Abstract
Perchlorate exposure occurs from ingestion of natural or manmade perchlorate in food or water. Perchlorate is used in a variety of industrial products including missile fuel, fireworks, and fertilizers, and industrial contamination of drinking water supplies has occurred in a number of areas. Perchlorate blocks iodide uptake into the thyroid, and decreases the production of thyroid hormone, a critical hormone for metabolism, neurodevelopment, and other physiologic functions. Occupational and clinical dosing studies have not identified clear adverse effects, but may be limited by small sample sizes, short study durations, and the inclusion of mostly healthy adults. Expanding evidence suggests that young children, pregnant women, fetuses, and people co-exposed to similarly acting agents may be especially susceptible to perchlorate. Given the ubiquitous nature of perchlorate exposure, and the importance of thyroid hormone for brain development, studying the impact of perchlorate on human health could have far-reaching public health implications.
Keywords: perchlorate, drinking water, thyroid, iodine
Introduction
Perchlorate is a chemical anion that occurs from both anthropogenic and natural sources. The chemical structure and other characteristics of perchlorate are shown in Table 1. Perchlorate is a strong oxidizing agent and has been used in a variety of industrial and consumer products including missile fuel, fireworks, vehicle airbags, fertilizers, and other products (1). Perchlorate also occurs naturally and has been found in high concentrations in soil and water in certain arid areas such as northern Chile and the southwest US (2, 3). Perchlorate can also occur as a natural impurity in nitrate salts from the desert regions in northern Chile, which have been imported and used in the production of nitrate fertilizers used in the US and elsewhere.
Table 1.
Chemical characteristics of perchlorate
| Chemical formula | ClO4− |
| Chemical structure |
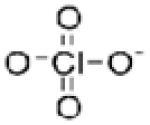
|
| CAS number | 14797-73-0 |
| Molecular weight | 99.45 g/mole |
| Physical form | White crystalline powder or clear liquid |
| Common chemical forms |
Ammonium perchlorate, sodium perchlorate, potassium perchlorate, and perchloric acid |
Perchlorate is highly stable in the environment and can be found in water, soil, and plants. Exposure to perchlorate is widespread and essentially everyone is exposed (4). Human exposure can occur from eating contaminated foods, inhalation or ingestion in occupationally-exposed workers, or from drinking water contaminated with perchlorate from either natural or man-made origins. In recent analyses of urinary perchlorate concentrations in the US National Health and Nutrition Survey (NHANES), detectable levels of perchlorate were found in every sample tested (detection limit=0.05 ppb) (5). Further analyses of NHANES participants’ diets and water sources suggested that food was the major source of perchlorate exposure in most people, with common sources being dark leafy green vegetables, milk products, fruits, and eggs (6). In 2005-2006 market basket analysis of 280 foods in the US food supply by the US Food and Drug Administration, detectable levels of perchlorate were found in at least one sample of 74% of the foods tested, with some of the highest levels reported in milk products (e.g. sour cream, milk, ice cream), certain meat products (e.g. cured ham, bologna, shrimp), and vegetables (collards, tomatoes, green beans, summer squash, potatoes) (7). It is important to note that while food is probably the major source of perchlorate in the population as a whole, this is not the case for every individual. In people living in areas where drinking water perchlorate concentrations are elevated, such as those living near industrially contaminated sites, water may become a more predominant exposure source than food and may be the primary determinant of perchlorate toxicity in these populations.
Contamination of drinking water
A number of water supplies in the US are contaminated with elevated levels of perchlorate. In an analysis of water samples from 3,262 residences of NHANES subjects, detectable concentrations of perchlorate (>0.100 ppb) were identified in 83% of samples, with a median concentration of 1.16 ppb (8). As part of the 2001–2005 US Unregulated Contaminant Monitoring Regulation (UCMR) survey data, perchlorate concentrations were assessed in 3,865 public water supplies throughout the US (9). Of the systems tested, approximately 160 (4.1%) had at least one reading above the detection limit of 4 ppb. These detections were identified in 26 states and two territories, and involved systems supplying approximately 11 million people. Of those with detectable levels, the mean and median concentrations were 9.85 ppb and 6.40 ppb, respectively. The current California regulatory standard for perchlorate in water is 6 ppb. This standard is based on evidence from a human intervention trial linking perchlorate to decreases in iodide uptake into the thyroid gland and evidence suggesting an enhanced susceptibility in certain potentially susceptible groups (10). Currently, the US Environmental Protection Agency does not have a regulatory standard for perchlorate in drinking water.
In a number of areas with elevated drinking water perchlorate concentrations, local water supplies have been contaminated with perchlorate from industrial sources. Perchlorates are soluble in water and generally have high mobility in soils (1). Perchlorate manufacturing, usually involving the production of ammonium perchlorate, sodium perchlorate, potassium perchlorate, and perchloric acid has taken place in facilities in a variety of locations in the US and elsewhere, and contamination of soil and water sources has occurred at some of these sites. For example, soil contamination at a perchlorate manufacturing facility near Henderson, Nevada lead to a plume of perchlorate and other chemicals that eventually contaminated local aquifers and the nearby Lake Mead, which feeds into the Colorado River. This river is a major source of drinking water for much of the southwest US including Los Angeles and San Diego Counties. Perchlorate concentrations as high as 9 ppb were reported in the Colorado River but have been gradually reduced with recent clean-up efforts (11). A study involving over 1,800 pregnant women in San Diego County, which receives much of its drinking water from the Colorado River, reported urinary perchlorate concentrations that were approximately two-times higher than national averages (12).
