Abstract
Purpose
Gemtuzumab ozogamicin (GO), a calicheamicin-conjugated monoclonal antibody against CD33, has been used in the treatment of acute myeloid leukemia (AML). We evaluated the impact of the addition of GO to standard chemotherapy and hematopoietic stem cell transplant (HCT) in patients with FLT3/ITD.
Experimental Design
We analyzed children with FLT3/ITD-positive AML (n=183) treated on 2 consecutive Children’s Oncology Group (COG) AML trials (NCT00070174 and NCT00372593). Outcomes were assessed for FLT3/ITD patients receiving standard chemotherapy with or without GO (GO vs. No-GO respectively), and the impact of consolidation HCT for high-risk FLT3/ITD patients (high FLT3/ITD allelic ratio [ITD-AR]).
Results
For all FLT3/ITD patients, complete remission (CR) rates for the GO vs. No-GO cohorts were identical (64% vs. 64%; p=0.98). Relapse rate (RR) after initial CR was 37% for GO recipients vs. 59% for No-GO recipients (p=0.02), disease free survival (DFS) was similar (47% vs. 41%; p=0.45), with higher treatment related mortality (TRM) in GO recipients (16% vs. 0%; p=0.008). Among high risk FLT3/ITD patients with high ITD-AR, those who received HCT in first CR with prior exposure to GO had a significant reduction in RR (15% vs. 53%; p=0.007), with a corresponding DFS of 65% vs. 40% (p=0.079), and higher TRM (19% vs. 7%; p=0.08).
Conclusions
CD33 targeting with HCT consolidation may be an important therapeutic strategy in high risk FLT3/ITD AML and its efficacy and associated toxicity warrant further investigation.
Keywords: CD33, AML, FLT3/ITD, gemtuzumab ozogamicin
Introduction
CD33 is a cell surface myeloid antigen that is variably expressed on the majority of blasts in patients with acute myeloid leukemia (AML), but is absent from early hematopoietic progenitor cells.(1, 2) CD33 is the target of the calicheamicin toxin-conjugated humanized monoclonal antibody gemtuzumab ozogamicin (GO). High expression of CD33 is associated with high-risk disease features, such as internal tandem duplications of FLT3 (FLT3/ITD), and poor prognosis in pediatric AML.(3, 4) In vitro studies have shown a correlative relationship between CD33 expression and GO-mediated cytotoxicity.(5) The safety of combining GO with conventional chemotherapy in adult trials for relapsed AML ultimately resulted in the accelerated FDA approval of GO in 2000 by the United States (US) Food and Drug Administration.(6–8) However, the initial phase III study of GO combined with conventional chemotherapy in adults failed to demonstrate improved outcomes,(9) and the drug was withdrawn from the US market. Subsequent randomized controlled trials have showed that GO improves outcomes in certain subsets of patients with AML, particularly those with low or intermediate-risk cytogenetics.(8, 10, 11) The Children’s Oncology Group (COG) phase III pilot trial AAML03P1 and the subsequent phase III trial AAML0531 confirmed the safety and benefit of adding GO to intensive chemotherapy in pediatric AML.(6, 12)
Therapy intensification and advancements in supportive care have led to improvement in overall outcomes in pediatric AML. However, patients with FLT3/ITD have a poor prognosis with chemotherapy alone, especially those with a high allelic ratio (ITD-AR; >0.4) of mutant to wild type FLT3.(13–18) Although allogeneic hematopoietic stem cell transplant (HCT) increases the survival to approximately 65% for this cohort, alternative therapeutic approaches are needed to improve long-term survival in a significant number of children with this lesion.(13, 19) Because the limits to which conventional treatment can be intensified have been reached, the use of alternative approaches such as immunotherapy are necessary to further improve outcomes. In this study, we investigated the impact of CD33 targeting with GO in FLT3/ITD patients as a method to improve outcomes for this high-risk group of patients.
Methods
Patients and treatment
Pediatric patients (ages 1 month to 30 years) with de novo AML enrolled on the COG phase III pilot study AAML03P1 and the subsequent phase III trial AAML0531 were eligible for this study. Between December 2003 and November 2005, 30 of the 339 eligible patients treated on AAML03P1 were positive for the FLT3/ITD mutation. Between August 2006 and June 2010, 153 of 1,022 eligible patients treated on AAML0531 were positive for the FLT3/ITD mutation. This trial was conducted in accordance with the Declaration of Helsinki. Complete eligibility criteria, risk stratification based on cytogenetic and molecular features as well as randomization and complete treatment details have been previously reported.(6, 12) In brief, patients treated on AAML0531 were randomly assigned to one of two study arms, Arm A which included standard therapy alone (No-GO) or Arm B which included GO (dose 3 mg/m2) administered once during induction course I on day 6 and again during intensification course II on day 7. All patients on AAML03P1 received GO on the same schedule as patients on Arm B of AAML0531. Initially, all patients with FLT3/ITD were treated under a biologic randomization; wherein patients with a matched sibling donor (MSD) underwent HCT and those without a MSD continued along in the assigned chemotherapy arm, with patients on Arm B receiving a second dose of GO during intensification. Following definitive recognition of HAR FLT3/ITD as a high-risk disease feature, AAML0531 was amended and all patients with ITD-AR>0.4 enrolled after April 14, 2008, were allocated to receive consolidation HCT from a suitable donor.
