Abstract
Streptococcus pneumoniae infections are an important cause of morbidity and mortality in older patients. Uncontrolled neutrophil-driven pulmonary inflammation exacerbates this disease. To test whether the α-tocopherol (α-Toc) form of vitamin E, a regulator of immunity, can modulate neutrophil responses as a preventive strategy to mitigate the age-associated decline in resistance to S. pneumoniae, young (4 mo) and old (22–24 mo) C57BL/6 mice were fed a diet containing 30-PPM (control) or 500-PPM (supplemented) α-Toc for 4 wk and intratracheally infected with S. pneumoniae. Aged mice fed a control diet were exquisitely more susceptible to S. pneumoniae than young mice. At 2 d postinfection, aged mice suffered 1000-fold higher pulmonary bacterial burden, 2.2-fold higher levels of neutrophil recruitment to the lung, and a 2.25-fold higher rate of lethal septicemia. Strikingly, α-Toc supplementation of aged mice resulted in a 1000-fold lower bacterial lung burden and full control of infection. This α-Toc–induced resistance to pneumococcal challenge was associated with a 2-fold fewer pulmonary neutrophils, a level comparable to S. pneumoniae–challenged, conventionally fed young mice. α-Toc directly inhibited neutrophil egress across epithelial cell monolayers in vitro in response to pneumococci or hepoxilin-A3, an eicosanoid required for pneumococcus-elicited neutrophil trans-epithelial migration. α-Toc altered expression of multiple epithelial and neutrophil adhesion molecules involved in migration, including CD55, CD47, CD18/CD11b, and ICAM-1. These findings suggest that α-Toc enhances resistance of aged mice to bacterial pneumonia by modulating the innate immune response, a finding that has potential clinical significance in combating infection in aged individuals through nutritional intervention.
Streptococcus pneumoniae is a bacterium that typically resides asymptomatically in the nasopharynx, but particularly in older patients it can cause invasive pneumococcal diseases such as pneumonia, meningitis, and bacteremia, resulting in ~1.6 million deaths worldwide annually (1, 2). Immunosenescence, the dysregulation of some aspects of immune responsiveness and overall decline in immunity that occurs with aging, strongly contributes to the increased susceptibility of older people to invasive pneumococcal diseases (3, 4). Several features of the age-related decline in adaptive immunity (5–7) that are critical for protection against S. pneumoniae have been identified, but less is known about potential defects in the innate immune responses. Rapidly responding innate defenses allow for and promote the development of adaptive immune responses; they are also crucial for prevention and initial control of S. pneumoniae infection (8). In fact, innate resistance of mice to this pathogen has been shown to decrease with age (9, 10), and has been associated with increased expression of host proteins that function as pneumococcal receptors during the establishment of infection (11) and defects in cytokine responses by alveolar macrophages (12).
An important innate cell type that plays an initial role in host defense against S. pneumoniae is the neutrophil (polymorphonuclear leukocyte [PMN]). Neutropenic patients are at increased risk for pneumonia (13). PMNs appear in the lung of infected mice within hours of pulmonary challenge (14), and depletion of PMNs increases bacterial burdens in the lungs and decreases survival of S. pneumoniae–infected mice (15, 16). However, although the presence of PMNs is required for clearance of pneumococci, overly exuberant PMN influx into the lung airways can result in tissue destruction, obstruction of gaseous exchange, and lung failure (17). In mice, survival of pneumococcal pneumonia is associated with the resolution of acute PMN-driven pulmonary inflammation (18, 19). Intriguingly, multiple models suggest that aging is associated with an increase in PMN influx into tissues (20, 21). The lungs of normal elderly individuals had increased numbers of PMNs in the respiratory tract (22) and elderly S. pneumoniae patients were reported to have a higher percentage of neutrophilic infiltrates in lung tissue specimens as compared with younger patients (23). Furthermore, in mice, persistent neutrophilic influx into the nasal cavities of aged mice was associated with prolonged colonization of the nasopharyngeal niche with S. pneumoniae (10), suggesting that the increased susceptibility of older patients to pneumococcal pneumonia could be due to overly exuberant recruitment of PMNs to sites of infection.
Acute pulmonary inflammation involves the recruitment of PMNs from the vasculature, into the interstitial space and then across the lung epithelium into the airways (24). Previous studies showed that PMN migration into the lung airways in response to pneumococcal infection required the production of the lipid chemoattractant hepoxilin A3 (HXA3), an eicosanoid derived from arachidonic acid via the action of 12-lipoxygenases (LOX) in lung epithelial cells (25). Importantly, pharmacologic inhibition or genetic ablation of 12-LOX activity dramatically decreased PMN influx into the lungs of S. pneumoniae–infected mice and resulted in uniform survival of mice to an otherwise lethal pneumococcal pulmonary challenge (25).
In addition to chemoattractant production, PMN transepithelial migration involves several adhesion ligand–receptor interactions. During PMN basal to apical transepithelial migration in response to the chemoattractant fMLF, migration is initiated by the interaction of β-2 integrins (CD18/CD11b) on PMNs with uncharacterized receptors on the basolateral face of the epithelium (26, 27) and is then facilitated by CD47, a glycoprotein expressed on the epithelium and PMNs (28, 29). Given that PMNs must release from the mucosal surface to migrate to distal sites of infection, the regulation of adhesiveness is central for transmigration. For example, once PMNs breach the epithelial barrier, the antiadhesive epithelial surface molecule CD55, also known as decay-accelerating factor, is thought to promote the release of the PMNs to the apical space (30). Blocking Abs directed against CD18, CD47, and CD55 inhibit HXA3-mediated PMN migration across polarized respiratory epithelial monolayers (31).
Vitamin E, an antioxidant for which older people are at risk for inadequate intake (32, 33), has potent immunoregulatory activities (34). Vitamin E deficiency impairs humoral and cell-mediated immune responses (34, 35). There are eight naturally occurring forms of vitamin E and dietary supplementation of the most bioavailable form (36), α-tocopherol (α-Toc), was shown to enhance adaptive immune responses, particularly in older people (37–39). Less is known about the effects of α-Toc on innate immunity (36, 40), but α-Toc treatment of mice prevents lung injury in response to LPS by reducing PMN migration into the lung airway space (41). α-Toc can decrease the production of 12-LOX metabolites by diminishing 12-LOX protein expression in pulmonary cells (42). In addition, α-Toc exposure of cells in vitro or oral α-Toc supplementation of healthy adults reduces the expression of CD11b/CD18 on PMNs (43), indicating that α-Toc is an attractive candidate to therapeutically regulate PMN movement. Unfortunately, the effects of α-Toc on innate immunity and inflammation during bacterial infection remain unexplored. The objective of this study was to test the potential of α-Toc in mitigating the age-associated decline in resistance to pneumococcal pneumonia and to gain insight into its underlying mechanism.
Materials and Methods
Mice
Young (2 mo) and aged (22–26 mo) male C57BL/6 mice were purchased from Charles River Laboratories and the National Institute on Aging colonies, respectively. Mice were maintained in a specific-pathogen free facility at Tufts University, and all procedures were performed in accordance with Institutional Animal Care and Use Committee guidelines. Mice displaying any significant signs of pathology, including tumors, enlarged spleens, or visible skin lesions, were excluded from the study.
Animal diets and in vivo α-Toc supplementation
Mice were fed water ad libitum and either a standard chow or a semisynthetic diet for 4 wk prior to infection. For the standard chow, autoclaved Harlan Teklad 7012 mouse chow was used. For in vivo α-Toc supplementation, mice were provided with semisynthetic nutritionally balanced diets (Custom Animal Diets, Bangor, PA) containing 30 (sufficient) or 500 (supplemented) parts per million (ppm) d-α-tocopheryl acetate (DSM Nutritional Products, Parsippany, NJ) as described previously (38). This supplementation level was previously shown to mimic the effects of vitamin E on immune responses in older humans consuming 200 IU/d vitamin E (39).
Bacteria
Midexponential growth phase aliquots of S. pneumoniae TIGR4 strain (serotype 4), grown at 37°C in 5% CO2 in Todd-Hewitt broth (BD Biosciences, San Jose, CA) supplemented with 0.5% yeast extract and Oxyrase (Oxyrase, Mansfield, OH), were frozen at −80°C in the growth media with 25% (v/v) glycerol. Prior to use, bacterial aliquots were thawed on ice, washed once, and diluted in PBS to the appropriate concentrations. Bacterial titers were then confirmed by plating on Tryptic Soy Agar plates supplemented with 5% sheep blood agar (Northeast Laboratory Services, Winslow, ME).
Murine infections
To initially compare the response of young or aged mice to a high-dose challenge that is capable of causing a lethal infection in a small fraction of young mice, young and aged mice were challenged intratracheally with 1–2 × 106 CFU of S. pneumoniae (Figs. 1, 2) in 50 µl PBS as described previously (25). After we determined that a large fraction (≥50%) of aged mice suffered lethal infection after this pneumococcal challenge, we used a low-dose challenge of 2 × 104 CFU that permitted the survival of most aged mice (Figs. 3, 4). “Uninfected” mice received PBS only. For enumeration of bacterial numbers, the mice were euthanized at day 2 postinfection. The lungs and brains were aseptically removed and homogenized in sterile PBS for 30 s. Bacterial spread into the blood was followed over time by collecting 10-µl blood samples from the lateral vein of mice every 24 h postinfection for 2 d. Dilutions of each sample were then prepared in sterile PBS and plated on blood agar plates. After pulmonary challenge, mice were monitored daily for weight loss and signs of sickness for 7 d.
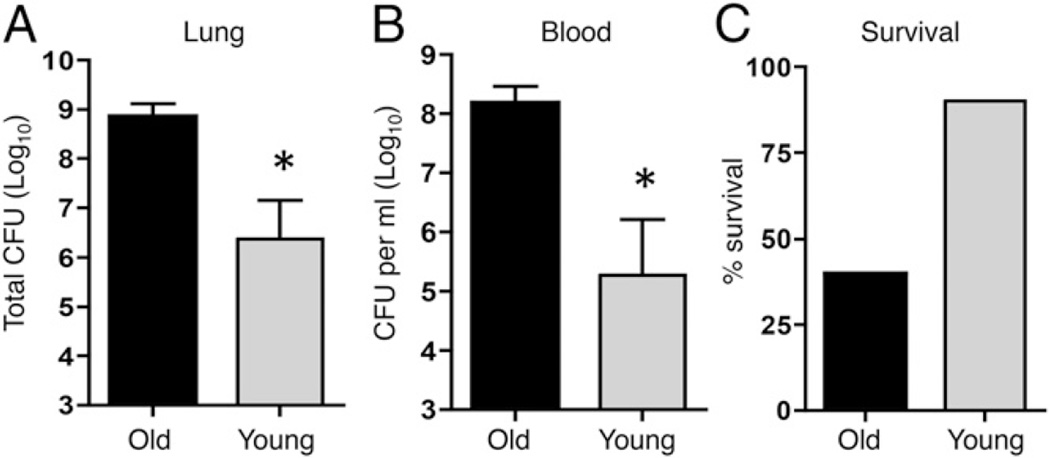
FIGURE 1.
