Abstract
Background
Older adults with dementia experience frequent transitions in care
Objectives
To describe transitions in care among older adults with dementia identified from a nationally representative cohort and to describe transition rates among subjects with more severe levels of cognitive and functional impairment
Design
Longitudinal cohort study
Setting
Health and Retirement Study (HRS)
Participants
16,186 HRS respondents aged 65 years or over whose survey data were linked with Medicare claims from 1999-2008
Measurements
Transitions in care between home, home with formal services, hospital, and nursing facility care, as well as cognitive function, activities of daily living, and mortality.
Results
The 3,447 (21.3%) HRS subjects who were ever diagnosed with dementia experienced frequent transitions. Among subjects transitioning from a hospital stay, 52.2% returned home without home care services while 33.8% transitioned to a nursing facility. Among subjects transitioning from a nursing facility, 59.2% transitioned to the hospital while 25.3% returned home without services. There were 2,139 transitions to death and 58.7% of HRS subjects with dementia died at home. Even among persons with moderate to severe dementia, we documented multiple transitions in care, including transitions from the hospital to home and back to the hospital.
Conclusion
In this nationally representative sample of older adults, subjects diagnosed with dementia experience frequent transitions. Those persons with dementia who are cared for at home and who transition back to home often have moderate to severe impairments in both function and cognition.
INTRODUCTION
Improving the quality and efficiency of care for older adults with dementia is a national priority.1, 2 On average, older adults with dementia accrue greater health care costs than persons without dementia.3, 4 A substantial proportion of these health care costs are attributed to hospitalizations and long-term care as well as to transitions between these two high-cost sites of care and the emergency department.3, 5-7 However, not all older adults with dementia accrue high health care costs and many older adults with dementia do not accrue substantial long-term care costs.3 Transitions in care have become an important target for improvements in quality and efficiency – for health care providers, regulators and policymakers -- because some transitions to higher cost settings of care are preventable or unnecessary.6, 8-16 Persons with dementia may be particularly at risk for preventable transitions in care because of the large number of their care transitions, comorbid medical conditions, and severity of cognitive impairment.5, 7, 17 Transitions may represent a particularly high-risk setting for medical errors, patient and family burden, and medical treatments that are not concordant with goals of care.18
In a prior study reporting on the frequency of care transitions among a large cohort of community-dwelling older adults in central Indiana, we merged subjects' electronic medical records with Medicare claims, Medicaid claims, the Minimum Dataset (MDS), and the Outcome and Assessment Information Set (OASIS) from 2001-2008.5 Compared to those without dementia, older adults with prevalent and those with incident dementia had more transitions in care per person year of follow-up. We documented a dynamic movement of older adults with dementia across multiple sites of care including into and out of skilled nursing care. The dataset for this prior study did not include information on subjects' cognitive or functional status (beyond the diagnosis of dementia) and the subjects were drawn from a single urban public health system.
In an editorial accompanying that prior study, Kane and Ouslander noted the limitations of the dataset as described above. They also hypothesized that many of the older adults with dementia who were hospitalized and eventually returned home, either with or without an intervening nursing facility stay, may have had milder dementia and were perhaps hospitalized for comorbid medical conditions rather than their dementing illness.19 The editorial further suggested that: "Some assessment of the severity of the dementia would greatly strengthen future studies of dementia transitions." The goal of the present study is two-fold. First, we sought to determine if the rates and patterns of transitions we observed in the local cohort study would be similar to those seen in the more nationally-representative sample of subjects enrolled in the Health and Retirement Study. Second, we sought to describe the severity of dementia and functional impairment among subjects with different patterns of transitions to test the hypothesis that older adults with dementia who transition to home are less impaired than those who transition to higher cost settings.
METHODS
This study was approved by the Indiana University Purdue University-Indianapolis Institutional Review Board and the Centers for Medicare and Medicaid Services Privacy Board. We used the nationally representative Health and Retirement Study (HRS) data linked with Medicare claims for all analyses in this study. The HRS and its associated databases have been used in a variety of studies of cognitive aging and dementia.4, 20-25 A key methodological consideration for the present study was to replicate the methods we had used in our prior study of transitions that used a local longitudinal cohort.5 There are three important differences between the prior and current databases. First, the HRS contains data on subjects' cognitive and functional status. Second, the HRS does not contain data on Medicaid claims, Minimum Dataset Data (MDS) or home health care claims. Third, rather than actual claims, the HRS contains data on self-reported and proxy-reported use of nursing facilities. Thus, we developed a method, described below, to harmonize Medicare claims for skilled nursing facility use with self-reported nursing home use so that we could capture both Medicare-paid nursing facility utilization (documented in Medicare claims data) and nursing facility use paid for by Medicaid or out-of-pocket (as self- or proxy-reported by HRS respondents) to approximate data available in our prior study which relied on Medicare, Medicaid, and MDS data to document nursing facility use.
