p>0.05, Fig 2A), but exhibited significant higher feed efficiency (38.0±3.2 mg BW/Kcal, p<0.05, Fig 2B) when compared with the group of NFD fed rats (64.5±4.1 Kcal/day and 27.8±2.8 mg BW/Kcal). Accordingly, 3 week HFD feeding resulted in a significant increase in BW (HFD, 58.2±5.2 gm vs. NFD 38.5±4.3 gm, p<0.05, Fig 2C). During E, with all groups receiving the NFD and the Ang II infusion, caloric intake was comparable in rats that had been induced with NFD or HFD (p>0.05) and the rates of BW gain were significantly reduced in all groups (p<0.05, Fig. 2C).
Figure 2.
Changes in caloric intake (A), feed efficiency (B) and body weight (C) in normal fat diet (NFD) rats and high fat diet (HFD) rats treated with central (icv) TNF-α, synthesis inhibitor pentoxifylline (PTX), AT1-R blocker irbesartan (Irbe) or inhibitor of microglial activation minocycline (Mino) before and after systemic infusion of a pressor dose of Ang II. (* significant difference vs. before Ang II; # significant difference vs. NFD rats; § significant difference vs. HFD groups).
Effect of icv PTX, Irbe or Mino on HFD-induced mRNA Expression of RAS and Inflammatory Elements in the LT
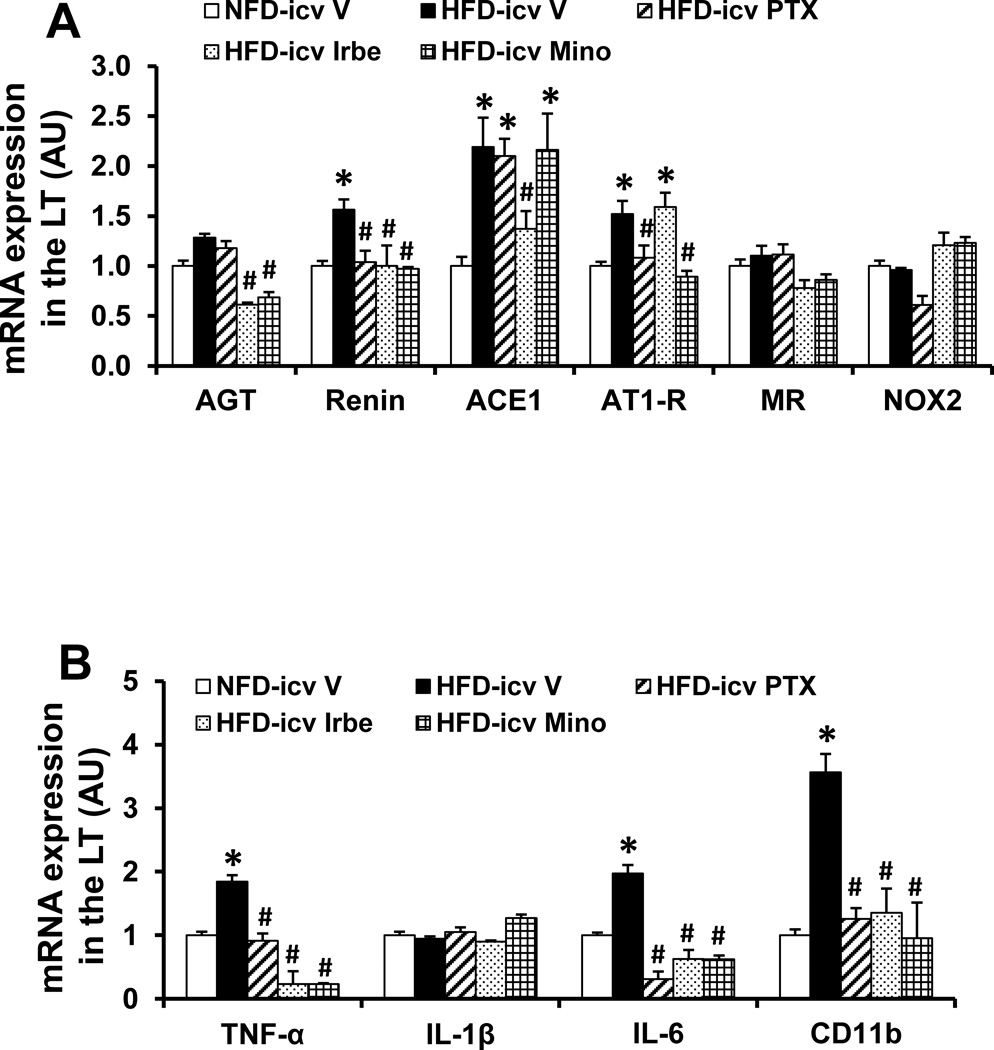
In LT tissue collected at the end of HFD feeding, HFD induced a significant increase in the mRNA expression of the inflammatory elements (i.e., TNF-α, IL-6, the microglial marker CD11b) and some (i.e., renin, AT1-R, ACE1), but not all, RAS components in the LT when compared with controls (p<0.05). The expression of AGT, mineralocorticoid receptor (MR), NOX2 and IL-1β in the LT was not affected by HFD (p>0.05, Fig 3A, 3B).
