Abstract
The duration of immunosuppressive effects following oral cyclosporine in dogs is unknown. This study used flow cytometry and quantitative reverse transcription–polymerase chain reaction (qRT-PCR) to evaluate the effects of high-dose oral cyclosporine across a 12-h dosing interval. Expression of interleukin-2 (IL-2) and interferon-gamma (IFN-γ) was compared before and after 8 days of cyclosporine at 10 mg/kg every 12 h in six healthy dogs. Samples were collected at 0, 2, 4, and 8 h postdosing for analysis of unactivated and activated T-cell and whole blood cytokine expression using flow cytometry and qRT-PCR, respectively, and at 0, 2, 4, 6, 8, and 10 h postdosing for measurement of cyclosporine concentrations. Flow cytometry and qRT-PCR both demonstrated significant marked reductions in IL-2 and IFN-γ levels at 0, 2, 4, and 8 h after dosing compared to pretreatment levels (P < 0.05) for activated samples, with less consistent effects observed for unactivated samples. Both flow cytometry and qRT-PCR are viable techniques for measuring cyclosporine pharmacodynamics in dogs, yielding comparable results with activated samples. Two hours postdrug administration is the preferred time for concurrent assessment of peak drug concentration and cytokine expression, and T-cell activation is needed for optimal results.
INTRODUCTION
Cyclosporine, a calcineurin inhibitor, is an important immunosuppressive agent in both dogs and humans. A potent inhibitor of T-cell activation, cyclosporine decreases the expression of nuclear factor of activated T-cell (NFAT) -regulated cytokines, including interleukin-2 (IL-2), interferon-gamma (IFN-γ), and tumor necrosis factor-alpha (TNF-α) (Rao et al., 1997). Cyclosporine is commonly used in the treatment of inflammatory and immune-mediated diseases in dogs, but there is substantial controversy regarding appropriate dosage regimens and how to best assess response to therapy. Pharmacokinetic monitoring is available, but there is limited information regarding appropriate therapeutic cyclosporine blood concentrations for various disease states in dogs (Archer et al., 2014). Veterinarians generally evaluate trough cyclosporine concentrations while, in human transplant medicine, peak concentrations collected 2 h postdosing are better correlated with both transplant rejection and the development of cyclosporine toxicity (Cantarovich et al., 1998; Citterio et al., 2001; Kahan, 2004; Mathias et al., 2005; Davies et al, 2007).
Blood cyclosporine concentrations in people do not predict patient response in all situations, and much work in human medicine has therefore been directed at pharmacodynamic monitoring of cyclosporine therapy (Hartel et al., 2002; Giese et al., 2004; Barten et al., 2006, 2007; Sommerer et al., 2008; Kuzuya et al., 2009). Pharmacodynamic studies of cyclosporine typically involve assays of either calcineurin activity or lymphocyte function. Lymphocyte proliferation, surface antigen expression, and cytokine production have all been evaluated after cyclosporine therapy, with most work in veterinary medicine focusing on reductions in peripheral blood and affected tissue cytokine levels (Kyles et al., 2000; Kobayashi et al., 2007; Kuga et al., 2008; Tivers et al., 2008). Flow cytometry and quantitative reverse transcription–polymerase chain reaction (qRT-PCR) are two commonly used techniques to measure T-cell cytokine responses, but assess different outcomes. Flow cytometry measures actual protein levels, while qRT-PCR looks only at messenger ribonucleic acid (mRNA) expression, which does not necessarily correlate with protein production.
Previous work in our laboratory has both evaluated and validated techniques to measure cytokine levels in dogs treated with cyclosporine using flow cytometry and qRT-PCR (Archer et al., 2011; Fellman et al., 2011; Riggs et al., 2013). Our initial work demonstrated cyclosporine-mediated suppression of cytokines and activation-related surface antigens for T cells incubated with cyclosporine (Fellman et al., 2011), and subsequent work confirmed that IL-2 and IFN-γ are suppressed after oral cyclosporine administration in dogs (Archer et al., 2011). However, it has not been established if cyclosporine-mediated suppression of NFAT-regulated cytokines is consistent across the dosing interval, or if there is T-cell recovery as the next dose is approached. This study evaluated the levels of the cytokines IL-2 and IFN-γ measured using both flow cytometry and qRT-PCR across a 12-h oral cyclosporine dosing interval. Blood cyclosporine concentrations were also measured. The goals of this study were to compare results obtained using both flow cytometry and qRT-PCR, and to determine the optimal time and method for pharmacodynamic measurement of cyclosporine’s effects on T cells.