Environmental perchlorate contamination may also occur at sites used for missile recycling, propellant or munitions disposal, or other military operations (13). The Department of Defense reported detectable levels of perchlorate at 284 (almost 70 percent) of its installations sampled from 1997 to 2009, with levels ranging from <1 ppb to 2.6 million ppb (14). Perchlorate has also been used in the manufacturing of fireworks. Although the extent of contamination from this source is unknown, environmental contamination can potentially occur at sites of fireworks manufacturing or displays.
Human pharmacokinetics and toxicity
Once ingested, perchlorate is fairly readily absorbed. In humans, perchlorate undergoes little metabolism and is excreted mostly unchanged. The primary route of perchlorate excretion is urination, and urinary levels of perchlorate are commonly used as a biomarker of perchlorate exposure (4). Following oral dosing in humans, perchlorate is fairly rapidly excreted with a half-life of about 8 hours (15). In lactating women, perchlorate is taken up into breast milk and this may be another site of perchlorate excretion as well as a source of exposure to breast fed infants (16).
Thyroid inhibition
The major toxic effect of perchlorate in humans is thyroid disruption. Perchlorate competitively inhibits the sodium-iodide symporter (NIS), an intrinsic membrane glycoprotein responsible for the uptake of iodide into the thyroid and other organs. Perchlorate is a potent inhibitor of this transporter with an affinity of about 30-times that of iodide (17). Iodide, the ion form of iodine, is a key component in the structure of the main forms of thyroid hormone (thyroxine (T4) and triiodothyronine (T3)) (Figure 1). As such, inhibition of its uptake into thyroid follicular cells, the cells responsible for the production of thyroid hormone, can lead to decreased production and secretion of thyroid hormone. In fact, in the past, perchlorate at high doses (600-1000 mg/day) was used as a treatment for hyperthyroidism, an overproduction of thyroid hormone. Since the 1950’s its medicinal use has diminished dramatically after several cases of aplastic anemia were reported in patients taking high doses of perchlorate (18, 19).
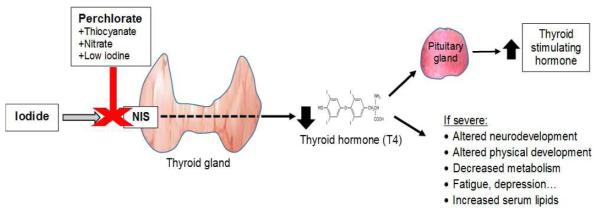
Figure 1.
Competitive inhibition of the sodium iodide symporter (NIS) by perchlorate and subsequent impacts on thyroid hormones and symptoms.
Thyroid hormone is involved in a wide variety of physiologic processes including metabolism, growth and development. Decreased production of this hormone can lead to hypothyroidism and clinical symptoms such as fatigue, muscle weakness, sensitivity to cold, constipation, weight gain, depression, and impaired memory. In the fetus and child, thyroid hormone is required for proper brain and neurological development (20). The fetal thyroid does not begin producing significant amounts of thyroid hormone until later in pregnancy, so in early pregnancy maternal T4 that crosses the placenta is the major source to serum T4 in the fetus (21). Severe decreases this hormone in utero and in early-life can lead to cretinism, a condition of severely arrested mental and physical growth commonly associated with a missing or underdeveloped thyroid or severe iodine deficiency during pregnancy (22). In addition, the results of several studies have suggested that even moderate decrements in maternal thyroid hormone levels or increases in thyroid stimulating hormone (TSH) during pregnancy, including those occurring within normal reference ranges, may also be associated with significant declines in offspring IQ or other cognitive measures (23-25). TSH is a hormone that is produced by the pituitary in response to diminished production of thyroid hormone, and increases in its serum level is sometimes a more sensitive indicator of thyroid stress than serum T4 or T3 levels (Figure 1).
Human studies of perchlorate and thyroid hormone
Studies in laboratory animals have investigated the relationship between perchlorate exposure and iodide uptake inhibition and decreases in thyroid hormone production (26). However, because major differences exist between humans and other species in terms of thyroid hormone storage, excretion, and clearance; co-exposures; regulatory feedback mechanisms; and thyroid hormone protein binding, the relevance of these findings to humans is unknown (27, 28). As discussed above, high doses of perchlorate were used in the past for medicinal purposes in humans and are known to decrease iodide uptake into the thyroid and decrease thyroid hormone production (15, 19, 29). However, the exact impacts of lower perchlorate exposures, such as those that may occur from occupational or environmental exposures, have been somewhat controversial.
A number of studies have assessed the toxicity of low to moderate doses of perchlorate in human populations. In a series of studies of workers in an ammonium perchlorate production facility, pre-shift to during-shift increases in urinary perchlorate concentrations (e.g. average concentrations of 0.16 mg/g creatinine pre-shift to 43 mg/g creatinine during-shift), and pre-shift to during-shift decreases in thyroidal radioactive iodine uptake (e.g. from 21.5 percent pre-shift to 13.5 percent during-shift) were reported (30-32). However, statistically significant decreases in serum thyroxine or increases in TSH were not seen. A number of clinical trials have also been done, in which human volunteers have been given known doses of perchlorate, and as a whole these have suggested that while lower exposures (e.g. 1.4 to 10 mg/day) for limited periods of time may impact thyroidal iodide uptake, they are not clearly associated with serum thyroid hormone concentrations in these study subjects (15, 33-35). Importantly though, in most of these studies, the daily doses of perchlorate were only given for a relatively short period of time (e.g. 14 days). This may be too short to overcome the compensation and maintenance of serum thyroid hormone levels due to the release of thyroid hormone stored in the thyroid (27). In one investigation, a double blinded, randomized clinical dosing study, 13 healthy volunteers received either placebo, or 0.5 mg or 3 mg of potassium perchlorate once per day for six months. This study also found no significant changes in serum T4 or TSH concentrations with perchlorate dosing, either during or after the dosing period (33). Interestingly though, decreases in thyroid radioactive iodide uptake were also not seen, even at dose levels similar to those where they had been reported in another study (15). The reason for this is unknown, although the authors noted that this could be due to the small number of participants, differences in dosing regimens, or the possibility that the NIS may be up-regulated as an adaptive response to long-term exposure.
