Abstract
Residual kidney function contributes substantially to solute clearance in dialysis patients but cannot be assessed without urine collection. We used serum filtration markers to develop dialysis-specific equations to estimate urinary urea clearance without the need for urine collection. In our development cohort, we measured 24-hour urine clearances under close supervision in 44 patients and validated these equations in 826 patients from the Netherlands Cooperative Study on the Adequacy of Dialysis. For the development and validation cohorts, median urinary urea clearance was 2.6 and 2.4 mL/min, respectively. During the 24-hour visit in the development cohort, serum β-trace protein concentrations remained in steady state but concentrations of all other markers increased. In the validation cohort, bias (median measured minus estimated clearance) was low for all equations. Precision was significantly better for β-trace protein and β2-microglobulin equations and the accuracy was significantly greater for β-trace protein, β2-microglobulin and cystatin C equations, compared with the urea plus creatinine equation. Area under the receiver operator characteristic curve for detecting measured urinary urea clearance by equation-estimated urinary urea clearance (both 2 mL/min or more) were 0.821, 0.850 and 0.796 for β-trace protein, β2-microglobulin and cystatin C equations, respectively; significantly greater than the 0.663 for the urea plus creatinine equation. Thus, residual renal function can be estimated in dialysis patients without urine collections.
Keywords: peritoneal dialysis, hemodialysis
Background
Residual kidney function (RKF) is associated with improved survival in dialysis patients.1–4 Even at the low levels of glomerular filtration rate (GFR) in dialysis patients, RKF is a major contributor to solute and volumeclearance.5–7 Dialysis patients with preserved RKF also have lower concentrations of uremic toxins, less volume overload, lower left ventricular mass, less inflammation, lower requirements for erythropoietin and better quality of life.4, 8,9 Consequently, loss of RKF after starting dialysis is associated with increased risk of death.10
RKF is generally expressed as urinary clearance of urea (CLUREA) or the average of urea and creatinine (CLUREA, CREAT). Current guidelines recommend assessment of RKF at regular intervals for adjustment of the dialysis prescription and including CLUREA in hemodialysis adequacy if it is ≥ 2 mL/min.7, 11, 12 However, there are no simple methods for assessing RKF, similar to GFR estimation from serum creatinine in non-dialysis patients. In clinical practice, RKF is assessed by timed 24–48 hour urine collection with calculation of urea and creatinine clearance.7 Urine collections, however, are cumbersome for the patients and the dialysis unit staff and prone to errors leading to overestimation or underestimation of RKF.7 Serum concentrations of low molecular weight proteins, such as β-trace protein (BTP), β2-microglobulin (B2M) and cystatin C are highly correlated with measured GFR.13–16 Hemodialysis clearance during conventional (diffusive) high-flux hemodialysis, is minimal for BTP (~25,000 Daltons)17–19 and partial for B2M (11,600 Daltons)18, 20 and cystatin C (13,300 Daltons).18, 21 Peritoneal dialysis clearance of B2M and cystatin C is lower than that of urea and creatinine22–26 whereas that of BTP has not been reported. High correlation with measured GFR and low or no removal by dialysis makes these markers attractive candidates for assessment of RKF.
The goal of our study was to use serum endogenous filtration markers to develop dialysis-specific equations to assess RKF and replace timed-urine collections. We developed these equations in a cohort of dialysis patients in Baltimore, Maryland that underwent careful, closely supervised and monitored 24-hour urine clearance measurements designed to minimize measurement error. We then validated the equations in an external cohort, the Netherland Cooperative Study on Adequacy of Dialysis (NECOSAD).
Results
Clinical Characteristics
In the development cohort (RKF Study; n=44), mean age was 55 years, 64% were male and 21% White (Table 1). None of the patients were vegetarian or had undergone limb amputation. Urinary clearance measurements in the RKF Study were performed on an interdialytic day with the following distribution of the study visit days: Monday, 8 (13.1%); Tuesday, 23 (37.7%); Wednesday, 11 (18%); Thursday, 15 (24.6%); Friday, 2 (3.3%); and Sunday, 2 (3.3%). Patients in the validation cohort (NECOSAD; n=826) were older and more likely to be White. In the development and validation cohorts, median 24-hour urine volume was 799 mL and 720 mL, median CLUREA was 2.6 and 2.4 mL/min and median CLUREA, CREAT was 3.1 and 3.2 mL/min/1.73 m2, respectively.
Table 1.
Clinical Characteristics of the Patients in the Residual Kidney Function Study and the Netherlands Cooperative Study on Adequacy of Dialysis
| Development Cohort | Validation Cohort | ||||||
|---|---|---|---|---|---|---|---|
| RKF Study, Baltimore, Maryland, USA | NECOSAD, Netherlands | ||||||
| All Participants | Hemodialysis | Peritoneal Dialysis | |||||
| Characteristics | Mean (SD) or N (%) | Mean (SD) or N (%) | P (vs. RKF Study) | Mean (SD) or N (%) | P (vs. RKF Study) | Mean (SD) or N (%) | P (vs. RKF Study) |
| Number | 44 | 826 | 587 | 239 | |||
| Demographics | |||||||
| Age, years | 55.43 (11.29) | 60.22 (14.45) | 0.03 | 63.44 (13.33) | <0.001 | 52.28 (14.03) | 0.16 |
| Male, % | 28 (64%) | 496 (60%) | 0.75 | 334 (57%) | 0.43 | 162 (67.78%) | 0.60 |
| White, % | 9 (21%) | 724 (88%) | <0.001 | 539 (91%) | <0.001 | 185 (77.41%) | <0.001 |
| Clinical Characteristics | |||||||
| Cause of ESRD, % | 0.10 | 0.08 | 0.04 | ||||
| Diabetes | 12 (27%) | 128 (16%) | 96 (16%) | 32 (13%) | |||
| Glomerulonephritis | 6 (14%) | 118 (14%) | 60 (10%) | 58 (24%) | |||
| Other | 26 (59%) | 580 (70%) | 431 (73%) | 149 (62%) | |||
| Diabetes, % | 23 (52%) | 174 (21%) | <0.001 | 137 (24%) | <0.001 | 37 (16%) | <0.001 |
| Hemodialysis, % | 40 (91%) | 587 (71%) | 0.003 | 587 (100%) | <0.001 | 0 (0%) | <0.001 |