Aged mice are more susceptible to pneumococcal infection. Aged (black bars) and young (gray bars) male mice were infected with 106 CFU S. pneumoniae and bacterial burdens in the lungs and blood (A and B) as well as survival (C) were assessed 48 h postinfection. Combined data from two separate experiments (n = 6 mice per group) are shown. *p < 0.05 indicates means are significantly different by Mann–Whitney test.
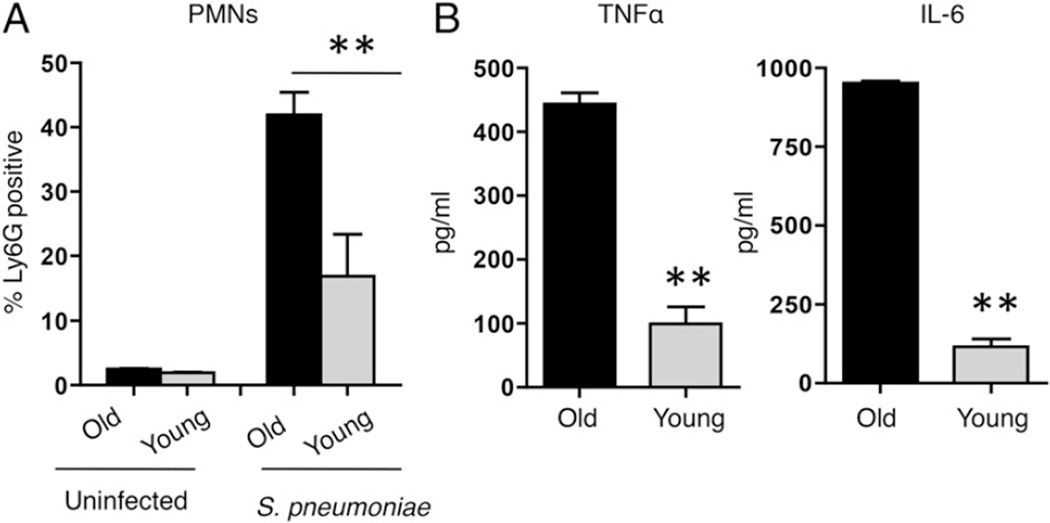
FIGURE 2.
Aged mice suffer higher levels of pulmonary inflammation upon S. pneumoniae challenge. PMN recruitment (A) and cytokine levels (B) in the lungs of aged (black bars) and young (gray bars) mice were measured 48 h after intratracheal challenge with 106 CFU S. pneumoniae. Combined data from two separate experiments (n = 6 mice per group) are shown. ** p < 0.001 indicates means are significantly different by Mann–Whitney U test.
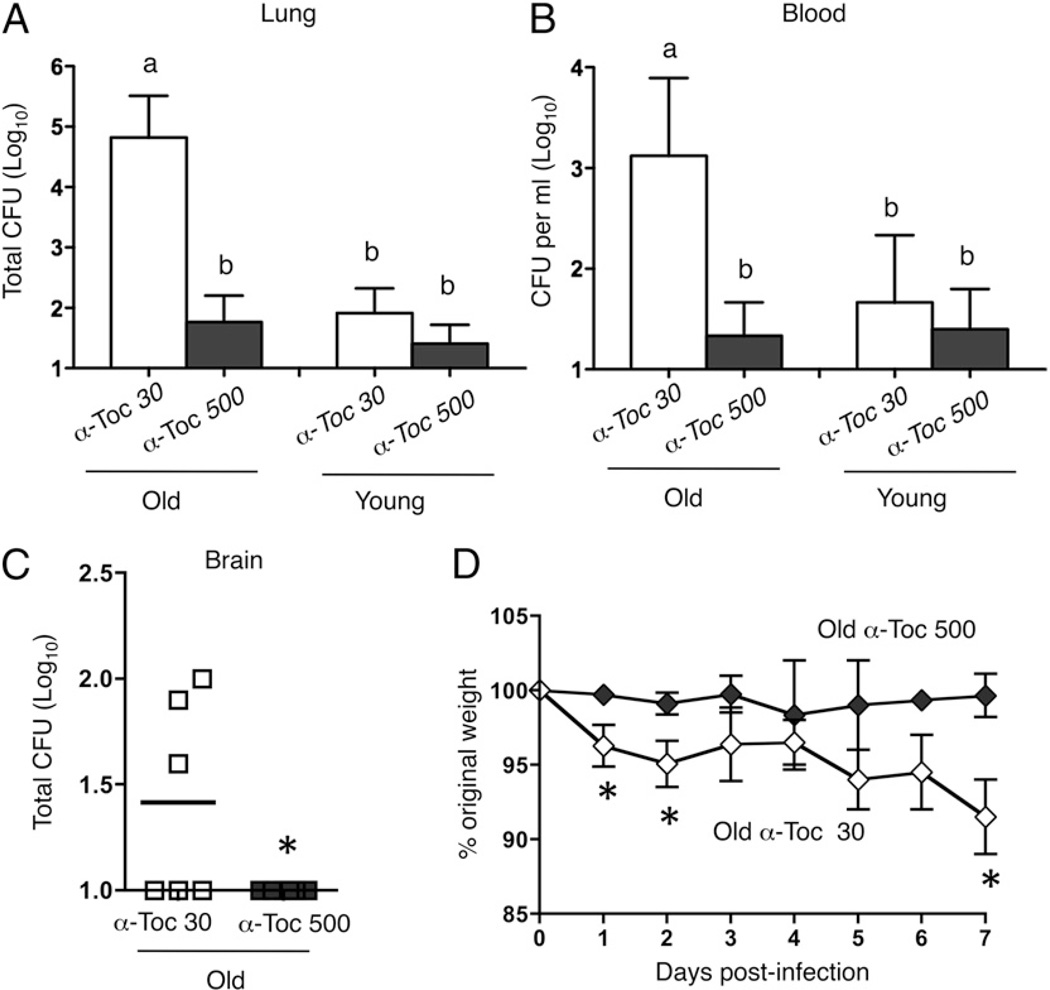
FIGURE 3.
α-Toc increases resistance of aged mice to pneumococcal infection. Mice were fed a control α-Toc 30 (white symbols) or α-Toc–supplemented (α-Toc 500; black symbols) diet for 1 mo and then infected with 104 S. pneumoniae. The bacterial loads in the lung (A), blood (B), and brain (C) were determined 48 h postinfection, and the weights (D) were measured daily for 7 d. (A and B) Combined data from four separate experiments (n = 12 mice per group) are shown. Groups were analyzed using a two-way ANOVA followed by Kruskal–Wallis test, and groups marked “a” are significantly (p < 0.05) different from those marked “b,” whereas groups marked “b” are statistically indistinguishable from each other. (C and D) Combined data from two separate experiments (n = 6 mice per group) are shown. *p < 0.05 indicates means are significantly different by Mann–Whitney U test.
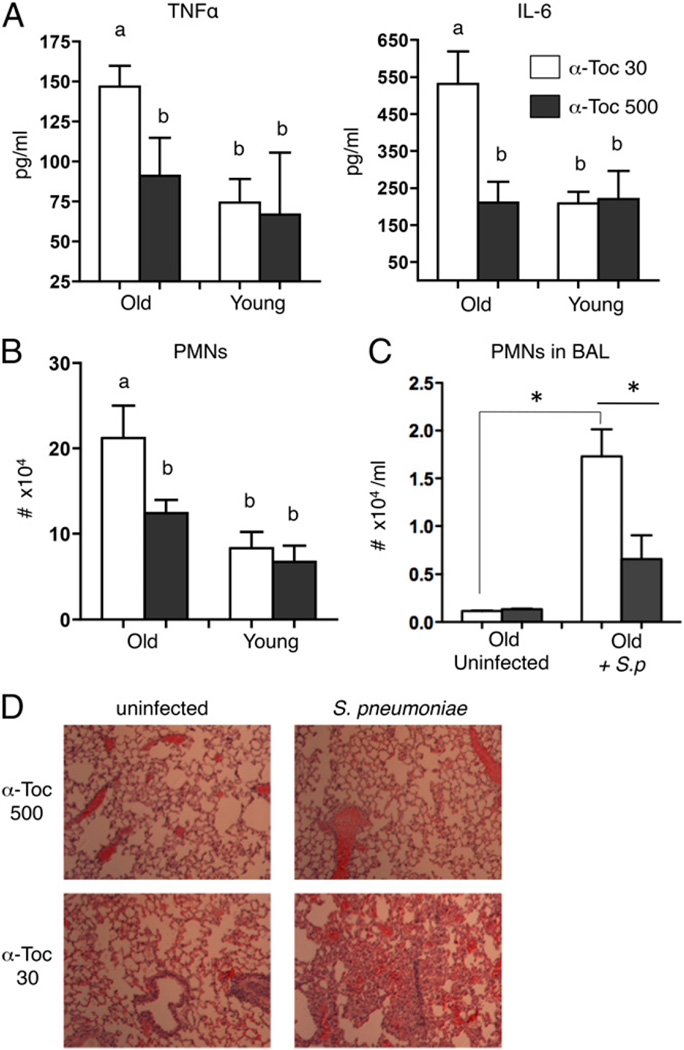
FIGURE 4.
α-Toc supplementation abolishes pulmonary inflammation in aged mice after S. pneumoniae infection. Young and aged mice fed a control α-Toc 30 (white bars) or α-Toc–supplemented (α-Toc 500; black bars) diet were infected intratracheally with 104 S. pneumoniae and inflammatory cytokine levels in lung homogenates (A) as well as the total number of PMNs (B) (Ly6G+) in the lungs were compared 48 h postinfection. Pooled data from four separate experiments (n = 12 per group) are shown, and were analyzed by two-way ANOVA followed by Kruskal–Wallis test. Groups marked “a” are significantly (p < 0.001) different from those marked “b,” whereas groups marked “b” are statistically indistinguishable from each other. (C) The number of PMNs in the bronchoalveolar lavage fluid of aged mice was also enumerated. (D) The lungs were also sectioned stained with H&E and examined by light microscopy. Original magnification ×10. Pooled data from two separate experiments (n = 6 per group) are shown. *p < 0.05 indicates means are significantly different by Mann–Whitney U test.