The HRS is an ongoing nationally representative study among adults aged 50 years and older. We focused on respondents aged 65 years and older whose HRS data are linked with Medicare claims. To match the time period targeted in our previous study, we relied on HRS interviews conducted between 1999-2008. In our prior study, we identified cases of dementia based on International Classification of Disease (ICD) codes contained in claims data, including 290.0-290.43, 291.2, 294.0-294.9, 331.0-331.9, 333.0, and 797.5 Thus, the current project focuses on the 16,186 HRS participants from 1999-2008 with linked Medicare claims data. HRS subjects without Medicare claims data are excluded. Notably, Medicare claims data include the actual dates of services. HRS survey data begin in 1992 and surveys are repeated every two years.
We used the following hierarchy of data to determine a subject's physical location on any given day over the 10-year follow-up period if they were not deceased: (a) Medicare claims place subject in hospital on that day; (b) Medicare claims place subject in nursing facility; (c) Medicare claims place subject at home with home health care; (d) self-reports place subject in nursing facility; (e) self-reports place subject in home health care; or (f) if in no other category, patient at home without home health care. Self-report nursing home dates were based upon a combination of RAND-HRS and exit interview data files from the AHEAD-HRS website (Health and Retirement Study, 1998-2010). The RAND-HRS data file includes measures from across the 12 waves of the HRS (1992, 1993, 1994, 1995, 1996, 1998, 2000, 2002, 2004, 2006, 2008, and 2010) in an easy-to-use format with consistent variable names. For respondents living in the nursing home at the time of the interview, the month and year of nursing home placement were extracted from each source from 1998 through 2010. We assumed the 15th day of the month for the day of placement for everyone and also assumed the respondent was in the nursing home until the time of the interview. Exit interview data supplemented the RAND-HRS for respondents who died while in the nursing home. Again, month and year of nursing home placement were extracted assuming the 15th day of the month for the day with date of death as the nursing home end date. In the transitions figures, subjects with dementia contribute to the dementia group's transition data only from the time they are diagnosed with dementia. Thus, a subject diagnosed with dementia in 2004 would only contribute to the transition figures from 2004-2008.
Building from methods used in published reports utilizing the HRS, self-reported outcome measures at the last assessment prior to transition and their scoring included activities of daily living (ADLs), instrumental activities of daily living (IADLs), cognitive status, and vital status.20-25 ADLs were assessed by respondents indicating they needed help with dressing, bathing, eating, getting in/out of bed and using the toilet. A sum score was used for ADLs ranging from 0-5. IADLs were indicated by having difficulty preparing meals, shopping for groceries, using the telephone, taking medications and managing money. A sum score was also used for IADLs ranging from 0-5. Vital status was determined according to the Medicare claims death date.
Cognitive status was based upon self- and proxy-reports. To determine cognitive status in self-respondents, we used the modified Telephone Interview for Cognitive Status (TICS). The HRS-modified TICS is a 27-point cognitive index developed from three interviewer-administered items that represent short-term memory, working memory, and speed of processing.26 The 27-point cognitive index includes: 1) an immediate and delayed 10-noun free recall test to measure short-term memory (0 to 20 points possible); 2) a serial seven subtraction test to measure working memory (0 to 5 points); and 3) a counting backwards test to measure speed of mental processing (0 to 2 points). To determine cognitive status in proxy-respondents, questions adapted from 16-item short form Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE)27, 28 were used to capture respondent change in memory over the past 2 years. Each item response was coded as 1=much improved, 2=a bit improved, 3=not much change, 4=a bit worse and 5=much worse resulting in a total cognitive impairment score with a possible range of 16-80. We defined subjects with moderate to severe dementia using the following criteria: (a) at any time during the observation period, subject had a diagnosis of dementia and had a TIC score ≤ 6 and needed help on at least one IADL; or (b) at any time during the observation period, subject had a diagnosis of dementia and had a IQCODE score ≥ 60 and needed help on at least one IADL. If subjects had a diagnosis of dementia, but did not meet the criteria for moderate to severe dementia, then they were placed in the mild dementia group. No dementia, mild dementia, and moderate to severe dementia groups are mutually exclusive.
Chi-square and t-tests were used to compare sample characteristics between ever and never demented groups. We then quantified the frequencies of various transitions from inpatient and skilled nursing facilities to other states as transition probabilities, which accounted for multiple transitions of the same type within a given subject. For example, it’s possible that over a 10-year period the occurrence of a subject placed in a hospital and then transferred to home health or nursing home could occur several times. Thus, to compare outcomes between outbound transition sites from inpatient and skilled nursing facilities, a generalized mixed effect linear model was used to account for the within-subject correlation arising from multiple records per subject. Estimates of inbound and outbound transition probabilities were presented graphically in diagrams showing directions and frequencies of all transitions among different care states. For compound transitions, we calculated the per-person annual transition rates for initial transitions originating from nursing facilities or hospitals. Rates for the most frequently observed compound transitional events out of hospital and nursing facility were presented in a tabular form together with associated 95% confidence intervals. The difference in transition rates between demented and non-demented groups was characterized as a transition rate ratio (RR); the estimated values of the RR and the corresponding 95% confidence intervals were obtained from generalized linear mixed effect models with a negative-binomial data assumption. Logarithmic-transformed lengths of observation (in years) were used as an offset in the model to account for varying lengths of follow-up per subject observed in the comparison groups. Confidence intervals with lower limits greater than one indicate significantly increased transition rates. All analyses were conducted using SAS/STAT software, Version 9.3 of the SAS System for Windows using a .05 level of significance.