MATERIALS AND METHODS
Dogs
This project involved six healthy, purpose-bred, adult female Walker hounds. Prior to the study, each dog received a physical examination, complete blood count, serum biochemistry profile, urinalysis, fecal flotation, and heartworm testing, with no significant abnormalities noted. Study protocols and animal care regimens were approved by the Mississippi State University Institutional Animal Care and Use Committee. Mississippi State University is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Cyclosporine administration
A repeated-measures design was used. Before drug administration, blood was collected from all dogs for pretreatment evaluation of IL-2 and IFN-γ using both flow cytometry and qRT-PCR. The dogs were divided into two groups of three dogs to simplify sample processing. The first three dogs were then given oral microenaulsified cyclosporine (Atopica®, Elanco, Greenfield, IL, USA), at a dose of 10 mg/kg every 12 h for 8 days. On day 8, blood was collected immediately prior to the next dose (0 h or ‘trough’ sample), and at two-hour intervals (2, 4, 6, 8, and 10 h) after drug administration, for cyclosporine blood concentration determination. Additional blood was collected predosing (O h), and at 2, 4, and 8 h after dosing for cytokine analysis using flow cytometry and qRT-PCR. Six days after the first three dogs began drug administration, the other three dogs began oral cyclosporine at the same dose and had blood collected at the previously described time points after 8 days of treatment. All blood was collected using jugular venipuncture with a 20-gauge needle and syringe, with collected blood immediately transferred to heparinized vacutainers for cytokine evaluation, and ethylenediaminete-traacetic acid (EDTA) tubes for cyclosporine blood concentration determination.
Cyclosporine blood concentrations
Blood was collected into EDTA anticoagulant tubes, and shipped to the Auburn University Veterinary Clinical Pharmacology Laboratory on ice for analysis within 48 h of collection. Samples were thawed to room temperature and then mixed by inversion to assure homogeneity. Cyclosporine was detected in canine EDTA whole blood using the Siemens (New York, NY, USA) Cyclosporine Immunoassay® (CSA) and the Siemens Cyclosporine Extended Range Immunoassay® (CSAE) on a Siemens (New York, NY, USA) Dimension Xpand Plus® general chemistry analyzer. For CSA, the upper limit of quantitation was 500 ng/mL, and the lower limit of quantitation was 25 ng/mL. CSA was calibrated using the Siemens CSA Calibrator®, and quality control was performed using More Diagnostics (Los Osos, CA, USA) RAP/TAC/CSA Controls®. For CSAE, the upper limit of quantitation was 2000 ng/mL, and the lower limit of quantitation was 350 ng/mL, with the ability to dilute up to 6000 ng/mL. CSAE was calibrated with the Siemens CSAE Calibrator®, and quality control was performed using More Diagnostics Cyclosporine C2 Controls®.
Cytoldne analysis
Flow cytometry
Flow cytometric analysis was performed as previously described by our laboratory (Archer et al., 2011; Fellman et al., 2011), with modifications as described below. Antibodies used were as follows: FITC-conjugated monoclonal anti-dog cluster of differentiation 3 (CD3) (MCA1774F, AbD Serotec, Raleigh, NC, USA), RPE-conjugated monoclonal anti-bovine IFN-γ (MCA1783PE, AbD Serotec), and biotinylated anti-canine IL-2 (BAF1815, R&D Systems, Minneapolis, MN, USA). The secondary label for IL-2 was RPE-conjugated streptavidin (#60669, Anaspec, San Jose, CA, USA). Heparinized blood was diluted with complete media at a ratio of one part blood, nine parts media. Half of the diluted whole blood samples were not activated (‘unactivated’), and the other half were activated with 12.5 ng/mL phorbol 12-myristate 13-acetate (PMA, cat. P8139) and 0.8 μm ionomycin (cat. 10634), both purchased from Sigma-Aldrich (St. Louis, MO, USA). All samples were then incubated for 7 h at 37 °C in a 5% C02 incubator. Brefeldin A (cat. 555029, BD Biosciences, San Jose, CA, USA) was added 1 h after activation at a final concentration of 1 μg/mL to stop cytokine secretion from T cells. After incubation, 350 μL of cell suspension was collected per sample, and anti-CD3 monoclonal antibody was added directly to the whole blood mixture and incubated for 25 min at room temperature in the dark. Red blood cells were lysed with BD PharmLyse (cat. 555899, BD Biosciences, San Jose, CA, USA) as recommended by the manufacturer. Cells were then fixed and permeabilized using the Becton Dickinson (BD) Cytofix/Cytoperm™ Plus Kit (cat. 554714, BD Biosciences, San Jose, CA, USA), stained for IL-2 and IFN-γ, and prepared for flow cytometry as previously described (Archer et al., 2011; Fellman et al., 2011).
A BD FACSCalibur™ flow cytometer was used for staining evaluation, with data analyzed using bd cellqcest™ pro software (BD Biosciences, San Jose, CA, USA). Lymphocytes were identified using forward scatter and side scatter, and 5000 lymphocytes were collected per sample. An additional gate identified CD3+ cells, and cytokine expression was measured from cells within the lymphocyte and CD3+ gates. Cell staining was measured using mean fluorescence intensity (MFI) values with single histogram statistics, and isotype controls and unstained samples were used as negative controls.
Quantitative RT-PCR
Heparinized whole blood was activated using 12.5 ng/mL PMA and 0.8 μM ionomycin. Another set of blood samples was left unactivated, and all samples were incubated for 5 h at 37 °C and 5% CO2. Total RNA was extracted and qRT-PCR analysis of IL-2 and IFN-γ expression performed as previously described by our laboratory (Riggs et al., 2013), with the minor modification of using 1.5 ng/μL RNA template in a 20 μL final reaction volume. RNA was frozen at −80 °C until analysis, and samples from each dog were analyzed on a single qPCR plate. Reactions were analyzed on a Stratagene™ Mx3005P using Stratagene™ mxpro qpcr software v4.10 for analysis (Agilent Technologies, Santa Clara, CA, USA).