Figure 3.
Quantitative comparison of the mRNA expression of renin-angiotensin system components (A), proinflammatory cytokines and microglial marker (B) in the lamina terminalis (LT) of rats fed normal fat diet (NFD) or high fat diet (HFD) and treated with central (icv) infusion of TNF-α synthesis inhibitor pentoxifylline (PTX), AT1-R blocker irbesartan (Irbe) or inhibitor of microglial activation minocycline (Mino) during induction (I). (* significant difference vs. NFD; # significant difference vs. HFD with icv vehicle).
Central infusion of PTX, Irbe or Mino normalized the increased mRNA expression of renin, TNF-α, IL-6 and CD11b produced by HFD (p<0.05, Fig 3A, 3B). Among the RAS components whose expression was upregulated by HFD, the increased AT1-R expression was attenuated by icv PTX, the elevated expression of AGT and ACE1 were inhibited by icv Irbe, while the increased expression of AGT and AT1-R was attenuated by icv Mino (p<0.05, Fig 3A, 3B). Message for MR, NOX2 and IL-1β remained unchanged.
Icv TNF-α- and low dose Ang II-induced Sensitization of Ang II hypertension
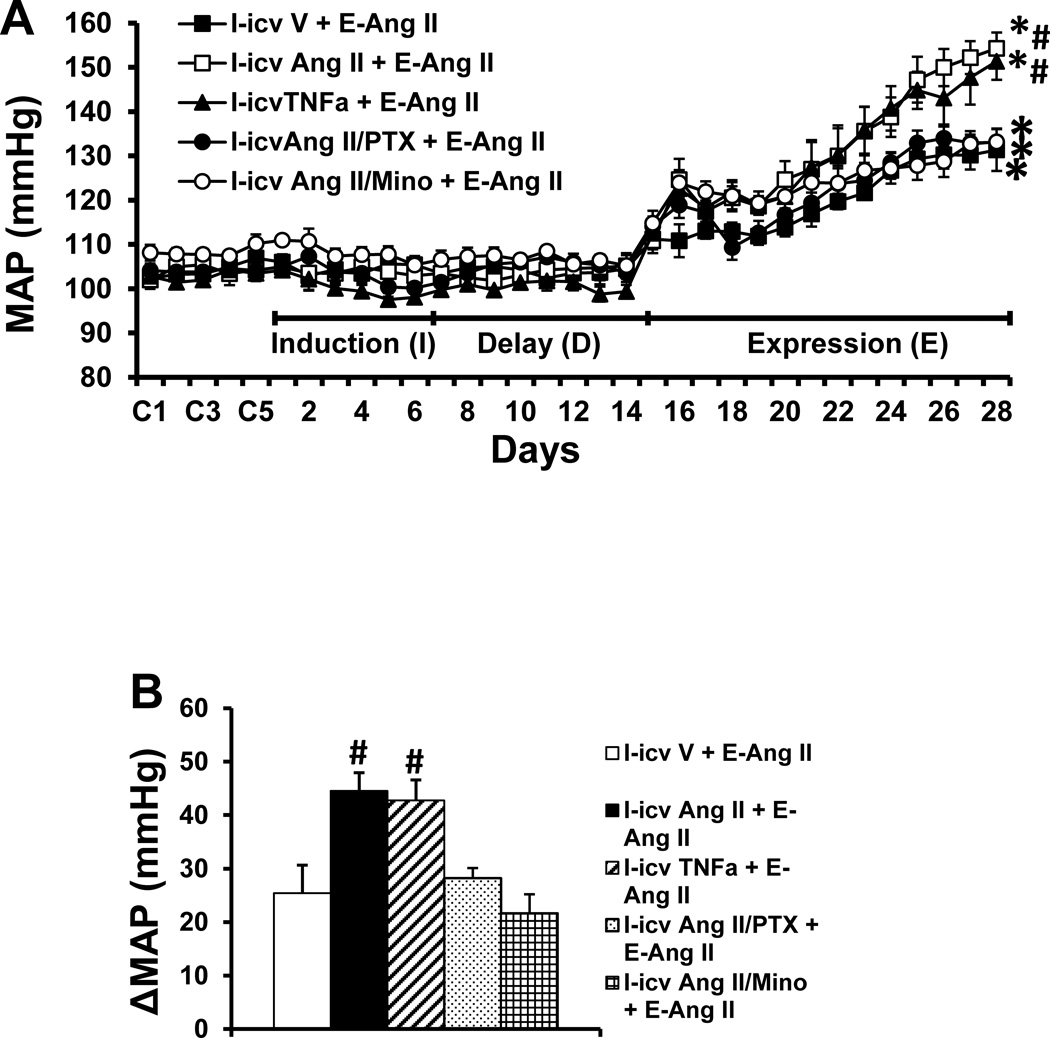
To confirm the direct sensitizing actions of central TNF-α and its mediation of central Ang II-induced sensitization, TNF-α or a supressor dose of Ang II combined with either a TNF-α synthesis inhibitor or an inhibitor of microglial activation was infused icv for 1 week during I. These treatments had no effect on MAP during I (Fig. 4A). The icv infusion of TNF-α mimicked the icv subpressor dose of Ang II-induced augmentation of the pressor effect produced by a subsequent pressor dose of Ang II during E [TNF-α, Δ44.5±3.4 mmHg, two-way ANOVA: effect of icv TNF-α, F(1, 30)= 6.51, p=0.016; effect of Ang II, F(2, 30)=168.3, p<0.0001. Ang II, Δ42.8±5.3 mmHg, two-way ANOVA: effect of icv Ang II, F(1, 30)= 6.65, p=0.015; effect of Ang II, F(2, 30)=120.0, p<0.0001] as compared to that of icv saline treated rats (Δ25.7±4.1 mmHg, Fig. 4A, 4B). This augmentation of the hypertensive response induced by the pressor dose of Ang II was abolished by concurrent icv infusion of the TNF-α synthesis inhibitor PTX or by the inhibitor of microglial activation Mino along with the icv subpressor dose of Ang II administrated during I [PTX Δ28.2±1.9 mmHg, two-way ANOVA: effect of icv PTX, F(1, 30)= 5.04, p=0.0324; effect of Ang II, F(2, 30)=137.7, p<0.0001. Mino Δ21.7±3.5 mmHg, two-way ANOVA: effect of icv Mino, F(1, 30)=4.97, p=0.0334; effect of Ang II, F(2, 30)=141.6, p<0.0001, Fig. 4A, 4B]. Systemic Ang II infusions produced slight, but comparable decreases in HR in all groups (Fig. S3).
Figure 4.