RESULTS
Table 1 shows the characteristics of the total HRS sample at baseline and compares the characteristics of subjects never diagnosed with dementia with those diagnosed with mild dementia or moderate to severe dementia. We highlight that both subjects we defined as mild dementia and those we defined with moderate to severe dementia had all been diagnosed with dementia according to CMS claims data. The self-report indicators of chronic conditions are taken from the HRS survey closest to the first dementia diagnosis or the last available survey if the subject never had a dementia diagnosis. Persons diagnosed with dementia were substantially older. All groups suffer from multiple comorbid medical conditions but subjects diagnosed with dementia were more likely to report a diagnosis of stroke. Despite a lower mean number of years of observation, persons with dementia had more transitions in care than those without dementia. The highest frequency of transitions were seen among those with moderate to severe dementia and these subjects were the least likely to have no transitions in care. HRS subjects diagnosed with dementia were more likely to have a hospitalization, more likely to have a nursing facility stay, and more likely to die. Subjects ever diagnosed with dementia had earlier times to first nursing facility use [Hazard ratio=5.14, 95% CI=(4.82-5.49)] and earlier times to death [Hazard ratio=2.76, 95% CI=(2.62-2.91).
Table 1.
HRS sample characteristics
| Total sample (N=16,186) |
Never Demented (N=12,739) |
Mild Dementia (N=2,278) |
Moderate to severe Dementia (N=1,169) |
p-value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, mean (SD)* | 70.1 (9.9) | 68.2 (9.4) | 75.7 (8.9) | 79.5 (8.5) | <.0001 |
| Female, n (%) | 9201 (56.9) | 7056 (55.4) | 1331 (58.4) | 814 (69.6) | <.0001 |
| Black, n (%) | 2178 (13.5) | 1646 (12.9) | 291 (12.8) | 241 (20.6) | <.0001 |
| Education, mean (SD) | 11.7 (3.5) | 11.9 (3.3) | 11.6 (3.5) | 9.8 (3.9) | <.0001 |
| Comorbid conditions | |||||
| Hypertension, n (%) | 10367 (65.1) | 8262 (65.7) | 1394 (63.8) | 711 (60.9) | .0018 |
| Diabetes, n (%) | 3890 (24.4) | 3121 (24.8) | 519 (23.8) | 250 (21.4) | .0250 |
| Cancer, n (%) | 3290 (20.7) | 2711 (21.6) | 408 (18.7) | 171 (14.6) | <.0001 |
| Lung disease, n (%) | 2356 (14.8) | 1916 (15.2) | 319 (14.6) | 121 (10.4) | <.0001 |
| Heart disease, n (%) | 4667 (29.3) | 3645 (29.0) | 671 (30.7) | 351 (30.0) | .2148 |
| CHF, n (%) | 1195 (7.5) | 885 (7.0) | 196 (9.0) | 114 (9.8) | <.0001 |
| Stroke, n (%) | 1947 (12.2) | 1252 (9.9) | 413 (18.9) | 282 (24.1) | <.0001 |
| Arthritis, n (%) | 11051 (69.4) | 8748 (69.5) | 1508 (69.1) | 795 (68.0) | .5228 |
| Transitions in Care | |||||
| Person years observation, mean (SD) |
8.3 (2.9) | 8.6 (2.8) | 7.6 (3.0) | 6.6 (3.0) | <.0001 |
| Total number of transitions, mean (SD) |
5.6 (7.8) | 4.2 (6.5) | 10.8 (9.8) | 11.2 (9.7) | <.0001 |
| Number of transitions per year of follow-up, mean (SD) |
1.3 (4.6) | 1.1 (4.6) | 2.1 (5.1) | 2.2 (2.7) | <.0001 |
| Number with zero transitions (%) over observation period |
4831 (29.8) | 4689 (36.8) | 124 (5.4) | 18 (1.5) | <.0001 |
| Health care utilization | |||||
| Number of hospital stays per year of follow-up, mean (SD) |
0.4 (1.5) | 0.3 (1.5) | 0.7 (1.1) | 0.8 (1.1) | <.0001 |
| Number with one or more hospital stays, (%) |
9861 (60.9) | 6786 (53.3) | 2040 (89.6) | 1035 (88.5) | <.0001 |
| Number of nursing home stays per year of follow- up, mean (SD) |
0.1 (0.4) | 0.1 (0.3) | 0.3 (0.8) | 0.3 (0.6) | <.0001 |
| Number with one or more nursing home stays, (%) |
3719 (23.0) | 1825 (14.3) | 1159 (50.9) | 735 (62.9) | <.0001 |
| Number of home health encounters per year of follow-up, mean (SD) |
0.1 (0.5) | 0.1 (0.5) | 0.2 (0.4) | 0.2 (0.4) | <.0001 |
| Number with one or more home health care days, (%) |
4754 (29.4) | 2985 (23.4) | 1195 (52.5) | 574 (49.1) | <.0001 |
| Vital Status | |||||
| Died, n (%) | 5035 (31.1) | 3122 (24.5) | 1117 (49.0) | 796 (68.1) | <.0001 |
Age as of January 1, 1999
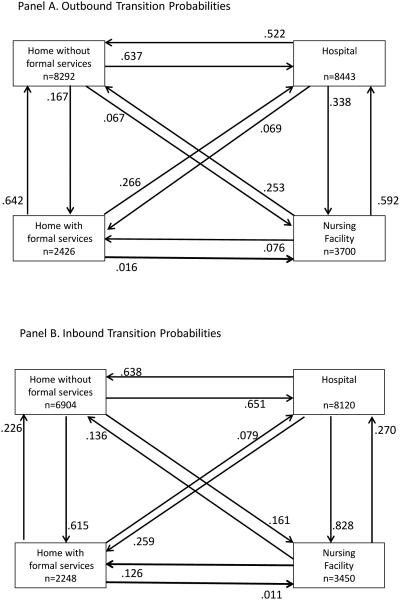