| Duration of Prior Dialysis, months | 26.7 (29.0) | 6.73 (4.49) | <0.001 | 5.42 (3.96) | <0.001 | 9.96 (4.07) | <0.001 |
| Height, cm | 171.6 (10.1) | 171.3 (9.9) | 0.83 | 170.4 (9.8) | 0.42 | 173.6 (9.8) | 0.23 |
| Weight, kg | 93.00 (27.46) | 73.81 (14.21) | <0.001 | 72.50 (14.02) | <0.001 | 77.04 (14.20) | <0.001 |
| Body Mass Index, Kg/m2 | 31.27 (7.76) | 25.10 (4.14) | <0.001 | 24.92 (4.17) | <0.001 | 25.54 (4.05) | <0.001 |
| Body Surface Area, m2 | 2.04 (0.31) | 1.86 (0.20) | <0.001 | 1.83 (0.20) | <0.001 | 1.91 (0.20) | <0.001 |
| Urea Volume of Distribution, L | 44.9 (11.6) | 38.0 (6.7) | <0.001 | 37.1 (6.4) | <0.001 | 40.1 (7.0) | <0.001 |
| 24-Hour Urine Volume, mL [Median (25th, 75th Percentiles)] | 799 (319, 1154) | 720 (389, 1217) | 0.33 | 679.71 (350, 1097) | 0.75 | 889 (472, 1522) | 0.02 |
| Urine Volume ≥250 mL, % | 34 (77%) | 718 (87%) | 0.07 | 501 (85%) | 0.19 | 217 (91%) | 0.02 |
| Measured Urinary Clearances, Median (25th, 75th Percentiles) | |||||||
| Urea Clearance, mL/min | 2.59 (0.92, 4.07) | 2.43 (1.31, 3.94) | 0.84 | 2.37 (1.28, 3.81) | 0.92 | 2.59 (1.41, 4.48) | 0.40 |
| Urea Clearance, mL/min/1.73 m2 | 2.42 (0.70, 3.31) | 2.30 (1.23, 3.66) | 0.24 | 2.24 (1.21, 3.57) | 0.33 | 2.36 (1.30, 3.97) | 0.13 |
| Creatinine Clearance, mL/min | 4.32 (1.62, 7.12) | 4.24 (2.33, 6.97) | 0.48 | 4.14 (2.21, 6.87) | 0.37 | 4.51 (2.73, 7.57) | 0.83 |
| Creatinine Clearance, mL/min/1.73 m2 | 3.71 (1.35, 5.96) | 4.03 (2.19, 6.57) | 0.61 | 3.98 (2.11, 6.52) | 0.68 | 4.05 (2.39, 7.09) | 0.48 |
| Average of Urea and Creatinine Clearance, mL/min | 3.58 (1.36, 5.50) | 3.42 (1.91, 5.42) | 0.67 | 3.38 (1.77, 5.19) | 0.51 | 3.55 (2.13, 6.11) | 0.88 |
| Average of Urea and Creatinine Clearance, mL/min/1.73 m2 | 3.09 (1.05, 4.82) | 3.17 (1.77, 5.13) | 0.45 | 3.14 (1.71, 4.95) | 0.54 | 3.24 (1.95, 5.62) | 0.30 |
| Weekly renal standard Kt/VUREA | 0.58 (0.16, 0.90) | 0.65 (0.36, 1.06) | 0.11 | 0.65 (0.35, 1.04) | 0.13 | 0.65 (0.37, 1.12) | 0.08 |
| Weekly renal standard Kt/VCREAT | 1.01 (0.33, 1.73) | 1.15 (0.62, 1.92) | 0.27 | 1.15 (0.60, 1.90) | 0.29 | 1.15 (0.67, 1.94) | 0.282 |
| Weekly renal standard Kt/VUREA, CREAT | 0.83 (0.25, 1.47) | 0.90 (0.50, 1.50) | 0.19 | 0.90 (0.49, 1.48) | 0.21 | 0.88 (0.53, 1.56) | 0.18 |
| Endogenous Serum Filtration Markers | |||||||
| Urea Nitrogen, mg/dL | 52.50 (15.74) | 64.92 (17.09) | <0.001 | 66.91 (16.67) | <0.001 | 60.05 (17.17) | 0.007 |
| Creatinine, mg/dL | 8.57 (3.07) | 8.73 (2.77) | 0.70 | 8.53 (2.63) | 0.93 | 9.22 (3.03) | 0.19 |
| β-Trace Protein, mg/L | 7.36 (3.13) | 6.93 (2.58) | 0.28 | 6.77 (2.45) | 0.13 | 7.32 (2.86) | 0.93 |
| β2 Microglobulin, mg/L | 24.28 (9.50) | 25.5 (9.49) | 0.42 | 25.55 (9.56) | 0.40 | 25.23 (9.34) | 0.53 |
| Cystatin C, mg/L | 5.24 (1.26) | 5.07 (1.10) | 0.35 | 5.02 (1.07) | 0.20 | 5.21 (1.17) | 0.91 |
Abbreviations: RKF, Residual Kidney Function; NECOSAD, The Netherlands Cooperative Study on the Adequacy of Dialysis; SD, Standard Deviation; ESRD, End Stage Renal Disease; Kt/V, Weekly clearance (Kt) divided by volume of total body water (V).
Conversion factors for units: creatinine in mg/dL to μmol/L, X 88.4; urea nitrogen in mg/dL to mmol/L, X 0.357.
RKF and Serum Concentrations of Endogenous Filtration Markers
In the development cohort with serial measurements of serum markers over 24 hours (n=44 patients with 61 visits), the rate of increase in markers was as follows: urea 10.8 mg/dL/day (95% CI, 8.1 to 13.5 ; p<0.001), creatinine 1.3 mg/dL/day (95% CI, 0.9 to 1.7; p<0.001), BTP 0.09 mg/L/day (95% CI, −0.40 to 0.58; p=0.71), B2M 1.27 mg/L/day (95% CI, 0.01 to 2.53 ; p=0.05) and cystatin C 0.30 mg/L/day (95% CI, 0.09 to 0.52; p=0.005). In both cohorts, filtration markers were negatively correlated with CLUREA (or CLUREA, CREAT) and positively correlated with each other (Figure 1, Figure S1 and Table S1). BTP, B2M and cystatin C were highly correlated with each other with the highest correlation between BTP and B2M (RKF Study, 0.807; NECOSAD, 0.759).
Figure 1. Association between Measured Urinary Clearances and Endogenous Filtration Markers in 826 Dialysis Patients of the Validation Cohort, The Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD).
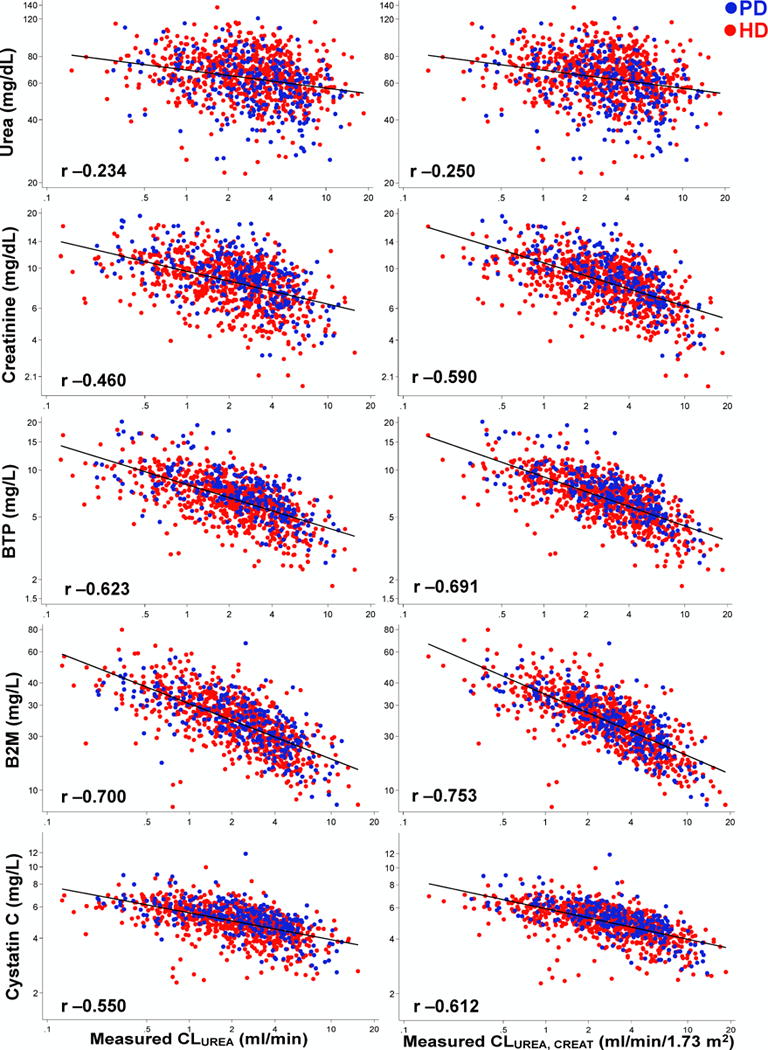
Scatterplots of natural log transformed urea, creatinine, β-trace protein (BTP), β2-microglobulin (B2M) and cystatin C on the vertical (Y) axis and measured urinary clearances on the horizontal (X) axis. Data from patients on peritoneal dialysis are displayed as blue dots and data from patients on hemodialysis are displayed as red dots. Black line is the linear fit. Pearson’s correlation coefficients are displayed in the bottom left corner of each scatterplot. Panel A (left): Measured urinary urea clearance (CLUREA) in mL/min; and Panel B (Right): Measured average of urinary urea and creatinine clearance (CLUREA, CREAT) in mL/min/1.73 m2.
Abbreviations: CLUREA, urinary urea clearance (mL/min); CLUREA, CREAT, average of urinary urea and creatinine clearance (in ml/min/1.73 m2); PD, peritoneal dialysis; HD, hemodialysis.
In the RKF Study, the concentrations of BTP, B2M and cystatin C were similar in patients with (n=5) or without a history of liver failure or hepatitis. In a subset of NECOSAD patients with previously measured CRP (n=543), there was no association between CRP and endogenous filtration markers (Table S2).
Equation Development in RKF Study
Using a prespecified variable selection procedure for equation development (see Methods), we found coefficients for sex to be significant in the models with BTP and B2M estimating CLUREA or CLUREA, CREAT (Table S3). In models that included all three low molecular weight proteins, BTP, B2M and cystatin C, the coefficients for BTP and B2M became smaller and coefficients for cystatin C were no longer significant. Forced addition of age, sex and race to all models, or excluding patients treated with peritoneal dialysis minimally changed the values of the markers’ coefficients and did not improve estimation (Table S4). Based on these data, we selected the parsimonious equations (without forced variables) presented in Table 2 for testing in the external validation cohort.
Table 2.