Relative gene expression was assessed for threshold cycle (Ct) using the 2−ΔΔCt method where ΔΔCt = (CtGOI – Ctnorm)trated – (CtGOI – Ctnorm)untreated, GOI is the gene of interest, and norm is the reference gene (GAPDH) (Livak & Schmittgen, 2001).
Statistical analysis
A repeated-measures design was utilized in this study. Visual assessment of the data using histograms with UNIVARIATE procedure of SAS® for Windows® 9.3 (SAS Institute, Inc., Cary, NC, USA) indicated that the data were not normally distributed. Consequently, the data were transformed by talcing the reciprocal square root of each value. Histograms indicated the transformed data were approximately normally distributed. A separate mixed-effects model for each outcome was used to test for a time effect using the MIXED procedure of SAS® for Windows® 9.3. A first-order autoregressive covariance structure was specified in the repeated statement to accommodate the repeated measures. For outcomes in which time had a significant effect (P ≤ 0.05), comparisons were made between each pair of time points using differences in least square means. Tukey Kramer adjustment was used for adjustment of P values for the multiple comparisons. A P value of less than or equal to 0.05 was considered to be significant for all analyses.
RESULTS
Cyclosporine blood concentrations
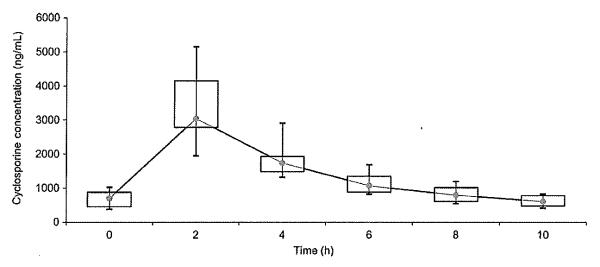
Blood cyclosporine concentrations are presented in Fig. 1. After 8 days of cyclosporine at 10 mg/kg every 12 h, peak concentrations were found at two hours postdosing. At two hours postdosing, the range was 1944–5148 ng/mL, and the median was 3040 ng/mL. Trough concentrations (0 h) ranged from 375 to 1021 ng/mL, with a median of 701 ng/mL.
Fig. 1.
Blood cyclosporine concentrations in six dogs measured on Day 8 after 7 days of oral cyclosporine dosed at 10 mg/kg every 12 h. Each box represents the interquartile range (IQR) from the 25th to 75th percentile. The point inside each box represents the median, and whiskers extend to maximum and minimum values. Cyclosporine concentrations were measured using an immunoassay.
Cytokine analysis
Flow cytometry
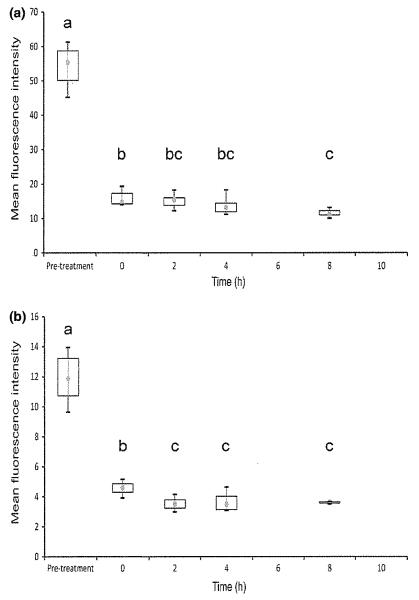
Flow cytometry results are shown in Figs 2 and 3. Activated sample expression of IL-2 and IFN-γ was significantly reduced relative to pretreatment values at 0, 2, 4, and 8 h after dosing for both cytokines (P < 0.05). There was also significantly reduced expression for the 2, 4, and 8 h samples relative to the 0 h (trough cyclosporine blood concentration) sample for IFN-γ, and for the 8 h sample relative to the 0 h sample for IL-2. All activated cyclosporine samples in all dogs at all post-treatment time points showed markedly lower protein expression than pretreatment samples (Fig. 2).
Fig. 2.
Activated T-cell expression of IL-2 (a) and IFN-γ (b) measured using flow cytometry in 6 dogs on Day 8 after oral cyclosporine dosing at 10 mg/kg every 12 h. Each box represents the IQR. The point inside each box represents the median, and whiskers extend to maximum and minimum values. Times that do not share a letter are significantly different (P < 0.05) based on mixed model analysis of data transformed by taking the reciprocal square root.
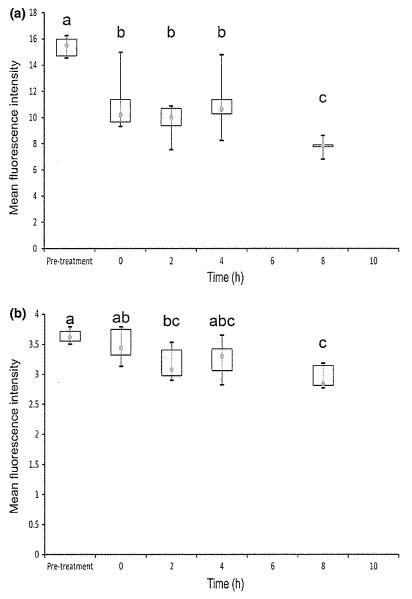
Fig. 3.
