Abstract
Proximal spinal muscular atrophy (SMA) is a childhood-onset degenerative disease resulting from the selective loss of motor neurons in the spinal cord. SMA is caused by the loss of SMN1 (survival motor neuron 1) but retention of SMN2. The number of copies of SMN2 modifies disease severity in SMA patients as well as in mouse models, making SMN2 a target for therapeutics development. Sodium butyrate (BA) and its analogue (4PBA) have been shown to increase SMN2 expression in SMA cultured cells. In this study, we examined the effects of BA, 4PBA as well as two BA prodrugs—glyceryl tributyrate (BA3G) and VX563—on the phenotype of SMNΔ7 SMA mice. Treatment with 4PBA, BA3G and VX563 but not BA beginning at PND04 significantly improved the lifespan and delayed disease end stage, with administration of VX563 also improving the growth rate of these mice. 4PBA and VX563 improved the motor phenotype of SMNΔ7 SMA mice and prevented spinal motor neuron loss. Interestingly, neither 4PBA nor VX563 had an effect on SMN expression in the spinal cords of treated SMNΔ7 SMA mice; however, they inhibited histone deacetylase (HDAC) activity and restored the normal phosphorylation states of Akt and glycogen synthase kinase 3β, both of which are altered by SMN deficiency in vivo. These observations show that BA-based compounds with favourable pharmacokinetics ameliorate SMA pathology possibly by modulating HDAC and Akt signaling.
INTRODUCTION
Proximal spinal muscular atrophy (SMA) is an autosomal recessive neurodegenerative disease resulting from a selective loss of α motor neurons in the anterior horn of the spinal cord leading to atrophy of limb and trunk muscles (Tisdale and Pellizzoni, 2015). SMA is one of the leading genetic causes of infant death in the world. In humans, the SMN (survival motor neuron) gene is duplicated and the two SMN genes (SMN1 and SMN2) differ functionally by a single nucleotide (C→T) within exon 7 (Lorson et al., 1999; Monani et al., 1999). SMN1 transcripts produce full-length SMN (FL-SMN) protein. Most of the transcripts from SMN2 lack exon 7 (SMNΔ7) and produce a truncated, unstable SMNΔ7 protein; about 10–20% of SMN2 transcripts are correctly spliced and produce FL-SMN protein. SMA results from ubiquitous SMN deficiency due to homozygous deletions or mutations of SMN1 and retention of SMN2 (Lefebvre et al., 1995). Additionally, the severity of SMA depends on the copy number of SMN2 and the consequent levels of the SMN protein (Coovert et al., 1997; Elsheikh et al., 2009; Lefebvre et al., 1997; McAndrew et al., 1997; Prior et al., 2005; Stabley et al., 2015; Swoboda et al., 2005; Tiziano et al., 2007; Wirth et al., 2006).
There is only one SMN gene (mSmn) in mice which is orthologous to SMN1 in humans (DiDonato et al., 1997; Viollet et al., 1997); embryonic lethality results from complete knockout of mSmn (Schrank et al., 1997). Conditional knockout of mSmn in specific cell types including neurons, muscle and hepatocytes also leads to death of those cells (Cifuentes-Diaz et al., 2002; Nicole et al., 2003; Vitte et al., 2004) indicating that SMN is essential for cellular viability. While transgenic insertion of the complete SMN2 genomic region into mSmn knockout mice rescues the embryonic lethal phenotype (Hsieh-Li et al., 2000; Monani et al., 2000), mice with low SMN2 copy numbers (i.e. 1 or 2) develop severe SMA and die within a few days after birth (Hsieh-Li et al., 2000; Michaud et al., 2010; Monani et al., 2000). In contrast, mSmn nullizygous mice with higher SMN2 copy numbers (from 4–16 copies) are indistinguishable from their non-transgenic littermates (Michaud et al., 2010; Monani et al., 2000), demonstrating that the SMN2 gene product can correct the SMA phenotype and that SMN2 copy number modifies the severity of disease in mice as it does in humans. SMNΔ7 SMA mice that also contain an exon 7-lacking SMN develop a slightly less severe SMA phenotype and die at 14–15 days (Le et al., 2005).Replacement of SMN in SMA neurons using adeno-associated virus-mediated gene delivery markedly ameliorates the SMA phenotype in mouse models (Dominguez et al., 2011; Foust et al., 2010; Passini et al., 2010; Valori et al., 2010). Taken together, these experiments show that modulating SMN levels can influence disease severity in mouse models as is the case in humans.