Residual Kidney Function Study Equations for Estimating CLUREA or CLUREA, CREAT
| CLUREA, mL/min | |
|---|---|
| Urea, Creatinine | CLUREA (mL/min) = 1.1 × UN0.949 × Creatinine−1.544 |
| BTP | CLUREA (mL/min) = 69 × BTP−2.114 × 1.677 if male |
| B2M | CLUREA (mL/min) = 1711 × B2M−2.328 × 1.610 if male |
| Cystatin C | CLUREA (mL/min) = 64 × Cystatin C−2.211 |
| BTP, B2M | CLUREA (mL/min) = 385 × BTP−1 450 × B2M−0.965 × 1.694 if male |
| CLUREA, CREAT, mL/min/1.73 m2 | |
| Urea, Creatinine | CLUREA, CREAT (mL/min/1.73 m2) = 2.4 × UN0.984 × Creatinine−1.868 |
| BTP | CLUREA, CREAT (mL/min/1.73 m2) = 95 × BTP−2.16 × 1.652 if male |
| B2M | CLUREA, CREAT (mL/min/1.73 m2) = 2852 × B2M−2.417 × 1.592 if male |
| Cystatin C | CLUREA, CREAT (mL/min/1.73 m2) = 123 × Cystatin C−2.468 |
| BTP, B2M | CLUREA, CREAT (mL/min/1.73 m2) = 673 × BTP−1 406 × B2M−1.096 × 1.670 if male |
Abbreviations: CLUREA, urinary urea clearance (mL/min); CLUREA, CREAT, average of urinary urea and creatinine clearance (mL/min/1.73 m2); UN, serum urea nitrogen; BTP, β-trace protein; B2M, β2 microglobulin
Note: Coefficients for urea nitrogen and creatinine are expressed for concentrations in mg/dL. Conversion factors for units: creatinine in mg/dL to μmol/L, X 88.4; urea nitrogen in mg/dL to mmol/L, X 0.357.
Equation Performance in NECOSAD
Estimating CLUREA
CLUREA estimation using RKF Study equations had low bias for all equations (Table 3). In general, all equations underestimated CLUREA and CLUREA, CREAT (Figure 2). Bias was higher (underestimation of measured CLUREA) using BTP, B2M and cystatin C equations compared with urea+creatinine equation. However, precision and accuracy were better using BTP, B2M and BTP+B2M equations compared with urea+creatinine equation. The combined BTP+B2M equation had the highest precision [lowest interquartile range (IQR)]. Bias was lower and accuracy was higher in patients treated with hemodialysis compared with those treated with peritoneal dialysis (Table S5). The diagnostic accuracy for detecting measured CLUREA ≥2 mL/min by equation-estimated CLUREA ≥2 mL/min was significantly higher for BTP, B2M and cystatin C equations compared with urea+creatinine equation (p<0.001; Figure 3 and Table S6).
Table 3.
Residual Kidney Function Study CLUREA and CLUREA, CREAT Estimating Equations’ Performance in 826 Patients from the Validation Cohort (NECOSAD)
| Equation | Markers | Other Variables | RMSE | Median Bias (95%CI)2, 3 | Precision (95% CI)4, 5 | Accuracy (95% CI)6, 7 |
|---|---|---|---|---|---|---|
| Measured Clearance – Estimates Clearance | IQR of Bias | Estimated within ± 2 mL/min of CLUREA | ||||
| CLUREA mL/min | mL/min | mL/min | % | |||
| RKF Study | Urea, Creatinine | 0.753 | 0.2 (0.01, 0.3) | 2.2 (2.1, 2.4) | 75 (72, 78) | |
| RKF Study | BTP | Sex | 0.612 | 0.4 (0.3, 0.5)a | 1.8 (1.6, 2.0)b | 81 (78, 83) b*** |
| RKF Study | B2M | Sex | 0.584 | 0.7 (0.6, 0.8)a | 1.6 (1.5, 1.7) b | 79 (76, 81) b* |
| RKF Study | Cystatin C | 0.667 | 0.5 (0.4, 0.6)a | 2.0 (1.8, 2.1) | 79 (76, 82) b*** | |
| RKF Study | BTP, B2M | Sex | 0.569 | 0.5 (0.4, 0.6)a | 1.5 (1.4, 1.7) b | 81 (79, 84) b*** |
| CLUREA, CREAT, mL/min/1.73 m2 | mL/min/1.73 m2 | mL/min/1.73 m2 | Estimates within ± 2 mL/min/1.73 m2 of CLUREA, CREAT, % | |||
| RKF Study Equations | ||||||
| RKF Study | Urea, Creatinine | 0.669 | 0.3 (0.2, 0.5)a | 2.7 (2.5, 2.9) | 68 (65, 72) | |
| RKF Study | BTP | Sex | 0.556 | 0.6 (0.5, 0.7)a | 2.1 (2.0, 2.3) | 71 (68, 74) |
| RKF Study | B2M | Sex | 0.553 | 1.0 (0.9, 1.1)a | 1.9 (1.7, 2.1) | 69 (66, 72) |
| RKF Study | Cystatin C | 0.605 | 0.7 (0.5, 0.9)a | 2.3 (2.1, 2.5) | 72 (69, 75) b* | |
| RKF Study | BTP, B2M | Sex | 0.506 | 0.7 (0.6, 0.8)a | 1.8 (1.6, 1.9) | 75 (72, 78) b*** |
Abbreviations: CLUREA, urinary urea clearance (mL/min); CLUREA, CREAT, average of urinary urea and creatinine clearance (mL/min/1.73 m2); BTP, β-trace protein; B2M, β2 microglobulin; IQR, Interquartile Range; RMSE, root mean squared error; NECOSAD, The Netherlands Cooperative Study on the Adequacy of Dialysis; RKF, Residual Kidney Function
Note: In the NECOSAD Study, 826 participants had 989 clearance measurements. Performance data are only for the first measurement (n=826).
Root mean squared error (RMSE) from linear regression of natural log transformed measured urinary clearance on natural log transformed estimated urinary clearance. A smaller RMSE implies a better model fit.
Bias is defined as the median difference between measured clearance and estimated clearance. Confidence intervals are calculated by bootstrapping with 2000 replicates.
Significance of bias in estimation using BTP, B2M and cystatin C equations compared with urea+creatinine equation was determined by Wilcoxon matched-pairs signed-ranks test (a and b indicate worse and better than the urea+creatinine equation, respectively). All p-values are <0.001.
Precision is defined as interquartile range of the median bias. Confidence intervals are calculated by bootstrapping with 2000 replicates.
Significance of the precision of estimation using BTP, B2M and cystatin C equations compared with urea+creatinine equation was determined by interquartile range regression with bootstrapped (2000 replicates) standard errors (a and b indicate worse and better than the urea+creatinine equation, respectively). All significant differences have p-values <0.001.
Accuracy is defined as estimates within ± 2 mL/min of CLUREA or ± 2.5 mL/min/1.73 m2 of CLUREA, CREAT. Confidence intervals are calculated by bootstrapping with 2000 replicates.
Significance of the accuracy of estimation using BTP, B2M and cystatin C equations compared with urea+creatinine equation was determined by McNemar’s chi-squared test. (a and b indicate worse and better than the urea+creatinine equation, respectively).
p<0.05;
p<0.01;
p<0.001
Figure 2. Association between Estimated and Measured Clearances in 826 Dialysis Patients of the Validation Cohort, The Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD).