Augmented pressor effects induced by Ang II during the expression (E) period in rats after central treatment with TNF-α or subpressor dose of Ang II during the induction (I) period (Fig. 4A). The sensitizing effect of subpressor dose of Ang II was attenuated by central inhibition of either TNF-α synthesis or microglia activation. Figure 4B shows the changes in MAP after infusion of Ang II during E in all groups. icv V = central treatment with vehicle during I; icv TNF-α = central treatment with TNF-α during I; icv Ang II = central treatment with subpressor dose of Ang II during I; I-icv Ang II/PTX = central concurrent treatment with subpressor dose of Ang II and TNF-α synthesis inhibitor pentoxifylline during I; I-icv Ang II/Mino = central concurrent treatment with subpressor dose of Ang II and inhibitor of microglial activation minocycline during I; E-Ang II = peripheral treatment with a pressor dose of Ang II during E. (* significant difference vs. baseline; # significant difference vs. I-icv V + E-Ang II and other groups treated with central blocker during I).
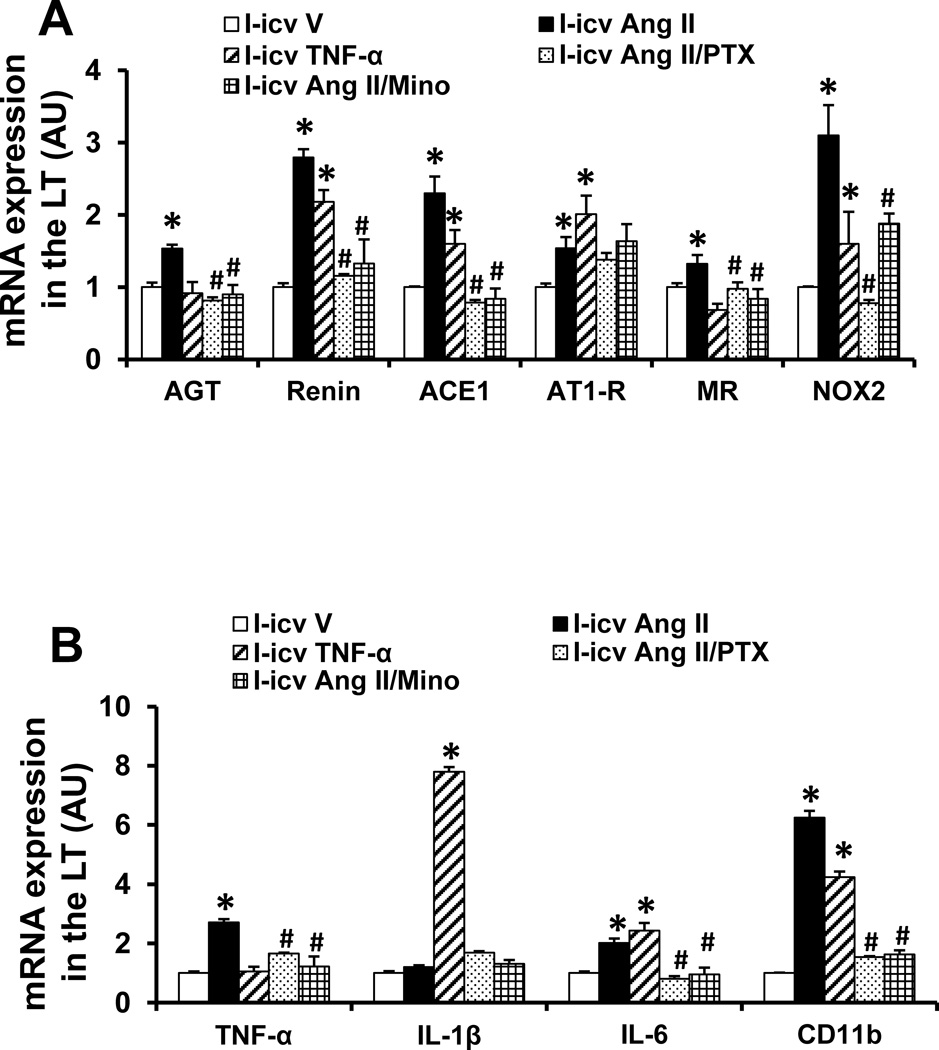
Effect of icv PTX or Mino on central subpressor dose of Ang II-induced mRNA Expression of RAS Components, Proinflammatory Cytokines and microglial marker in the LT
In LT tissue collected at the end of D period, the icv subpressor dose of Ang II given during I produced a significant increase in the mRNA expression of renin, AGT, AT1-R, ACE1, MR, NOX2, TNF-α, IL-6 and CD11b (p<0.05), but had no effect on the mRNA expression of IL-1β when compared with controls. The icv infusion of TNF-α during I similarly elicited most of the increases in the mRNA expression induced by Ang II except the mRNA expression of AGT, MR, TNF-α, but had a significant potentiating effect on the mRNA expression of IL-1β. Concurrent central infusion of PTX or Mino along with a sensitizing dose of Ang II blocked the enhanced increase in mRNA expression in most cases (p<0.05) with the exception of AT1-R (Fig 5A, 5B).
Figure 5.
Quantitative comparison of the mRNA expression of renin-angiotensin system components (A), proinflammatory cytokines and microglial marker (B) in the lamina terminalis (LT) of rats receiving central (icv) infusion of TNF-α, subpressor dose of Ang II or subpressor dose of Ang II plus TNF-α synthesis inhibitor pentoxifylline (PTX) or inhibitor of microglial activation minocycline (Mino) during induction (I). (* significant difference vs. icv vehicle; # significant difference vs. icv Ang II)
Discussion