While Table 1 contains data on the frequency of transitions, Figure 1 provides data on the probability of transitions between specific sites of care. Panel A displays the outbound (transitioning out of the site) and Panel B the inbound (transitioning into the site) probabilities of a transfer between two sites of care for older adults following their initial diagnosis of dementia. This figure includes both subjects with mild and those with moderate to severe dementia. Probabilities are conditional on the subject moving from one site to another among all transitions to or from that site. Subjects with no transitions from or to the site are not represented in the figure. The figure also does not include transitions to death. Notably, these figures do not purport to display a full clinical course of all transitions for any given subject, the figures do not display compound or multiple transitions, and the figures do not imply causation.
Figure 1. Outbound and Inbound Transition Probabilities.
Panel A. Each box represents one of four mutually exclusive sites of care. The number within each box represents the total number of transitions out of this site of care. For each site of care there are three lines that lead out of it and into the other three sites of care. The number next to each arrow represents the conditional probability of the transition out of the site of care and into the other site of care. When combined with the conditional probability of a transition out of the site due to death (data not shown), the sum of the three conditional probabilities out of each site of care equals 1.
Panel B. Each box represents one of four mutually exclusive sites of care. The number within each box represents the total number of transitions into this site of care. For each site of care there are three lines that lead to it from the other three sites of care. The number next to each arrow represents the conditional probability of the transition into the site of care from the other site of care. The sum of the three conditional probabilities into each site of care equals 1.
As shown in Figure 1, the largest number of transitions was between the home and the hospital; 52.2% of subjects with dementia transitioning out of the hospital returned home without formal services and 33.8% transitioned to the nursing home. Among subjects with a nursing facility transition, 59.2% transitioned to the hospital while 25.3% transitioned to home without formal services. Among subjects with a hospital stay, 65.1% transitioned to the hospital from home without formal services while 27% arrived from a nursing facility. Among subjects with a nursing facility stay, 82.8% arrived in the nursing facility from the hospital. With regard to older adults receiving formal home care services, we note that 61.5% of these services are started among persons already at home while only 25.9% are initiated among persons transitioning from the hospital.
There were 2139 transitions to death among the 3447 patients with dementia; 58.7% died at home with or without formal services, 27.8% died in the hospital, and only 13.5% died in a nursing facility if we define place of death as the last site of care prior to the death date.
While Table 1 shows that persons with moderate to severe dementia continue to experience multiple transitions in care, Table 2 explores the hypothesis that persons with moderate to severe dementia are less likely to experience specific types of transitions in care compared to persons with mild dementia. For all three groups, the hospital is the most likely site of a care transition from home and the home is the most likely site of transition from the hospital. Persons with moderate to severe dementia are more likely to transition to a nursing facility from the hospital and more likely to transition from a nursing facility to a hospital. However, even among subjects transitioning from a nursing facility who have moderate to severe dementia, 28.3% of the transitions were to home. Data in this table do not support the hypothesis that those subjects with moderate to severe dementia are substantially less likely to experience transitions from nursing facility care.
Table 2.