Statistical analysis
The significance of observed difference in proportions was tested using Pearson’s χ2 test and Fisher’s exact test when data were sparse. Complete remission (CR) was defined as <5% blasts on morphologic examination and no evidence of extramedullary disease following the first course of induction therapy. The Kaplan-Meier method was used to estimate overall survival (OS) and disease free survival (DFS). Data were current on AAML03P1 and AAML0531 as of March 31, 2014. Estimates of OS and DFS at 5 years were reported along with corresponding two times Greenwood standard errors. Overall survival was defined as time from study entry until death, DFS as the time from end of induction I for patients in CR until relapse or death. Relapse risk was defined as the time from either end of induction I for patients in CR or from end of intensification I to relapse where deaths without a relapse were considered competing events. Treatment-related mortality (TRM) was defined as the time from end of induction I for patients in CR to death without relapse where relapses were considered competing events. Estimates of RR and TRM at 5 years were obtained by methods that account for competing events. For results that compare patients who receive HCT or consolidation chemotherapy alone, analyses are defined as time from completion of one course of intensification for patients who continue on chemotherapy. Patients lost to follow up were censored at their date of last known contact. The significance of predictor variables was tested with the log-rank statistic for OS and DFS and with Gray’s statistic for RR and TRM.(20) Cox proportional hazard models were used to estimate hazard rations (HR) for univariate and multivariate analyses of OS, EFS, and DFS.(21) Competing risk regression models were used to estimate HRs for analyses of RR and TRM.(22)
Results
GO and disease response
A total 1,214 patients with de novo AML treated on COG AAML03P1 and AAML0531 were evaluated for inclusion and we identified 183 patients positive for FLT3/ITD and their clinical outcome data were included in further analysis. The median follow-up time (and range) for patients alive at last contact 7.45 (3.96–8.94) years for 30 patients on AAML03P1 and 4.59 (0.3–6.44) years for 153 patients on AAML0531. Of these 183 patients with FLT3/ITD, 112 patients received GO in addition to standard chemotherapy (GO arm), treated on AAML0531 Arm B (n=82) and AAML03P1 (n=30). The remaining 71 patients all treated on AAML0531 Arm A received chemotherapy only on the No-GO arm. There were no significant differences by age, gender, race, ethnicity, extramedullary involvement, cytogenetic features, presenting white blood cell count, and bone marrow blast percentages between the GO and No-GO cohorts (Table 1). There were significantly more patients with NPM1 mutations in the No-GO vs. GO cohort (p=0.01). Although NPM1 mutations have subsequently been demonstrated to be a low-risk mutation and confer a favorable prognosis,(23, 24) patients treated on AAML03P1 and AAML0531 were not stratified by NPM1 status and some received HCT in first CR per protocol stratification. There were no significant differences between FLT3/ITD high vs. low allelic ratios between the two treatment cohorts (Table 1).
Table 1.
Clinical and biological characteristics of FLT3/ITD patients in the GO and No-GO cohorts.
| No-GO (n=71) n (%) |
GO (n=112) n (%) |
p-value | ||
|---|---|---|---|---|
| Gender | ||||
| Male | 41 (58%) | 61 (54%) | 0.66 | |
| Age, Median (range) | 13.2 (0.7–20.4) | 12.1 (1.6–20.9) | 0.49 | |
| 0–2y | 2 (3%) | 4 (4%) | 1 | |
| 3–10y | 20 (48%) | 41 (37%) | 0.24 | |
| 11–21y | 49 (69%) | 67 (60%) | 0.21 | |
| Race | ||||
| Asian | 3 (5%) | 12 (12%) | 0.13 | |
| Black or African | 7 (11%) | 13 (13%) | 0.77 | |
| White | 53 (84%) | 78 (76%) | 0.20 | |
| CNS Disease | ||||
| Yes | 5 (7%) | 7 (6%) | 1 | |
| Chloroma | ||||
| Yes | 9 (13%) | 9 (8%) | 0.30 | |
| WBC ×103/µL, median (range) | 66.5 (0.2–470) | 53.4 (1.2–827.2) | 0.94 | |
| BM Blasts % | 80 (3–98) | 79 (23–100) | 0.90 | |
| Cytogenetics | ||||
| Normal | 29 (58%) | 57 (54%) | 0.61 | |
| t(8;21) | 2 (3%) | 3 (3%) | 1 | |
| inv(16) | 3 (4%) | 2 (2%) | 0.38 | |
| Abnormal 11 | 4 (6%) | 2 (2%) | 0.21 | |
| t(6;9)(p23;q34) | 5 (7%) | 10 (10%) | 0.64 | |
| Del 7q | 0 (0%) | 0 (0%) | 1 | |
| Trisomy 8 | 9 (13%) | 21 (20%) | 0.27 | |
| Other | 4 (6%) | 9 (9%) | 0.53 | |
| NPM1 mutant | 17 (27%) | 12 (11%) | 0.01 | |
| CEBPA mutant | 4 (6%) | 7 (7%) | 1 | |
| FLT3/ITD allelic ratio | ||||
| ≤0.4 (Low) | 30 (42%) | 38 (34%) | 0.26 | |
| >0.4 (High) | 41 (58%) | 74 (66%) | ||
Patients in the GO cohort received the initial dose of GO during induction I, we therefore analyzed the impact of GO on CR rates at the end of induction (EOI) I. After the initial induction course, patients with FLT3/ITD had a CR rate of 64% vs. 77% for the FLT3/ITD negative patients (p<0.001). Among FLT3/ITD-positive cohort, patients with or without GO exposure had an identical CR rate of 64% (p=0.98). Minimal residual disease (MRD) as detected by flow cytometry was also analyzed and 56% of GO recipients achieved MRD negative status at EOI1 vs. 49% in the No-GO cohort (p=0.44), with 63% of both cohorts achieving MRD negative status at EOI2 (p=0.96).