Obesity-related hypertension is a major risk factor for the development of cardiovascular diseases. These studies provide evidence that a short-term exposure to HFD activates the RAS and PICs in the LT and sensitizes the pressor actions of Ang II. A similar sensitization of the Ang II-induced hypertensive response can be produced by icv administration of TNF-α or a subpressor dose of Ang II. These observations indicate that activation of the RAS and PICs and the interaction between these two systems are involved in the process of sensitization. The sensitizing action of HFD is also associated with activation of microglia, which express AT1-R and PIC receptors and are a potential source of PICs.24,25 Taken together, these results suggest that eating a HFD for a short time can induce remarkable changes in CNS function that increase the vulnerability to hypertension.
Overproduction of Ang II or excessive activation of the brain RAS contributes to the genesis of cardiovascular diseases including hypertension.26 Ang II can act as a potent proinflammatory agent and stimulate the production of PICs such as TNF-α, IL-6 and IL-1β in the brain that augment hypertensive responses.1,24,27 The possible cellular sources of brain PICs include CD11b+ microglia and neurons.25 A recent study demonstrated that inflammation and microglial activation within the PVN are associated with elevated BP and augmented sympathetic activity induced by Ang II infusion.24 Furthermore, icv TNF-α injection elevates BP and sympathetic activity in a dose-dependent manner.4 PICs act within the SFO to upregulate the expression of components of the RAS and mediators of inflammation that elicit a sympathoexcitatory response mediated by the RAS.3 Central inhibition of TNF-α reverses alterations in RAS components and attenuates Ang II-induced hypertension.1 Moreover, both Ang II and TNF-α can activate NADPH oxidase, leading to enhanced oxidative stress and decreased bioavailability of NO that combine to contribute to increases in SNA and BP.26 These studies confirm that the brain RAS and PICs can mutually facilitate each other’s expression in the CNS as well as the sympathoexcitatory and pressor effects that generate hypertension.1,2 Previous studies from our laboratory have shown that subpressor doses of Ang II and aldosterone given during I produced upregulation of components of the brain RAS resulting in a sensitized hypertensive response to a subsequently administered, pressor dose of Ang II.17,18 The present study extends our previous work by showing that central pretreatment with TNF-α mimicked the sensitizing actions of Ang II, upregulating mRNA expression of the RAS components, PICs, microglial marker and NADPH oxidase in the LT and augmenting the hypertensive response to a pressor dose of Ang II. Inhibition of either TNF-α synthesis or microglia activation abolished these changes. These results indicate that Ang II upregulation of PICs and microglial activation mediate Ang II-induced sensitization, and suggest that low grade central inflammation induced by different classes of stimuli can sensitize the brain to be predisposed to the expression of frank hypertension.