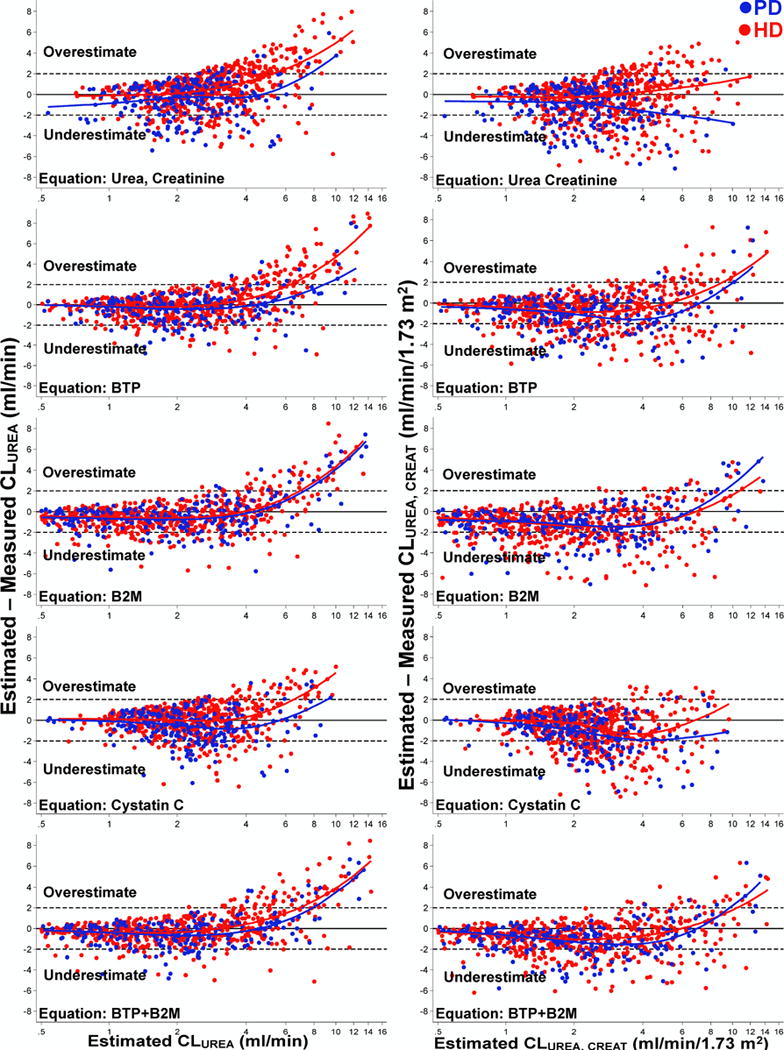
The difference between estimated and measured clearance is presented on the vertical (Y) axis and estimated clearance on the horizontal (X) axis. Positive numbers on the Y axis represent overestimation of measured clearance and negative numbers represent underestimation. Excluding extreme observations, defined as estimated clearance (X axis) >99th percentile or <0.5 mL/min for CLUREA (0.5 mL/min/1.73 m2 for CLUREA, CREAT) and difference between estimated and measured clearance (Y axis) >99th percentile or <1st percentile. Data from patients on peritoneal dialysis are displayed as blue dots and data from patients on hemodialysis are displayed as red dots. Blue and red lines are model fits from median quantile regression of bias on measured clearance modeled as restricted cubic spline with 4 quantile knots. Solid black line represents bias=0. Panel A (left): Results for urinary urea clearance (CLUREA) in mL/min; and Panel B (Right): Results for average of urinary urea and creatinine clearance (CLUREA, CREAT) in mL/min/1.73 m2.
Abbreviations: CLUREA, urinary urea clearance (mL/min); CLUREA, CREAT, average of urinary urea and creatinine clearance (in ml/min/1.73 m2); PD, peritoneal dialysis; HD, hemodialysis; BTP, β-trace protein; B2M, β2 microglobulin.
Figure 3. Receiver Operating Characteristic (ROC) Curves for the Diagnostic Accuracy of Estimating Equations in 826 Dialysis Patients of the Validation Cohort, The Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD).
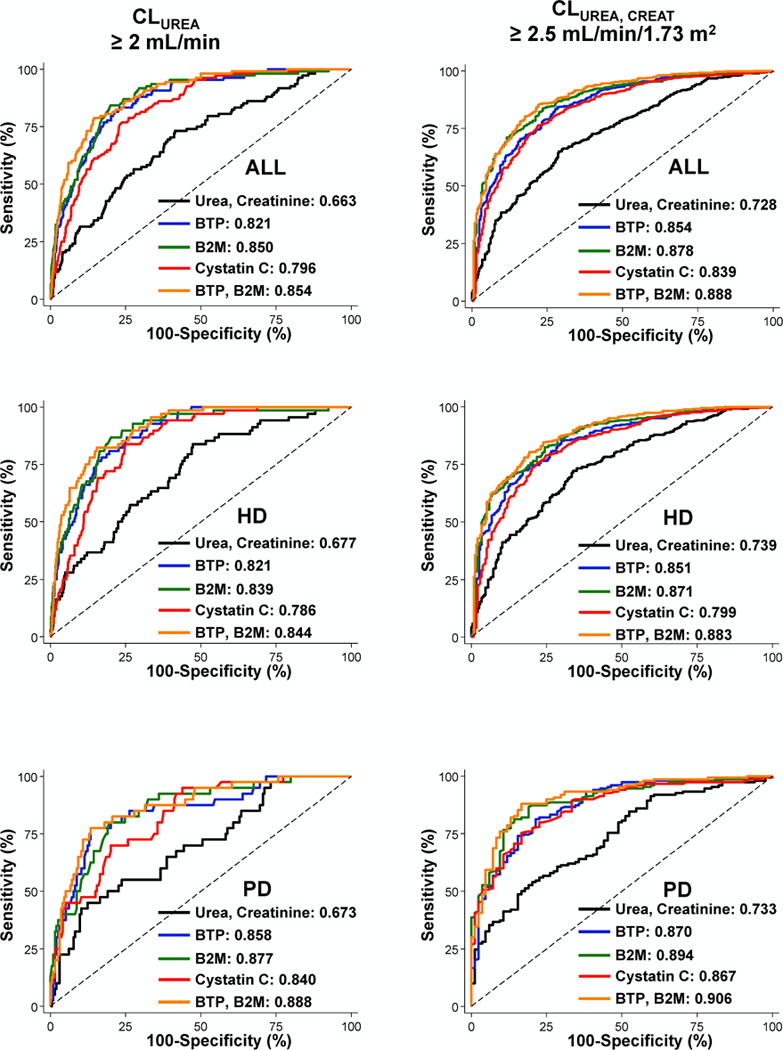
Sensitivity (%) is presented on the Y axis and 100-Specificity (%) is presented on the X axis. Solid black line is urea and creatinine equation, solid blue line is β-trace protein (BTP) equation, solid green line is β2-microglobulin (B2M) equation, solid red line is cystatin C equation and orange line is BTP and B2M equation. Results are presented overall and stratified by patients treated with hemodialysis or peritoneal dialysis. Area under the ROC is presented as numbers in each panel. Panel A (left): Diagnostic accuracy for estimating urinary urea clearance (CLUREA) ≥ 2mL/min; and Panel B: Diagnostic accuracy for estimating the average of urinary urea and creatinine clearance (CLUREA, CREAT) ≥ 2.5 mL/min/1.73 m2.
Abbreviations: CLUREA, urine urea clearance (mL/min); CLUREA, CREAT, average of urinary urea and creatinine clearance (in ml/min/1.73 m2); PD, peritoneal dialysis; HD, hemodialysis; BTP, β-trace protein; B2M, β2 microglobulin.
Estimating CLUREA, CREAT
Compared with previously published equations in non-dialysis patients, the RKF study equations estimating CLUREA, CREAT had significantly lower bias (p<0.001), higher accuracy (p<0.001) but similar precision (Table S7). The Hoek cystatin C equation, developed in NECOSAD, overestimated CLUREA, CREAT (as compared to underestimation by RKF Study equation) but had similar precision and accuracy compared with the RKF Study cystatin C equation.
Repeat Measurements
There were 162 repeat measurements over a median of 9.1 months (IQR: 8.9, 9.3). The median (IQR) change in CLUREA was −0.7 mL/min (−1.4, −0.01) and in CLUREA, CREAT was −1.1 mL/min/1.73 m2 (−1.9, −0.05). The decline in RKF over time was associated with increase in serum concentrations of filtration markers (Table S8 and Figure S2). There was moderate correlation between the initial and repeat clearance measurements and estimations, although the estimating equations underestimated the change in measured clearances over time (Table S9).
Discussion
In this report, we present dialysis-specific equations to estimate CLUREA and CLUREA, CREAT using serum filtration markers. These equations do not require timed-urine collection. We developed these equations in a cohort of dialysis patients in Baltimore, Maryland (RKF Study), with carefully monitored urine clearance measurements and then validated them in an external cohort of dialysis patients in The Netherlands (NECOSAD). The low molecular weight protein (BTP, B2M and cystatin C) equations had better performance than those including metabolites (urea, creatinine). BTP, B2M and cystatin C equations also had high diagnostic accuracy for identifying patients with CLUREA ≥ 2 mL/min, the K/DOQI threshold for considering urinary CLUREA in hemodialysis adequacy. These equations are valid for use in dialysis patients with self-reported urine volume ≥1 cup/day and could be considered for use in place of urine collections.