SMN2 expression can be increased by small molecule drugs in vivo at different levels of gene regulation including promoter activation (Gogliotti et al., 2013; Thurmond et al., 2008; Van Meerbeke et al., 2013), increased inclusion of exon 7 in SMN2 mRNA transcripts (Cherry et al., 2013; Naryshkin et al., 2014; Palacino et al., 2015) and translational read-through of SMNΔ7 mRNAs (Heier and DiDonato, 2009; Mattis et al., 2012; Mattis et al., 2009a; Mattis et al., 2009b). Splice-correcting antisense oligonucleotides of differing chemistries have been shown by numerous studies to increase SMN expression in vivo and improve the survival of SMA mice (Hua et al., 2010; Hua et al., 2011; Mitrpant et al., 2013; Osman et al., 2014; Passini et al., 2011; Porensky et al., 2012; Sahashi et al., 2013; Staropoli et al., 2015; Williams et al., 2009; Zhou et al., 2013). Many of these compounds are in various stages of preclinical and clinical development for SMA.
Inhibitors of histone deacetylase (HDAC) activity have been identified as inducers of SMN2 transcription (Lunke and El-Osta, 2009). By increasing histone acetylation, these HDAC inhibitors increase SMN2 promoter activity which results in elevated SMN protein levels (Andreassi et al., 2004; Avila et al., 2007; Brahe et al., 2005; Brichta et al., 2003; Brichta et al., 2006; Chang et al., 2001; Garbes et al., 2009; Hahnen et al., 2006; Harahap et al., 2012; Kernochan et al., 2005; Riessland et al., 2010; Riessland et al., 2006; Sumner et al., 2003). Sodium butyrate (BA) and its analogue sodium 4-phenylbutyrate (4PBA) inhibit histone deacetylation in vitro (Boffa et al., 1978; Davis et al., 2000). BA and 4PBA increase the expression of SMN in cultured cells from SMA patients (Andreassi et al., 2004; Chang et al., 2001). Furthermore, continuous administration of BA to severe SMA mice (SMN2;mSmnΔ7/ Δ7) moderately increased their survival (Chang et al., 2001). While BA has poor plasma pharmacokinetics in rodents (Egorin et al., 1999), 4PBA has better pharmacokinetics in vivo (Berg et al., 2001). Similarly, glyceryl tributyrate (BA3G; tributyrin) is a BA prodrug with a glycerol backbone and improved pharmacokinetics (Edelman et al., 2003; Egorin et al., 1999). BA3G inhibits tumor growth in various cancer models (Kuefer et al., 2004) and had undergone phase I clinical trials in patients with solid tumors (Conley et al., 1998). Lastly, the orally bioavailable BA prodrug VX563 has a significantly extended plasma half-life in primates relative to BA (McCaffrey et al., 1996). In this study, we sought to examine the effects of BA, 4PBA, BA3G and VX563 on the survival and motor phenotype as well as on SMN expression in SMNΔ7 SMA mice.
MATERIALS AND METHODS
Animals and Ethical Statement
SMNΔ7 SMA mice (SMN2+/+;SMNΔ7+/+;mSmn−/−) were generated from male and female carrier mice of the genotype SMN2+/+;SMNΔ7+/+;mSmn+/− (line 4299; FVB.Cg-Tg(SMN2*delta7)4299Ahmb Tg(SMN2)89Ahmb Smn1tm1Msd). These mice originated from our colony but can be obtained from The Jackson Laboratory (#005025). As diet can affect the survival and phenotype of these study mice (Butchbach et al., 2010a) as well as responsiveness to drugs like trichostatin A and D156844 (Butchbach et al., 2014; Narver et al., 2008), the mice were fed the Harlan-Teklad 22/5 Rodent Diet. Neonatal offspring were genotyped using a PCR-based assay on genomic DNA from tail biopsies as described previously (Butchbach et al., 2007b; Le et al., 2005). All experiments were conducted in accordance with the protocols described in the National Institutes of Health Guide for the Care and Use of Animals and were approved by the Ohio State University Institutional Laboratory Animal Care and Use Committee.