Potentially susceptible populations
An important aspect of the clinical trial and occupational studies on perchlorate and thyroid function that have been done to date is that they have involved mostly healthy adults. A gradually expanding body of evidence suggests certain sub-populations could be more susceptible to perchlorate than healthy adult subjects (28). Table 2 shows several of the key epidemiologic studies of perchlorate and thyroid function. Supporting this evidence is the fact that healthy adults have an estimated several months of thyroid hormone stored in the thyroid gland, and these stores likely help maintain serum thyroid hormone concentrations at normal levels during relatively short periods of thyroid hormone disruption. In comparison, the half-life of T4 in the serum of neonates is only three days (36), and thyroidal stores of thyroidal hormone are estimated to be less than one day (37). Because of these low stores, neonates and other young children may not be able to compensate for intermittent short-term perchlorate exposures as well as adults. Another factor is that pregnancy is a time of increased stress on the thyroid, and an increased demand for iodine intake (38). Additional exposures like perchlorate during this time of stress could potentially lead to toxic effects that may not otherwise occur in healthy, non-pregnant people. Some data also suggests that many young children in the U.S. may not have an adequate iodine intake. In a study of 57 women from the Boston area, Pearce et al. estimated that 47 percent of the breast milk samples did not contain enough iodine to meet the infant iodine intake recommended by the Institute of Medicine (39). Low levels of circulating iodide could lead to a reduced iodide uptake by the thyroid that may be exacerbated by perchlorate. As a whole, all of these factors provide a biological basis to suggest that fetuses, young children, or pregnant women might be especially susceptible to perchlorate.
Table 2.
Selected key epidemiologic studies of perchlorate in and impacts on the thyroid gland
| Author (ref) | Design | Exposure | Sample size | Results | Comments |
|---|---|---|---|---|---|
| Greer et al., 2002 (15) |
Intervention trial in healthy US adults |
Daily doses of perchlorate in drinking water: 0.007, 0.02, 0.1, and 0.5 mg/kg- day for two weeks |
7-10 subjects at each dose level |
Dose dependent decreases in radioactive iodine uptake into the thyroid of 2 to 67%. No changes in serum thyroid hormone levels |
Decreases in radioactive iodine uptake were statistically significant at the three highest dose levels. |
| Blount et al., 2006 (61) |
Cross-sectional study in the 2001-2 US National Health and Nutrition Examination Survey (NHANES) |
A single urinary concentration of perchlorate measured in each subject |
1,111 women age 12 years and older |
A statistically significant association between increasing perchlorate and decreasing serum levels of total T4 and increasing serum levels of TSH in women with urinary iodine concentrations <100 μg/L |
Associations with total T4 were not seen in women with urinary iodine concentrations ≥100 μg/L |
| Steinmaus et al., 2015 (12) |
Longitudinal study in pregnant women in San Diego County, CA in the years 2000-2003 |
A single urinary concentration of perchlorate measured in each subject collected at an average of 8.6 weeks of pregnancy |
1,880 pregnant women |
A statistically significant association between increasing perchlorate and decreasing serum levels of total T4 and free T4, and increasing serum levels of TSH measured at an average of 17 weeks gestation |
Greater associations seen in women with elevated levels of nitrate and thiocyanate, and with anti-thyroid antibody positivity |
| Taylor et al.,
2014 (52) |
Longitudinal study of perchlorate exposure during pregnancy in Cardiff, UK and Turin, Italy in 2002-2006 and offspring IQ at age 3 years |
A single urinary concentration of perchlorate measured in each mother during the first trimester of pregnancy |
487 mother-child pairs who were hypothyroid/ hypothyroxinemic during pregnancy |
Maternal perchlorate levels in the highest 10% increased the odds of child IQ being in the lowest 10% (OR=3.14; 95% CI, 1.38-7.13) |
|
| Horton et al., 2015 (63) |
Cross-sectional study in pregnant women in New York City, NY in the years 2009-2010 |
A single urinary concentration of perchlorate, nitrate, and thiocyanate measured in each subject collected at 12 weeks gestation |
284 pregnant women | A statistically significant positive association between the weighted sum of urinary concentrations of perchlorate, thiocyanate, and nitrate and increased serum TSH, with perchlorate having the largest weight in the weighted index. |
No association seen when perchlorate analyzed separately. |
CI, confidence interval; OR, odds ratio; T4, thyroxine; TSH, thyroid stimulating hormone
To date, the results of human epidemiologic studies on perchlorate and thyroid function in pregnant women have been mixed. For example, in a series of studies done in Wales, Greece, Italy, and Argentina, no clear associations between maternal urinary levels of perchlorate and serum thyroid levels during pregnancy were seen (40-42). However, in a recent analysis of 1,880 pregnant women from a more highly exposed area in southern California, a statistically significant association was identified between increasing urinary perchlorate concentrations and decreasing serum T4 and increasing serum TSH levels (12). Similar associations were also seen in a cross-sectional study in 200 pregnant women from Thailand (43). A number of studies have also examined associations between perchlorate concentrations in drinking water during pregnancy, and measurements of TSH or T4 bloodspot concentrations in newborns (2, 44-51). These T4 or TSH levels are often collected as part of mandatory screening programs for congenital hypothyroidism. The results of these studies have also been mixed, with some but not all identifying statistically significant associations between increasing perchlorate exposure and increasing newborn TSH. Few studies have assessed associations between perchlorate exposure in pregnancy and subsequent offspring IQ or other cognitive measures. In a retrospective analysis of 487 mother-child pairs in mothers from Cardiff, UK and Turin, Italy who were pregnant from 2000-2006 and who were hypothyroid or hypothyroxinemic during pregnancy, first trimester urinary perchlorate levels in the highest 10% of the study population were associated with a greater than three-fold increased odds of offspring IQ being in the lowest 10% at 3 years of age (odds ratio=3.14; 95% CI 1.38, 7.13; p=0.006) (52).