BTP is a 168 amino acid glycoprotein with varying molecular weight between 23,000 to 29,000 Daltons.13 The major sources of circulating BTP are leptomeninges, arachnoid cells, choroid plexus and oligodendrocytes of the central nervous system.13 Serum BTP can be used to estimate GFR in non-dialysis patients.15, 27, 28 BTP is not removed by conventional low or high-flux dialysis.17, 19 BTP clearance by peritoneal dialysis has not been reported. In our development cohort, serum concentrations of BTP appeared to be in steady state with a non-significant change during the interdialytic period (0.09 mg/L/day; p=0.71). BTP equations had good performance in the validation cohort and are unlikely to be subject to same caveats as urea+creatinine equations. We found that sex coefficients were significant in our development models suggesting that the association between GFR and BTP differs by sex. BTP can also decrease with corticosteroids and its use may not be reliable in patients receiving these medications.29, 30 B2M is a 11,600 Dalton protein which is a component of the major histocompatibility molecules present on all nucleated cells.15 B2M is removed by high-flux hemodialysis and its concentrations increase in patients with malignancy and inflammation.20 In our development cohort, there was a small interdialytic rise in B2M (1.27 mg/L/day; p=0.05) which may affect interpretation of results after the long interdialytic interval or in patients with varying dialysis dose. Cystatin C is a 13,300 Daltons nonglycosylated protein that is expressed in all nucleated cells.14 Cystatin C is also removed partially by high-flux hemodialysis; its concentrations can be affected by corticosteroid use, but probably not by inflammation.21, 29, 30,31In our development cohort, there was a significant interdialytic rise in cystatin C (0.30 mg/L/day; p=0.005). Based on these findings, serum BTP equations may be the most reliable for assessing RKF in dialysis patients. However, while B2M and cystatin C assays are available for clinical use in the US, BTP assay is not yet commercially available in the US but was recently launched in Europe. Further studies are needed to carefully characterize the kinetics of these low molecular weight proteins in diverse dialysis populations and validate our findings.
In previous studies, cystatin C is reported to be correlated with CLUREA, CREAT in dialysis patients and a cystatin C equation to estimate CLUREA, CREAT was developed in a subset of the NECOSAD.32 Other equations using BTP and cystatin C, including the CKD-EPI creatinine and cystatin C equations, performed poorly for estimating CLUREA, CREAT in our study. A number of reasons may underlie this poor performance. First, since GFR estimating equations in non-dialysis patients were developed in patients with higher GFR than in our study, the equation performance is not optimized at low GFR. Second, the coefficients for the serum markers in non-dialysis studies reflect the influence of only endogenous non-GFR determinants and not the dialysis determinants. It is also possible that these non-GFR determinants change as GFR declines. Third, we did not standardize cystatin C measurement to International Federation of Clinical Chemistry standards. As a result, differences in performance of cystatin C RKF Study versus CKD-EPI equations, may reflect differences in assay calibration. Similarly, variability in cystatin C assay over time could also partially account for differences in performance. There are no published equations for estimating CLUREA in dialysis patients.
The performance of RKF Study urea+creatinine equation was significantly better than the CKD-EPI creatinine equation. This improved performance may reflect optimization of urea and creatinine coefficients in RKF Study equation to reflect non-renal (dialytic) clearance. However, urea (60 Daltons) and creatinine (113 Daltons) are not in steady state between dialysis treatments. In RKF Study, the interdialytic rise in serum urea nitrogen and creatinine was 10.8 and 1.3 mg/dL/day, respectively, while the patients were receiving a standardized diet. The rate of rise may be significantly different outside of this controlled environment and will the use of urea+creatinine equations in clinical practice. Nevertheless, since urea and creatinine are measured routinely in dialysis patients, the RKF Study urea+creatinine equation might be used as a screening tool to estimate RKF in patients with self-reported urine output ≥1 cup/day, without additional cost. Low molecular weight proteins, and in particular BTP, may then be used for more reliable RKF estimation and clinical decision making.
RKF is strongly associated with improved survival in hemodialysis and peritoneal dialysis patients; each 1 mL/min/1.73 m2 higher CLUREA, CREAT is associated with an 11–48% lower risk of death.1–4, 20 Besides excretion of freely filtered solutes (e.g., urea, creatinine), RKF also enables excretion of protein-bound solutes (e.g., p-cresol sulfate, indoxyl sulfate) which are cleared by tubular secretion but not effectively removed by dialysis.9 Continuous volume excretion reduces volume overload and left ventricular hypertrophy.6, 8 Preserving RKF may improve survival in dialysis patients but the cumbersome nature of urine collections has greatly impeded advances in this area. RKF is also a strong confounder in dialysis studies and incomplete adjustment for RKF can lead to biased results. Our results now present a new opportunity to assess RKF without urine collections and can potentially overcome challenges to incorporating RKF in dialysis care and research.
In routine clinical practice, hemodialysis adequacy is assessed by equation-estimated dialyzer urea clearance for 1 dialysis session (spKt/VUREA; target ≥1.4)7 which can be used to calculate a cumulative weekly standard Kt/VUREA (stdKt/VUREA; target ≥2.3).7 Calculation of stdKt/VUREA allows comparison of dose across different hemodialysis regimens such as 3/week in-center hemodialysis and 5–7/week home/frequent hemodialysis.33 Peritoneal dialysis adequacy is assessed by quarterly peritoneal urea clearance measurement and expressed as weekly stdKt/VUREA (target ≥1.7).11 CLUREA from a timed urine collection is routinely incorporated into peritoneal dialysis prescription. Equations to incorporate CLUREA into the stdKt/VUREA calculations for hemodialysis patients are also available.33 The 2015 NKF K/DOQI Guidelines for Hemodialysis Adequacy recommend that CLUREA can be included in adequacy calculations provided it is measured periodically.34 The K/DOQI 2006 Peritoneal Dialysis guidelines recommend that CLUREA can be incorporated in dialysis dose if urine volume is ≥ 200 mL per day.11 RKF Study equations will allow estimation of CLUREA from serum markers without urine collection. The estimated CLUREA can then be used to adjust dialysis dose by incorporating it in stdKt/VUREA. This strategy can used for peritoneal dialysis patients to adjust dialysate volume or the number of fills. For home/frequent hemodialysis patients, lower dialysate volume can be used for patients treated with NxStage System One and, for patients without volume overload, the frequency of treatments can be reduced to 3–4/week from 5–7/week. Similarly, in-center hemodialysis patients without volume overload that have substantial CLUREA can be dialyzed less frequently or for shorter duration. Further studies are needed to validate the safety and effectiveness of these strategies.
The focus of our study was on cross-sectional estimation of RKF. However, we recognize the clinical importance of repeated measurements over time in a single individual. In the subgroup of NECOSAD participants with repeated measurements (N=162), measured clearances declined over time; CLUREA by −0.7 mL/min (IQR: −1.4, −0.01) and CLUREA, CREAT by −1.1 mL/min/1.73 m2 (IQR: −1.9, −0.05). The decline in measured clearance correlated with increased concentrations of endogenous filtration makers, particularly BTP, B2M and cystatin C (Figure S2). Equation-estimated clearance also declined over time (Table S9). Although the first and repeat equation-estimated clearances were moderately highly correlated, the equations underestimated the change in clearance over time. For example, the median change in equation-estimated CLUREA with BTP and B2M equations was −0.2 mL/min and −0.4 mL/min, respectively, compared with change in measured CLUREA of −0.7 mL/min. These findings should be kept in mind while monitoring individual patients and highlight the need for improving performance of estimation equations.
Strengths of our study include careful urine collections in the development cohort (RKF Study), under near ideal conditions, which allowed reliable measurements of CLUREA and CLUREA, CREAT; a highly-rigorous pre-specified analytic plan for equation development; use of multiple endogenous filtration markers; and a large external validation cohort of hemodialysis and peritoneal dialysis patients in a different country with a different racial-ethnic composition and body weight than the validation cohort, which greatly improves the generalizability of our results. Limitations include few patients treated with peritoneal dialysis in the development cohort and urine collections at home in NECOSAD which may have introduced measurement error and reduced equations’ performance. In the development cohort, we did not perform bladder ultrasound to check for bladder emptying which could contribute to underestimation of urinary clearance. GFR can vary during the interdialytic interval in dialysis patients.35 We did not standardize the day of the week for clearance measurements which could contribute to underestimation of CLUREA. The relatively large root mean squared error (RMSE)suggests presence of high relative variability (log scale measures variation as a fraction of the absolute value of the gold standard) that is not completely captured by the variables in the estimating equations, highlighting the need to improve estimates for individual prediction and clinical decision making. However, we must also recognize that the gold standard itself has error which limits how well it can be predicted.36 As we did not standardize cystatin C measurements, the internal comparisons remain valid but differences in performance with external equations and future studies may also be due to laboratory measurement error. However, impact on estimation is likely to be minimal when measured in units of ml/min/1.73 m2. We did not exclude patients with thyroid disease or steroid use in the development cohort and this may also affect the performance of cystatin C equation.
In conclusion, we have developed equations to estimate CLUREA and CLUREA, CREAT in dialysis patients without requiring urine collections (Table 2). These equations have good performance and diagnostic accuracy. In particular, serum BTP appears to be in steady state during the interdialytic interval and BTP equations may not be influenced by diet and dialysis schedules compared with equations using other filtration markers. These RKF estimation equations are valid for patients with self-reported urine output ≥1 cup/day that are treated with peritoneal dialysis or conventional (non-convective) hemodialysis. Further research is needed to determine if dialysis dose can be safely modified using estimating equations instead of timed urine collections.