Conditional transitional probabilities by level of dementia severity
| No Dementia |
Mild Dementia |
Moderate- Severe Dementia |
p-value* | |
|---|---|---|---|---|
| Among subjects with a transition from home, the conditional probability that the transition was to: |
<.0001 | |||
| home health care | .126 | .174 | .168 | |
| hospital | .762 | .709 | .638 | |
| nursing facility | .031 | .056 | .099 | |
| death | .081 | .061 | .095 | |
| Among subjects with a transition from home health care, the conditional probability that the transition was to: |
<.0001 | |||
| to home | .742 | .703 | .709 | |
| to hospital | .194 | .239 | .225 | |
| to nursing facility | .008 | .018 | .015 | |
| to death | .056 | .040 | .051 | |
| Among subjects with a transition from the hospital, the conditional probability that the transition was to: |
<.0001 | |||
| to home | .742 | .607 | .553 | |
| to home health care | .093 | .089 | .079 | |
| to nursing facility | .118 | .261 | .325 | |
| to death | .048 | .043 | .043 | |
| Among subjects with a transition from a nursing facility, the conditional probability that the transition was to: |
<.0001 | |||
| to home | .408 | .288 | .283 | |
| to home health care | .178 | .117 | .072 | |
| to hospital | .341 | .535 | .585 | |
| to death | .073 | .060 | .060 |
p-value from chi-square test
Table 3 displays the functional and cognitive status of subjects with dementia based on their transitional site of care. Rather than using categories of dementia status, this table shows the mean functional and cognitive status of subjects with dementia who follow different transitional patterns of care. The hypothesis of these analyses is that subjects with dementia who are discharged to home are less cognitively and functionally impaired than subjects discharged to a nursing facility or discharged to home with home health care services. While this hypothesis is supported by these data, these data also show that persons with dementia who are discharged home from the hospital or the nursing facility have substantial functional and cognitive impairment.
Table 3.
Clinical characteristics by outbound transition site of care among hospital and nursing facility subjects with dementia.
| Proxy respondent, n (%)* |
IADL, mean (SD)* |
ADL, mean (SD)* |
Modified TICS, mean (SD)** |
IQCODE, mean (SD)*** |
Died by 2008, n (%) |
|
|---|---|---|---|---|---|---|
| Hospitalized subjects’ outbound transition site of care |
||||||
| Home without formal services |
1187 (27.8) | 1.7 (1.9) | 1.0 (1.6) | 10.5 (4.7) | 63.9 (13.5) | 2447 (55.5) |
| Home with formal services |
123 (21.8) | 1.4 (1.7) | 0.8 (1.4) | 10.9 (4.7) | 61.1 (13.3) | 335 (57.5) |
| Nursing facility | 962 (34.6) | 2.1 (1.9) | 1.3 (1.7) | 9.5 (4.4) | 65.7 (12.6) | 1854 (64.9) |
| p-value**** | <.0001 | <.0001 | <.0001 | <.0001 | .0245 | <.0001 |
| Nursing facility subjects’ outbound transition site of care |
||||||
| Home without formal services |
369 (40.8) | 2.4 (2.0) | 1.6 (1.8) | 9.9 (4.5) | 67.4 (12.5) | 523 (55.8) |
| Home with formal services |
50 (18.5) | 1.5 (1.7) | 0.9 (1.5) | 10.7 (4.5) | 61.8 (11.5) | 143 (50.5) |
| Hospital | 891 (41.3) | 2.4 (2.0) | 1.6 (1.8) | 9.1 (4.3) | 67.0 (12.7) | 1575 (71.9) |
| Only transition to death |
127 (45.2) | 2.4 (2.0) | 1.7 (1.9) | 8.6 (4.4) | 67.5 (12.6) | NA |
| No transitions, no death |
55 (32.7) | 2.3 (2.0) | 1.4 (1.8) | 9.8 (4.1) | 71.8 (9.6) | NA |
| p-value**** | <.0001 | <.0001 | <.0001 | <.0001 | .1558 | <0.001 |
at time of last assessment prior to transition
among self-respondents aged>=65, high score indicates better cognitive function; possible range=0-27
among proxies aged >=65, high score indicates worse cognitive function; possible range=16-80
a generalized linear model for repeated measures was used to make comparisons across location with either a logit (proxy respondent, died by 2008), Poisson (IADL and ADL scores) or identity (Modified TICS, IQCode) link function.
Table 4 displays the rates of common “compound” transitions in care among the subjects who had at least one such compound transition. These analyses address the clinical scenario sometimes referred to as “ping-pong” transitions when, for example, a person transitions between the hospital to the nursing facility and then back to the hospital. Because there are innumerable potential possible compound transitions, we limit the presentation of data here to the most commonly occurring patterns involving high cost settings of care and two transitions. Subjects with dementia are more likely to experience compound transitions, as indicated by the estimated transition rate ratios. The data again demonstrate that subjects with moderate to severe dementia are as likely as those will mild dementia to experience compound transitions involving hospital and nursing home transfers. We highlight that these compound transitions include transitions to home without home health care. In the analyses represented in Table 4, the time between transitions could be days to years so we conducted additional analyses specifically examining 30-day re-hospitalizations. Among those subjects with moderate to severe dementia with a 30-day re-hospitalization, 47.7% had been discharged to home without home health care services from the index hospitalization compared to 53.0% for those with mild dementia, and 45.0% with moderate to severe dementia had been discharged to a nursing facility compared to 37.8% for those with mild dementia.
Table 4.