The reasons for most of the inconsistencies in results across the different studies of perchlorate in pregnant women and newborns are not known, but many could be related to differences in confounding factors (i.e. other factors that may impact thyroid hormone levels like age, thyroid or other disease, use of certain medications, or low iodine intake), the specific methods used to assess perchlorate exposure or thyroid hormone status, the presence of various levels of potential effect modifiers (e.g. the presence of thyroid disease or high nitrate or thiocyanate intake), differences in statistical analyses, or sample sizes and statistical power (26). One key aspect in the design of these studies is the methods used to estimate perchlorate exposure. For example, several studies have estimated perchlorate exposures based on the perchlorate concentrations in the drinking water supplies where the subjects have lived, without knowledge of whether or not the subject actually drank from this source, how much they drank, or the presence of perchlorate intake from food (46, 47, 49, 53, 54). In addition, in almost all studies thyroid hormone status was based on only a single serum measurement, despite the fact that thyroid hormone levels can vary throughout the day and from day to day. Similarly, some studies have based perchlorate exposure on only a single urinary measurement, and these concentrations can also vary somewhat from day to day (55). Potentially, each of these factors could cause either false positive or false negative findings. However, in most studies measurements of exposure and outcome were done independently (e.g. the methods used to classify perchlorate exposure were independent of whether a subject had high or low thyroid hormone levels). As such, in most studies these measurement errors would most likely bias results towards underestimating any true perchlorate-thyroid hormone associations that may exist (26, 56). This bias, and the resulting underestimated effect sizes, could result in reduced statistical power and may have led to some null study results.
In addition to pregnant women and young children, another group that could be particularly susceptible to perchlorate are people who are exposed to other factors that also cause thyroid disruption. As with perchlorate, both nitrate and thiocyanate also competitively inhibit the NIS (57). Thiocyanate (SCN−) is an anion or a salt or ester containing this ion that is produced in the metabolism of cysteine and detoxification of cyanide, and is commonly found in cruciferous vegetables (e.g. cabbage, broccoli, Brussel sprouts) or as a result of tobacco smoking (58, 59). Nitrate (NO3 ) is a chemical commonly found in drinking water contaminated by agricultural use of nitrate fertilizers or by livestock grazing (60). It also occurs naturally in foods such as leafy green vegetables, and is used as a preservative in processed meats. In analyses of data from the 2001-2 NHANES study, perchlorate-thyroid hormone associations were found to be strongest in women with low urinary iodine levels (<100 µg/L) and elevated urinary thiocyanate concentrations (in the upper tertile) (61, 62). And, in a study of 284 women from New York City who were pregnant from 2009-2010, while statistically significant associations with serum thyroid hormone levels were not seen for urinary concentrations of perchlorate, thiocyanate, or nitrate individually, the weighted sum of all three of these analytes was associated with increased maternal serum TSH, with perchlorate having the largest contribution (63).
An in vitro study involving human NIS transfected into Chinese hamster ovary cells has shown that perchlorate, thiocyanate, and nitrate, in combination with low iodine, can have additive effects at inhibiting iodide uptake by the NIS (17, 64). In this study, the relative potency of perchlorate on a molar basis at inhibiting iodide uptake was found to be 15, 30, and 240 times that of thiocyanate, iodide, and nitrate. Importantly, these results are based on potency per moles, and although perchlorate is more potent on this basis, human exposure levels on a molar basis are typically many times higher for nitrate and thiocyanate than for perchlorate. Given this, some authors have suggested that in human populations the iodide uptake inhibiting effects of thiocyanate and nitrate should far overwhelm those of perchlorate (65, 66). Importantly though, these potency estimates are based on in vitro data, without consideration of the complex compensatory mechanisms, lower exposure levels, chronic exposures, co-exposures, nitrate or thiocyanate metabolism, and other factors that can complicate their interpretation and limit their relevance to actual human conditions (26). In addition, as reviewed above, a number of studies have identified perchlorate-thyroid hormone associations in human populations at perchlorate, thiocyanate, and nitrate exposure levels that are inconsistent with these in vitro potency estimates. Given all of this, the ability of in vitro potency studies to mimic real-life human conditions and to accurately predict perchlorate toxicity in humans cannot be firmly established at this time.