Post-induction clinical outcome was evaluated based on induction exposure to GO. FLT3/ITD patients treated with GO had a reduction in RR of 37 ± 12% vs. 59 ± 16% for the No-GO cohort (p=0.02; Figure 1A). However, this GO associated improvement in relapse did not translate into an improvement in survival, as the GO vs. No-GO cohorts had a DFS of 47 ± 12% vs. 41± 15% (p=0.5; Figure 1B) and an OS of 50 ± 10% vs. 49 ± 13%, respectively (p=0.74). Evaluation of TRM demonstrated that patients who received GO had a significantly higher rate of TRM compared to the No-GO recipients (16 ± 9% vs. 0%; p=0.008; Figure 1C).
Figure 1. Clinical outcomes for FLT3/ITD patients in the No-GO and GO cohorts.
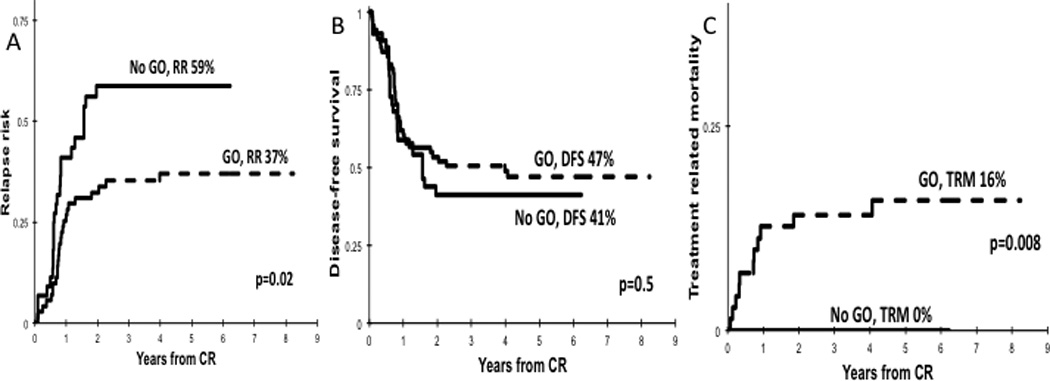
(A) RR, (B) DFS, and (C) TRM.
In univariable and multivariable cox regression analyses that included treatment arm, NPM1 mutation status, diagnostic white blood cell count, and race, treatment with GO was associated with lower RR compared to No-GO (HR 0.4, p=0.01; Supplementary Table 1). There were no differences among the GO vs. No-GO cohorts in OS (HR=0.99, p=0.99), EFS (HR=0.91, p=0.64) or DFS (HR=0.77, p=0.36; Supplementary Table 1). Given that all TRM events occurred in the GO arm, there was no convergence for further analysis, again demonstrating higher TRM for patients treated with GO.
GO and hematopoietic stem cell transplant
Our group and others have demonstrated the poor prognostic impact of high ITD-AR on outcome, and that these patients may benefit from intensification of consolidation therapy with allogeneic HCT.(13, 15, 25) As a result, an amendment was introduced into AAML0531 that allocated FLT3/ITD patients with high ITD-AR to the high risk arm of therapy and to receive allogeneic HCT in first CR. Of the 58 patients with FLT3/ITD who received HCT in first CR, 33 had received induction GO and 25 were treated on the No-GO arm. Cumulative incidence of relapse for the GO recipients was 22 ± 15% vs. 56 ± 21% for the No-GO cohort (p=0.003; Figure 2A) with a corresponding DFS of 56 ± 18% vs. 40 ± 20% (p=0.09; Figure 2B). TRM for GO recipients compared to No-GO recipients was 22 ± 15% vs. 4 ± 8% (p=0.08; Figure 2C). The OS for patients in the GO and No-GO cohorts was 65 ± 17% vs. 49 ± 22% (p=0.21).
Figure 2. Outcomes for FLT3/ITD patients in the No-GO and GO cohorts who received consolidation HCT in first CR.
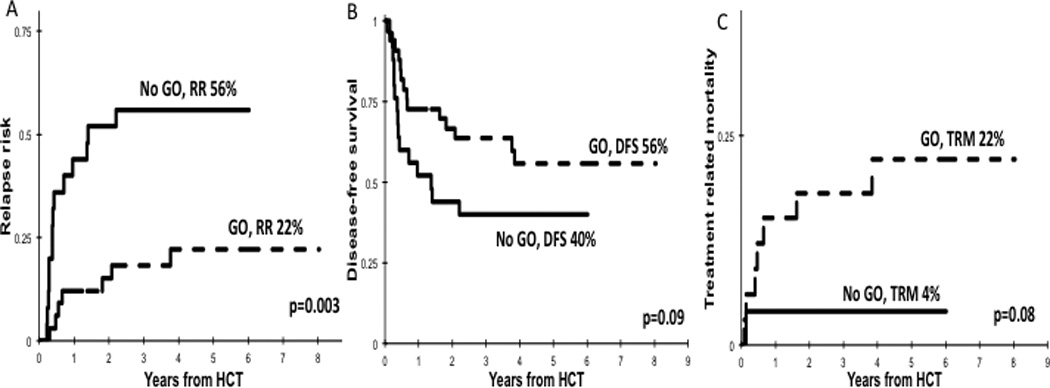
(A) RR, (B) DFS, and (C) TRM.
Among FLT3/ITD patients who received consolidation chemotherapy alone (n=57), patients who received GO (n=25) vs. No-GO (n=22) had similar RR (43 ± 17% vs. 58 ± 23%, p=0.28), OS (59 ± 17% vs. 60% ± 22%, p=0.90), DFS (45 ± 17% vs. 42 ± 22%; p=0.80), and TRM (12 ± 11% vs. 0%; p=0.11; Supplementary Figure 1).