Isolation of cells from the lungs and airway spaces
Mice were euthanized 2 d postinfection and the lungs were removed, minced into small pieces, and then digested for 1 h with 1 mg/ml Type II collagenase (Worthington, Lakewood, NJ) and 50 U/ml DNase (Worthington). Single-cell suspensions were obtained by mashing the digested lungs through sterile mesh screens using the plunger of a 3-ml syringe. To collect the cells infiltrating the airway spaces, the bronchoalveolar lavage fluid was obtained by washing the lungs with PBS. RBCs were removed from cell suspensions by treatment with a hypotonic lysis buffer (Lonza, Allendale, NJ). Cells were analyzed using flow cytometry.
Histology
For histologic analysis, whole lungs were harvested from aged mice on α-Toc–sufficient or α-Toc–supplemented diet 2 d postinfection and fixed in 10% buffered formalin (Sigma-Aldrich, St. Louis, MO). The tissues were then embedded in paraffin, sectioned at 5 µm, and stained with H&E at the Animal Histology Core at Tufts University. Three whole lung sections from three mice per group were analyzed using a Nikon eclipse TE2000-U microscope.
Cytokine ELISAs
Two d postinfection, the lungs were harvested and homogenized in sterile PBS, and the resulting supernatants were used to measure IL-6 and TNF-α concentrations using the murine IL-6 and TNF-α ELISA kits (eBioscience, San Diego, CA), respectively, following the manufacturer’s protocol.
Migration of human neutrophils across epithelial cell monolayers
As the first step to translate the results to humans and to determine the underlying mechanism of α-Toc–induced effects, in vitro studies were conducted using human neutrophils and human pulmonary derived epithelial cell line. Human pulmonary mucoepidermoid carcinoma–derived NCI-H292 (H292; ATCC, Manassas, VA) cells were grown and polarized on the underside of collagen-coated Transwell filters (0.33 cm2; Corning Life Sciences, Tewksbury, MA) in RPMI 1640 medium (ATCC) with 2 mM l-glutamine, 10% FBS (Invitrogen, Grand Island, NY), and 100 U penicillin–streptomycin (H292 media) as described previously (25). To isolate human PMNs, whole blood was obtained from healthy human volunteers. The volunteers ranged in age from 22 to 55 y; 60% were male and 40% were female; and 34% were white, 44% were Asian, and 22% were considered “other.” Subjects were recruited through the Department of Molecular Biology and Microbiology, Tufts University School of Medicine. Volunteers were asked a set of questions prior to donating blood. Individuals who were pregnant, taking medication, or reporting symptoms indicative of infection within the prior 3 wk were excluded from the study. Blood was drawn using acid citrate–dextrose as an anti-coagulant, and PMNs were then enriched using a 2% gelatin sedimentation technique as described previously (44), which allows for rapid isolation of functionally active PMNs at 90% purity. All study procedures were approved by Tufts Medical Center Human Investigation Review Board. All subjects signed a board-approved consent form prior to enrollment in the study.
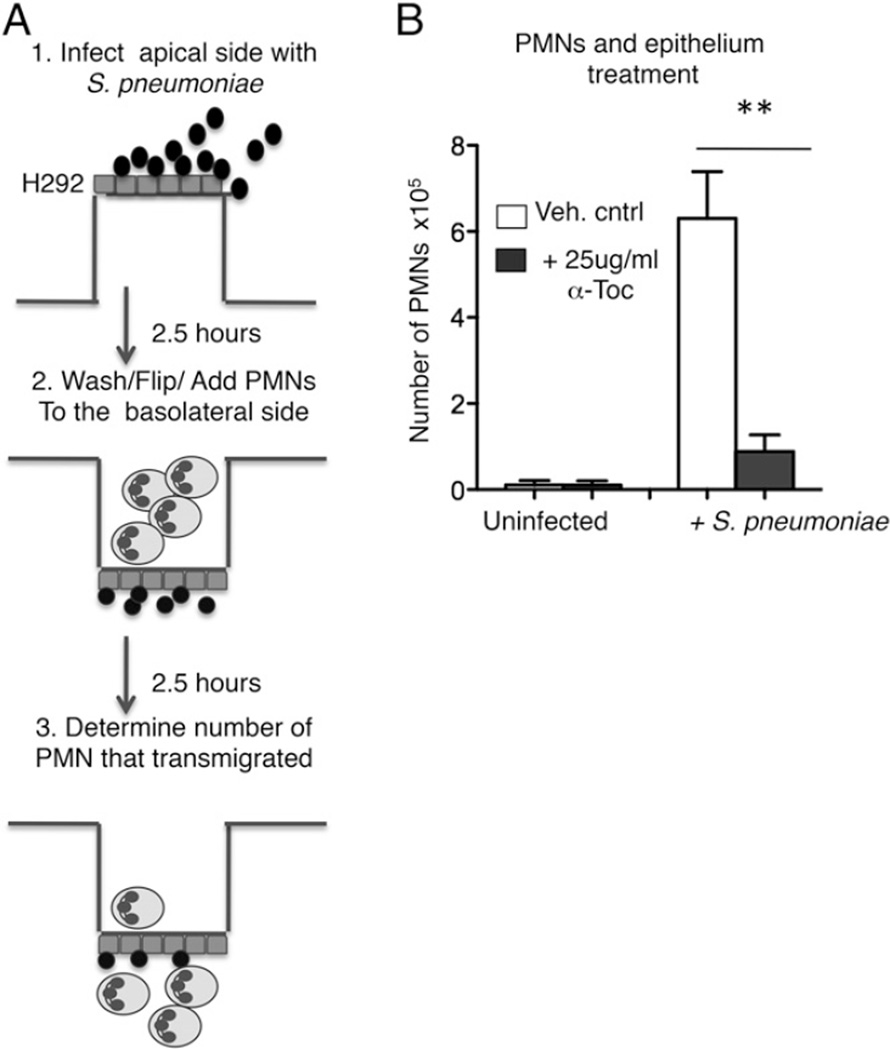
The PMN trans-epithelial migration assay was performed using a previously described protocol (25). In summary, epithelial monolayers grown on inverted Transwell filters (Fig. 5A) were washed and equilibrated in HBSS for 30 min. The apical side of monolayers were infected with 25 µl of pneumococci at a multiplicity of infection of 5 or were treated with HBSS (uninfected) only; 2.5 h after treatment, the monolayers were washed and flipped over into 24-well plates. Six hundred microliters of HBSS was added to the lower (apical) chamber and 100 µl of PMNs (1 × 106) were added to the top (i.e., basolateral) chamber. When indicated, 10−8 µM formylated-Met-Leu-Phe (fMLF) or 200 ng/ml hepoxilin A3 (Enzo, Farmingdale, NY) was added to the bottom (apical) chamber of uninfected monolayers. Migration was allowed to occur at 37°C/ CO2 for 2.5 h, unless indicated otherwise. PMNs that transmigrated into the lower apical chamber were measured by the myeloperoxidase (MPO) ELISA (25).
FIGURE 5.
α-Toc reduces human PMN transepithelial migration in response to pneumococcal infection. (A) Outline of the in vitro migration assay. (B) Polarized H292 epithelial cells were pretreated overnight, whereas PMNs (which are short-lived cells) were pretreated for 1 h with α-Toc or vehicle control (Veh. cntrl; see Materials and Methods). The cells were then washed to remove any free α-Toc before the start of the migration assay. The number of PMNs that migrated from the basolateral to the apical side in response to pneumococcal infection (+S. pneumoniae) or media only (uninfected) were measured by MPO ELISA. Representative data from one of three separate experiments performed, where each condition was tested in triplicate per experiment, are shown. **p < 0.001 indicates means significantly different by unpaired Student t test.
In vitro α-Toc supplementation of epithelial cells and PMNs
A α-Toc stock solution (30 mg/ml) was prepared by dissolving d-α-to-copherol (ADM, Decatur, IL) in ethanol (38). To enhance cellular uptake (38), the stock solution was then diluted in FBS to a final concentration of 2 mg/ml and incubated for 30 min at 37°C with gentle intermittent vortexing. For pretreatment of PMNs with α-Toc, freshly isolated human PMNs (2 × 107 cells/ml) were incubated with α-Toc at the indicated final concentrations, or 0.06% ethanol as vehicle control, in HBSS lacking calcium and magnesium on ice for 1 h (a duration found in pilot experiments to be sufficient to significantly diminish transmigration but short enough to permit experimentation with these short-lived cells; data not shown), washed, and then added to the Transwells for the migration assay (detailed below). For endothelial cells and lymphocytes, intracellular levels of α-Toc peak after ~20 h incubation. Hence, for pretreatment of epithelial cells with α-Toc, both the upper and lower chambers of Transwells seeded with polarized human lung epithelial cells (2 × 106 cells/insert) were incubated overnight at 37°C/5% CO2 in H292 media supplemented with the indicated (Figs. 5, 6, 7C) final concentrations of α-Toc or vehicle control. The final concentrations of α-Toc in epithelial cells or PMNs were not determined, but were sufficient in both cell types to result in observable effects (see Results). The inserts were then washed to remove the excess α-Toc that was not incorporated by the cells prior to starting the migration assay. For the combined treatment, each cell type was treated with α-Toc as outlined above, and the cells were then combined in the migration assay (discussed next).