Conclusion
In conclusion, perchlorate exposure is ubiquitous in humans. Although food may be the greatest source of exposure in most people, drinking water is likely the greatest source in those drinking from water sources that have been industrially or naturally contaminated with elevated concentrations of perchlorate. It has been well established that perchlorate can block iodide uptake into the thyroid gland, and result in decreased production and secretion of thyroid hormone. A number of studies have also linked relatively common lower, environmental levels of perchlorate to decrements in thyroid function, although findings are not consistent across all studies. Most positive findings have been reported in potentially susceptible groups including pregnant women, newborns, and people exposed to multiple thyroid disrupting agents. To date, major regulatory agencies such as the US Environmental Protection Agency have not established regulatory standards for perchlorate in drinking water, although a few states in the US have developed their own regulations. For example, California and Massachusetts have set maximum contaminant levels of 6 and 2 ppb, respectively, for perchlorate in public drinking water supplies (67). Given the widespread nature of perchlorate exposure, and the importance of thyroid hormone for proper brain and neurodevelopment and other physiologic functions, further research on the long-term toxicity of perchlorate, especially in susceptible populations, could have far-reaching public health implications.
Footnotes
Conflict of Interest
Craig M. Steinmaus declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.ATSDR . Toxicological Profile for Perchlorates. Agency for Toxic Substances and Disease Registry. Atlanta, GA: 2008. Available from: http://www.atsdr.cdc.gov/toxprofiles/tp162-c3.pdf. [PubMed] [Google Scholar]
- 2.Tellez Tellez R, Michaud Chacon P, Reyes Abarca C, Blount BC, Van Landingham CB, Crump KS, et al. Long-term environmental exposure to perchlorate through drinking water and thyroid function during pregnancy and the neonatal period. Thyroid. 2005;15(9):963–75. doi: 10.1089/thy.2005.15.963. [DOI] [PubMed] [Google Scholar]
- 3.Rao B, Anderson TA, Orris GJ, Rainwater KA, Rajagopalan S, Sandvig RM, et al. Widespread natural perchlorate in unsaturated zones of the southwest United States. Environ Sci Technol. 2007;41(13):4522–8. doi: 10.1021/es062853i. [DOI] [PubMed] [Google Scholar]
- 4.Blount BC, Valentin-Blasini L. Biomonitoring as a method for assessing exposure to perchlorate. Thyroid. 2007;17(9):837–41. doi: 10.1089/thy.2007.0106. [DOI] [PubMed] [Google Scholar]
- 5.Blount BC, Valentin-Blasini L, Osterloh JD, Mauldin JP, Pirkle JL. Perchlorate exposure of the US population, 2001-2002. J Expo Sci Environ Epidemiol. 2007;17(4):400–7. doi: 10.1038/sj.jes.7500535. [DOI] [PubMed] [Google Scholar]
- 6.Lau FK, deCastro BR, Mills-Herring L, Tao L, Valentin-Blasini L, Alwis KU, et al. Urinary perchlorate as a measure of dietary and drinking water exposure in a representative sample of the United States population 2001-2008. J Expo Sci Environ Epidemiol. 2013;23(2):207–14. doi: 10.1038/jes.2012.108. [DOI] [PubMed] [Google Scholar]
- 7.U.S. FDA . Survey Data on Perchlorate in Food: 2005/2006 Total Diet Study Results. U.S. Food and Drug Administration. Center for Food Safety and Applied Nutrition; 2008. Available from: http://www.cfsan.fda.gov/~dms/clo4dat2.html. [Google Scholar]
- 8.Blount BC, Alwis KU, Jain RB, Solomon BL, Morrow JC, Jackson WA. Perchlorate, nitrate, and iodide intake through tap water. Environ Sci Technol. 2010;44(24):9564–70. doi: 10.1021/es1025195. [DOI] [PubMed] [Google Scholar]
- 9.U.S. EPA . Interim Drinking Water Health Advisory For Perchlorate. Office of Science and Technology. Office of Water. U.S. Environmental Protection Agency; 2008. EPA 822-R-08-025. [Google Scholar]
- 10.California State Water Resources Control Board. California Environmental Protection Agency Perchlorate in Drinking Water. 2016 Retrieved 2/18/2016, 2016, from http://www.waterboards.ca.gov/drinking_water/certlic/drinkingwater/Perchlorate.shtml.