The RAS and PICs have both been implicated in the regulation of energy balance and obesity-induced hypertension. Not only does adipose tissue have a local RAS12,28 but serum levels of major the components of the RAS (renin, AGT, ACE) are elevated in obesity.10 TNF-α is commonly considered as one of the initiators of the pro-inflammatory cascade, which can induce the production of other cytokines. Inhibition of TNF-α during inflammatory events abolishes many of the ensuing responses, including increased IL-1β and IL-6.29 TNF-α levels are increased in obesity and serve as a marker for obesity.30 Recent studies have revealed that HFD feeding increases hypothalamic PIC expression including IL-1β, IL-6 and TNF-α, and several components of the RAS such as AT1-R.14,31 The increased gene expression for the RAS and for PICs is accompanied by increased microglial activation in the hypothalamus including the arcuate nucleus (ARC), SFO and PVN.13,32,33 Some of these responses were reversed upon deletion of AT1a specially within the PVN.31
It has been shown that systemic RAS and TNF-α, which are both increased in obesity, play a role in promoting energy storage, whereas these two systems act within the CNS to increase energy expenditure.11,34,35 The difference between the central and peripheral effects of the RAS and TNF-α suggests the presence of a negative feedback mechanism that is activated when peripheral RAS activity and TNF-α levels are high and gain access to the brain.11 However, this putative central negative feedback pathway for energy balance triggers the cardiovascular consequences of activation of the RAS and PICs with resultant elevations of SNA and BP. Consistent with the activating effect of obesity on the brain, we found in the present study that the HFD feeding significantly elicited increases in mRNA expression of several RAS components, PICs and a microglial marker in the LT. These changes might reflect a mechanism for sensitizing the brain cardiovascular nuclei and enhance their reactivity, which is evident by the enhanced hypertensive response to the subsequent Ang II infusion in the HFD fed rats. Furthermore, central inhibition of AT1-R and inflammation during HFD feeding abolished the HFD-induced sensitizing effect on hypertension. Combined with the findings that a low dose of TNFα or Ang II administered centrally directly sensitizes Ang II-induced hypertension, these observations suggest that the HFD-induced upregulation of the RAS and PICs in the CNS participates in the sensitization process. The capacity of the RAS or PICs to mutually up-regulate their expression may be indicative of actions within central sensitization-related positive feed-forward systems which can accelerate the onset and rate of development of hypertension.
It should be noted that many previous studies have employed long term HFD feeding (8 weeks or more longer time) to produce obesity and elevated levels of RAS components and of PICs (e.g. TNF-α) in both brain and peripheral tissues.11,14,31,34 However, one recent study showed that in rats predisposed to DIO, the expression of proinflammatory biomarkers was increased in the mediobasal hypothalamus within 24 h of HFD onset,13 suggesting that hypothalamic inflammation occurs prior to obesity onset. This raises questions as to what factor(s) or through which pathway HFD activates the CNS RAS and inflammation so that the brain is sensitized to circulating hypertensive agents in the initial stages of overnutrition before the advent of increased systemic inflammation and RAS activation.
It has been shown that the hypothalamic structures residing inside blood-brain barrier (BBB), including the ARC, PVN, supraoptic nucleus and lateral hypothalamus, are major sites for the regulation of autonomic and energy processes.4,11,36 The LT including the SFO, a sensory circumventricular organ lacking the normal BBB, has also been documented as a CNS structure involved in both cardiovascular regulation and energy homeostasis.16,37 The SFO sends efferent projections to communicate with these hypothalamic nuclei inside BBB.16 In the present study, we found that 3 weeks of HFD feeding significantly increased the mRNA expression of the RAS and PICs in the LT. This result suggests that the components of the LT may first sense factors produced by HFD feeding and respond to the changes in the energy homeostasis by producing increased activation of the RAS and PICs in the cardiovascular neural network without apparent changes in the BP and sympathetic activity. Further study is needed to investigate the hypothalamic nuclei involving the HFD-induced sensitization process, as well as looking more specifically at the pathway between the SFO and the hypothalamic nuclei involved in processing signals associated with the RAS and PICs that are initiated in the course of developing obesity.
Recently, a series of studies from Head and colleagues demonstrated in rabbits that 3 weeks of HFD feeding rapidly produced increased MAP, HR and RSNA.6–8 This obesity-induced hypertension could be blocked by a central leptin antagonist after 3 weeks of HFD, which is a time when body weight, visceral white adipose tissue and plasma leptin levels were markedly elevated.38 They also found that the hypertension and high RSNA persisted long after stopping HFD and normalizing of circulating levels of leptin. These findings raise the possibility that HFD produces factors in addition to leptin that contribute to the increases in BP and SNA. It is likely that these other factors are the RAS components and PICs.39,40 Results of the current study showing that 3 weeks of HFD feeding significantly increased body weight and the mRNA expression of the RAS and PICs in the LT point to these as additional important mediating factors. Combining the results of Head et al. studies6–8 with the current findings, it can be hypothesized that either local or systemic RAS components and PICs, probably stimulated by the HFD itself or by leptin,41 are the critical mediators of HFD-induced sensitization of hypertension.