Annual transition rates of common compound transitions among subjects with at least one compound transition
| Total sample (N=16,186) |
Never Demented (N=12,739) |
Mild Dementia (N=2,278) |
Moderate to Severe Dementia (N=1,169) |
|
|---|---|---|---|---|
| Number (%) of subjects with >=1 compound transitions out of the nursing facility |
3238 (20.0%) | 1525 (12.0%) | 1042 (45.7%) | 671 (57.4%) |
| Transition Type | Annual transition rate and corresponding 95% confidence interval (CI) of compound transition; Rate ratio (RR) and 95% CI of RR as compared to the never demented group |
|||
| nursing facility to hospital to nursing facility |
0.17, (CI: 0.15-0.18) | 0.10, (CI: 0.09-0.12) | 0.21, (CI: 0.18-0.24) RR: 2.29 (CI: 1.99-2.64) |
0.24, (CI: 0.21-0.27) RR: 2.85 (CI: 2.43-3.33) |
| nursing facility to home to hospital |
0.06, (CI: 0.05-0.06) | 0.06, (CI: 0.05-0.06) | 0.06, (CI: 0.05-0.06) RR: 1.07 (CI: 0.93-1.24) |
0.07, (CI: 0.06-0.08) RR: 1.33 (CI: 1.13-1.55) |
| nursing facility to home to nursing facility |
0.01, (CI: 0.01-0.01) | 0.01, (CI: 0.01-0.01) | 0.01, (CI: 0.01-0.01) RR: 1.02 (CI: 0.73-1.41) |
0.02, (CI: 0.01-0.03) RR: 2.03 (CI: 1.49-2.76) |
| nursing facility to home health care to hospital |
0.01, (CI: 0.01-0.0)1 | 0.01, (CI: 0.01-0.02) | 0.01, (CI: 0.01-0.02) RR: 1.14 (CI: 0.84-1.55) |
0.01, (CI: 0.00-0.01) RR: 0.65 (CI: 0.43-1.00) |
| Number (%) of subjects with >=1 compound transitions out of the hospital |
7927 (49.0%) | 5085 (39.9%) | 1857 (81.5%) | 985 (84.2%) |
| Transition Type | Annual transition rate and corresponding 95% confidence interval (CI) of compound transition; Rate ratio (RR) and 95% CI of RR as compared to the never demented group |
|||
| hospital to home to hospital | 0.30, (CI: 0.29-0.32) | 0.31, (CI: 0.29-0.33) | 0.31, (CI: 0.28-0.33) RR: 1.08 (CI: 1.01-1.15) |
0.27, (CI: 0.24-0.30) RR: 0.97 (CI: 0.88-1.06) |
| hospital to nursing facility to hospital |
0.07, (CI: 0.07-0.08) | 0.04, (CI: 0.03-0.05) | 0.12, (CI: 0.11-0.14) RR: 4.54 (CI: 3.88-5.31) |
0.16, (CI: 0.13-0.18) RR: 6.19 (CI: 5.13-7.48) |
| hospital to nursing facility to home |
0.05, (CI: 0.04-0.05) | 0.04, (CI: 0.03-0.04) | 0.06, (CI: 0.05-0.07) RR: 2.10 (CI: 1.88-2.36) |
0.08, (CI: 0.06-0.09) RR: 2.68 (CI: 2.34-3.06) |
| hospital to home health care to hospital |
0.02, (CI: 0.02-0.03) | 0.02, (CI: 0.02-0.03) | 0.02, (CI: 0.02-0.03) RR: 1.37 (CI: 1.12-1.67) |
0.02, (CI: 0.01-0.02) RR: 1.22 (CI: 0.93-1.59) |
DISCUSSION
The two goals of this study were: (1) to determine if the rates and patterns of care transitions we observed in a local cohort of older adults would be similar to those seen in the more nationally-representative sample of subjects enrolled in the Health and Retirement Study and (2) to describe the severity of dementia and functional impairment among subjects with different patterns of transitions. With respect to our first goal, the analysis of the nationally representative HRS sample confirms four findings we reported from the Indianapolis cohort. First, older adults with dementia experience numerous transitions in care over the course of 10 years, including frequent transitions out of nursing facility care to other sites of care. Second, we confirmed that the time to first nursing facility use and time to death among HRS participants with dementia mirror those in the Indianapolis cohort as well as other cohorts reported in the literature.29 Subjects with dementia clearly have earlier times to nursing facility use and death compared to subjects without dementia. Third, for many subjects, the hospital serves as the "front door" for the nursing facility. Even though many subjects with dementia transitioned to home, the most frequent route to the nursing facility was via the hospital. Care in these three sites of care is inter-connected and a nursing facility stay or a return home may often be an extension of the hospitalization and may often be due to a comorbid condition. Fourth, most of the HRS sample diagnosed with dementia died at home rather than in nursing homes.