- 11.U.S. EPA . Perchlorate monitoring results. Henderson, Nevada to the lower Colorado River. U.S. Environmental Protection Agency; 2005. Available from: http://www.epa.gov/fedfac/pdf/perrpt12_05.pdf. [Google Scholar]
- 12••.Steinmaus C, Pearl M, Kharrazi M, Blount BC, Miller MD, Pearce EN, et al. Thyroid hormones and moderate exposure to perchlorate during pregnancy in women in Southern California. Environ Health Perspect. 2015 doi: 10.1289/ehp.1409614. [In press]. Largest study to date of perchlorate during pregnancy and maternal serum thyroid hormone levels. Identified associations between increasing urinary perchlorate and decreasing thyroxine, decreasing free thyroxine, and increasing thyroid stimulating hormone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trumpolt CW, Crain M, Cullison GD, Flanagan SJP, Siegel L, Lathrop S. Perchlorate: sources, uses, and occurrences in the environment. Remediation. 2005:65–89. Winter. [Google Scholar]
- 14.U.S. GAO . Occurrence Is Widespread but at Varying Levels; Federal Agencies Have Taken Some Actions to Respond to and Lessen Releases. U.S. Government Accountability Office; 2010. GAO-10-769. Available from: www.gao.gov/assets/310/308652.pdf. [Google Scholar]
- 15.Greer MA, Goodman G, Pleus RC, Greer SE. Health effects assessment for environmental perchlorate contamination: the dose response for inhibition of thyroidal radioiodine uptake in humans. Environ Health Perspect. 2002;110(9):927–37. doi: 10.1289/ehp.02110927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirk AB, Martinelango PK, Tian K, Dutta A, Smith EE, Dasgupta PK. Perchlorate and iodide in dairy and breast milk. Environ Sci Technol. 2005;39(7):2011–7. doi: 10.1021/es048118t. [DOI] [PubMed] [Google Scholar]
- 17.Tonacchera M, Pinchera A, Dimida A, Ferrarini E, Agretti P, Vitti P, et al. Relative potencies and additivity of perchlorate, thiocyanate, nitrate, and iodide on the inhibition of radioactive iodide uptake by the human sodium iodide symporter. Thyroid. 2004;14(12):1012–9. doi: 10.1089/thy.2004.14.1012. [DOI] [PubMed] [Google Scholar]
- 18.Hobson QT. Aplastic anaemia due to treatment with potassium perchlorate. Br Med J. 1961;1(5236):1368–9. doi: 10.1136/bmj.1.5236.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung AM, Pearce EN, Braverman LE. Perchlorate, iodine and the thyroid. Best Pract Res Clin Endocrinol Metab. 2010;24(1):133–41. doi: 10.1016/j.beem.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zoeller RT. Transplacental thyroxine and fetal brain development. J Clin Invest. 2003;111(7):954–7. doi: 10.1172/JCI18236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Escobar GM, Ares S, Berbel P, Obregon MJ, del Rey FE. The changing role of maternal thyroid hormone in fetal brain development. Semin Perinatol. 2008;32(6):380–6. doi: 10.1053/j.semperi.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Cao XY, Jiang XM, Dou ZH, Rakeman MA, Zhang ML, O'Donnell K, et al. Timing of vulnerability of the brain to iodine deficiency in endemic cretinism. N Engl J Med. 1994;331(26):1739–44. doi: 10.1056/NEJM199412293312603. [DOI] [PubMed] [Google Scholar]
- 23.Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341(8):549–55. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- 24.Pop VJ, Brouwers EP, Vader HL, Vulsma T, van Baar AL, de Vijlder JJ. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol (Oxf) 2003;59(3):282–8. doi: 10.1046/j.1365-2265.2003.01822.x. [DOI] [PubMed] [Google Scholar]
- 25.Pop VJ, Kuijpens JL, van Baar AL, Verkerk G, van Son MM, de Vijlder JJ, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf) 1999;50(2):149–55. doi: 10.1046/j.1365-2265.1999.00639.x. [DOI] [PubMed] [Google Scholar]
- 26.OEHHA . Office of Environmental Health Hazard Assessment. California Environmental Protection Agency; Oakland, CA: 2015. Public Health Goal. Perchlorate in Drinking Water. Available from: http://oehha.ca.gov/water/phg/2015perchlorate.html. [Google Scholar]
- 27.Zoeller RT. Interspecies differences in susceptibility to perturbation of thyroid hormone homeostasis requires a definition of "sensitivity" that is informative for risk analysis. Regul Toxicol Pharmacol. 2004;40(3):380. doi: 10.1016/j.yrtph.2004.08.008. author reply 381-2. [DOI] [PubMed] [Google Scholar]
- 28.NAS . Health Implications of Perchlorate Ingestion. National Academy of Science; Washington, DC; 2005. Available from: http://dels.nas.edu/Report/Health-Implications-Perchlorate-Ingestion/11202. [Google Scholar]
- 29.Godley AF, and Stanbury JB. Preliminary experience in the treatment of hyperthyroidism with potassium perchlorate. J Clin Endocrinol Metab. 1954;14(1):70–8. doi: 10.1210/jcem-14-1-70. [DOI] [PubMed] [Google Scholar]
- 30.Braverman LE, He X, Pino S, Cross M, Magnani B, Lamm SH, et al. The effect of perchlorate, thiocyanate, and nitrate on thyroid function in workers exposed to perchlorate long-term. J Clin Endocrinol Metab. 2005;90(2):700–6. doi: 10.1210/jc.2004-1821. [DOI] [PubMed] [Google Scholar]