Unactivated T-cell expression of IL-2 (a) and IFN-γ (b) measured using flow cytometry in 6 dogs on Day 8 after oral cyclosporine dosing at 10 mg/kg every 12 h. Each box represents the IQR. The point inside each box represents the median, and whiskers extend to maximum and minimum values. Times that do not share a letter are significantly different (P < 0.05) based on mixed model analysis of data transformed by taking the reciprocal square root.
Unactivated sample analysis (Fig. 3) revealed significant reduction in IL-2 cytokine levels for hours 0, 2, 4, and 8 relative to pretreatment. IL-2 cytokine levels at hour 8 were also significantly lower than at hours 0, 2, and 4. Only hours 2 and 8 were significantly lower than pretreatment for IFN-γ levels, and hour 8 was also significantly lower than hour 0. There was an overall smaller difference in MFI across the sampling times for unactivated samples than for activated samples, largely due to unactivated pretreatment samples having much lower fluorescence than activated pretreatment samples.
Quantitative RT-PCR
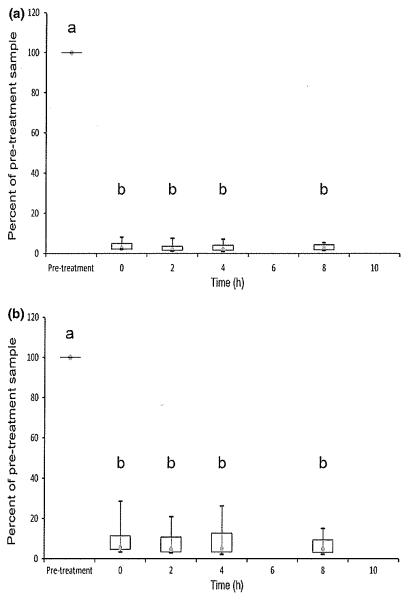
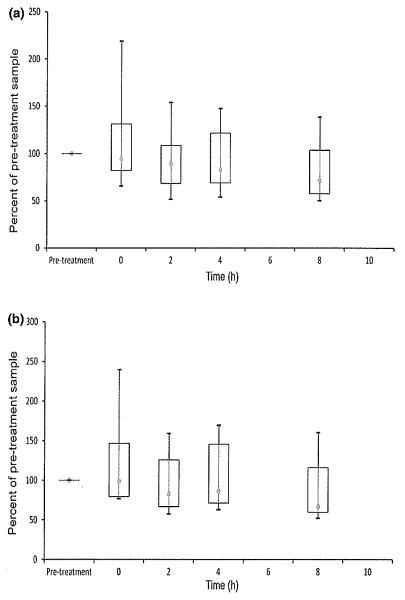
Quantitative RT-PCR results are shown in Figs 4 and 5. Activated expression of IL-2 and IFN-γ mRNA was significantly reduced relative to pretreatment values at 0, 2, 4, and 8 h after dosing for both cytokines (P < 0.05). In contrast to flow cytometry, there was no statistically significant variation in the degree of suppression across the dosing interval. All activated cyclosporine samples in all dogs at all post-treatment time points showed markedly lower gene expression than pretreatment samples (Fig. 4). There were no significant differences in mRNA expression for unactivated samples (Fig. 5).
Fig. 4.
Activated whole blood mRNA expression of IL-2 (a) and IFN-γ (b) measured using qRT-PCR in six dogs on Day 8 after oral cyclosporine dosing at 10 mg/kg every 12 h. Relative quantification was performed using the 2−ΔΔCt method where ΔΔCt = (CtGOI – Ctnorm)treated – (CtGOI – Ctnorm)untreated, GOI is the gene of interest, and norm is the reference gene GAPDH. Data are expressed as a percentage of cytokine expression in pretreatment samples, which are given a value of 100%. Each box represents the IQR. The point inside each box represents the median, and whiskers extend to maximum and minimum values. Times that do not share a letter are significantly different (P < 0.05) based on mixed model analysis of data transformed by taking the reciprocal square root.
Fig. 5.
Unactivated whole blood mRNA expression of IL-2 (a) and IFN-γ (b) measured using qRT-PCR in six dogs on Day 8 after oral cyclosporine dosing at 10 mg/kg every 12 h. Relative quantification was performed using the 2−ΔΔCt method where ΔΔCt = (CtGOI – Ctnorm)treated – (CtGOI – Ctnorm)untreated, GOI is the gene of interest, and norm is the reference gene GAPDH. Data are expressed as a percentage of cytokine expression in pretreatment samples, which are given a value of 100%. Each box represents the IQR. The point inside each box represents the median, and whiskers extend to maximum and minimum values. No significant differences identified.
DISCUSSION
To the authors’ knowledge, this study provides the first paired evaluation of flow cytometry and qRT-PCR for measurement of cyclosporine pharmacodynamics in dogs. Our results show that high-dose oral cyclosporine markedly and consistently suppresses cytokine expression in healthy dogs in activated blood samples when evaluated using either technique. Flow cytometry did show an extra degree of suppression of protein expression at 2, 4, and 8 h relative to 0 h activated samples for IFN-γ, and a significant difference between hour 0 and hour 8 for activated IL-2. Overall, however, there was minimal variability seen in suppression across the oral dosing interval, and even at trough drug concentrations 12 h after the previous oral cyclosporine dose (hour 0), both IL-2 and IFN-γ were significantly reduced from pretreatment levels for activated samples using both methods.