Impact of GO and high allelic ratio FLT3/ITD
We evaluated the role of GO in this high-risk cohort with high ITD-AR who underwent HCT in first CR (n=41) according to treatment with induction GO (n=26) versus No-GO (n=15). Post transplant RR in GO recipients was 15 ± 15% vs. 53 ± 27% for the No-GO cohort (p=0.007; Figure 3A) with a corresponding DFS of 65 ± 19% vs. 40 ± 25% (p=0.08; Figure 3B) and OS of 68 ± 19% vs. 51 ± 27% (p=0.33). The TRM among GO vs. No-GO recipients treated with HCT was 19 ± 16% vs. 7 ± 13% (p=0.30; Figure 3C). Evaluation of the effect of GO on high ITD-AR patients who did not receive HCT demonstrated that GO recipients (n=17) had a RR 47 ± 25% vs. 64 ± 36% for the No-GO cohort (n=10; p=0.42), with a corresponding DFS of 47 ± 24% vs. 36 ± 32%, (p=0.66), OS of 64 ± 24% vs. 56 ± 33% (p=0.67), and a TRM of 6 ± 12% vs. 0% respectively (p=0.44).
Figure 3. Outcomes for HAR FLT3/ITD patients treated in the No-GO and GO cohorts who received HCT.
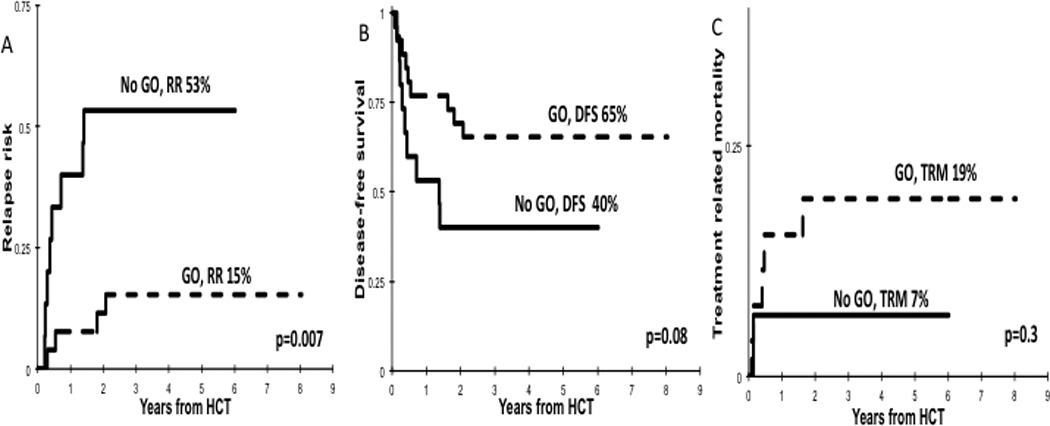
(A) RR, (B) DFS, (C) TRM.
We analyzed outcomes of low AR (≤0.4) patients and found GO recipients (n=25) had an OS of 53 ± 22% vs. 58 ± 22% for the No-GO cohort (n=22; p=0.99), with a corresponding DFS of 34 ± 21% vs. 44 ± 22% (p=0.87) and a RR of 42 ± 22% vs 56% ± 22% (p=0.19; Supplementary Table 2). High TRM was again noted in GO recipients compared to No-GO (24 ± 20% vs. 0%; p=0.04). Most patients with low AR received consolidation chemotherapy alone so these patients were not stratified further according to consolidation therapy.
GO and treatment-related mortality
The increase in TRM that was seen in the GO arm for FLT3/ITD patients was further analyzed, especially with regard to initial concerns that GO was associated with increased incidence of post-HCT sinusoidal obstructive syndrome (SOS). Among all patients treated on AAML03P1 and AAML0531, 31 had SOS of any grade that occurred after HCT. Among FLT3/ITD patients, 8 had SOS of any grade (GO, n=6; No-GO, n=2), although the overall numbers were too low to draw any significant conclusions. In almost all cases, SOS was not fatal. However, in 3 patients treated on the 2 studies, SOS was listed as the cause or significant contributing cause of death and occurred in the first 30 days post-HCT. Of the 3 patients, 2 were FLT3/ITD patients who received GO and 1 was a FLT3 wild-type patient who did not receive GO.
In an analysis of the causes of TRM among FLT3/ITD patients, we identified 14 cases of TRM in the GO cohort, equally divided between no-HCT (n=7) and HCT (n=7) recipients. Infections were the major cause of TRM among GO recipients (n=9, 64%). Among the no-HCT recipients, 6 of 7 deaths were attributed to infection, with 1 of these deaths occurring more than 1000 days after the end of the intensification course II, which included the last dose of GO. Among the HCT recipients in the GO cohort, infections were the cause of death in 4 patients. TRM was attributed to SOS in 1 patient and was the primary contributing cause where no primary cause was listed in 1 other patient. Among the GO cohort who received HCT, 5 of 7 deaths occurred more than 100 days from HCT and more than 250 days from the end of the induction I course which contained the only GO dose these patients received. Two of the deaths were very late and occurred 1.6 and 3.8 years post HCT. There was only case of TRM in the No-GO cohort and was attributed to infection.
Due to the higher rate of TRM observed among patients in the GO cohort compared to the No-GO cohort, we evaluated the toxicity on both treatment arms. We measured the time to neutrophil and platelet recovery only for patients treated on AAML0531 (n=153), because the complete data were not available for AAML03P1. After induction I, patients in the GO cohort showed a trend toward an increase in median time to neutrophil recovery (absolute neutrophil count [ANC]> 500/µL) compared with those in the No-GO cohort (33 vs. 30 days; p=0.06) and an increase in median time to platelet recovery (platelet count > 50×109/L; 29.5 vs. 26.5 days, respectively; p=0.003). Following HCT, there was no difference in median time to neutrophil recovery among the GO vs. No-GO cohort (30 vs. 30 days; p=0.66). However, the median time to post-HCT platelet recovery was higher for patients in the GO cohort compared to No-GO (44 vs. 39 day; p=0.05). Among FLT3/ITD patients who did not receive HCT, there were no differences in median time to recovery for ANC or platelets in intensification II or intensification course III among the GO and No-GO cohorts, with the GO cohort receiving an additional dose of GO in intensification II (Supplementary Table 3).