- 31.Gibbs JP, Ahmad R, Crump KS, Houck DP, Leveille TS, Findley JE, et al. Evaluation of a population with occupational exposure to airborne ammonium perchlorate for possible acute or chronic effects on thyroid function. J Occup Environ Med. 1998;40(12):1072–82. doi: 10.1097/00043764-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Lamm SH, Braverman LE, Li FX, Richman K, Pino S, Howearth G. Thyroid health status of ammonium perchlorate workers: a cross-sectional occupational health study. J Occup Environ Med. 1999;41(4):248–60. doi: 10.1097/00043764-199904000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Braverman LE, Pearce EN, He X, Pino S, Seeley M, Beck B, et al. Effects of six months of daily low-dose perchlorate exposure on thyroid function in healthy volunteers. J Clin Endocrinol Metab. 2006;91(7):2721–4. doi: 10.1210/jc.2006-0184. [DOI] [PubMed] [Google Scholar]
- 34.Lawrence J, Lamm S, Braverman LE. Low dose perchlorate (3 mg daily) and thyroid function. Thyroid. 2001;11(3):295. doi: 10.1089/105072501750159796. [DOI] [PubMed] [Google Scholar]
- 35.Lawrence JE, Lamm SH, Pino S, Richman K, Braverman LE. The effect of short-term low-dose perchlorate on various aspects of thyroid function. Thyroid. 2000;10(8):659–63. doi: 10.1089/10507250050137734. [DOI] [PubMed] [Google Scholar]
- 36.Vulsma T, Gons MH, de Vijlder JJ. Maternal-fetal transfer of thyroxine in congenital hypothyroidism due to a total organification defect or thyroid agenesis. N Engl J Med. 1989;321(1):13–6. doi: 10.1056/NEJM198907063210103. [DOI] [PubMed] [Google Scholar]
- 37.van den Hove MF, Beckers C, Devlieger H, de Zegher F, De Nayer P. Hormone synthesis and storage in the thyroid of human preterm and term newborns: effect of thyroxine treatment. Biochimie. 1999;81(5):563–70. doi: 10.1016/s0300-9084(99)80111-4. [DOI] [PubMed] [Google Scholar]
- 38.Institute of Medicine Food and Nutrition Board . Dietary Reference Intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. National Academy Press; Washington, D.C.: 2001. [PubMed] [Google Scholar]
- 39.Pearce EN, Leung AM, Blount BC, Bazrafshan HR, He X, Pino S, et al. Breast milk iodine and perchlorate concentrations in lactating Boston-area women. J Clin Endocrinol Metab. 2007;92(5):1673–7. doi: 10.1210/jc.2006-2738. [DOI] [PubMed] [Google Scholar]
- 40.Pearce EN, Alexiou M, Koukkou E, Braverman LE, He X, Ilias I, et al. Perchlorate and thiocyanate exposure and thyroid function in first-trimester pregnant women from Greece. Clin Endocrinol (Oxf) 2012;77(3):471–4. doi: 10.1111/j.1365-2265.2012.04407.x. [DOI] [PubMed] [Google Scholar]
- 41.Pearce EN, Lazarus JH, Smyth PP, He X, Dall'amico D, Parkes AB, et al. Perchlorate and thiocyanate exposure and thyroid function in first-trimester pregnant women. J Clin Endocrinol Metab. 2010;95(7):3207–15. doi: 10.1210/jc.2010-0014. [DOI] [PubMed] [Google Scholar]
- 42.Pearce EN, Spencer CA, Mestman JH, Lee RH, Bergoglio LM, Mereshian P, et al. Effect of environmental perchlorate on thyroid function in pregnant women from Cordoba, Argentina, and Los Angeles, California. Endocr Pract. 2011;17(3):412–7. doi: 10.4158/EP10293.OR. [DOI] [PubMed] [Google Scholar]
- 43••.Charatcharoenwitthaya N, Ongphiphadhanakul B, Pearce EN, Somprasit C, Chanthasenanont A, He X, et al. The association between perchlorate and thiocyanate exposure and thyroid function in first-trimester pregnant Thai women. J Clin Endocrinol Metab. 2014;99(7):2365–2371. doi: 10.1210/jc.2013-3986. One of the earliest studies to identify an association between maternal perchlorate exposure and maternal thyroid hormone levels during pregnancy. [DOI] [PubMed] [Google Scholar]
- 44.Amitai Y, Winston G, Sack J, Wasser J, Lewis M, Blount BC, et al. Gestational exposure to high perchlorate concentrations in drinking water and neonatal thyroxine levels. Thyroid. 2007;17(9):843–50. doi: 10.1089/thy.2006.0336. [DOI] [PubMed] [Google Scholar]
- 45.Brechner R, Brown M, Herman W. Re: Ammonium perchlorate contamination of Colorado River drinking water is associated with abnormal thyroid function in newborns in Arizona (Letter) J Occup Environ Med. 2001;43:308–9. doi: 10.1097/00043764-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Brechner RJ, Parkhurst GD, Humble WO, Brown MB, Herman WH. Ammonium perchlorate contamination of Colorado River drinking water is associated with abnormal thyroid function in newborns in Arizona. J Occup Environ Med. 2000;42(8):777–82. doi: 10.1097/00043764-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Buffler PA, Kelsh MA, Lau EC, Edinboro CH, Barnard JC, Rutherford GW, et al. Thyroid function and perchlorate in drinking water: an evaluation among California newborns, 1998. Environ Health Perspect. 2006;114(5):798–804. doi: 10.1289/ehp.8176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao Y, Blount BC, Valentin-Blasini L, Bernbaum JC, Phillips TM, Rogan WJ. Goitrogenic anions, thyroid-stimulating hormone, and thyroid hormone in infants. Environ Health Perspect. 2010;118(9):1332–7. doi: 10.1289/ehp.0901736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kelsh MA, Buffler PA, Daaboul JJ, Rutherford GW, Lau EC, Barnard JC, et al. Primary congenital hypothyroidism, newborn thyroid function, and environmental perchlorate exposure among residents of a Southern California community. J Occup Environ Med. 2003;45(10):1116–27. doi: 10.1097/01.jom.0000091683.25325.55. [DOI] [PubMed] [Google Scholar]