Unactivated cytokine expression showed similar findings as with activated samples for flow cytometry, although differences in fluorescence were of smaller magnitude. Activated samples had total separation of MFI values for pre- relative to post-treatment samples, while unactivated fluorescence was lower and had overlap in pre- and post-treatment MFI values. The most prominent suppression was seen for 8-h unactivated samples, which demonstrated significantly lower protein expression than all other times for IL-2, and were lower than pretreatment and 0-h samples for IFN-γ. Unactivated qRT-PCR samples, on the other hand, showed no significant differences between pretreatment and post-treatment expression levels. Activation of lymphocytes increases the production of cytokines, so lower expression and smaller differences among treatment times were expected for unactivated samples. Testing unactivated samples would decrease processing time, but because of the poorer separation in pre- and post-treatment cytokine levels, PMA and ionomycin activation of blood samples is recommended for pharmacodynamic evaluation of cyclosporine’s effects. The remainder of this discussion will focus on activated cell expression results.
Previous work in our laboratory has evaluated both flow cytometry and qRT-PCR for assessment of cyclosporine pharmacodynamics (Archer et al., 2011; Fellman et al., 2011; Riggs et al., 2013). Our earlier results suggested that both techniques provide comparable results, and this study confirms that both methods document similar marked suppression of T-cell function for an extended period after each oral dose of cyclosporine. A previous study by Flores and others evaluated the ability of flow cytometry and qRT-PCR to assess suppression of cytokine expression caused by in vitro exposure to cyclosporine and tacrolimus in blood from cynomolgus monkeys (Flores et al., 2004) and concluded that both techniques could be used interchangeably when evaluating cyclosporine pharmacodynamics in monkeys. Our study confirms that both methods appear to be similarly interchangeable in dogs, at least at high drug doses.
The additional degree of cytokine protein suppression shown using flow cytometry between trough drug concentrations and later time points in the dosing interval suggests that this method may be more discriminating and able to reflect subtle differences in suppression of T-cell function at high cyclosporine concentrations. As suppression of cytokine mRNA expression in our study was slightly more rapid, sustained and complete than protein expression, our results suggest that the qRT-PCR assay may be more sensitive to the effects of cyclosporine and may therefore be able to identify suppression of T-cell function at lower drug concentrations, but further studies at lower cyclosporine concentrations would be needed to evaluate this possibility.
Blood cyclosporine concentrations following oral cyclosporine dosing in the current study were similar to those reported in previous studies, with a peak at two hours and a gradual reduction in blood concentrations over subsequent hours. Previously, trough cyclosporine concentrations of 500 ng/mL have been suggested as a target for attainment of adequate immunosuppression in dogs (Daigle, 2002). However, although only four of the six dogs in this study reached a 500 ng/mL trough concentration, with one dog attaining a trough of only 375 ng/mL, all dogs showed marked suppression of cytokine expression. Although ideal peak cyclosporine concentrations for dogs have not been published, a target peak drug concentration of 800–1400 ng/mL is recommended by the Auburn University Veterinary Clinical Pharmacology Laboratory for dogs, and all of the dogs in the current study exceeded this level. In fact, the lowest peak in this study was 1944 ng/mL, and the median was 3040 ng/mL. Given the pronounced suppression of cytokines in our study, our results suggest that, in dogs as in people, it is likely that peak cyclosporine concentrations may correlate better with immunosuppressive effects compared to trough concentrations. Although our study only looked at two cytokines and therefore may not reflect all of the immune effects of cyclosporine, these two cytokines are known to reflect the drug’s main mechanism of action (Giese et al., 2004).
In human pharmacokinetic studies, cyclosporine area under the curve (AUC) has been shown to have the best correlation with clinical outcome (Kahan et al., 1995). Numerous studies have confirmed the lack of correlation of trough cyclosporine concentrations with AUC and clinical outcome and have identified improved outcomes when measuring peak drug concentrations as a surrogate measure for AUC (Cantarovich et al., 1998; Mahalati et al., 1999; Citterio et al., 2001; Halim et al., 2005). In humans, cyclosporine absorption occurs primarily in the first 4 h after oral administration, and drug absorption exhibits high inter- and intra-individual variability that is not adequately reflected by trough measurements (Halim et al., 2005). Temporally, calcineurin inhibition has been shown to closely follow cyclosporine blood concentrations in humans and mice, with little residual inhibition of calcineurin enzyme activity once blood drug concentrations drop below peak levels (Halloran et al., 1999). Interestingly, however, residual suppression of T-cell expression of NFAT-regulated cytokines can persist long beyond peak cyclosporine levels and the expected parallel transient inhibition of calcineurin, as demonstrated by our study. The results of our study, along with consideration of past studies, suggest that peak cyclosporine blood concentrations determine the degree of calcineurin inhibition but that, even when blood drug concentrations then drop markedly, the residual effects of transient calcineurin inhibition on NFAT-regulated cytokine expression persist for a sustained period of time. Pharmacodynamic monitoring of cytokine expression in dogs may therefore the best means of determining both the extent and duration of suppression of T-cell function. Further evaluation of cyclosporine pharmacokinetics and pharmacodynamics in dogs, and correlation of results with clinical outcome in canine patients treated with cyclosporine, is warranted.