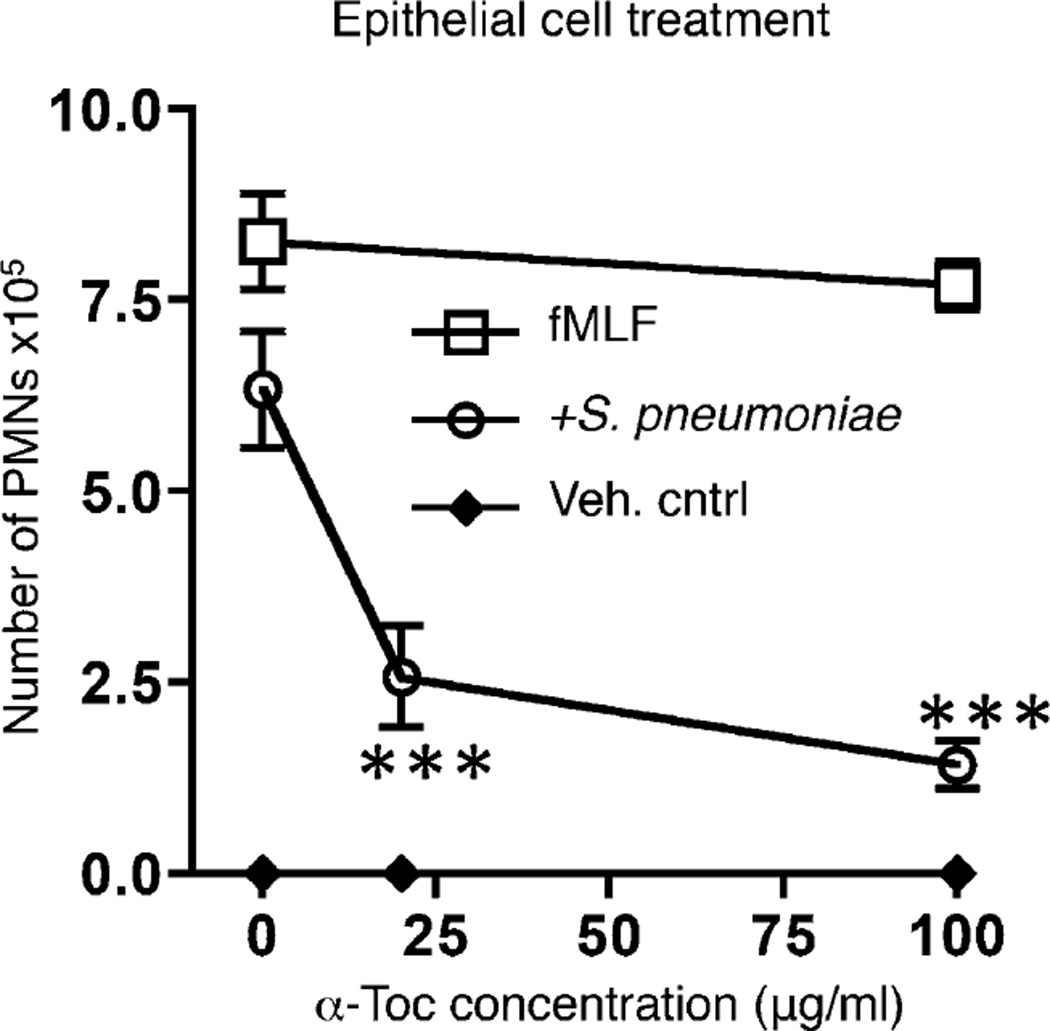
FIGURE 6.
α-Toc pretreatment of the epithelium is sufficient to decrease PMN trans-migration in response to pneumococcal infection. Polarized H292 epithelial cells were pretreated overnight with α-Toc at the concentrations indicated on the x-axis (see Materials and Methods). The number of PMNs that migrated from the basolateral to the apical side in response to pneumococcal infection (circles), fMLF (squares) or media only (Veh. cntrl; diamonds) were measured by MPO ELISA. ***p < 0.0001 indicates means significantly different from no α-Toc treatment within the same group unpaired Student t test. Representative data from six separate experiments, where each condition was tested in triplicate per experiment, are shown.
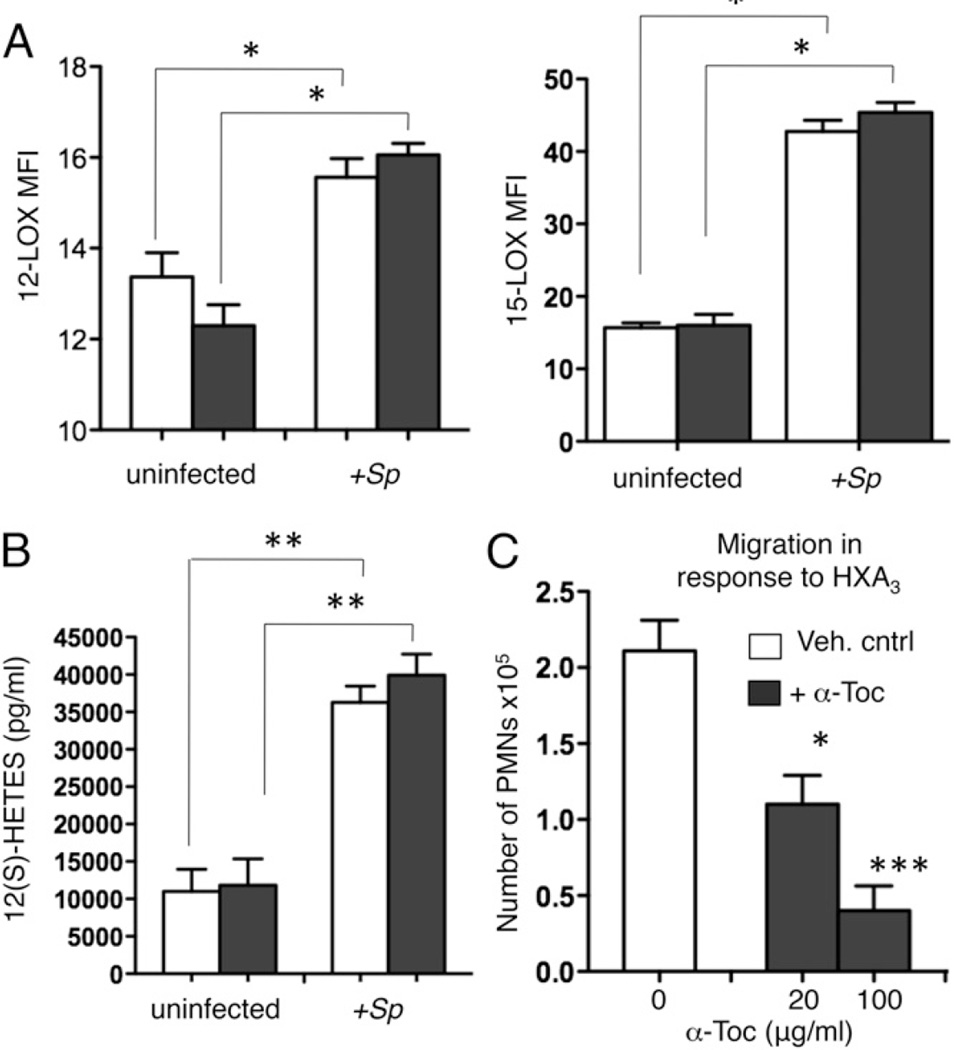
FIGURE 7.
α-Toc has no effect on 12-lipoxygenase levels or activity. Epithelial cells were treated overnight with vehicle control (Veh. cntrl; white bars) or α-Toc (black bars) at 100 µg/ml or the indicated concentrations. (A) 12- and 15-lipoxygenase protein levels were then assessed by intracellular staining followed by flow analysis of H292 cells treated with S. pneumoniae or left uninfected. Representative from one of four separate experiments, in which each condition was tested in triplicate per experiment, are shown. *p < 0.05 indicates means significantly different by unpaired Student t test. (B) The amount of the metabolite 12(S)-HETES released into the supernatants was measured by ELISA. **p < 0.001 by unpaired Student t test. Representative data from one of three separate experiments, in which each condition was tested in triplicate per experiment, are shown. (C) PMN migration in response to HXA3 (200 ng/ml) added to the lower chamber (apically) was measured by MPO ELISA. Representative data from one of two separate experiments, in which each condition was tested in triplicate per experiment, are shown. *p < 0.05, ***p < 0.0001 indicates means significantly different from vehicle control by unpaired Student t test.
12-(S)-HETE ELISA
Cell-free culture supernatants collected from the lower chamber (Apical side) at the end of the migration assay with human neutrophils were tested for hydroxyeicosatetraenoic acid (12-[S]-HETE) levels by ELISA (Enzo, Farmingdale, NY) following the manufacturer’s instructions.
Effect of α-Toc on bacterial viability and binding
To test the effect of α-Toc on bacterial viability, S. pneumoniae TIGR4 strain (serotype 4) were grown at 37°C in 5% CO2 in THY media supplemented with vehicle control or varying concentrations of α-Toc for 2.5 h (i.e., the duration of the infection in the Transwell assay). The bacterial cultures were then washed with PBS, diluted, and plated on blood agar plates for enumeration of live bugs. To test the effect of α-Toc on pneumococcal binding to lung epithelial cells, adherent layers of H292 cells grown in 24-well plates were infected with the bacteria at a multiplicity of infection of 10 for 2.5 h. The cells were then washed extensively with PBS to remove unbound bugs and then treated with trypsin and EDTA to lift off of the adherent wells. To measure the amount of bound bacteria, dilutions were then plated on blood agar plates.
Assessment of cell surface markers and intracellular 12-LOX by flow cytometry
Abs specific for murine Ly6G (clone 1A 8) and murine TCR-β (H57-597) were purchased from BD Biosciences (San Jose, CA). Anti-human CD11b (ICRF44), CD18 (TS1/18), CD55 (JS11), CD47 (CC2C6) Abs were purchased from BioLegend (San Diego, CA). ALOX 15/15-lipoxygenase-1 polyclonal Ab was purchased from Abs-online (Atlanta, GA). 12-LOX (anti platelet type p-12-LOX) mAb (C-5) was purchased from Santa Cruz (Dallas, TX). For surface staining, cells were harvested, stained, and analyzed. For detection of intracellular 12- and 15-LOX, cells were fixed and permeabilized using the Cytofix/Cytoperm Kit (BD Biosciences, San Jose, CA) prior to staining. Fluorescence intensities of live cells (at least 25,000 events) were measured on a FACSCalibur and analyzed using FlowJo.
Statistics
Statistical analysis was performed using Prism5 for Macintosh (Graph Pad, San Diego, CA). For the in vitro migration and cell surface phenotype studies, both paired and unpaired Student t test were used, as indicated in the figure legends. Mann–Whitney U test was used for the animal studies in comparing the differences between old and young mice on standard chow (Figs. 1, 2). In comparing the interaction of age and diet and their impact on susceptibility (Figs. 3, 4), data were analyzed with two-way ANOVA with significant differences between means evaluated by Kruskal-Wallis test. Bar graphs represent the mean values ± SEM. All comparisons with p < 0.05 were considered significantly different.
Results
Aged male mice are highly susceptible to S. pneumoniae infection
Previous investigations primarily used female BALB/c mice to model the increased susceptibility to S. pneumoniae that occurs with aging (9, 12), but male C57BL/6 mice have been used previously to characterize the effects of α-Toc supplementation of the diet on aging (38). To confirm that we can use male C57BL/6 mice to model the age-related susceptibility to S. pneumoniae lung infection, groups of young (2 mo) or aged (22–26 mo) mice were infected intratracheally with 106 CFU of S. pneumoniae TIGR4 strain. We found that aged mice were highly susceptible to pneumococcal infection. At 48 h postinfection, the aged mice suffered more than 1000-fold higher bacterial burdens in their lungs and blood compared with their young counterparts (Fig. 1A, 1B). While 90% of young mice were still alive at this time point, less than half of the aged mice survived (Fig. 1C). These findings confirm that experimental infection of aged C57BL/6 mice models the well-known susceptibility of older humans to invasive pneumococcal disease.