Methods
Study Design and Data Collection
We developed the equations in the RKF Study, a prospective cohort of dialysis patients in Baltimore, Maryland.37 From November 2011 to October 2014, we recruited dialysis patients from 8 outpatient dialysis units. Inclusion criteria were age ≥18 years, English speaking and self-reported ability to produce ≥1 cup/day (approximately 250 mL) of urine. Exclusion criteria included prior kidney transplant. Patients underwent carefully supervised urine clearance measurements at a baseline visit (n=44). Additionally, 9 patients underwent repeat measurements within 6 weeks of initial visit (median 33 days; IQR, 30–40) and 8 at 12 months (median 371 days; IQR, 285–385). We used data from these 61 clearance measurements (44 initial visits and 17 repeat visits), collected under near-ideal setting, for equation development.
We validated the equations in the NECOSAD, a large multicenter prospective cohort study of incident hemo- and peritoneal dialysis patients in the Netherlands that recruited patients from 38 dialysis centers from January 1997 to January 2005.2, 3 Inclusion criteria were age ≥18 years and starting renal replacement therapy for the first time. The present analysis includes 826 patients with stored specimens and available data on RKF.
The Johns Hopkins Medicine Institutional Review Board approved the study. The NECOSAD Study was approved by the Medical Ethics boards of all participating centers.
Renal Clearance Measurement
In both studies, RKF was assessed by a timed urine collection to measure urinary solute clearances. In the RKF study, we performed clearance measurements in hemodialysis patients on an interdialytic day, at least 12 hours or more after the last hemodialysis session (Tuesday or Thursday for patients dialyzing on a Monday, Wednesday, Friday schedule and Sunday, Wednesday or Friday for patients on a Tuesday, Thursday, Saturday dialysis schedule). We performed the clearance measurements under carefully supervised conditions, during a 24-hours inpatient research visit in the Johns Hopkins Bayview Clinical Research Unit in Baltimore, Maryland. Prior to the visit, we verified that patients were on a stable dose of antihypertensive medications. We instructed the patients to eat a light meal on the evening before the visit and a light breakfast on the morning of the visit. During the visit, we served food from standardized menus with the following average composition: protein, 64±1 g/day; potassium, 1.7±0.1 g/day; sodium 1.7±0.4 g/day and phosphate 0.8±0.5 g/day. We allowed daily fluid intake of 1000 ml per day and only allowed non-caffeinated drinks. During the visit, patients were allowed to ambulate in their room and an adjacent lounge. Patients were encouraged not to smoke but were allowed to smoke if they requested. We collected blood samples at the start of measurement (0 minutes), at 2 hours and at 24 hours when the urine collection ended. For the duration of the visit, trained nurses monitored and regularly reminded the patients to collect all voided urine. We calculated urinary CLUREA and CLCREAT from 24-hour urine collections as follows: urine concentration times urine volume divided by mean serum concentration (from measurements at 0, 2 hours and 24 hours). We expressed CLUREA in mL/min to allow incorporation in Kt/VUREA that uses urea volume of distribution rather than body surface area. We also calculated the average of urinary urea and creatinine clearance (CLUREA, CREAT) expressed it per 1.73 m2 of body surface area calculated by Dubois formula38, to allow comparability to GFR estimating equations in non-dialysis patients. We used CLUREA as the reference test for estimating urea clearance and CLUREA, CREAT as the reference test for estimating GFR.
In the peritoneal dialysis patients of the NECOSAD Study, we used timed 24-hour urine collections directly prior to a monitoring visit to the outpatient clinic, where a blood sample was taken. We used this sample to calculate urinary clearances. The hemodialysis patients collected all urine produced during the entire interdialytic interval and blood samples were drawn at the end of the preceding hemodialysis session and directly before the next hemodialysis. We used the mean of these two values for the urinary clearance calculations in hemodialysis patients.39 We analyzed data from samples obtained at 3 or 12 months after dialysis initiation in NECOSAD.
Laboratory Methods
We performed all laboratory measurements at the University of Minnesota’s Advanced Research and Diagnostic Laboratory. Serum and urine urea and creatinine were measured on a Roche COBAS 6000 Analyzer and BTP, B2M and cystatin C were measured on a Siemens ProSpec Nephelometer. Assay precision, characteristics and normal ranges are described in Table S10. In NECOSAD, creatinine (mainly using the alkaline picrate method) and urea had previously been measured at the local laboratories. Earlier analyses in NECOSAD had shown that the method of creatinine measurement had a negligible effect on creatinine concentrations in the presence of very high serum concentrations in dialysis patients.
Analyses in the Development Dataset – RKF Study
We developed separate equations to estimate CLUREA (in mL/min) and CLUREA, CREAT (in mL/min/1.73 m2) based on serum urea and creatinine (together) and BTP, B2M and cystatin C alone and in combination with each other. We used serum markers from 24 hour time point (predialysis values) as predictors in model development as the blood samples from this time point can be readily obtained in clinical practice. We prespecified a process for equation development similar to our methods for estimating glomerular filtration rate (GFR) in non-dialysis patients.14, 40–42 We transformed continuous variables to natural logarithms to stabilize variance. We compared the correlation between log-transformed markers and log-transformed CLUREA (or CLUREA, CREAT). We used least square linear regression to relate log-transformed measured CLUREA (or CLUREA, CREAT) to log serum markers assessing linearity from lowess smoothed plots (bandwidth=0.8). We then considered inclusion of age, sex and race in the models defining significance threshold for model entry as p<0.1 and for including interactions as p<0.01. We retained statistically significant variables in the model if they reduced the RMSE by ≥2%. RMSE measures the typical deviation of individual observations from the model prediction providing precision with which the dependent variable (CLUREA or CLUREA, CREAT) can be predicted. A smaller RMSE implies a better model fit. In sensitivity analyses, we forced age, sex and race into the estimating equations models and assessed if they improved equation performance. For equation building, we used least square linear regression on data from 61 visits for 44 participants with the cluster option in STATA calculating robust standard errors after allowing for within individual correlations. To assess the change in markers over time, we used a random effects model with a population-averaged estimator.
Analyses in the External Validation Dataset - NECOSAD
We compared the baseline characteristics of RKF Study participants with the NECOSAD participants, overall and by dialysis modality, using t-test for continuous variables and chi-squared test for categorical variables. We compared the performance of the RKF Study CLUREA and CLUREA, CREAT estimating equations overall and in subgroups of patients receiving peritoneal dialysis or hemodialysis. We excluded repeat measurements over time while assessing performance. We also compared the performance of the RKF Study CLUREA, CREAT equations with other published GFR estimating equations.14, 27, 28, 32, 40, 41 There are no published equations for estimating CLUREA. In the subset of patients with repeat measurements, we compared the correlations between repeat measurements and repeat estimations (excluding 1 patient with increase in CLUREA >9 mL/min on repeat measurement). We only tested RKF Study equations’ performance in NECOSAD and did not change RKF Study equations based on the NECOSAD data.
Metrics for Equation Performance
We compared measured and estimated CLUREA (or CLUREA, CREAT) graphically by plotting the difference (measured CLUREA – estimated CLUREA) against estimated CLUREA as the estimates are the metric observed in clinical practice (residual versus fitted values plot). We defined bias as the median difference and precision as the interquartile range of this difference. We defined accuracy as estimates within ±2 mL/min of measured CLUREA (or ±2 mL/min/1.73 m2 of measured CLUREA, CREAT). We choose this absolute difference of 2 mL/min rather than a relative percent change as this threshold is clinically relevant (used as a cut-off CLUREA for dialysis adequacy consideration by the 2006 K/DOQI Hemodialysis Adequacy Guidelines)7 and because at a low level of kidney function, small absolute differences in clearance can result in large relative difference. We calculated confidence intervals for the metrics using bootstrapping with 2000 replicates. We determined the significance of differences between equations using the Wilcoxon matched-pairs signed-ranks test for bias, interquartile range regression for precision and McNemar’s test for accuracy. We assessed differences in equation performance between dialysis modalities using median quantile regression for bias, interquartile range regression for precision and two-sample test of proportions for accuracy. We also assessed the sensitivity, specificity, positive and negative predictive values and area under the receiver operating characteristic (ROC) curve of the equations for estimating measured CLUREA ≥ 2 mL/min or CLUREA, CREAT ≥ 2.5 ml/min/1.73 m2 which is the mean CLUREA, CREAT in the development data when CLUREA is 2 mL/min.
We performed all analyses using STATA version 13.1 (StataCorp, www.stata.com).