Because mRNA gene expression results do not always reflect protein expression or activity levels, one limitation of the present study is that we did not determine activity and protein levels of the RAS components and PICs as the basis of the changes in the mRNA levels after HFD treatment. Therefore, further study to analyze the protein levels in cardiovascular nuclei and circulating concentrations of the RAS and PICs after short term HFD may uncover mechanisms through which the HFD modulates the brain nuclei sensitivity that participates in the pathophysiology of obesity-related hypertension. Another limitation is that TNF-α synthesis inhibitor PTX and inhibitor of microglial activation Mino used in the present study are not selective and affect multiple biochemical targets.42,43 However, using both PTX and Mino produced results that converge to be highly suggestive that inflammation and related factors are involved in mediating the sensitizing effects of HFD on the hypertensive response. The use of more selective inhibitors in the future studies should lend additional support to this interpretation of our current findings.
Perspectives
Clinical and experimental data indicate important interactions between obesity and hypertension. Activation of both the RAS and PICs in the CNS play a critical role in the development of obesity, hypertension or both. The present findings indicate that similar to the sensitizing effects of subpressor doses of Ang II or aldosterone,17,18 sensitization by HFD feeding is associated with maintained changes in the expression of “pressor” components of the brain RAS and PICs. These observations provide novel evidence and insight for the actions of RAS and of PICs on LT structures to mediate cardiovascular sensitizing effects of metabolic factors, even though these factors do not induce immediate abnormalities in the cardiovascular function. One implication of these and our demonstrations of sensitization and maintained neuroplasticity17–20 is that challenges to homeostasis can act to reprogram the neural network controlling BP to generate an enhanced pressor response when a hypertensinogenic stimulus is either sustained or encountered at a later time.
Supplementary Material
Novelty and Significance.
What is New?
These studies demonstrate that short term exposure to high fat diet (HFD) sensitizes the hypertensive response to angiotensin (Ang) II. Central inhibition either of proinflammatory cytokines (PICs) synthesis or of renin angiotensin system (RAS) activity abolishes HFD-elicited sensitization of Ang II hypertension.
What is Relevant?
The demonstration of HFD facilitating effect on the expression of the central RAS and PICs as well as the interaction between these two systems indicates that central nervous system RAS and PICs are likely to play an important role in the pathogenesis and progression of obesity-related hypertension.
Summary
The study indicates that HFD acts on the brain to sensitize the hypertensive response to Ang II and that sensitization is associated with maintained altered expression of RAS and PICs within components of a forebrain cardiovascular control network.
Acknowledgments
Sources of Funding
This work was supported by the NIH grants HL-14388 (AKJ), HL-98207 (AKJ), and MH-80241(AKJ), HL-096671 (RBF) HL-073986 (RBF), with partial support from the Department of Veterans Affairs (RBF).
Footnotes
Disclosures: None
References
- 1.Yu Y, Xue BJ, Zhang ZH, Wei SG, Beltz TG, Guo F, Johnson AK, Felder RB. Early interference with p44/42 mitogen-activated protein kinase signaling in hypothalamic paraventricular nucleus attenuates angiotensin II-induced hypertension. Hypertension. 2013;61:842–849. doi: 10.1161/HYPERTENSIONAHA.111.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sriramula S, Cardinale JP, Francis J. Inhibition of TNF in the brain reverses alterations in RAS components and attenuates angiotensin II-induced hypertension. PLoS One. 2013;8:e63847. doi: 10.1371/journal.pone.0063847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei SG, Yu Y, Zhang ZH, Felder RB. Proinflammatory cytokines upregulate sympathoexcitatory mechanisms in the subfornical organ of the rat. Hypertension. 2015;65:1126–1133. doi: 10.1161/HYPERTENSIONAHA.114.05112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Purkayastha S, Zhang G, Cai D. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-β and NF-κB. Nat Med. 2011;17:883–887. doi: 10.1038/nm.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Żera T, Ufnal M, Szczepańska-Sadowska E. TNF and angiotensin type 1 receptors interact in the brain control of blood pressure in heart failure. Cytokine. 2015;71:272–277. doi: 10.1016/j.cyto.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 6.Garrison RJ, Kannel WB, Stokes J, III, Castelli WP. Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Prev Med. 1987;16:235–251. doi: 10.1016/0091-7435(87)90087-9. [DOI] [PubMed] [Google Scholar]