Aging is associated with increased recruitment of PMNs during S. pneumoniae infection
The death of the aged mice within 2–3 d following pneumococcal infection suggested a defect in innate immune defense. Because PMNs are important innate immune cells with a crucial role during S. pneumoniae infection (15, 16), we compared the recruitment of these cells into the lungs of aged or young mice. The percentage of PMNs was comparable between uninfected controls of both ages, but upon infection, the aged mice had a 20-fold increase in PMNs recruited into the lungs compared with a 7-fold increase in the young mice (Fig. 2A). Pneumococcal infection was accompanied by significantly elevated levels of inflammatory cytokines (TNF-α, IL-6) in the lung homogenates of aged mice compared with young counterparts (Fig. 2B). These results show that compared with young mice, aged mice have a greater degree of inflammatory response to S. pneumoniae infection.
α-Toc supplementation boosts the resistance of aged mice to invasive S. pneumoniae infection
To test whether α-Toc can mitigate the susceptibility of aged mice to pneumococcal infection, young or aged mice were fed a diet containing 30 ppm (control) or 500 ppm (supplemented) α-Toc for 4 wk. This dose is roughly equivalent to a daily supplement of 200 IU vitamin E in humans (39), which has been shown to be substantially below the recommended upper tolerance limit for this vitamin and to enhance the immune response of older humans (39, 45). Previous work showed that the α-Toc–supplemented diet significantly elevated tissue levels of α-Toc in both young and old mice from a similar basal level, and that these elevated α-Toc concentrations were statistically indistinguishable between the two age groups (46). The mice were then intratracheally challenged with 104 CFU S. pneumoniae TIGR4, a (low) dose that we found empirically not to cause a rapidly lethal infection (e.g., see Fig. 3), thus permitting the survival and productive analysis of a significant fraction of aged mice. Similar to what we previously observed after high dose infection, aged mice on the control diet were significantly more susceptible to the infection than young mice on the same diet. Strikingly, α-Toc supplementation completely reversed the age-associated innate susceptibility to pneumococcal lung challenge (Fig. 3). Compared with aged mice on the control diet, supplemented aged mice had a 1000-fold fewer bacteria in their lungs (Fig. 3A), a bacterial burden statistically indistinguishable from that of young mice fed on the control or α-Toc–supplemented diet. Importantly, α-Toc supplementation also drastically decreased the systemic spread of the bacteria into the circulation of infected aged mice (Fig. 3B). Furthermore, while more than 50% of aged mice on the control diet experienced bacterial spread to the brain, the α-Toc–supplemented counterparts (Fig. 3C) and the young mice (data not shown) did not suffer brain infection. The aged mice on the control diet lost 10% of their original weight by day 7 (when the experiment was terminated), whereas those supplemented with α-Toc suffered no weight loss (Fig. 3D). Although α-Toc supplementation in young mice resulted in somewhat lower bacterial burdens in the lung and blood, the differences did not reach statistical significance. These results demonstrate that α-Toc supplementation of aged mice increases resistance to invasive pneumococcal disease.
α-Toc supplementation dramatically reduces lung inflammation in S. pneumoniae–infected aged mice
To improve the understanding of the mechanism of α-Toc–mediated resistance of aged mice to S. pneumoniae, we assessed the effect of α-Toc supplementation on lung inflammation of aged mice. Similar to our observation in aged mice challenged with high (106 CFU) dose of S. pneumoniae TIGR4, low-dose (104 CFU) challenge resulted in levels of TNF-α and IL-6 that were dramatically elevated compared with young mice (Fig. 4A). Strikingly, this aged-related difference was abolished by α-Toc supplementation. The age-associated higher production of proinflammatory cytokines coincided with increased numbers (Fig. 4B) and percentages (data not shown) of PMNs in lung homogenates of aged mice, all of which were reduced by α-Toc supplementation to the levels observed in young mice (Fig. 4B). The mitigating effect of α-Toc supplementation on inflammation was restricted to aged mice because supplementation of young mice had no significant effect on pulmonary PMNs.
Histologic analysis of lung sections showed that infected aged mice on the control diet suffered massive inflammatory cellular influx into the airways, but the pulmonary spaces of α-Toc–supplemented mice were clear and normal lung architecture was preserved (Fig. 4D). In fact, after pneumococcal challenge, aged mice fed the control diet but not those fed the α-Toc–supplemented diet showed a significant increase in bronchoalveolar lavage fluid PMNs upon infection compared with baseline (uninfected) levels (Fig. 4C). α-Toc supplementation resulted in a 3-fold reduction in the number of PMNs recruited into the airways upon S. pneumoniae challenge (Fig. 4C). These findings indicate that α-Toc supplementation reduces lung inflammation, and in particular the influx of inflammatory PMNs into lungs of aged mice during invasive pneumococcal infection.
α-Toc supplementation reduces PMN migration across lung epithelial cells in response to S. pneumoniae infection
To test whether the reduction of PMN influx into the lungs induced by α-Toc supplementation could be due to a direct effect on the migration process, rather than simply a reflection of reduced bacterial burdens, we modeled PMN movement across the lung epithelium in vitro using PMNs isolated from young (between 22 and 55 y old), healthy human donors (Fig. 5A). We used young donors because previous studies showed that vitamin E supplementation in people is equally efficient at altering the phagocytic and adherent characteristics of isolated PMN from aged or young individuals (47). In addition, we chose 20 µg/ml α-Toc because this concentration reflects the average plasma α-tocopherol levels measured in humans taking a daily supplement of 200 IU vitamin E (39). This concentration of α-Toc had no effect on bacterial viability (Supplemental Fig. 1A) or the ability of pneumococci to bind lung epithelial cells (Supplemental Fig. 1B).
As shown previously (25), apical S. pneumoniae infection of polarized monolayers of H292 human epithelial cells grown on Transwell filters elicited robust basolateral to apical PMN transmigration (Fig. 5B). Combined pretreatment of both the epithelial layer and PMNs with α-Toc (see Materials and Methods) resulted in a significant 2–3-fold reduction in PMN movement in response to S. pneumoniae (Fig. 5B), indicating that α-Toc may modulate pulmonary inflammation through a direct effect on the interaction of PMNs and lung epithelium.
α-Toc treatment of the epithelium is sufficient to decrease PMN transmigration
To determine whether the effect of α-Toc on PMN transmigration was due to an effect on the epithelial cells, polarized monolayers of H292 cells were treated with varying concentrations of α-Toc overnight then washed thoroughly before the addition of S. pneumoniae and (untreated) PMNs (see Materials and Methods). This pretreatment of the epithelium in isolation was sufficient to reduce PMN migration in response to pneumococcal infection in a dose dependent manner (Fig. 6, open circles). The effect of α-Toc was specific to migration elicited by pneumococcal infection, because the response to the positive control chemotactic peptide fMLF was not altered (Fig. 6, open squares).
α-Toc treatment of cultured pulmonary epithelium does not alter 12/15-LOX levels or activity and reduces PMN transmigration across polarized epithelial monolayers in response to exogenous HXA3
Because PMN transepithelial migration in response to S. pneumoniae infection is dependent on the production of the eicosanoid HXA3 by 12-LOX enzymes in the lung epithelium (25), we tested the effect of α-Toc on this pathway. Humans express several functional 12-LOX isoforms 15-LOX-1, 12R-LOX, and the platelet-type p12-LOX) (48). Of those, 15-LOX-1 and p12-LOX were shown to be expressed in epithelial cells and to increase upon pneumococcal infection (25). Upon flow cytometric detection of these enzymes inside permeabilized H292 cells, we found that the protein levels of both 15- and 12-LOX increased upon S. pneumoniae infection of H292 cells, consistent with previous findings (25) (Fig. 7A). Furthermore, the activity of the above enzymes, as measured by the production of the 12-LOX-dependent metabolite 12(S)-HETE was significantly increased upon infection (Fig. 7B). Interestingly, pretreatment of the epithelium with α-Toc had no effect on protein levels of either 12-LOX or 15-LOX in the H292 cells, detected either by flow cytometry as above or with Western blot analysis of cell lysates (data not shown; Fig. 7A). Furthermore, pretreatment with α-Toc did not affect the levels of 12(S)-HETE in uninfected or in pneumococcal-infected H292 cells (Fig. 7B).
Given that α-Toc did not alter the protein levels or the apparent activity of the enzymes required for HXA3 production, we tested whether pretreatment of epithelium with α-Toc might decrease PMN transmigration across monolayers in response to exogenously added HXA3. H292 cells were mock-treated or treated with α-Toc, and the transepithelial migration of PMNs from the basolateral side in response to HXA3 added to the apical chamber was measured. α-Toc treatment of the epithelium resulted in a dose-dependent reduction in the number of PMNs crossing this barrier in response to exogenously added HXA3 (Fig. 7C).
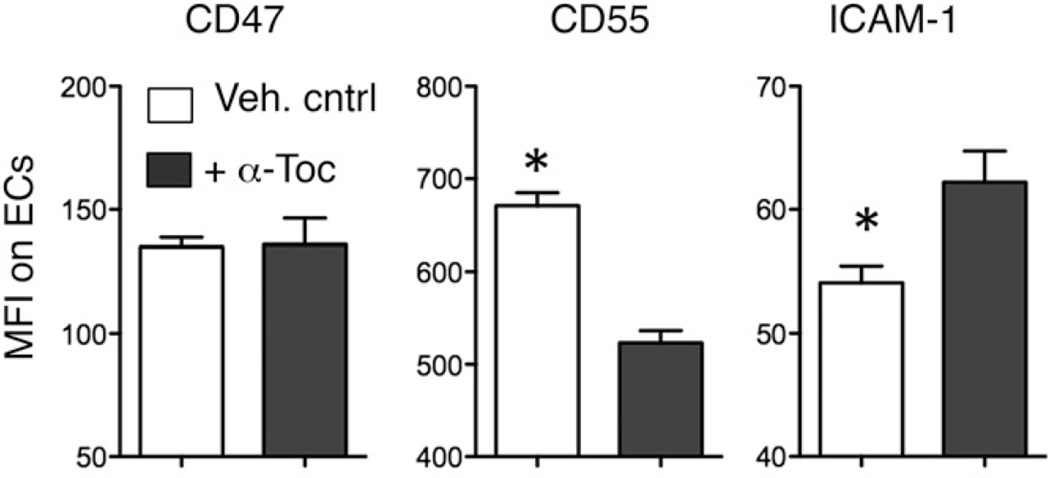
α-Toc treatment of the epithelium alters the expression of epithelial adhesion molecules
PMN migration across epithelial monolayers requires carefully orchestrated PMN interaction with several epithelial adhesion molecules, such as CD47 (28) and ICAM-1 (49), and CD55 (30). For example, the ubiquitously expressed CD47, by interacting with the PMN signal regulatory protein α (SIRPα), can promote PMN transepithelial migration (28, 29), and the apically expressed ICAM-1 can function to promote apical localization of PMNs during the transmigration process. Production of the antiadhesive molecule CD55, sometimes coupled with downregulation of ICAM-1 (50), can facilitate release of PMNs from the epithelial surface as the final step in transmigration (30). We found that whereas CD47 expression remained unchanged upon α-Toc treatment, expression of CD55 decreased and expression of ICAM-1 increased (Fig. 8), consistent with the hypothesis that α-Toc can diminish the release of PMNs from the apical epithelial surface.