One goal of the present study was to determine the ability of both flow cytometry and qRT-PCR to detect differences in T-cell cytokine expression across the cyclosporine dosing interval. Previous studies in human medicine used changes in cytokine expression from trough to peak cyclosporine blood concentrations as indicators of the degree of immunosuppression (Kon-standin et al., 2007; Sommerer et al., 2008). Although flow cytometry did show mildly increased suppression from trough to peak levels, overall the cytokine levels in our study were markedly suppressed at all time points after cyclosporine administration, and there was minimal change across the dosing interval. The high oral cyclosporine dose used in this study most likely caused maximal immunosuppression without allowing time for immune recovery between doses. We chose a 10 mg/kg twice daily dose of oral cyclosporine for this study because, based on our previous work, this dose tended to place trough drug levels at or around the previously published target immunosuppressive concentration of 500 ng/mL (Daigle, 2002; Archer et al., 2011). Lesser cyclosporine doses would be expected to cause more variation in cytokine levels over time and, in fact, previous work in our laboratory confirmed that when the much lower approved oral cyclosporine dose for canine atopic dermatitis (5 mg/kg once daily) was administered to healthy dogs, suppression of activated T-cell cytokine expression 8 h after drug dosing in individual dogs varied from minimal to marked (Archer et al., 2011). Based on studies in people, it is possible that marked suppression of T-cell function at the time of peak cyclosporine blood concentrations with subsequent partial recovery of suppression at the time of trough levels may be useful as an indicator of adequate but not excessive immunosuppression (Konstandin et al., 2007; Sommerer et al., 2008) and that the dose of cyclosporine used in our study caused a greater degree of immunosuppression than might be needed clinically. Further studies with various cyclosporine doses across the entire dosing interval will be needed to determine whether the degree of suppression of T-cell function varies postdosing in individual dogs at lower drug doses. For dogs on high doses of cyclosporine, however, the results of our present study suggest that, using qRT-PCR, cytokine analysis performed at any time point during therapy will likely reflect maximal immunosuppression. For flow cytometry, however, samples 2–8 h postdosing may be more representative.
To allow direct comparison with our previous work with cyclosporine, in which effects were assessed after 1 week of drug therapy, we chose to monitor the effects of cyclosporine on T-cell function after a full week of drug dosing, and our study confirmed that by this time cytokine expression was markedly suppressed. Undoubtedly, the immunosuppressive effects of cyclosporine will begin to manifest before completion of a full week of therapy, and further studies will be needed to determine how rapidly suppression of T-cell cytokine expression occurs after drug therapy is commenced.
Our study has confirmed that at high drug doses expression of the cytokines IL-2 and IFN-γ is consistently reduced by cyclosporine, and suggests that, as in humans, NFAT-regulated cytokine assays show great promise as biomarkers of drug-induced immunosuppression. In human medicine, the information provided by pharmacokinetic and pharmacodynamic monitoring is considered to be complementary, and the authors suggest that utilization of both techniques will be needed to develop optimal immunosuppressive regimens in canine patients.
Limitations of this study include the relatively small number of animals, the lack of an untreated control group, the single dog breed (Walker hound) used, and the use of only healthy animals. The sample size is typical for a standard pharmacokinetic study (Riviere, 1999), and results were consistent among all dogs, with marked differences noted between pretreatment and post-treatment results. A formal untreated control group was not included, and provision of a control group would have confirmed a lack of diurnal effect. However, a single untreated dog was included in the flow cytometry groups to ensure appropriate sample activation at all times (data not shown). The Walker hound is not known to have issues with cyclosporine metabolism or variations in activity of the efflux pumps that handle drug metabolism and is expected to serve as an acceptable model for all dog breeds. Cyclosporine is commonly used for inflammatory and immune-mediated diseases, and it is possible that the presence of these conditions will affect cytokine responses to cyclosporine. As results from this study reflect healthy dogs only, further work will be needed to confirm the relevance of these assays in diseased clinic patients.
In conclusion, our study describes the use of flow cytometry and qRT-PCR to measure expression of the cytokines IL-2 and IFN-γ after oral cyclosporine administration. Both techniques demonstrated marked reduction in activated cytokine levels at 2, 4, 8 and 12 h after administration of high doses of cyclosporine, and the degree of suppression of T-cell function varied only slightly across the 12-h dosing interval. Unactivated sample results were more variable, especially for qRT-PCR. Based on these results, pharmacodynamic monitoring of T-cell function could be performed using PMA and ionomycin activation at any time point in the 12-h interval between drug doses in dogs receiving chronic high-dose cyclosporine. Concurrent cyclosporine blood concentration measurement revealed 12-h trough concentrations that, based on previous studies, would be considered acceptable in only four of six dogs, but high (above reference laboratory ‘target’ range) 2 h peak concentrations in all dogs. As peak drug concentrations were attained in all dogs at 2 h postdosing, and cytokine expression was also markedly suppressed in all dogs at 2 h postdosing, 2 h after administration of high doses of cyclosporine is likely the most promising single time point for concurrent pharmacokinetic and pharmacodynamic assessment, although further study is needed to clarify the value of cytokine expression changes from trough to peak drug concentrations at lower drug doses. Both flow cytometry and qRT-PCR provided similar information, and our study suggests that both assays can be used interchangeably when monitoring cyclosporine therapy, at least at high drug doses. Further studies at different cyclosporine doses, and at earlier intervals after commencement of therapy, will be necessary to evaluate the full range of effects of the drug on T-cell function and to fully determine the best technique for cytokine assessment.