There were no differences between the cohorts for all grade 3 or higher toxicities (CTC v3.0 Toxicity) for any of the treatment cycles, including among patients who received HCT (Supplementary Table 4). Importantly, there were no differences among grade 3 or higher infection rates, incidence of hemorrhage, or liver dysfunction between both cohorts (Supplementary Table 4).
Discussion
In this retrospective study of de novo pediatric FLT3/ITD AML patients, we found that although the addition of GO to standard chemotherapy did not improve the initial CR rate, exposure to GO during induction led to significant reduction in relapse in this high-risk population. Furthermore, the impact of GO persisted into consolidation HCT, where those who had received induction GO had a significantly lower rate of relapse post HCT. The disease benefit was most pronounced in high ITD-AR patients, the cohort with the highest risk disease. Although the improvement in RR was very clear in both univariate and multivariate analyses, the findings suggest that an increase in TRM may have attenuated the direct translation of the reduction in RR into corresponding improvements in OS. Historically, patients with high ITD-AR have had the poorest outcomes with standard chemotherapy regimens. Data from COG as well as a number of adult trials have shown the benefit of HCT in first CR for this group of patients.(13, 15),(19, 26–28) For patients in the No-GO cohort, there were no differences in outcome measures according to HCT vs. no-HCT status, suggesting that GO may enhance the beneficial impact of HCT in reducing relapse risk. Our findings suggest that indeed the most optimal outcome for this high-risk cohort may be achieved with the use of induction GO followed by HCT in first CR.
The data we present is consistent with the increasing evidence from adult and pediatric clinical trials that targeting CD33 is a promising therapeutic strategy in AML and can enhance the effects of conventional chemotherapy and allogeneic HCT.(8, 10, 12) A recent meta-analysis on the use of GO in AML found that GO was associated with reduction in RR and improved OS, with the most significant survival benefit in patients with good and intermediate cytogenetic risk characteristics, but not in patients with FLT3/ITD.(11) Here, we show that patients with the high risk molecular lesion FLT3/ITD do experience significant disease benefit from GO, especially when combined with HCT. In addition to our study, Castaigne et al. have reported results of the French ALFA-0701 study which combined fractionated dosing of GO with conventional chemotherapy, and found that patients with FLT3/ITD derived a distinct benefit from the addition of GO to induction chemotherapy.(10, 29) The strategy of GO fractionated dosing is supported by data that the binding and internalization of GO results in renewed CD33 expression on myeloid blasts, which can be subsequently exploited to further saturate the blasts with additional GO and increase cell death.(30) Thus, fractionated dosing may provide further improvements in clinical outcomes for FLT3/ITD patients, especially when combined with consolidation HCT.
The single dose GO regimen utilized in the COG trials was in part selected due to initial concerns for GO-induced hepatotoxicity, particularly SOS when GO was given in close proximity to HCT.(31, 32) Although we did observe a few cases of SOS in the GO cohort, there was no apparent increase and the overall numbers are too low to draw any definitive conclusions. Patients in the GO cohort had significantly higher TRM overall, with the majority of deaths attributed to infection. It is important to note that the TRM in the No-GO cohort was very low, with only 1 death, which occurred post-HCT. This very low TRM rate is atypical for the intensity of standard AML therapy and lower than what was seen in AAML03P1 and AAML0531 overall, where there were no observed differences in overall TRM for patients in the GO vs. No-GO cohorts.(6, 12) Importantly, half of the TRM events in our study occurred over 100 days from the last dose of GO, and do not immediately seem to be directly attributable to GO. Even among patients who received GO prior to HCT, the majority of TRM events also occurred at over 100 days following HCT.
The GO recipients did have longer duration to platelet and neutrophil recovery in the courses prior to HCT, including post-induction I courses that did not include GO, as well as delayed platelet recovery following HCT. The delayed platelet recovery we observed correlates with that previously reported in adult AML patients receiving GO.(10) Our findings suggest that there may be significant GO-induced effects on normal CD33-positive myeloid progenitors, leading to potential marrow exhaustion, which may be exacerbated by exposure to subsequent cytotoxic therapy and lead to significantly prolonged myelosuppression. This delayed hematopoietic recovery and prolonged neutropenia could be a potential contributor to the higher TRM seen in the GO cohort, which was largely due to infectious causes. We observed no differences in infection rates between the two cohorts overall, however it is possible that prolonged myelosuppression could contribute to increased morbidity and mortality in the setting of infection. Further understanding of the lasting effects of CD33-targeting on myeloid progenitors could inform the safety of combining these agents with additional myelosuppressive therapies, including HCT.
We have previously shown that high-risk disease features, including FLT3/ITD, have high CD33 expression.(4) Studies by van der Velden et al. demonstrated that the level of bone marrow blast CD33 expression correlates with peripheral blood (PB) blast clearance by GO.(30) This suggests that high CD33 expression may mediate the anti-leukemic efficacy of GO in FLT3/ITD-positive AML. Adequate targeting of leukemia stem cells (LSCs) is essential to therapeutic efficacy and similar to bulk blasts, the sensitivity of LSCs to GO has been correlated with the level of CD33 expression.(33) Importantly, LSCs that harbor FLT3/ITD mutations are more sensitive to GO-mediated cytotoxicity when compared to non-ITD LSCs,(33) suggesting that additional biologic features may be involved. High levels of CD33 expression, which is often present on FLT3/ITD blasts, may be enhance the efficacy of GO. However it is likely that multiple biologic features, including overall disease burden and Pgp status, contribute to moderate the sensitivity to GO and other CD33 targeted agents.