FIGURE 8.
α-Toc alters the surface expression of various adhesion molecules required for transmigration on epithelial cells (ECs). Epithelial cells were pretreated overnight with 100 µg/ml α-Toc (dark bars) or vehicle control (Veh. cntrl; white bars). Surface expression of the indicated molecules was assessed by flow cytometry. Mean florescent intensities (MFI) of the various molecules on the surface were then measured. Representative data from one of three separate experiments, in which each condition was tested in triplicate per experiment, are shown. *p < 0.05 indicates means significantly different by paired Student t test.
α-Toc downregulates adhesion molecules on PMNs involved in transepithelial migration
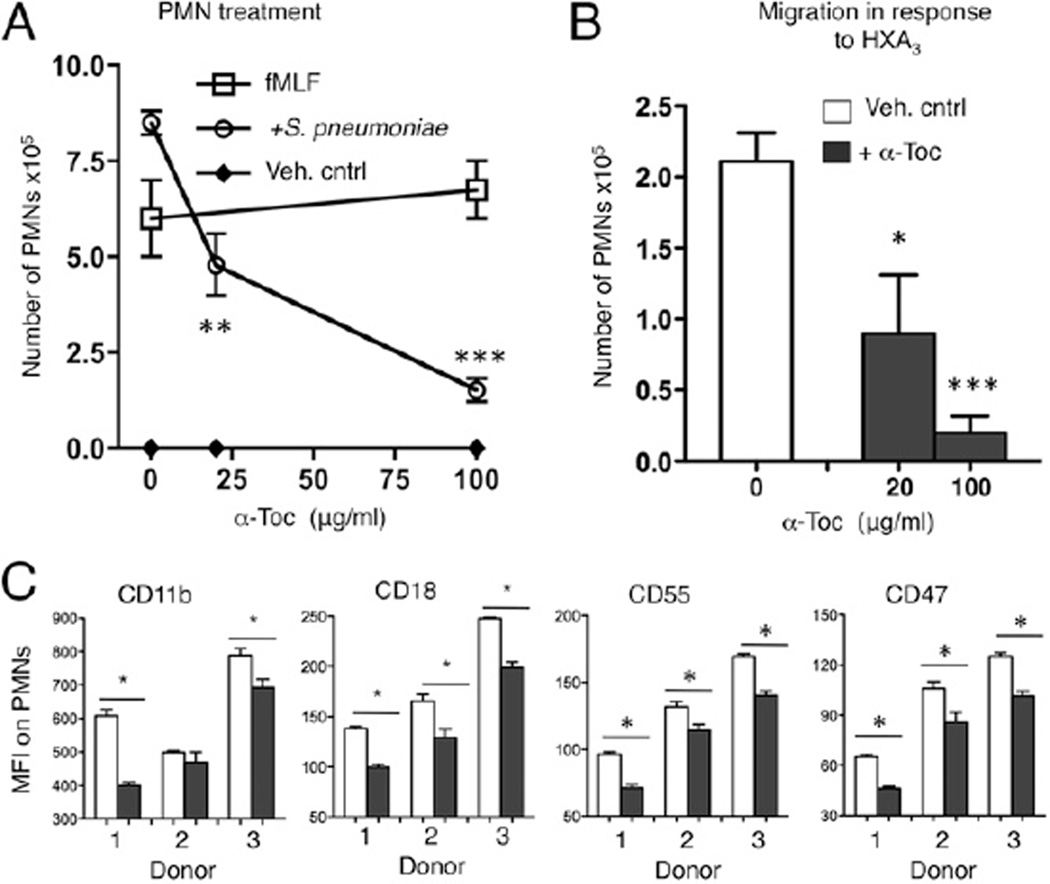
The addition of 20–25 µg/ml α-Toc to PMNs and epithelial cells appeared to have a slightly greater (~5-fold) inhibitory effect on transmigration than the addition of α-Toc to epithelial cells alone did (~3-fold; Figs. 5, 6), raising the possibility that α-Toc also influences PMN transmigration across monolayers by altering PMNs. In fact, treatment of only the PMNs with α-Toc (see Materials and Methods) also reduced transepithelial migration in response to pneumococcal infection (Fig. 9A). Inhibition was dose-dependent; at 20 µg/ml, α-Toc reduced migration ~2-fold, whereas 100 µg/ml α-Toc reduced migration ~9-fold. Not only was transmigration in response to S. pneumoniae diminished; as predicted, given that S. pneumoniae induces an HXA3-dependent chemotactic response (25), transmigration in response to HXA3 was also reduced in a dose-dependent manner (Fig. 9B).
FIGURE 9.
α-Toc treatment of PMNs inhibits their transepithelial movement in response to HXA3 and infection by reducing the surface expression of various adhesion molecules required for transmigration. PMNs were pretreated with α-Toc for 1 h at the concentrations indicated on the x-axis. (A) The number of PMNs that migrated from the basolateral to the apical side in response to pneumococcal infection (circles), fMLF (squares), media only (Veh. cntrl; diamonds) or (B) HXA3 were measured by MPO ELISA. **p < 0.001, ***p < 0.0001 indicates means significantly different from no α-Toc treatment within the same group by paired Student t test. Representative data from three separate experiments (A) and two separate experiments (B), in which each condition was tested in triplicate per experiment, are shown. (C) PMNs were pretreated with 100 µg/ml α-Toc (dark bars) or vehicle control (Veh. Cntrl; white bars). Surface expression of the indicated molecules was assessed by flow cytometry. Mean florescent intensities (MFI) of the various molecules on the surface were then measured. PMNs from each donor were tested in three separate experiments. Combined data are shown. *p < 0.05 indicates means significantly different by paired Student t test.
To investigate the mechanism by which α-Toc reduced the ability of PMNs to cross-pulmonary epithelial monolayers, we measured the surface expression of CD18, CD11b, CD47, and CD55, each previously demonstrated to be required for HXA3-mediated transepithelial movement (31). CD18/11b is a PMN-associated β2-integrin that interacts with an unknown epithelial molecule (27). The expression of CD18, CD11b, CD47, and CD55 on the surface of PMNs from three separate donors was determined by flow cytometry after treatment with either α-Toc or vehicle control. Except for Donor 2, PMNs from each of the individuals responded to α-Toc with a significant decrease in CD11b expression (Fig. 9C). The variable CD11b response is consistent with previous studies indicating that the response of PMNs to α-Toc treatment can vary from person to person (51, 52). Strikingly, α-Toc treatment resulted in a significant decrease of CD18, CD47, and CD55 on the surface of PMNs from all donors (Fig. 9C). Given that function-blocking Abs against each of these molecules has been shown to inhibit PMN transmigration across pulmonary epithelium (31), our data suggest that the downregulation of these molecules on the PMN surface contributes to the mitigation of a harmful acute inflammatory process during S. pneumoniae infection.
Discussion
We present a murine pneumococcal lung infection model in which aged mice suffer excessive inflammatory responses after S. pneumoniae lung challenge and fail to control the infection, thus mimicking aspects of S. pneumoniae lung infection in older humans. We also show that α-Toc dietary supplementation of aged mice results in the control of pulmonary infection and prevention of systemic dissemination, likely by targeting the overly exuberant recruitment of neutrophils into the lungs. Although several studies have examined the role of vitamin E in reversing the impairment of immune cell functions that accompany aging (36), to our knowledge, this study is the first to demonstrate that dietary α-Toc supplementation dramatically modulates pulmonary inflammation and significantly enhances resistance to S. pneumoniae infection by aged hosts.
Previous reports indicated that aged mice are exquisitely susceptible to pneumococcal lung infection (9, 12), and we found that aged mice suffered approximately a 1000-fold higher bacterial loads in the lungs and blood than young mice. Dysregulated inflammatory responses and low-grade chronic inflammation that accompany aging are thought to contribute to the increased susceptibility of older adults to infections (4), and aged female BALB/cBy mice have elevated basal levels of inflammatory cytokines such as IL-1β, IL-6, and TNF-α in the lungs (11). We found at 48 h postinfection that IL-6 and TNF-α levels were elevated in the lungs of aged compared with young mice. Interestingly, lower levels of these cytokines in the lungs of aged compared with young mice at 6 h postinfection have been documented previously (12), suggesting that in aged mice, the immediate proinflammatory cytokine response to pneumococcal infection can be initially delayed before an enhanced response occurs as the infection progresses. In addition, we found that aged mice suffered significantly higher numbers of PMNs in their lungs 48 h postinfection, consistent with previous studies (12).
The stronger pulmonary inflammatory response of aged mice likely contributes to poorer control of infection. In fact, administration of TNF-α to young mice mimicked the enhanced susceptibility to S. pneumoniae observed in old mice (9). TNF-α elevated the expression of the platelet-activating factor receptor and the polymeric Ig receptor, two receptors for S. pneumoniae, suggesting that inflammatory signaling promotes an environment conducive to the establishment of infection (3, 9). The higher bacterial loads in the bloodstream of aged mice could simply reflect their enhanced pulmonary pneumococcal burden, but they could also be a result in a breakdown of the epithelial barrier associated with acute inflammation. Although PMNs are required to control S. pneumoniae infection (15, 16), overly exuberant recruitment of PMNs leads to tissue damage and exacerbates disease (18, 19). In addition, PMN migration across the lung epithelium in itself disrupts epithelial integrity and can facilitate breach of this barrier by pneumococci (25).
A key finding of this study is that α-Toc dietary supplementation resulted in 50–500-fold lower bacteria in the lungs and bloodstream after intratracheal challenge of aged mice, completely reversing the age-associated susceptibility to pneumococcal infection. α-Toc–supplemented aged mice also did not suffer bacterial spread to the brain, a potentially important finding given the 30% mortality rate of pneumococcal meningitis (1). α-Toc had no direct effect on bacterial viability or attachment to lung epithelial cells in vitro. Rather, consistent with the hypothesis that the robust acute inflammatory response triggered by S. pneumoniae infection of old mice hinders bacterial clearance, we found that α-Toc supplementation reduced the proinflammatory cytokine and PMN responses to S. pneumoniae infection to levels indistinguishable from those of young mice. Given that it is possible that the diminished inflammatory response in α-Toc–supplemented aged mice was indirect (i.e., simply a reflection of their diminished bacterial burden), we measured the effects of α-Toc on PMN transepithelial migration, a key step in the acute inflammatory response. α-Toc reduced the movement of PMNs across a respiratory epithelial monolayer, indicating that α-Toc directly modulates the inflammatory response to S. pneumoniae infection.