- 7.Prior LJ, Eikelis N, Armitage JA, Davern PJ, Burke SL, Montani JP, Barzel B, Head GA. Exposure to a high-fat diet alters leptin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension. 2010;55:862–868. doi: 10.1161/HYPERTENSIONAHA.109.141119. [DOI] [PubMed] [Google Scholar]
- 8.Armitage JA, Burke SL, Prior LJ, Barzel B, Eikelis N, Lim K, Head GA. Rapid onset of renal sympathetic nerve activation in rabbits fed a high-fat diet. Hypertension. 2012;60:163–171. doi: 10.1161/HYPERTENSIONAHA.111.190413. [DOI] [PubMed] [Google Scholar]
- 9.Lim K, Burke SL, Head GA. Obesity-related hypertension and the role of insulin and leptin in high-fat-fed rabbits. Hypertension. 2013;61:628–634. doi: 10.1161/HYPERTENSIONAHA.111.00705. [DOI] [PubMed] [Google Scholar]
- 10.de Kloet AD1, Krause EG, Woods SC. The renin angiotensin system and the metabolic syndrome. Physiol Behav. 2010;100:525–534. doi: 10.1016/j.physbeh.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Kloet AD, Pati D, Wang L, Hiller H, Sumners C, Frazier CJ, Seeley RJ, Herman JP, Woods SC, Krause EG. Angiotensin type 1a receptors in the paraventricular nucleus of the hypothalamus protect against diet-induced obesity. J Neurosci. 2013;33:4825–4833. doi: 10.1523/JNEUROSCI.3806-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thaler JP, Guyenet SJ, Dorfman MD, Wisse BE, Schwartz MW. Hypothalamic inflammation: marker or mechanism of obesity pathogenesis? Diabetes. 2013;62:2629–2634. doi: 10.2337/db12-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschöp MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maric T, Woodside B, Luheshi GN. The effects of dietary saturated fat on basal hypothalamic neuroinflammation in rats. Brain Behav Immun. 2014;36:35–45. doi: 10.1016/j.bbi.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Johnson AK, Gross PM. Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 1993;7:678–686. doi: 10.1096/fasebj.7.8.8500693. [DOI] [PubMed] [Google Scholar]
- 16.Mimee A, Smith PM, Ferguson AV. Circumventricular organs: targets for integration of circulating fluid and energy balance signals? Physiol Behav. 2013;121:96–102. doi: 10.1016/j.physbeh.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Xue B, Zhang Z, Johnson RF, Johnson AK. Sensitization of slow pressor angiotensin II (Ang II)-initiated hypertension: induction of sensitization by prior AngII treatment. Hypertension. 2012;59:459–466. doi: 10.1161/HYPERTENSIONAHA.111.185116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xue B, Zhang Z, Roncari CF, Guo F, Johnson AK. Aldosterone acting through the central nervous system sensitizes angiotensin II-induced hypertension. Hypertension. 2012;60:1023–1030. doi: 10.1161/HYPERTENSIONAHA.112.196576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clayton SC, Zhang Z, Beltz T, Xue B, Johnson AK. CNS neuroplasticity and salt-sensitive hypertension induced by prior treatment with subpressor doses of ANG II or aldosterone. Am J Physiol Regul Integr Comp Physiol. 2014;306:R908–R917. doi: 10.1152/ajpregu.00010.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue B, Zhang Z, Guo F, Johnson AK. Either sodium deprivation or sodium excess sensitizes angiotensin (ANG) II-induced hyeprtension. FASEB J. 2013 p695.612. [Google Scholar]
- 21.Xue B, Beltz TG, Yu Y, Guo F, Gomez-Sanchez CE, Hay M, Johnson AK. Central interactions of aldosterone and angiotensin II in aldosterone- and angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol. 2011;300:H555–H564. doi: 10.1152/ajpheart.00847.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leenen FH, Yuan B. Prevention of hypertension by irbesartan in Dahl S rats relates to central angiotensin II type 1 receptor blockade. Hypertension. 2001;37:981–984. doi: 10.1161/01.hyp.37.3.981. [DOI] [PubMed] [Google Scholar]
- 23.Kang YM, Zhang ZH, Xue B, Weiss RM, Felder RB. Inhibition of brain proinflammatory cytokine synthesis reduces hypothalamic excitation in rats with ischemia-induced heart failure. Am J Physiol Heart Circ Physiol. 2008;295:H227–H236. doi: 10.1152/ajpheart.01157.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK. Brain microglial cytokines in neurogenic hypertension. Hypertension. 2010;56:297–303. doi: 10.1161/HYPERTENSIONAHA.110.150409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clausen BH, Lambertsen KL, Babcock AA, Holm TH, Dagnaes-Hansen F, Finsen B. Interleukin-1beta and tumor necrosis factor-alpha are expressed by different subsets of microglia and macrophages after ischemic stroke in mice. J Neuroinflammation. 2008;5:46. doi: 10.1186/1742-2094-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan SH, Chan JY. Angiotensin-generated reactive oxygen species in brain and pathogenesis of cardiovascular diseases. Antioxid Redox Signal. 2013;19:1074–1084. doi: 10.1089/ars.2012.4585. [DOI] [PubMed] [Google Scholar]