Our second goal was to explore the hypothesis that many older adults with dementia who appear to be discharged to home without formal services, either from the hospital or the nursing facility, have milder severity of dementia compared to those without these transitions. We examined this hypothesis from two perspectives. First, we demonstrate that persons with moderate to severe dementia have the highest frequency of transitions and that their transition probabilities to other sites of care are very similar to those with mild dementia. Persons with moderate to severe dementia also have similar rates of compound transitions, even when our analyses control for lower observation periods among those with moderate to severe dementia due to death. We did not find that persons with moderate to severe dementia simply stayed in nursing facilities with little or no transitions in care. Second, we report the cognitive and functional impairment among persons transitioning out of high cost sites of care. Those subjects with dementia transitioning to home from the hospital or the nursing facility had lower levels of impairment but were impaired. Indeed, those returning home but without services from the nursing facility had impairments that mirrored the high level of impairment of subjects transitioning from the hospital to the nursing facility. We found little evidence that those persons with dementia cared for at home are limited to persons with only milder forms of dementia. While the subject may have been hospitalized for a condition unrelated to dementia, it is still likely that self-management of the condition responsible for the acute care would be more difficult among those subjects with cognitive impairment.
This study has important limitations despite the strengths of a nationally representative sample. The measures of nursing facility use and the measures of function in the HRS sample all rely on self-reports or proxy-respondents both which are subject to recall and other biases. However, we do not have reason to believe that these biases would be differentially severe depending on the subject’s transitional site of care. Also, the combined method we used to assess nursing facility use resulted in findings consistent with our prior study which did not rely on self-reports. Another limitation in self-reports is that, even when accurate, the assessment of functional and cognitive function are taken from surveys prior to the index transition and subjects might have improved or worsened in the intervening period. For example, persons who received subacute rehabilitation might have improved more than those who did not. Also, persons with dementia who have impairments may have those impairments due to comorbid conditions. We highlight, however, that the potential for error would not be expected to vary based on the patient’s transitions patterns.
In conclusion, persons with dementia identified from a nationally representative cohort of older Americans followed for a decade experienced multiple transitions in care. We find little evidence for a global picture of dementia characterized by a terminal placement in a nursing facility although such placements may be the path for some persons with dementia. We replicate findings in this representative sample that demonstrate multiple transitions in care for persons with dementia including even transitions to home among those with significant physical and cognitive limitations and moderate to severe dementia. The reality of this "network of care" which includes both formal and informal settings and providers is unlikely to change. Indeed, evidence would suggest that the medical acuity of persons with dementia can be expected to increase both at home and in nursing facilities. Recognizing the expensive inefficiencies in the current system, the Federal Government is investing significant resources in testing models of care designed to improve transitions and reduce unnecessary hospitalizations of older adults, particularly among those with dementia.1, 30 This is necessary work to improve support at home and in nursing homes, especially as we prepare for growing numbers of patients with dementia transitioning across settings with increasing levels of acute and chronic care needs.
Acknowledgments
Supported by NIA grant R01 AG031222 and K24 AG024078
References
- 1.Callahan CM, Sachs GA, Lamantia MA, et al. Redesigning systems of care for older adults with Alzheimer's disease. Health Aff (Millwood) 2014;33(4):626–632. doi: 10.1377/hlthaff.2013.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Workgroup on NAPA's scientific agenda for a national initiative on Alzheimer’s disease Alzheimer's & Dementia. 2012;8(4):357–371. doi: 10.1016/j.jalz.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Arling G, Tu W, Stump TE, et al. Impact of dementia on payments for long-term and acute care in an elderly cohort. Med Care. 2013;51(7):575–581. doi: 10.1097/MLR.0b013e31828d4d4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hurd MD, Martorell P, Delavxande A, et al. Monetary costs of dementia in the United States. The New England journal of medicine. 2013;368(14):1326–1334. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callahan CM, Arling G, Tu W, et al. Transitions in Care for Older Adults with and without Dementia. Journal of the American Geriatrics Society. 2012;60(5):813–820. doi: 10.1111/j.1532-5415.2012.03905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LaMantia MA, Scheunemann LP, Viera AJ, et al. Interventions to improve transitional care between nursing homes and hospitals: a systematic review. Journal of the American Geriatrics Society. 2010;58(4):777–782. doi: 10.1111/j.1532-5415.2010.02776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teno JM, Gozalo PL, Bynum JP, et al. Change in end-of-life care for Medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470–477. doi: 10.1001/jama.2012.207624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coleman EA, Smith JD, Frank JC, et al. Preparing patients and caregivers to participate in care delivered across settings: the Care Transitions Intervention. Journal of the American Geriatrics Society. 2004;52(11):1817–1825. doi: 10.1111/j.1532-5415.2004.52504.x. [DOI] [PubMed] [Google Scholar]