In a mouse model of S. pneumoniae infection, PMN migration across the epithelium into the airways is blocked by chemical inhibition or genetic ablation of the murine 12/15-lipoxygenase 12/15-LOX (25), the enzymes required for the production of the eicosanoid PMN chemoattractant HXA3. α-Toc has been shown to bind to soybean 12-LOX, inhibiting its activity in vitro (53), and to decrease both 12-LOX protein levels and 12(S)-HETE, a downstream metabolite of 12-lipoxygenases, in the lungs of OVA-sensitized BALB/c mice (42). However, we found that α-Toc treatment had no effect on 12-LOX levels or 12(S)-HETE production by cultured respiratory epithelial cells, and exogenous HXA3 did not reverse the inhibitory effect of α-Toc on PMN migration in our model, suggesting that α-Toc alters the responsiveness of PMNs or epithelial cells, or both, to HXA3.
α-Toc, which is incorporated into the cell membrane, has been described to alter the localization and function of several molecules involved in signaling cascades (38, 54). In fact, we found that on both the respiratory epithelium and PMNs, α-Toc influenced the expression of cell surface molecules that are critical in regulating PMN transmigration. α-Toc diminished the epithelial expression of the antiadhesive molecule CD55 and increased the expression of the adhesive molecule ICAM-1 (31). In gut and oral epithelium, CD55 has been postulated to promote the apical release of the PMNs once they breach the epithelial barrier by competing with ICAM-1, which would otherwise retain the PMNs at the apical surface (50). It is tempting to speculate that apical localization of PMNs also provides a strategically placed epithelial defense against S. pneumoniae in the lungs, without triggering the frank inflammatory response that could result in tissue damage and airway obstruction. We also found that α-Toc diminished the PMN surface expression of CD18, CD47, and CD55, which notably have been shown to promote HXA3-mediated PMN migration across the epithelium (31). Interestingly, we found that α-Toc did not alter PMN migration in response to fMLF, which is consistent with previous work indicating that although fMLF is a bacterial derived product that is produced by S. pneumoniae, it does not contribute to pulmonary recruitment of PMNs during pneumococcal infection of mice (55). In addition, fMLF activates G-protein coupled formyl peptide receptors on the surface of PMNs that, when stimulated, result in pleiotropic downstream effects, including the release of free radical and degradative enzymes and enhanced chemotaxis (56). In fact, the latter is in part due to fMLF-mediated increases the expression of CD18, CD47, and CD55 on the PMN surface (31), an effect that can negate the inhibitory effect of α-Toc on the expression of these same adhesion molecules.
In summary, our study shows that α-Toc supplementation reverses the age-driven susceptibility of aged mice to invasive S. pneumoniae infections at least in part by modulating the dysregulated pulmonary recruitment of PMNs that accompanies aging, raising the potential application of this vitamin in combating infections in older people. In addition, the demonstration that α-Toc inhibits HXA3-mediated transepithelial migration, a process implicated in diverse intestinal and respiratory infections (25, 44, 48, 57), as well as in pathologic inflammatory conditions (48, 58), suggests that α-Toc could also form the basis of therapeutic applications against diverse and important human conditions.
Supplementary Material
Acknowledgments
This work was supported in part by Tufts Collaborates Grant M230169 (to A.C., J.M.L., and S.N.M.), U.S. Department of Agriculture Contract 58-1950-0-014 (to S.N.M.), and the A.S.P.E.N. Rhoads Research Foundation 2013 and 2014 Abbott Nutrition grant (to E.N.B.G.).
Abbreviations used in this article
- HETES
hydroxyeicosatetraenoic acid
- HXA3
hepoxilin A3
- LOX
lipoxygenase
- MPO
myeloperoxidase
- PMN
polymorphonuclear leukocyte
- ppm
parts per million
- α-Toc
α-tocopherol
Footnotes
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Chong CP, Street PR. Pneumonia in the elderly: a review of the epidemiology, pathogenesis, microbiology, and clinical features. South Med. J. 2008;101:1141–1145. doi: 10.1097/SMJ.0b013e318181d5b5. quiz 1132, 1179. [DOI] [PubMed] [Google Scholar]
- 2.Wroe PC, Finkelstein JA, Ray GT, Linder JA, Johnson KM, Rifas-Shiman S, Moore MR, Huang SS. Aging population and future burden of pneumococcal pneumonia in the United States. J. Infect. Dis. 2012;205:1589–1592. doi: 10.1093/infdis/jis240. [DOI] [PubMed] [Google Scholar]
- 3.Krone CL, van de Groep K, Trzcinski K, Sanders EA, Bogaert D. Immunosenescence and pneumococcal disease: an imbalance in host-pathogen interactions. The lancet. Respir. Med. 2014;2:141–153. doi: 10.1016/S2213-2600(13)70165-6. [DOI] [PubMed] [Google Scholar]
- 4.Paterson GK, Orihuela CJ. Pneumococci: immunology of the innate host response. Respirology. 2010;15:1057–1063. doi: 10.1111/j.1440-1843.2010.01814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer KC. The role of immunity in susceptibility to respiratory infection in the aging lung. Respir. Physiol. 2001;128:23–31. doi: 10.1016/S0034-5687(01)00261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park S, Nahm MH. Older adults have a low capacity to opsonize pneumococci due to low IgM antibody response to pneumococcal vaccinations. Infect. Immun. 2011;79:314–320. doi: 10.1128/IAI.00768-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sen G, Chen Q, Snapper CM. Immunization of aged mice with a pneumococcal conjugate vaccine combined with an unmethylated CpG-containing oligodeoxynucleotide restores defective immunoglobulin G anti-polysaccharide responses and specific CD4+-T-cell priming to young adult levels. Infect. Immun. 2006;74:2177–2186. doi: 10.1128/IAI.74.4.2177-2186.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koppe U, Suttorp N, Opitz B. Recognition of Streptococcus pneumoniae by the innate immune system. Cell. Microbiol. 2012;14:460–466. doi: 10.1111/j.1462-5822.2011.01746.x. [DOI] [PubMed] [Google Scholar]
- 9.Hinojosa E, Boyd AR, Orihuela CJ. Age-associated inflammation and toll-like receptor dysfunction prime the lungs for pneumococcal pneumonia. J. Infect. Dis. 2009;200:546–554. doi: 10.1086/600870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krone CL, Trzciński K, Zborowski T, Sanders EA, Bogaert D. Impaired innate mucosal immunity in aged mice permits prolonged Streptococcus pneumoniae colonization. Infect. Immun. 2013;81:4615–4625. doi: 10.1128/IAI.00618-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shivshankar P, Boyd AR, Le Saux CJ, Yeh IT, Orihuela CJ. Cellular senescence increases expression of bacterial ligands in the lungs and is positively correlated with increased susceptibility to pneumococcal pneumonia. Aging Cell. 2011;10:798–806. doi: 10.1111/j.1474-9726.2011.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyd AR, Shivshankar P, Jiang S, Berton MT, Orihuela CJ. Age-related defects in TLR2 signaling diminish the cytokine response by alveolar macrophages during murine pneumococcal pneumonia. Exp. Gerontol. 2012;47:507–518. doi: 10.1016/j.exger.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rolston KV. The spectrum of pulmonary infections in cancer patients. Curr. Opin. Oncol. 2001;13:218–223. doi: 10.1097/00001622-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Nieminen J, St-Pierre C, Bhaumik P, Poirier F, Sato S. Role of galectin-3 in leukocyte recruitment in a murine model of lung infection by Streptococcus pneumoniae. J. Immunol. 2008;180:2466–2473. doi: 10.4049/jimmunol.180.4.2466. [DOI] [PubMed] [Google Scholar]
- 15.Garvy BA, Harmsen AG. The importance of neutrophils in resistance to pneumococcal pneumonia in adult and neonatal mice. Inflammation. 1996;20:499–512. doi: 10.1007/BF01487042. [DOI] [PubMed] [Google Scholar]
- 16.Hahn I, Klaus A, Janze AK, Steinwede K, Ding N, Bohling J, Brumshagen C, Serrano H, Gauthier F, Paton JC, et al. Cathepsin G and neutrophil elastase play critical and nonredundant roles in lung-protective immunity against Streptococcus pneumoniae in mice. Infect. Immun. 2011;79:4893–4901. doi: 10.1128/IAI.05593-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehrer RI, Ganz T, Selsted ME, Babior BM, Curnutte JT. Neutrophils and host defense. Ann. Intern. Med. 1988;109:127–142. doi: 10.7326/0003-4819-109-2-127. [DOI] [PubMed] [Google Scholar]
- 18.Marks M, Burns T, Abadi M, Seyoum B, Thornton J, Tuomanen E, Pirofski LA. Influence of neutropenia on the course of serotype 8 pneumococcal pneumonia in mice. Infect. Immun. 2007;75:1586–1597. doi: 10.1128/IAI.01579-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burns T, Abadi M, Pirofski LA. Modulation of the lung inflammatory response to serotype 8 pneumococcal infection by a human immunoglobulin m monoclonal antibody to serotype 8 capsular polysaccharide. Infect. Immun. 2005;73:4530–4538. doi: 10.1128/IAI.73.8.4530-4538.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murciano C, Yáñez A, O’Connor JE, Gozalbo D, Gil ML. Influence of aging on murine neutrophil and macrophage function against. Candida albicans. FEMS Immunol. Med. Microbiol. 2008;53:214–221. doi: 10.1111/j.1574-695X.2008.00418.x. [DOI] [PubMed] [Google Scholar]
- 21.Ito Y, Betsuyaku T, Nasuhara Y, Nishimura M. Lipopolysaccharide-induced neutrophilic inflammation in the lungs differs with age. Exp. Lung Res. 2007;33:375–384. doi: 10.1080/01902140701634843. [DOI] [PubMed] [Google Scholar]
- 22.Pignatti P, Ragnoli B, Radaeli A, Moscato G, Malerba M. Age-related increase of airway neutrophils in older healthy nonsmoking subjects. Rejuvenation Res. 2011;14:365–370. doi: 10.1089/rej.2010.1150. [DOI] [PubMed] [Google Scholar]