Drug Formulations
Butyrate (BA; sodium salt, Sigma-Aldrich, St. Louis, MO) was dissolved in ddH2O. Sodium 4-phenylbutyrate (4PBA; Lancaster Synthesis Inc., Ward Hill, MA) was dissolved in ddH2O and solubilized by adjusting the pH to ~7.0 with concentrated NaOH. In most cases, the 4PBA solution (sodium salt) was heated to facilitate solubilization; the pH of the 4PBA solution was readjusted to 7.0 after cooling to room temperature. Glyceryl butyrate (BA3G, tributyrin; Sigma-Aldrich) and VX563 (Vertex Pharmaceuticals, Cambridge, MA) were used as supplied by the manufacturer. All aqueous solutions were filter sterilized prior to injections.
Drug Administration
Beginning at 4 days after birth (PND04), SMA mice and their non-SMA littermates were treated with either BA (5 g/kg/d t.i.d.), 4PBA (500 mg/kg/d t.i.d.), BA3G (5 g/kg/d b.i.d.), VX563 (6 g/kg/d b.i.d.) or H2O by oral administration using a curved 18-gauge feeding needle (Harvard Apparatus, Holliston, MA) as described previously (Butchbach et al., 2007b). The selection of drug dose was based on either the dose used for similar studies or the maximum tolerable dose provided by the drug manufacturer. For b.i.d. dosing, the drugs were administered at 09.00 and 17.00 daily while those drugs with a t.i.d. dosing were administered at 09.00, 13.00 and 17.00 daily without fail. We have found that variable time intervals between dosings negate any therapeutic benefit (unpublished observations). Drugs were administered to mice until the last SMA pup in the litter perished. Due to the physicochemical properties of these compounds, it was not possible for the person dosing the neonatal mice to be blinded to drug treatment. For all of the subsequent analyses, however, the persons completing these analyses were blinded with respect to treatment cohort.
Behavior Analysis
A cohort of drug-treated SMNΔ7 SMA mice was assessed for changes in vectorial movement duration, spontaneous locomotor activity and pivoting activity as described (Butchbach et al., 2007a). All of the measures were collected using Stopwatch+ (Center for Behavioral Neuroscience, Atlanta, GA). For vectorial movement duration, the amount of time each mouse was in vectorial movement, either crawling at PND07 or walking at PND11 and PND14, within the viewing timeframe was collected. For spontaneous locomotor activity, each pup was placed in the center of a gridded (with 28 2.5-cm2 grids) arena and the number of grids crossed in 60 sec was counted. For pivoting, each pup was placed in the center of a gridded arena and the number of times the pup turned 90°C (pivots) during a 60-sec time frame was counted. Spontaneous locomotor activity and pivoting were monitored on PND07, PND11 and PND14. To minimize the stress on the pup, all motor phenotype assays were conducted simultaneously.
Detection of BA Levels
Neonatal mice received a single dose of each drug or vehicle; the forebrain was dissected and rapidly frozen in liquid nitrogen one hour after injection. Tissue BA levels were determined using high-performance liquid chromatography (HPLC) to detect 2-nitrophenylhydrazide derivatives as described (Miwa and Yamamoto, 1987). Briefly, 50 µL methanol were added to 50 µL forebrain extracts and centrifuged at 14,000×g for 15 min at 4°C to precipitate protein. 100 µL of butyrate standard (125-10,000 pmol butyric acid; Sigma-Aldrich) or tissue extract containing 500 pmol 2-ethylbutyric acid (2-EtBA; Sigma-Aldrich) as an internal standard were mixed with 400 µL 125 mM 1-EDC·HCl (N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride; Sigma-Aldrich) + 1.5 % (v/v) pyridine (Sigma-Aldrich) in ethanol and 200 µL 20 mM 2-NPH·HCl (2-nitrophenylhydrazine hydrochloride; Sigma-Aldrich) and incubated for 20 min at 60°C. 100 µL 15% (w/v) KOH in methanol:water (4:1) were added to each sample and incubated for 15 min at 60°C. The samples were then cooled to room temperature. Separation of fatty acid derivatives was accomplished using a Shimadzu liquid chromatograph with a UV-visible detector. A Prevail C8 column (particle size of 5 µm; 250 mm × 4.6 mm; Grace Davison Discovery Sciences, Columbia, MD) fitted with an Adsorbosphere C8 guard cartridge (particle size of 5 µm; 7.5 mm × 4.6 mm; Grace Davison Discovery Sciences) was used. Separation was carried out isocratically using methanol:water (58%:42%) at room temperature. A representative chromatogram showing the elution profiles of BA and 2-EtBA derivatives is shown in the Supplementary Figure.
Quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR)
Total RNA was isolated from spinal cords of SMNΔ7 SMA mice treated with drugs for 5 days using RNeasy Mini columns (QIAGEN, Germantown, MD) according to manufacturer’s directions. Quantification of FL-SMN mRNA transcript levels was accomplished using real time RT-PCR, or qRT-PCR, as described previously (Simard et al., 2007) with modifications. The quantities of FL-SMN transcript were then normalized to a geometric mean of three different reference transcripts (Vandesompele et al., 2002): hypoxanthine phosphorylribosyltransferase 1 (Hprt1), β-actin (Actb) and RNA polymerase IIA (Pol2A). These normalized quantities were then compared to vehicle-treated carrier mRNA samples.
Immunoblot
Spinal cords from animals treated with drugs for 5 days were dissected and homogenized in lysis buffer (0.1% Triton X-100 and Complete Protease Inhibitor cocktail (Roche Life Sciences, Indianapolis, IN) dissolved in PBS, pH 7.4). These samples were resolved through SDS-PA gels via electrophoresis and transferred onto PVDF membranes via electroblotting as described previously (Butchbach et al., 2010b). For the detection of SMN protein, 100 µg of tissue protein extract was added to each lane of a midi-gel; 10 µg of protein extract was added to each lane of a mini-gel for detection of all other proteins. Immunoblotting was completed as described in (Butchbach et al., 2010b). The following primary antibodies were used in this study: mouse anti-SMN mAb (1:500; MANSMA2, (Young et al., 2000), Developmental Studies Hybridoma Bank, Iowa City, IA), mouse anti-β-actin mAb (1:20000; clone AC-15, Sigma-Aldrich), rabbit anti-histone H3 pAb (1:1000; Cell Signaling Technology, Beverly, MA), rabbit anti-acetyl-histone H3(K9) pAb (1:1000; Cell Signaling Technology), rabbit anti-Akt pAb (1:1000; Cell Signaling Technology), rabbit anti-phospho-Akt (S473) mAb (1:1000; clone 193H12, Cell Signaling Technology), rabbit anti-GSK3β mAb (1:1000; clone 27C10, Cell Signaling Technology), rabbit anti-phospho-GSK3β (S9) mAb (1:1000; clone 5B3, Cell Signaling Technology) and rabbit anti-GAPDH pAb (1:10000; Sigma-Aldrich). Band intensities were measured using ImageJ 1.45s (National Institutes of Health).
SMN Enzyme-linked Immunosorbent Assay (ELISA)
Quantitation of SMN protein levels in spinal cord extracts was measured using the SMN (human) Enzyme Immunometric Assay from Enzo Life Sciences (Farmingdale, NY) as described in (Nguyen thi Man et al., 2008) except that 40 µg of spinal cord extract were used for each sample. SMN concentrations were expressed as pg SMN per mL extract.
snRNP Assembly
In vitro snRNP assembly assays were run on tissue extracts from normal and SMNΔ7 SMA mice treated with drugs for 5 days. Preparation of mouse tissue extracts and snRNP assembly experiments were carried out essentially as previously described (Gabanella et al., 2007; Gabanella et al., 2005). The amount of immunoprecipitated U1 snRNAs was quantified using a STORM 860 Phosphorimager (GE Healthcare, Piscataway, NJ) and the ImageQuant version 4.2 software.
Histology
SMNΔ7 SMA mice and carrier littermates were treated with BA, 4PBA, VX563 or vehicle beginning at PND04 until PND11. Treated mice were anesthetized with 2.5% Avertin and then transcardially perfused with ice-cold PBS followed by 4% paraformaldehyde in Sørensen’s phosphate buffer (100 mM Na2HPO4 and 100 mM NaH2PO4, pH 7.4). The lumbar spinal cords were postfixed with 4% paraformaldehyde in Sørensen’s phosphate buffer overnight at 4°C followed by cryoprotection with 30% sucrose in ddH2O overnight at 4°C. The lumbar spinal cords were sectioned coronally at a thickness of 25 µm using the MultiBrain® Technology by NeuroScience Associates (Knoxville, TN). Every 12th section block was mounted onto a glass slide and stained with 1% Cresyl violet as described in (Le et al., 2005).
Statistical Analysis
Data are expressed as means ± standard error and were analyzed using one-way ANOVA with a Bonferonni post hoc test or unpaired t-tests. Kaplan-Meier analysis was performed on lifespan and onset of body mass loss data using the Mantel-Cox log rank post hoc test. All statistical analyses were performed with SPSS v.22.0.