- 27.Cardinale JP, Sriramula S, Mariappan N, Agarwal D, Francis J. Angiotensin II-induced hypertension is modulated by nuclear factor-κB in the paraventricular nucleus. Hypertension. 2012;59:113–121. doi: 10.1161/HYPERTENSIONAHA.111.182154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Claflin KE, Grobe JL. Control of energy balance by the brain Renin-Angiotensin system. Curr Hypertens Rep. 2015;17:38. doi: 10.1007/s11906-015-0549-x. [DOI] [PubMed] [Google Scholar]
- 29.Turnbull AV, Pitossi FJ, Lebrun JJ, Lee S, Meltzer JC, Nance DM, del Rey A, Besedovsky HO, Rivier C. Inhibition of tumor necrosis factor-alpha action within the CNS markedly reduces the plasma adrenocorticotropin response to peripheral local inflammation in rats. J Neurosci. 1997;17:3262–3273. doi: 10.1523/JNEUROSCI.17-09-03262.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Margoni A, Perrea DN, Vlachos I, Prokopaki G, Pantopoulou A, Fotis L, Kostaki M, Papavassiliou AG. Serum leptin, adiponectin and tumor necrosis factor-α in hyperlipidemic rats with/without concomitant diabetes mellitus. Mol Med. 2011;17:36–40. doi: 10.2119/molmed.2010.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Kloet AD, Pioquinto DJ, Nguyen D, Wang L, Smith JA, Hiller H, Sumners C. Obesity induces neuroinflammation mediated by altered expression of the renin-angiotensin system in mouse forebrain nuclei. Physiol Behav. 2014;136:31–38. doi: 10.1016/j.physbeh.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yi CX, Al-Massadi O, Donelan E, Lehti M, Weber J, Ress C, Trivedi C, Müller TD, Woods SC, Hofmann SM. Exercise protects against high-fat diet-induced hypothalamic inflammation. Physiol Behav. 2012;106:485–490. doi: 10.1016/j.physbeh.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 33.Shi P, Grobe JL, Desland FA, Zhou G, Shen XZ, Shan Z, Liu M, Raizada MK, Sumners C. Direct pro-inflammatory effects of prorenin on microglia. PLoS One. 2014;9:e92937. doi: 10.1371/journal.pone.0092937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Kloet AD1, Pacheco-López G, Langhans W, Brown LM. The effect of TNFα on food intake and central insulin sensitivity in rats. Physiol Behav. 2011;103:17–20. doi: 10.1016/j.physbeh.2010.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grobe JL, Grobe CL, Beltz TG, Westphal SG, Morgan DA, Xu D, de Lange WJ, Li H, Sakai K, Thedens DR, Cassis LA, Rahmouni K, Mark AL, Johnson AK, Sigmund CD. The brain Renin-angiotensin system controls divergent efferent mechanisms to regulate fluid and energy balance. Cell Metab. 2010;12:431–442. doi: 10.1016/j.cmet.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harlan SM, Morgan DA, Agassandian K, Guo DF, Cassell MD, Sigmund CD, Mark AL, Rahmouni K. Ablation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circ Res. 2011;108:808–812. doi: 10.1161/CIRCRESAHA.111.240226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young CN, Morgan DA, Butler SD, Mark AL, Davisson RL. The brain subfornical organ mediates leptin-induced increases in renal sympathetic activity but not its metabolic effects. Hypertension. 2013;61:737–744. doi: 10.1161/HYPERTENSIONAHA.111.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barzel B, Weir JM, Meikle PJ, Burke SL, Armitage JA, Head GA. Short term fat feeding rapidly increases plasma insulin but does not result in dyslipidaemia. Front Physiol. 2014;5:469. doi: 10.3389/fphys.2014.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purkayastha S, Cai D. Neuroinflammatory basis of metabolic syndrome. Mol Metab. 2013;2:356–363. doi: 10.1016/j.molmet.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalupahana NS, Moustaid-Moussa N. The renin-angiotensin system: a link between obesity, inflammation and insulin resistance. Obes Rev. 2012;13:136–149. doi: 10.1111/j.1467-789X.2011.00942.x. [DOI] [PubMed] [Google Scholar]
- 41.Hilzendeger AM, Morgan DA, Brooks L, Dellsperger D, Liu X, Grobe JL, Rahmouni K, Sigmund CD, Mark AL. A brain leptin-renin angiotensin system interaction in the regulation of sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2012;303:H197–H206. doi: 10.1152/ajpheart.00974.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang M, Xu Y-J, Mengi SA, Arneja AS, Dhalla NS. Therapeutic potentials of pentoxifylline for treatment of cardiovascular diseases. Exp Clin Cardiol. 2004;9:103–111. [PMC free article] [PubMed] [Google Scholar]
- 43.Plane JM, Shen Y, Pleasure DE, Deng W. Prospects for minocycline neuroprotection. Arch Neurol. 2010;67:1442–1448. doi: 10.1001/archneurol.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.