ACKNOWLEDGMENTS
This study was funded by a grant from the Office of Research and Graduate Studies and by the Dr. Hugh G. Ward Chair Discretionary Fund, both at the Mississippi State University College of Veterinary Medicine. Many thanks to Jenica Haraschak for her help with sample preparation, and to Dawn Boothe and Jameson Sofge of the Auburn Clinical Pharmacology Lab for cyclosporine blood concentration determination.
REFERENCES
- Archer TM, Fellman CL, Stokes JV, Pinchuk LM, Lunsford KV, Pruett SB, Langston VC, Mackin AJ. Pharmacodynamic monitoring of canine T-cell cytokine responses to oral cyclosporine. Journal of Veterinary Internal Medicine. 2011;25:1391–1397. doi: 10.1111/j.1939-1676.2011.00797.x. [DOI] [PubMed] [Google Scholar]
- Archer TM, Boothe DM, Langston VC, Fellman CL, Lunsford KV, Mackin AJ. Oral cyclosporine treatment in dogs: a review of the literature. Journal of Veterinary Internal Medicine. 2014;28:1–20. doi: 10.1111/jvim.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barten MJ, Rahmel A, Bocsi J, Boldt A, Garbade J, Dhein S, Mohr FW, Gummert JF. Cytokine analysis to predict immunosuppression. Cytometry A. 2006;69:155–157. doi: 10.1002/cyto.a.20215. [DOI] [PubMed] [Google Scholar]
- Barten MJ, Tarnok A, Garbade J, Bittner HB, Dhein S, Mohr FW, Gummert JF. Pharmacodynamics of T-cell function for monitoring immunosuppression. Cell Proliferation. 2007;40:50–63. doi: 10.1111/j.1365-2184.2007.00413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantarovich M, Barkun J, Besner JG, Metrakos P, Alpert E, Deschenes M, Aalamian Z, Tchervenkov JI. Cyclosporine peak levels provide a better correlation with the area-under-the-curve than trough levels in liver transplant patients treated with neoral. Transplantation Proceedings. 1998;30:1462–1463. doi: 10.1016/s0041-1345(98)00316-9. [DOI] [PubMed] [Google Scholar]
- Citterio F, Scata MC, Borzi MT, Pozzetto U, Castagneto M. C2 single-point sampling to evaluate cyclosporine exposure in long-term renal transplant recipients. Transplantation Proceedings. 2001;33:3133–3136. doi: 10.1016/s0041-1345(01)02336-3. [DOI] [PubMed] [Google Scholar]
- Daigle JC. More economical use of cyclosporine through combination drug therapy. Journal of the American Animal Hospital Association. 2002;38:205–208. doi: 10.5326/0380205. [DOI] [PubMed] [Google Scholar]
- Davies RA, Veinot JP, Williams K, Haddad H, Baker A, Donaldson J, Pugliese C, Struthers C, Masters RG, Hendry PJ, Mesana T. Assessment of cyclosporine pharmacokinetic parameters to facilitate conversion from CO to C2 monitoring in heart transplant recipients. Transplantation Proceedings. 2007;39:3334–3339. doi: 10.1016/j.transproceed.2007.08.109. [DOI] [PubMed] [Google Scholar]
- Fellman CL, Stokes JV, Archer TM, Pinchuk LM, Lunsford KV, Mackin AJ. Cyclosporine A affects the in vitro expression of T cell activation-related molecules and cytokines in dogs. Veterinary Immunology and Immunopathology. 2011;140:175–180. doi: 10.1016/j.vetimm.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Flores MG, Zhang S, Ha A, Holm B, Reitz BA, Morris RE, Borie DC. In vitro evaluation of the effects of candidate immunosuppressive drugs: flow cytometry and quantitative real-time PCR as two independent and correlated read-outs. Journal of Immunological Methods. 2004;289:123–135. doi: 10.1016/j.jim.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Giese T, Zeier M, Meuer S. Analysis of NFAT-regulated gene expression in vivo: a novel perspective for optimal individualized doses of calcineurin inhibitors. Nephrology, Dialysis, Transplantation. 2004;19(Suppl 4):iv55–iv60. doi: 10.1093/ndt/gfh1043. [DOI] [PubMed] [Google Scholar]
- Halim MA, Nampoory MR, Johny KV, Donia F, Hamid MH, Said T, Nair MP, Mansour M, Al-Muzairai I, Samhan M, Al-Mou-sawi M. The area under the concentration-time curve versus trough and peak blood level monitoring in renal transplant recipients on cyclosporine. Transplantation Proceedings. 2005;37:3019–3021. doi: 10.1016/j.transproceed.2005.07.061. [DOI] [PubMed] [Google Scholar]
- Halloran PF, Helms LM, Kung L, Noujaim J. The temporal profile of calcineurin inhibition by cyclosporine in vivo. Transplantation. 1999;68:1356–1361. doi: 10.1097/00007890-199911150-00023. [DOI] [PubMed] [Google Scholar]