FLT3/ITD is one of the first genomic lesions in AML to be targeted for therapeutic benefit with tyrosine kinase inhibitors (TKI), as the blasts are uniquely susceptible to apoptotic effects of FLT3 inhibition.(34–37) To date, FLT3 inhibitors have demonstrated the ability to favorably affect initial disease response, but have thus far failed to achieve sustained remissions and improvements in overall outcomes.(38–42) For many patients, FLT3 inhibition might be an inadequate therapeutic strategy, even when combined with conventional chemotherapy, as FLT3 is often not the only driver mutation and can be a later event absent in the founding clone.(43),(44) Thus additional therapeutic strategies, including those that target the LSC population, are necessary. The strategy of bulk reduction, a setting wherein TKIs can be very effective, might be combined with CD33-directed therapeutics, which can effectively target BM blasts, to create more effective therapeutic regimens. TKIs can also be used for prolonged periods of time whereas GO or anti-CD33 agents may have myelosuppressive effects limiting the timing and duration that they can be safely used.
Our findings confirm that CD33 targeting is an effective therapeutic strategy in AML and FLT3/ITD patients may derive a distinct disease benefit from this therapy, especially when combined with HCT for high ITD-AR patients. The increased TRM in this population warrants further investigation regarding optimal dosing and timing, especially in conjunction with HCT, to improve overall outcomes. Newly developed CD33-targeted agents, which may have distinct therapeutic profiles, are currently under investigation in clinical trials.(45, 46) FLT3/ITD patients might experience significant benefit from this treatment strategy, especially when combined with additional therapies such HCT and FLT3 inhibition.
Supplementary Material
Statement of Translational Relevance.
In this study we assess the impact of combining gemtuzumab ozogamicin (GO) with conventional chemotherapy in pediatric patients with FLT3/ITD positive AML and show that the addition of GO in induction results in reduction of relapse. This anti-leukemic effect was even more pronounced in high allelic ratio FLT3/ITD patients. Ours is the first study to examine the impact of CD33 targeting and GO in pediatric FLT3/ITD AML and assess its impact by allelic ratio, which is recognized as a marker of poor prognosis. This high-risk group of patients is in need of new therapeutic strategies as a significant number patients experience relapse despite intensive chemotherapy and hematopoietic stem cell transplant. Our study finds that FLT3/ITD patients experience significant benefit from CD33 targeting, and these results may be further improved when combined with additional therapies such HCT and FLT3 inhibition.
Acknowledgments
Financial Support: This work was supported by grants to the Children’s Oncology Group including U10 CA98543 (Chair’s grant) and U10 CA98413 (Statistical Center). K.T. is an Alex’s Lemonade Stand Foundation Young Investigator.
Footnotes
Conflicts of Interest: The authors declare no competing financial interests.
Authorship
Contributions: K.T. designed and performed research, analyzed data and wrote the manuscript. T.A.A. and R.B.G. performed statistical analysis and edited the manuscript. S.C.R., B.H., L.S., J.A.P, M.L., R.A, and A.S.G analyzed the data and edited the manuscript. S.M. designed and performed research, analyzed data and edited the manuscript.
References
- 1.Walter RB, Appelbaum FR, Estey EH, Bernstein ID. Acute myeloid leukemia stem cells and CD33-targeted immunotherapy. Blood. 2012;119:6198–6208. doi: 10.1182/blood-2011-11-325050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernstein ID. CD33 as a target for selective ablation of acute myeloid leukemia. Clinical lymphoma. 2002;2(Suppl 1):S9–S11. doi: 10.3816/clm.2002.s.002. [DOI] [PubMed] [Google Scholar]
- 3.Dinndorf PA, Buckley JD, Nesbit ME, Lampkin BC, Piomelli S, Feig SA, et al. Expression of myeloid differentiation antigens in acute nonlymphocytic leukemia: increased concentration of CD33 antigen predicts poor outcome--a report from the Childrens Cancer Study Group. Medical and pediatric oncology. 1992;20:192–200. doi: 10.1002/mpo.2950200303. [DOI] [PubMed] [Google Scholar]
- 4.Pollard JA, Alonzo TA, Loken M, Gerbing RB, Ho PA, Bernstein ID, et al. Correlation of CD33 expression level with disease characteristics and response to gemtuzumab ozogamicin containing chemotherapy in childhood AML. Blood. 2012;119:3705–3711. doi: 10.1182/blood-2011-12-398370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walter RB, Raden BW, Kamikura DM, Cooper JA, Bernstein ID. Influence of CD33 expression levels and ITIM-dependent internalization on gemtuzumab ozogamicin-induced cytotoxicity. Blood. 2005;105:1295–1302. doi: 10.1182/blood-2004-07-2784. [DOI] [PubMed] [Google Scholar]
- 6.Cooper TM, Franklin J, Gerbing RB, Alonzo TA, Hurwitz C, Raimondi SC, et al. AAML03P1, a pilot study of the safety of gemtuzumab ozogamicin in combination with chemotherapy for newly diagnosed childhood acute myeloid leukemia: a report from the Children's Oncology Group. Cancer. 2012;118:761–769. doi: 10.1002/cncr.26190. [DOI] [PubMed] [Google Scholar]