- 9.Intrator O, Zinn J, Mor V. Nursing home characteristics and potentially preventable hospitalizations of long-stay residents. Journal of the American Geriatrics Society. 2004;52(10):1730–1736. doi: 10.1111/j.1532-5415.2004.52469.x. [DOI] [PubMed] [Google Scholar]
- 10.Grabowski DC, O'Malley AJ, Barhydt NR. The costs and potential savings associated with nursing home hospitalizations. Health Aff (Millwood) 2007;26(6):1753–1761. doi: 10.1377/hlthaff.26.6.1753. [DOI] [PubMed] [Google Scholar]
- 11.Arling G, Kane RL, Bershadsky J. Targeting criteria and quality indicators for promoting resident transitions from nursing home to community. Report to the Minnesota Department of Human Services. 2008:56. [Google Scholar]
- 12.Ouslander JG, Lamb G, Perloe M, et al. Potentially avoidable hospitalizations of nursing home residents: frequency, causes, and costs: [see editorial comments by Drs. Jean F. Wyman and William R. Hazzard, pp 760-761] Journal of the American Geriatrics Society. 2010;58(4):627–635. doi: 10.1111/j.1532-5415.2010.02768.x. [DOI] [PubMed] [Google Scholar]
- 13.Ouslander JG, Lamb G, Tappen R, et al. Interventions to reduce hospitalizations from nursing homes: evaluation of the INTERACT II collaborative quality improvement project. Journal of the American Geriatrics Society. 2011;59(4):745–753. doi: 10.1111/j.1532-5415.2011.03333.x. [DOI] [PubMed] [Google Scholar]
- 14.Cooke V, Arling G, Lewis T, et al. Minnesota's Nursing Facility Performance-Based Incentive Payment Program: an innovative model for promoting care quality. The Gerontologist. 2010;50(4):556–563. doi: 10.1093/geront/gnp140. [DOI] [PubMed] [Google Scholar]
- 15.Walsh EG, Wiener JM, Haber S, et al. Potentially avoidable hospitalizations of dually eligible Medicare and Medicaid beneficiaries from nursing facility and Home- and Community-Based Services waiver programs. Journal of the American Geriatrics Society. 2012;60(5):821–829. doi: 10.1111/j.1532-5415.2012.03920.x. [DOI] [PubMed] [Google Scholar]
- 16.Kessler C, Williams MC, Moustoukas JN, et al. Transitions of care for the geriatric patient in the emergency department. Clin Geriatr Med. 2013;29(1):49–69. doi: 10.1016/j.cger.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Gozalo P, Teno JM, Mitchell SL, et al. End-of-Life Transitions among Nursing Home Residents with Cognitive Issues. N Engl J Med. 2011;365(13):1212–1221. doi: 10.1056/NEJMsa1100347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coleman EA. Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs. Journal of the American Geriatrics Society. 2003;51(4):549–555. doi: 10.1046/j.1532-5415.2003.51185.x. [DOI] [PubMed] [Google Scholar]
- 19.Kane RL, Ouslander JG. Dementia as a moving target. Journal of the American Geriatrics Society. 2012;60(5):980–982. doi: 10.1111/j.1532-5415.2012.03918.x. [DOI] [PubMed] [Google Scholar]
- 20.Clark DO, S TE, Tu W, et al. Hospital and nursing home use from 2002-2008 among US older adults with cognitive impairment not dementia in 2002. Alzheimer Dis Assoc Disord. 2012 doi: 10.1097/WAD.0b013e318276994e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crimmins EM, Kim JK, Langa KM, et al. Assessment of cognition using surveys and neuropsychological assessment: the health and retirement study and the aging, demographics, and memory study. J Gerontol B Psychol Sci Soc Sci. 2011;66(Suppl 1):i162–i171. doi: 10.1093/geronb/gbr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher GG, Franks MM, Plassman BL, et al. Caring for individuals with dementia and cognitive impairment, not dementia: findings from the aging, demographics, and memory study. Journal of the American Geriatrics Society. 2011;59(3):488–494. doi: 10.1111/j.1532-5415.2010.03304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plassman BL, Langa KM, McCammon RJ, et al. Incidence of dementia and cognitive impairment, not dementia in the United States. Ann Neurol. 2011 doi: 10.1002/ana.22362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Annals of internal medicine. 2008;148(6):427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langa KM, Plassman BL, Wallace RB, et al. The Aging, Demographics, and Memory Study: study design and methods. Neuroepidemiology. 2005;25(4):181–191. doi: 10.1159/000087448. [DOI] [PubMed] [Google Scholar]
- 26.Langa K, Kabeto M, Weir D. Task 1 for Report on Race and Cognitive Impairment using HRS 2006. University of Michigan; 2009. [Google Scholar]
- 27.Jorm AF. The Informant Questionnaire on cognitive decline in the elderly (IQCODE): a review. Int Psychogeriatr. 2004;16(3):275–293. doi: 10.1017/s1041610204000390. [DOI] [PubMed] [Google Scholar]
- 28.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24(1):145–153. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- 29.Larson EB, Shadlen MF, Wang L, et al. Survival after initial diagnosis of Alzheimer disease. Annals of internal medicine. 2004;140(7):501–509. doi: 10.7326/0003-4819-140-7-200404060-00008. [DOI] [PubMed] [Google Scholar]
- 30.Reuben DB, Evertson LC, Wenger NS, et al. The university of california at los angeles Alzheimer's and dementia care program for comprehensive, coordinated, patient-centered care: preliminary data. Journal of the American Geriatrics Society. 2013;61(12):2214–2218. doi: 10.1111/jgs.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]