- 23.Menter T, Giefing-Kroell C, Grubeck-Loebenstein B, Tzankov A. Characterization of the inflammatory infiltrate in Streptococcus pneumoniae pneumonia in young and elderly patients. Pathobiol. 2014;81:160–167. doi: 10.1159/000360165. [DOI] [PubMed] [Google Scholar]
- 24.Craig A, Mai J, Cai S, Jeyaseelan S. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect. Immun. 2009;77:568–575. doi: 10.1128/IAI.00832-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhowmick R, Tin Maung NH, Hurley BP, Ghanem EB, Gronert K, McCormick BA, Leong JM. Systemic disease during Streptococcus pneumoniae acute lung infection requires 12-lipoxygenase-dependent inflammation. J. Immunol. 2013;191:5115–5123. doi: 10.4049/jimmunol.1300522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zen K, Liu Y, Cairo D, Parkos CA. CD11b/CD18-dependent interactions of neutrophils with intestinal epithelium are mediated by fucosylated proteoglycans. J. Immunol. 2002;169:5270–5278. doi: 10.4049/jimmunol.169.9.5270. [DOI] [PubMed] [Google Scholar]
- 27.Zen K, Parkos CA. Leukocyte-epithelial interactions. Curr. Opin. Cell Biol. 2003;15:557–564. doi: 10.1016/s0955-0674(03)00103-0. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Merlin D, Burst SL, Pochet M, Madara JL, Parkos CA. The role of CD47 in neutrophil transmigration. Increased rate of migration correlates with increased cell surface expression of CD47. J. Biol. Chem. 2001;276:40156–40166. doi: 10.1074/jbc.M104138200. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Bühring HJ, Zen K, Burst SL, Schnell FJ, Williams IR, Parkos CA. Signal regulatory protein (SIRPalpha), a cellular ligand for CD47, regulates neutrophil transmigration. J. Biol. Chem. 2002;277:10028–10036. doi: 10.1074/jbc.M109720200. [DOI] [PubMed] [Google Scholar]
- 30.Lawrence DW, Bruyninckx WJ, Louis NA, Lublin DM, Stahl GL, Parkos CA, Colgan SP. Antiadhesive role of apical decay-accelerating factor (CD55) in human neutrophil transmigration across mucosal epithelia. J. Exp. Med. 2003;198:999–1010. doi: 10.1084/jem.20030380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hurley BP, Sin A, McCormick BA. Adhesion molecules involved in hepoxilin A3-mediated neutrophil transepithelial migration. Clin. Exp. Immunol. 2008;151:297–305. doi: 10.1111/j.1365-2249.2007.03551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryan AS, Craig LD, Finn SC. Nutrient intakes and dietary patterns of older Americans: a national study. J. Gerontol. 1992;47:M145–M150. doi: 10.1093/geronj/47.5.m145. [DOI] [PubMed] [Google Scholar]
- 33.Panemangalore M, Lee CJ. Evaluation of the indices of retinol and alpha-tocopherol status in free-living elderly. J. Gerontol. 1992;47:B98–B104. doi: 10.1093/geronj/47.3.b98. [DOI] [PubMed] [Google Scholar]
- 34.Meydani SN, Han SN, Wu D. Vitamin E and immune response in the aged: molecular mechanisms and clinical implications. Immunol. Rev. 2005;205:269–284. doi: 10.1111/j.0105-2896.2005.00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kowdley KV, Mason JB, Meydani SN, Cornwall S, Grand RJ. Vitamin E deficiency and impaired cellular immunity related to intestinal fat malabsorption. Gastroenterology. 1992;102:2139–2142. doi: 10.1016/0016-5085(92)90344-x. [DOI] [PubMed] [Google Scholar]
- 36.Pae M, Meydani SN, Wu D. The role of nutrition in enhancing immunity in aging. Aging Dis. 2012;3:91–129. [PMC free article] [PubMed] [Google Scholar]
- 37.Adolfsson O, Huber BT, Meydani SN. Vitamin E-enhanced IL-2 production in old mice: naive but not memory T cells show increased cell division cycling and IL-2-producing capacity. J. Immunol. 2001;167:3809–3817. doi: 10.4049/jimmunol.167.7.3809. [DOI] [PubMed] [Google Scholar]
- 38.Marko MG, Ahmed T, Bunnell SC, Wu D, Chung H, Huber BT, Meydani SN. Age-associated decline in effective immune synapse formation of CD4(+) T cells is reversed by vitamin E supplementation. J. Immunol. 2007;178:1443–1449. doi: 10.4049/jimmunol.178.3.1443. [DOI] [PubMed] [Google Scholar]
- 39.Meydani SN, Meydani M, Blumberg JB, Leka LS, Siber G, Loszewski R, Thompson C, Pedrosa MC, Diamond RD, Stollar BD. Vitamin E supplementation and in vivo immune response in healthy elderly subjects. A randomized controlled trial. JAMA. 1997;277:1380–1386. doi: 10.1001/jama.1997.03540410058031. [DOI] [PubMed] [Google Scholar]
- 40.Wu D, Meydani SN. Age-associated changes in immune and inflammatory responses: impact of vitamin E intervention. J. Leukoc. Biol. 2008;84:900–914. doi: 10.1189/jlb.0108023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rocksén D, Ekstrand-Hammarström B, Johansson L, Bucht A. Vitamin E reduces transendothelial migration of neutrophils and prevents lung injury in endotoxin-induced airway inflammation. Am. J. Respir. Cell Mol. Biol. 2003;28:199–207. doi: 10.1165/rcmb.4899. [DOI] [PubMed] [Google Scholar]
- 42.Mabalirajan U, Aich J, Leishangthem GD, Sharma SK, Dinda AK, Ghosh B. Effects of vitamin E on mitochondrial dysfunction and asthma features in an experimental allergic murine model. J. Appl. Physiol. 2009;107:1285–1292. doi: 10.1152/japplphysiol.00459.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshida N, Yoshikawa T, Manabe H, Terasawa Y, Kondo M, Noguchi N, Niki E. Vitamin E protects against polymorphonuclear leukocyte-dependent adhesion to endothelial cells. J. Leukoc. Biol. 1999;65:757–763. doi: 10.1002/jlb.65.6.757. [DOI] [PubMed] [Google Scholar]
- 44.Hurley BP, Siccardi D, Mrsny RJ, McCormick BA. Polymorphonuclear cell transmigration induced by Pseudomonas aeruginosa requires the eicosanoid hepoxilin A3. J. Immunol. 2004;173:5712–5720. doi: 10.4049/jimmunol.173.9.5712. [DOI] [PubMed] [Google Scholar]
- 45.Meydani SN, Meydani M, Blumberg JB, Leka LS, Pedrosa M, Diamond R, Schaefer EJ. Assessment of the safety of supplementation with different amounts of vitamin E in healthy older adults. Am. J. Clin. Nutr. 1998;68:311–318. doi: 10.1093/ajcn/68.2.311. [DOI] [PubMed] [Google Scholar]
- 46.Meydani SN, Shapiro AC, Meydani M, Macauley JB, Blumberg JB. Effect of age and dietary fat (fish, corn and coconut oils) on tocopherol status of C57BL/6Nia mice. Lipids. 1987;22:345–350. doi: 10.1007/BF02534004. [DOI] [PubMed] [Google Scholar]
- 47.De la Fuente M, Hernanz A, Guayerbas N, Victor VM, Arnalich F. Vitamin E ingestion improves several immune functions in elderly men and women. Free Radic. Res. 2008;42:272–280. doi: 10.1080/10715760801898838. [DOI] [PubMed] [Google Scholar]
- 48.McCormick BA. Bacterial-induced hepoxilin A3 secretion as a proinflammatory mediator. FEBS J. 2007;274:3513–3518. doi: 10.1111/j.1742-4658.2007.05911.x. [DOI] [PubMed] [Google Scholar]
- 49.Parkos CA, Colgan SP, Diamond MS, Nusrat A, Liang TW, Springer TA, Madara JL. Expression and polarization of intercellular adhesion molecule-1 on human intestinal epithelia: consequences for CD11b/CD18-mediated interactions with neutrophils. Mol. Med. 1996;2:489–505. [PMC free article] [PubMed] [Google Scholar]
- 50.Campbell EL, Louis NA, Tomassetti SE, Canny GO, Arita M, Serhan CN, Colgan SP. Resolvin E1 promotes mucosal surface clearance of neutrophils: a new paradigm for inflammatory resolution. FASEB J. 2007;21:3162–3170. doi: 10.1096/fj.07-8473com. [DOI] [PubMed] [Google Scholar]
- 51.Belisle SE, Hamer DH, Leka LS, Dallal GE, Delgado-Lista J, Fine BC, Jacques PF, Ordovas JM, Meydani SN. IL-2 and IL-10 gene polymorphisms are associated with respiratory tract infection and may modulate the effect of vitamin E on lower respiratory tract infections in elderly nursing home residents. Am. J. Clin. Nutr. 2010;92:106–114. doi: 10.3945/ajcn.2010.29207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Belisle SE, S.Leka L, Dallal GE, Jacques PF, Delgado-Lista J, Ordovas JM, Meydani SN. Cytokine response to vitamin E supplementation is dependent on pre-supplementation cytokine levels. Biofactors. 2008;33:191–200. doi: 10.1002/biof.5520330305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grossman S, Waksman EG. New aspects of the inhibition of soybean lipoxygenase by alpha-tocopherol. Evidence for the existence of a specific complex. Int. J. Biochem. 1984;16:281–289. doi: 10.1016/0020-711x(84)90101-0. [DOI] [PubMed] [Google Scholar]
- 54.Marko MG, Pang HJ, Ren Z, Azzi A, Huber BT, Bunnell SC, Meydani SN. Vitamin E reverses impaired linker for activation of T cells activation in T cells from aged C57BL/6 mice. J. Nutr. 2009;139:1192–1197. doi: 10.3945/jn.108.103416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gauthier JF, Fortin A, Bergeron Y, Dumas MC, Champagne ME, Bergeron MG. Differential contribution of bacterial N-formyl-methionyl-leucyl-phenylalanine and host-derived CXC chemokines to neutrophil infiltration into pulmonary alveoli during murine pneumococcal pneumonia. Infect. Immun. 2007;75:5361–5367. doi: 10.1128/IAI.02008-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dalpiaz A, Spisani S, Biondi C, Fabbri E, Nalli M, Ferretti ME. Studies on human neutrophil biological functions by means of formyl-peptide receptor agonists and antagonists. Curr. Drug Targets Immune Endocr. Metabol. Disord. 2003;3:33–42. doi: 10.2174/1568008033340333. [DOI] [PubMed] [Google Scholar]
- 57.Boll EJ, Struve C, Sander A, Demma Z, Krogfelt KA, McCormick BA. Enteroaggregative Escherichia coli promotes trans-epithelial migration of neutrophils through a conserved 12-lipoxygenase pathway. Cell. Microbiol. 2012;14:120–132. doi: 10.1111/j.1462-5822.2011.01706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pazos M, Siccardi D, Mumy KL, Bien JD, Louie S, Shi HN, Gronert K, Mrsny RJ, McCormick BA. Multidrug resistance-associated transporter 2 regulates mucosal inflammation by facilitating the synthesis of hepoxilin A3. J. Immunol. 2008;181:8044–8052. doi: 10.4049/jimmunol.181.11.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.