- Hartel C, Fricke L, Schumacher N, Kirchner H, Muller-Steinhardt M. Delayed cytokine mRNA expression kinetics after T-lymphocyte costimulation: a quantitative measure of the efficacy of cyclosporin A-based immunosuppression. Clinical Chemistry. 2002;48:2225–2231. [PubMed] [Google Scholar]
- Kahan BD. Therapeutic drug monitoring of cyclosporine: 20 years of progress. Transplantation Proceedings. 2004;36(2 Suppl):3788–3918. doi: 10.1016/j.transproceed.2004.01.091. [DOI] [PubMed] [Google Scholar]
- Kahan BD, Welsh M, Rutzky LP. Challenges in cyclosporine therapy: the role of therapeutic monitoring by area under the curve monitoring. Therapeutic Drug Monitoring. 1995;17:621–624. doi: 10.1097/00007691-199512000-00013. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Momoi Y, Iwasaki T. Cyclosporine A inhibits the mRNA expressions of IL-2, IL-4 and IFN-gamma, but not TNF-alpha, in canine mononuclear cells. Journal of Veterinary Medical Science. 2007;69:887–892. doi: 10.1292/jvms.69.887. [DOI] [PubMed] [Google Scholar]
- Konstandin MH, Sommerer C, Doesch A, Zeier M, Meuer SC, Katus HA, Dengler TJ, Giese T. Pharmacodynamic cyclosporine A-monitoring: relation of gene expression in lymphocytes to cyclosporine blood levels in cardiac allograft recipients. Transplant International. 2007;20:1036–1043. doi: 10.1111/j.1432-2277.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- Kuga K, Nishifuji K, Iwasaki T. Cyclosporine A inhibits transcription of cytokine genes and decreases the frequencies of IL-2 producing cells in feline mononuclear cells. Journal of Veterinary Medical Science. 2008;70:1011–1016. doi: 10.1292/jvms.70.1011. [DOI] [PubMed] [Google Scholar]
- Kuzuya T, Kobayashi T, Katayama A, Nagasaka T, Miwa Y, Uchida K, Nakao A, Yamada K. Evaluation of inter-leukin-2 mRNA in whole blood as a parameter for monitoring cyclosporine pharmacodynamics. Biological and Pharmaceutical Bulletin. 2009;32:604–608. doi: 10.1248/bpb.32.604. [DOI] [PubMed] [Google Scholar]
- Kyles AE, Gregory CR, Craigmill AL. Comparison of the in vitro antiproliferative effects of five immunosuppressive drugs on lymphocytes in whole blood from cats. American Journal of Veterinary Research. 2000;61:906–909. doi: 10.2460/ajvr.2000.61.906. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C (T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mahalati IC, Belitsky P, Sketris I, West K, Panek R. Neoral monitoring by simplified sparse sampling area under the concentration-time curve: its relationship to acute rejection and cyclosporine nephrotoxicity early after kidney transplantation. Transplantation. 1999;68:55–62. doi: 10.1097/00007890-199907150-00011. [DOI] [PubMed] [Google Scholar]
- Mathias HC, Ozalp F, Will MB, Borland W, Payne C, Kerr M, Lockhart J, Murday AJ. A randomized, controlled trial of CO- Vs C2-guided therapeutic drug monitoring of cyclosporine in stable heart transplant patients. Journal of Heart and Lung Transplantation. 2005;24:2137–2143. doi: 10.1016/j.healun.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Rao A, Luo C, Hogan PG. Transcription factors of the NPAT family: regulation and function. Annual Review of Immunology. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- Riggs C, Archer T, Fellman C, Figueiredo AS, Follows J, Stokes J, Wills R, Mackin A, Bulla C. Analytical validation of a quantitative reverse transcriptase polymerase chain reaction assay for evaluation of T-cell targeted immunosuppressive therapy in the dog. Veterinary Immunology and Immunopathology. 2013;156:229–234. doi: 10.1016/j.vetimm.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviere JE. Comparative Pharmacokinetics: Principles, Techniques, and Application. Iowa State University Press; Ames: 1999. [Google Scholar]
- Sommerer C, Giese T, Schmidt J, Meuer S, Zeier M. Ciclosporin A tapering monitored by NFAT-regulated gene expression: a new concept of individual immunosuppression. Transplantation. 2008;85:15–21. doi: 10.1097/01.tp.0000296824.58884.55. [DOI] [PubMed] [Google Scholar]
- Tivers MS, Catchpole B, Gregory SP, House AK. Interleukin-2 and interferon-gamma mRNA expression in canine anal furunculosis lesions and the effect of ciclosporin therapy. Veterinary Immunology and Immunopathology. 2008;125:31–36. doi: 10.1016/j.vetimm.2008.04.018. [DOI] [PubMed] [Google Scholar]