- 50.Li FX, Byrd DM, Deyhle GM, Sesser DE, Skeels MR, Katkowsky SR, et al. Neonatal thyroid-stimulating hormone level and perchlorate in drinking water. Teratology. 2000;62(6):429–31. doi: 10.1002/1096-9926(200012)62:6<429::AID-TERA10>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 51.Li Z, Li FX, Byrd D, Deyhle GM, Sesser DE, Skeels MR, et al. Neonatal thyroxine level and perchlorate in drinking water. J Occup Environ Med. 2000;42(2):200–5. doi: 10.1097/00043764-200002000-00020. [DOI] [PubMed] [Google Scholar]
- 52••.Taylor PN, Okosieme OE, Murphy R, Hales C, Chiusano E, Maina A, et al. Maternal perchlorate levels in women with borderline thyroid function during pregnancy and the cognitive development of their offspring: data from the Controlled Antenatal Thyroid Study. J Clin Endocrinol Metab. 2014;99(11):4291–8. doi: 10.1210/jc.2014-1901. First study to identify associations between urinary perchlorate levels during pregnancy and subsequent decreases in IQ in the offspring. [DOI] [PubMed] [Google Scholar]
- 53.Lamm SH, Doemland M. Has perchlorate in drinking water increased the rate of congenital hypothyroidism? J Occup Environ Med. 1999;41(5):409–11. doi: 10.1097/00043764-199905000-00011. [DOI] [PubMed] [Google Scholar]
- 54.Steinmaus C, Miller MD, Smith AH. Perchlorate in drinking water during pregnancy and neonatal thyroid hormone levels in California. J Occup Environ Med. 2010;52(12):1217–1224. doi: 10.1097/JOM.0b013e3181fd6fa7. [DOI] [PubMed] [Google Scholar]
- 55.Mervish N, Blount B, Valentin-Blasini L, Brenner B, Galvez MP, Wolff MS, et al. Temporal variability in urinary concentrations of perchlorate, nitrate, thiocyanate and iodide among children. J Exp Sci Environ Epidemiol. 2011;22(2):212–8. doi: 10.1038/jes.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Greenland S. In: Basic methods of sensitivity analysis and external adjustment, in Modern Epidemiology. 2nd Rothman K, Greenland S, editors. Lippincott Raven; Philadelphia: 1998. pp. 343–57. [Google Scholar]
- 57.Wyngaarden JB, Stanbury JB, Rapp B. The effects of iodine, perchlorate, thiocyanate, and nitrate administration upon the iodide concentrating mechanism of the rat thyroid. Endocrinology. 1953;52(5):568–74. doi: 10.1210/endo-52-5-568. [DOI] [PubMed] [Google Scholar]
- 58.Foss OP, Lund-Larsen PG. Serum thiocyanate and smoking: interpretation of serum thiocyanate levels observed in a large health study. Scand J Clin Lab Invest. 1986;46(3):245–51. doi: 10.3109/00365518609083666. [DOI] [PubMed] [Google Scholar]
- 59.Sanchez CA, Blount BC, Valentin-Blasini L, Krieger RI. Perchlorate, thiocyanate, and nitrate in edible cole crops (Brassica sp. Bull Environ Contam Toxicol. 2007;79(6):655–9. doi: 10.1007/s00128-007-9292-6. [DOI] [PubMed] [Google Scholar]
- 60.Ward MH, deKok TM, Levallois P, Brender J, Gulis G, Nolan BT, et al. Workgroup report: Drinking-water nitrate and health--recent findings and research needs. Environ Health Perspect. 2005;113(11):1607–14. doi: 10.1289/ehp.8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blount BC, Pirkle JL, Osterloh JD, Valentin-Blasini L, Caldwell KL. Urinary perchlorate and thyroid hormone levels in adolescent and adult men and women living in the United States. Environ Health Perspect. 2006;114(12):1865–71. doi: 10.1289/ehp.9466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steinmaus C, Miller MD, Howd R. Impact of smoking and thiocyanate on perchlorate and thyroid hormone associations in the 2001-2002 National Health and Nutrition Examination Survey. Environ Health Perspect. 2007;115(9):1333–8. doi: 10.1289/ehp.10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63•.Horton MK, Blount BC, Valentin-Blasini L, Wapner R, Whyatt R, Gennings C, et al. 2015;143:1–9. doi: 10.1016/j.envres.2015.09.013. Pt A. Identified combined impacts of perchlorate, nitrate, and thiocyanate on serum thyroid stimulating hormone levels in pregnant women. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Groef B, Decallonne BR, Van der Geyten S, Darras VM, Bouillon R. Perchlorate versus other environmental sodium/iodide symporter inhibitors: potential thyroid-related health effects. Eur J Endocrinol. 2006;155(1):17–25. doi: 10.1530/eje.1.02190. [DOI] [PubMed] [Google Scholar]
- 65.Kimbrough DE. 2011;53(5):465–6. doi: 10.1097/JOM.0b013e31821aa4ac. author reply 466-7. [DOI] [PubMed] [Google Scholar]
- 66.Tarone RE, Lipworth L, McLaughlin JK. The epidemiology of environmental perchlorate exposure and thyroid function: a comprehensive review. J Occup Environ Med. 2010;52(6):653–60. doi: 10.1097/JOM.0b013e3181e31955. [DOI] [PubMed] [Google Scholar]
- 67.ASTSWMO Perchlorate Policy Update Final; Association of State and Territorial Solid Waste Management Officials; 444 North Capitol Street, N.W., Suite 315 Washington, D.C. 20001. Apr, 2011. 2011. http://www.astswmo.org/Files/Policies_and_Publications/Federal_Facilities/2011.04_FIN AL_Perchlorate_Policy_Update.pdf. [Google Scholar]