Supplementary Material
Figure S1: Association between Measured Urinary Clearances and Endogenous Filtration Markers in 44 Dialysis Patients in Development Cohort, The Residual Kidney Function Study. Scatterplots of natural log transformed urea, creatinine, β-trace protein (BTP), β2-microglobulin (B2M) and cystatin C on the vertical (Y) axis and measured urinary clearances on the horizontal (X) axis. Data from patients on peritoneal dialysis are displayed as blue dots and data from patients on hemodialysis are displayed as red dots. Black line is the linear fit. Pearson’s correlation coefficients are displayed in the bottom left corner of each scatterplot. Panel A (left): Measured urinary urea clearance (CLUREA) in mL/min; and Panel B: Measured average of urinary urea and creatinine clearance (CLUREA, CREAT) in mL/min/1.73 m2.
Abbreviations: CLUREA, urinary urea clearance (mL/min); CLUREA, CREAT, average of urinary urea and creatinine clearance (in ml/min/1.73 m2); PD, peritoneal dialysis; HD, hemodialysis.
Figure S2: Association between Change in Measured Urinary Clearances and Endogenous Filtration Markers in 162 Dialysis Patients in Validation Cohort, The Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD). Scatterplots of change in urea, creatinine, β-trace protein (BTP), β2-microglobulin (B2M) and cystatin C on the vertical (Y) axis and change in measured urinary clearances on the horizontal (X) axis. Data from patients on peritoneal dialysis are displayed as blue dots and data from patients on hemodialysis are displayed as red dots. Black line is the linear fit. Pearson’s correlation coefficients are displayed in the bottom left corner of each scatterplot. Panel A (left): Measured urinary urea clearance (CLUREA) in mL/min; and Panel B: Measured average of urinary urea and creatinine clearance (CLUREA, CREAT) in mL/min/1.73 m2.
Abbreviations: CLUREA, urinary urea clearance (mL/min); CLUREA, CREAT, average of urinary urea and creatinine clearance (in ml/min/1.73 m2); PD, peritoneal dialysis; HD, hemodialysis.
Acknowledgments
Dr. Shafi is supported by K23-DK-083514.
Research reported in this publication was supported by the National Institute Of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This publication was made possible by the Johns Hopkins Institute for Clinical and Translational Research (ICTR) which is funded in part by Grant Number UL1 TR 001079 from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the Johns Hopkins ICTR, NCATS or NIH.
We thank the patients, staff, and physicians of DaVita dialysis clinics and the Nephrology Center of Maryland for their support and participation in the RKF study. We thank Dr. Duvuru Geetha, Dr. Stephen M. Sozio, Dr. Deidra C. Crews, Dr. Bernard G. Jaar and Dr. Luis F. Gimenez for their support of the RKF Study.
We thank the nursing staff of the participating hospitals dialysis centers, the trial nurses and the staff of the NECOSAD trial office for their assistance in the collection and management of data for this study. The members of Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD) Study Group include A.J. Apperloo, J.A. Bijlsma, M. Boekhout, W.H. Boer, P.J.M. van der Boog, H.R. Büller, M. van Buren, F. Th. de Charro, C.J. Doorenbos, M.A. van den Dorpel, A. van Es, W.J. Fagel, G.W. Feith, C.W.H. de Fijter, L.A.M. Frenken, W. Grave, J.A.C.A. van Geelen, P.G.G. Gerlag, J.P.M.C. Gorgels, R.M. Huisman, K.J. Jager, K. Jie, W.A.H. Koning-Mulder, M.I. Koolen, T.K. Kremer Hovinga, A.T.J. Lavrijssen, A.J. Luik, J. van der Meulen, K.J. Parlevliet, M.H.M. Raasveld, F.M. van der Sande, M.J.M. Schonck, M.M.J. Schuurmans, C.E.H. Siegert, C.A. Stegeman, P. Stevens, J.G.P. Thijssen, R.M. Valentijn, G.H. Vastenburg, C.A. Verburgh, H.H. Vincent, and P.F. Vos.
Study data were collected and managed using REDCap electronic data capture tools hosted at Johns Hopkins University.1 REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources.
The reagents for β-trace protein, β2-microglobluin and cystatin C assays were provided by Siemens to the University of Minnesota, where the measurements were performed. Siemens had no role in the design, analysis, and interpretation of data or the preparation of this manuscript.
Parts of this work were presented at the 2014 Annual Meeting of the American Society of Nephrology in Philadelphia, PA, November 12–16, 2014.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of Competing Financial Interests
Dr. Shafi reports receiving speaker fees from Siemens.
Dr. Inker reports funding to Tufts Medical Center for research and contracts with the National Institutes of Health, National Kidney Foundation, Pharmalink AB and Gilead Sciences, a consulting agreement with Otsuka, and has a provisional patent [Coresh, Inker and Levey] filed 8/15/2014 – Precise estimation of glomerular filtration rate from multiple biomarkers (licensing under negotiation).
Dr. Levey reports funding to Tufts Medical Center for research and contracts with the National Institutes of Health, National Kidney Foundation, Amgen, Pharmalink AB, Gilead Sciences, and has a provisional patent [Coresh, Inker and Levey] filed 8/15/2014 – Precise estimation of glomerular filtration rate from multiple biomarkers (licensing under negotiation).
Dr. Coresh has a provisional patent [Coresh, Inker and Levey] filed 8/15/2014 – Precise estimation of glomerular filtration rate from multiple biomarkers (licensing under negotiation).
Dr. Eckfeldt reports being a consultant for Gentian which is a Norwegian manufacturer of reagents for clinical cystatin C measurement procedures and his research laboratory has received free or steeply discounted reagents from Siemens for measurement of β-trace protein, cystatin C, and β-2 microglobulin.
Paul A. Harris, Robert Taylor, Robert Thielke, Jonathon Payne, Nathaniel Gonzalez, Jose G. Conde, Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform. 2009 Apr;42(2):377–81
References
- 1.Bargman JM, Thorpe KE, Churchill DN. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol. 2001;12:2158–2162. doi: 10.1681/ASN.V12102158. [DOI] [PubMed] [Google Scholar]
- 2.Termorshuizen F, Dekker FW, van Manen JG, et al. Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol. 2004;15:1061–1070. doi: 10.1097/01.asn.0000117976.29592.93. [DOI] [PubMed] [Google Scholar]
- 3.Termorshuizen F, Korevaar JC, Dekker FW, et al. The relative importance of residual renal function compared with peritoneal clearance for patient survival and quality of life: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD )-2. Am J Kidney Dis. 2003;41:1293–1302. doi: 10.1016/s0272-6386(03)00362-7. [DOI] [PubMed] [Google Scholar]
- 4.Shafi T, Jaar BG, Plantinga LC, et al. Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am J Kidney Dis. 2010;56:348–358. doi: 10.1053/j.ajkd.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konings CJ, Kooman JP, Schonck M, et al. Fluid status in CAPD patients is related to peritoneal transport and residual renal function: evidence from a longitudinal study. Nephrol Dial Transplant. 2003;18:797–803. doi: 10.1093/ndt/gfg147. [DOI] [PubMed] [Google Scholar]
- 6.Wang AYM, Lai KN. The importance of residual renal function in dialysis patients. Kidney Int. 2006;69:1726–1732. doi: 10.1038/sj.ki.5000382. [DOI] [PubMed] [Google Scholar]
- 7.Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis. 2006;48(Suppl 1):S2–90. doi: 10.1053/j.ajkd.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 8.Wang AY-M, Wang M, Woo J, et al. A novel association between residual renal function and left ventricular hypertrophy in peritoneal dialysis patients. Kidney Int. 2002;62:639–647. doi: 10.1046/j.1523-1755.2002.00471.x. [DOI] [PubMed] [Google Scholar]
- 9.Marquez IO, Tambra S, Luo FY, et al. Contribution of residual function to removal of protein-bound solutes in hemodialysis. Clin J Am Soc Nephrol. 2011;6:290–296. doi: 10.2215/CJN.06100710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Wal WM, Noordzij M, Dekker FW, et al. Full loss of residual renal function causes higher mortality in dialysis patients; findings from a marginal structural model. Nephrol Dial Transplant. 2011;26:2978–2983. doi: 10.1093/ndt/gfq856. [DOI] [PubMed] [Google Scholar]
- 11.Clinical Practice Guidelines for Peritoneal Dialysis Adequacy. American Journal of Kidney Diseases. 2006;48:S98–S129. doi: 10.1053/j.ajkd.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Tattersall J, Martin-Malo A, Pedrini L, et al. EBPG guideline on dialysis strategies. Nephrol Dial Transplant. 2007;22:ii5–21. doi: 10.1093/ndt/gfm022. [DOI] [PubMed] [Google Scholar]