- 7.Aplenc R, Alonzo TA, Gerbing RB, Lange BJ, Hurwitz CA, Wells RJ, et al. Safety and efficacy of gemtuzumab ozogamicin in combination with chemotherapy for pediatric acute myeloid leukemia: a report from the Children's Oncology Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:2390–3295. doi: 10.1200/JCO.2007.13.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnett AK, Hills RK, Milligan D, Kjeldsen L, Kell J, Russell NH, et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:369–377. doi: 10.1200/JCO.2010.31.4310. [DOI] [PubMed] [Google Scholar]
- 9.Petersdorf SH, Kopecky KJ, Slovak M, Willman C, Nevill T, Brandwein J, et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013;121:4854–4860. doi: 10.1182/blood-2013-01-466706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castaigne S, Pautas C, Terre C, Raffoux E, Bordessoule D, Bastie JN, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet. 2012;379:1508–1516. doi: 10.1016/S0140-6736(12)60485-1. [DOI] [PubMed] [Google Scholar]
- 11.Hills RK, Castaigne S, Appelbaum FR, Delaunay J, Petersdorf S, Othus M, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014;15:986–996. doi: 10.1016/S1470-2045(14)70281-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gamis AS, Alonzo TA, Meshinchi S, Sung L, Gerbing RB, Raimondi SC, et al. Gemtuzumab Ozogamicin in Children and Adolescents With De Novo Acute Myeloid Leukemia Improves Event-Free Survival by Reducing Relapse Risk: Results From the Randomized Phase III Children's Oncology Group Trial AAML0531. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014 doi: 10.1200/JCO.2014.55.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meshinchi S, Alonzo TA, Stirewalt DL, Zwaan M, Zimmerman M, Reinhardt D, et al. Clinical implications of FLT3 mutations in pediatric AML. Blood. 2006;108:3654–3561. doi: 10.1182/blood-2006-03-009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zwaan CM, Meshinchi S, Radich JP, Veerman AJ, Huismans DR, Munske L, et al. FLT3 internal tandem duplication in 234 children with acute myeloid leukemia: prognostic significance and relation to cellular drug resistance. Blood. 2003;102:2387–2394. doi: 10.1182/blood-2002-12-3627. [DOI] [PubMed] [Google Scholar]
- 15.Schlenk RF, Kayser S, Bullinger L, Kobbe G, Casper J, Ringhoffer M, et al. Differential impact of allelic ratio and insertion site in FLT3-ITD positive AML with respect to allogeneic hematopoietic stem cell transplantation. Blood. 2014 doi: 10.1182/blood-2014-05-578070. [DOI] [PubMed] [Google Scholar]
- 16.Meshinchi S, Woods WG, Stirewalt DL, Sweetser DA, Buckley JD, Tjoa TK, et al. Prevalence and prognostic significance of Flt3 internal tandem duplication in pediatric acute myeloid leukemia. Blood. 2001;97:89–94. doi: 10.1182/blood.v97.1.89. [DOI] [PubMed] [Google Scholar]
- 17.Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–4335. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 18.Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–1759. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- 19.Schechter T, Gassas A, Chen H, Pollard J, Meshinchi S, Zaidman I, et al. The Outcome of Allogeneic Hematopoietic Cell Transplantation for Children with FLT3/ITD-Positive Acute Myelogenous Leukemia. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2014 doi: 10.1016/j.bbmt.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. New York: Wiley; 1980. [Google Scholar]
- 21.Cox DR. Regression Models and Life-Tables. Journal of the the Royal Statistical Society. 1972;34:187–220. [Google Scholar]
- 22.Fine JPaG, Robert J. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 23.Hollink IH, Zwaan CM, Zimmermann M, Arentsen-Peters TC, Pieters R, Cloos J, et al. Favorable prognostic impact of NPM1 gene mutations in childhood acute myeloid leukemia, with emphasis on cytogenetically normal AML. Leukemia. 2009;23:262–270. doi: 10.1038/leu.2008.313. [DOI] [PubMed] [Google Scholar]
- 24.Brown P, McIntyre E, Rau R, Meshinchi S, Lacayo N, Dahl G, et al. The incidence and clinical significance of nucleophosmin mutations in childhood AML. Blood. 2007;110:979–985. doi: 10.1182/blood-2007-02-076604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meshinchi S, Arceci RJ, Sanders JE, Smith FO, Woods WB, Radich JP, et al. Role of allogeneic stem cell transplantation in FLT3/ITD-positive AML. Blood. 2006;108:400. doi: 10.1182/blood-2005-12-4938. author reply-1. [DOI] [PubMed] [Google Scholar]
- 26.Brunet S, Labopin M, Esteve J, Cornelissen J, Socie G, Iori AP, et al. Impact of FLT3 internal tandem duplication on the outcome of related and unrelated hematopoietic transplantation for adult acute myeloid leukemia in first remission: a retrospective analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:735–741. doi: 10.1200/JCO.2011.36.9868. [DOI] [PubMed] [Google Scholar]
- 27.DeZern AE, Sung A, Kim S, Smith BD, Karp JE, Gore SD, et al. Role of allogeneic transplantation for FLT3/ITD acute myeloid leukemia: outcomes from 133 consecutive newly diagnosed patients from a single institution. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17:1404–1409. doi: 10.1016/j.bbmt.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bornhauser M, Illmer T, Schaich M, Soucek S, Ehninger G, Thiede C, et al. Improved outcome after stem-cell transplantation in FLT3/ITD-positive AML. Blood. 2007;109:2264–2265. doi: 10.1182/blood-2006-09-047225. author reply 5. [DOI] [PubMed] [Google Scholar]