- 13.White CA, Ghazan-Shahi S, Adams MA. beta-Trace Protein: A Marker of GFR and Other Biological Pathways. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2015;65:131–146. doi: 10.1053/j.ajkd.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 14.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woitas RP, Stoffel-Wagner B, Poege U, et al. Low-molecular weight proteins as markers for glomerular filtration rate. Clinical chemistry. 2001;47:2179–2180. [PubMed] [Google Scholar]
- 16.Rombach SM, Baas MC, ten Berge IJ, et al. The value of estimated GFR in comparison to measured GFR for the assessment of renal function in adult patients with Fabry disease. Nephrol Dial Transplant. 2010;25:2549–2556. doi: 10.1093/ndt/gfq108. [DOI] [PubMed] [Google Scholar]
- 17.Gerhardt T, Poge U, Stoffel-Wagner B, et al. Serum levels of beta-trace protein and its association to diuresis in haemodialysis patients. Nephrol Dial Transplant. 2008;23:309–314. doi: 10.1093/ndt/gfm510. [DOI] [PubMed] [Google Scholar]
- 18.Lindstrom V, Grubb A, Alquist Hegbrant M, et al. Different elimination patterns of beta-trace protein, beta2-microglobulin and cystatin C in haemodialysis, haemodiafiltration and haemofiltration. Scand J Clin Lab Invest. 2008;68:685–691. doi: 10.1080/00365510802047693. [DOI] [PubMed] [Google Scholar]
- 19.Melegos DN, Grass L, Pierratos A, et al. Highly elevated levels of prostaglandin D synthase in the serum of patients with renal failure. Urology. 1999;53:32–37. doi: 10.1016/s0090-4295(98)00453-1. [DOI] [PubMed] [Google Scholar]
- 20.Cheung AK, Rocco MV, Yan G, et al. Serum beta-2 microglobulin levels predict mortality in dialysis patients: results of the HEMO study. J Am Soc Nephrol. 2006;17:546–555. doi: 10.1681/ASN.2005020132. [DOI] [PubMed] [Google Scholar]
- 21.Huang SH, Tirona RG, Reid-Wilkinson F, et al. The kinetics of cystatin C removal by hemodialysis. Am J Kidney Dis. 2015;65:174–175. doi: 10.1053/j.ajkd.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Steubl D, Hettwer S, Dahinden P, et al. C-terminal agrin fragment (CAF) as a serum biomarker for residual renal function in peritoneal dialysis patients. Int Urol Nephrol. 2015;47:391–396. doi: 10.1007/s11255-014-0852-5. [DOI] [PubMed] [Google Scholar]
- 23.Yang Q, Li R, Zhong Z, et al. Is cystatin C a better marker than creatinine for evaluating residual renal function in patients on continuous ambulatory peritoneal dialysis? Nephrol Dial Transplant. 2011;26:3358–3365. doi: 10.1093/ndt/gfr045. [DOI] [PubMed] [Google Scholar]
- 24.Al-Wakeel JS, Hammad D, Memon NA, et al. Serum cystatin C: a surrogate marker for the characteristics of peritoneal membrane in dialysis patients. Saudi J Kidney Dis Transpl. 2009;20:227–231. [PubMed] [Google Scholar]
- 25.Montini G, Amici G, Milan S, et al. Middle molecule and small protein removal in children on peritoneal dialysis. Kidney Int. 2002;61:1153–1159. doi: 10.1046/j.1523-1755.2002.00216.x. [DOI] [PubMed] [Google Scholar]
- 26.Kabanda A, Goffin E, Bernard A, et al. Factors influencing serum levels and peritoneal clearances of low molecular weight proteins in continuous ambulatory peritoneal dialysis. Kidney Int. 1995;48:1946–1952. doi: 10.1038/ki.1995.495. [DOI] [PubMed] [Google Scholar]
- 27.Poge U, Gerhardt T, Woitas RP. Estimation of Glomerular Filtration Rate by Use of Beta-Trace Protein. Clinical chemistry. 2008;54:1403–1405. doi: 10.1373/clinchem.2007.101840. [DOI] [PubMed] [Google Scholar]
- 28.White CA, Akbari A, Doucette S, et al. A novel equation to estimate glomerular filtration rate using beta-trace protein. Clinical chemistry. 2007;53:1965–1968. doi: 10.1373/clinchem.2007.090126. [DOI] [PubMed] [Google Scholar]
- 29.White CA, Akbari A, Doucette S, et al. Effect of clinical variables and immunosuppression on serum cystatin C and beta-trace protein in kidney transplant recipients. Am J Kidney Dis. 2009;54:922–930. doi: 10.1053/j.ajkd.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Abbink FC, Laarman CA, Braam KI, et al. Beta-trace protein is not superior to cystatin C for the estimation of GFR in patients receiving corticosteroids. Clin Biochem. 2008;41:299–305. doi: 10.1016/j.clinbiochem.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 31.Grubb A, Bjork J, Nyman U, et al. Cystatin C, a marker for successful aging and glomerular filtration rate, is not influenced by inflammation. Scand J Clin Lab Invest. 2011;71:145–149. doi: 10.3109/00365513.2010.546879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoek FJ, Korevaar JC, Dekker FW, et al. Estimation of residual glomerular filtration rate in dialysis patients from the plasma cystatin C level. Nephrol Dial Transplant. 2007;22:1633–1638. doi: 10.1093/ndt/gfm027. [DOI] [PubMed] [Google Scholar]
- 33.Daugirdas JT, Depner TA, Greene T, et al. Standard Kt/Vurea: a method of calculation that includes effects of fluid removal and residual kidney clearance. Kidney Int. 2010;77:637–644. doi: 10.1038/ki.2009.525. [DOI] [PubMed] [Google Scholar]
- 34.National Kidney Foundation. KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. American Journal of Kidney Diseases. 2015;66 doi: 10.1053/j.ajkd.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 35.van Olden RW, van Acker BA, Koomen GC, et al. Time course of inulin and creatinine clearance in the interval between two haemodialysis treatments. Nephrol Dial Transplant. 1995;10:2274–2280. doi: 10.1093/ndt/10.12.2274. [DOI] [PubMed] [Google Scholar]
- 36.Kwong YT, Stevens LA, Selvin E, et al. Imprecision of urinary iothalamate clearance as a gold-standard measure of GFR decreases the diagnostic accuracy of kidney function estimating equations. Am J Kidney Dis. 2010;56:39–49. doi: 10.1053/j.ajkd.2010.02.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shafi T, Levey AS, Inker LA, et al. Plasma Iohexol Clearance for Assessing Residual Kidney Function in Dialysis Patients. Am J Kidney Dis. 2015 doi: 10.1053/j.ajkd.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dubois D, Dubois E. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–871. [Google Scholar]
- 39.Jansen MA, Hart AA, Korevaar JC, et al. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int. 2002;62:1046–1053. doi: 10.1046/j.1523-1755.2002.00505.x. [DOI] [PubMed] [Google Scholar]
- 40.Inker LA, Tighiouart H, Coresh J, et al. GFR Estimation Using beta-Trace Protein and beta-Microglobulin in CKD. Am J Kidney Dis. 2015 doi: 10.1053/j.ajkd.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Association between Measured Urinary Clearances and Endogenous Filtration Markers in 44 Dialysis Patients in Development Cohort, The Residual Kidney Function Study. Scatterplots of natural log transformed urea, creatinine, β-trace protein (BTP), β2-microglobulin (B2M) and cystatin C on the vertical (Y) axis and measured urinary clearances on the horizontal (X) axis. Data from patients on peritoneal dialysis are displayed as blue dots and data from patients on hemodialysis are displayed as red dots. Black line is the linear fit. Pearson’s correlation coefficients are displayed in the bottom left corner of each scatterplot. Panel A (left): Measured urinary urea clearance (CLUREA) in mL/min; and Panel B: Measured average of urinary urea and creatinine clearance (CLUREA, CREAT) in mL/min/1.73 m2.
Abbreviations: CLUREA, urinary urea clearance (mL/min); CLUREA, CREAT, average of urinary urea and creatinine clearance (in ml/min/1.73 m2); PD, peritoneal dialysis; HD, hemodialysis.
Figure S2: Association between Change in Measured Urinary Clearances and Endogenous Filtration Markers in 162 Dialysis Patients in Validation Cohort, The Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD). Scatterplots of change in urea, creatinine, β-trace protein (BTP), β2-microglobulin (B2M) and cystatin C on the vertical (Y) axis and change in measured urinary clearances on the horizontal (X) axis. Data from patients on peritoneal dialysis are displayed as blue dots and data from patients on hemodialysis are displayed as red dots. Black line is the linear fit. Pearson’s correlation coefficients are displayed in the bottom left corner of each scatterplot. Panel A (left): Measured urinary urea clearance (CLUREA) in mL/min; and Panel B: Measured average of urinary urea and creatinine clearance (CLUREA, CREAT) in mL/min/1.73 m2.
Abbreviations: CLUREA, urinary urea clearance (mL/min); CLUREA, CREAT, average of urinary urea and creatinine clearance (in ml/min/1.73 m2); PD, peritoneal dialysis; HD, hemodialysis.