- 29.Renneville A, Abdelali RB, Chevret S, Nibourel O, Cheok M, Pautas C, et al. Clinical impact of gene mutations and lesions detected by SNP-array karyotyping in acute myeloid leukemia patients in the context of gemtuzumab ozogamicin treatment: results of the ALFA-0701 trial. Oncotarget. 2014;5:916–932. doi: 10.18632/oncotarget.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Der Velden VH, te Marvelde JG, Hoogeveen PG, Bernstein ID, Houtsmuller AB, Berger MS, et al. Targeting of the CD33-calicheamicin immunoconjugate Mylotarg (CMA-676) in acute myeloid leukemia: in vivo and in vitro saturation and internalization by leukemic and normal myeloid cells. Blood. 2001;97:3197–3204. doi: 10.1182/blood.v97.10.3197. [DOI] [PubMed] [Google Scholar]
- 31.Sievers EL, Larson RA, Stadtmauer EA, Estey E, Lowenberg B, Dombret H, et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19:3244–3254. doi: 10.1200/JCO.2001.19.13.3244. [DOI] [PubMed] [Google Scholar]
- 32.Wadleigh M, Richardson PG, Zahrieh D, Lee SJ, Cutler C, Ho V, et al. Prior gemtuzumab ozogamicin exposure significantly increases the risk of veno-occlusive disease in patients who undergo myeloablative allogeneic stem cell transplantation. Blood. 2003;102:1578–1582. doi: 10.1182/blood-2003-01-0255. [DOI] [PubMed] [Google Scholar]
- 33.Jawad M, Seedhouse C, Mony U, Grundy M, Russell NH, Pallis M. Analysis of factors that affect in vitro chemosensitivity of leukaemic stem and progenitor cells to gemtuzumab ozogamicin (Mylotarg) in acute myeloid leukaemia. Leukemia. 2010;24:74–80. doi: 10.1038/leu.2009.199. [DOI] [PubMed] [Google Scholar]
- 34.Brown P, Meshinchi S, Levis M, Alonzo TA, Gerbing R, Lange B, et al. Pediatric AML primary samples with FLT3/ITD mutations are preferentially killed by FLT3 inhibition. Blood. 2004;104:1841–1849. doi: 10.1182/blood-2004-03-1034. [DOI] [PubMed] [Google Scholar]
- 35.Levis M, Tse KF, Smith BD, Garrett E, Small D. A FLT3 tyrosine kinase inhibitor is selectively cytotoxic to acute myeloid leukemia blasts harboring FLT3 internal tandem duplication mutations. Blood. 2001;98:885–887. doi: 10.1182/blood.v98.3.885. [DOI] [PubMed] [Google Scholar]
- 36.Levis M, Allebach J, Tse KF, Zheng R, Baldwin BR, Smith BD, et al. A FLT3-targeted tyrosine kinase inhibitor is cytotoxic to leukemia cells in vitro and in vivo. Blood. 2002;99:3885–3891. doi: 10.1182/blood.v99.11.3885. [DOI] [PubMed] [Google Scholar]
- 37.Mead AJ, Gale RE, Kottaridis PD, Matsuda S, Khwaja A, Linch DC. Acute myeloid leukaemia blast cells with a tyrosine kinase domain mutation of FLT3 are less sensitive to lestaurtinib than those with a FLT3 internal tandem duplication. British journal of haematology. 2008;141:454–460. doi: 10.1111/j.1365-2141.2008.07025.x. [DOI] [PubMed] [Google Scholar]
- 38.Ravandi F, Cortes JE, Jones D, Faderl S, Garcia-Manero G, Konopleva MY, et al. Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:1856–1862. doi: 10.1200/JCO.2009.25.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inaba H, Rubnitz JE, Coustan-Smith E, Li L, Furmanski BD, Mascara GP, et al. Phase I pharmacokinetic and pharmacodynamic study of the multikinase inhibitor sorafenib in combination with clofarabine and cytarabine in pediatric relapsed/refractory leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:3293–3300. doi: 10.1200/JCO.2011.34.7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wander SA, Levis MJ, Fathi AT. The evolving role of FLT3 inhibitors in acute myeloid leukemia: quizartinib and beyond. Therapeutic advances in hematology. 2014;5:65–77. doi: 10.1177/2040620714532123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fischer T, Stone RM, Deangelo DJ, Galinsky I, Estey E, Lanza C, et al. Phase IIB trial of oral Midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:4339–4345. doi: 10.1200/JCO.2010.28.9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cortes JE, Kantarjian H, Foran JM, Ghirdaladze D, Zodelava M, Borthakur G, et al. Phase I study of quizartinib administered daily to patients with relapsed or refractory acute myeloid leukemia irrespective of FMS-like tyrosine kinase 3-internal tandem duplication status. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:3681–3687. doi: 10.1200/JCO.2013.48.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee BH, Williams IR, Anastasiadou E, Boulton CL, Joseph SW, Amaral SM, et al. FLT3 internal tandem duplication mutations induce myeloproliferative or lymphoid disease in a transgenic mouse model. Oncogene. 2005;24:7882–7892. doi: 10.1038/sj.onc.1208933. [DOI] [PubMed] [Google Scholar]
- 44.Shih LY, Huang CF, Wu JH, Lin TL, Dunn P, Wang PN, et al. Internal tandem duplication of FLT3 in relapsed acute myeloid leukemia: a comparative analysis of bone marrow samples from 108 adult patients at diagnosis and relapse. Blood. 2002;100:2387–2392. doi: 10.1182/blood-2002-01-0195. [DOI] [PubMed] [Google Scholar]
- 45.Kung Sutherland MS, Walter RB, Jeffrey SC, Burke PJ, Yu C, Kostner H, et al. SGN-CD33A: a novel CD33-targeting antibody-drug conjugate using a pyrrolobenzodiazepine dimer is active in models of drug-resistant AML. Blood. 2013;122:1455–1463. doi: 10.1182/blood-2013-03-491506. [DOI] [PubMed] [Google Scholar]
- 46.Krupka C, Kufer P, Kischel R, Zugmaier G, Bogeholz J, Kohnke T, et al. CD33 target validation and sustained depletion of AML blasts in long-term cultures by the bispecific T-cell-engaging antibody AMG 330. Blood. 2014;123:356–365. doi: 10.1182/blood-2013-08-523548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