RESULTS
Chemically Stable BA Drugs Increase the Survival of SMNΔ7 SMA Mice
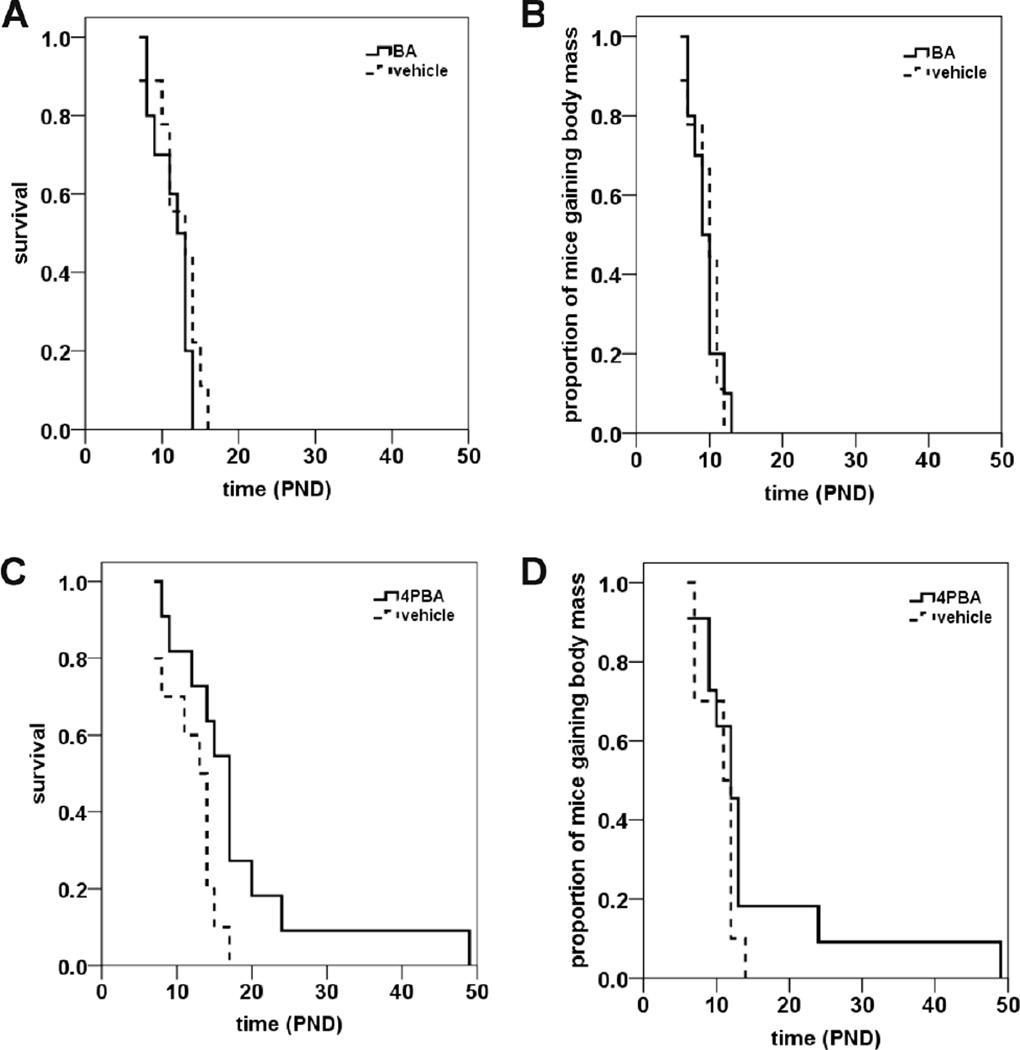
To determine if BA affected the lifespans of SMNΔ7 SMA mice, these mice were treated with BA (5 g/kg/d; t.i.d. (ter in die, three times a day)) beginning at postnatal day 4 (PND04) via oral delivery (Butchbach et al., 2007b). Two indices were used to assess the ameliorative effect of a given drug on SMNΔ7 SMA mice: lifespan and onset of body mass loss, which serves as a marker for disease end-stage in SMNΔ7 SMA mice (Butchbach et al., 2007a; Le et al., 2005). Oral administration of BA did not significantly increase the average lifespan of SMNΔ7 SMA mice (Figure 1A; mean survival for BA = 11.5 ± 0.7 d (n = 10); for vehicle mice = 12.1 ± 1.0 d (n = 9); p = 0.253, χ2 = 1.305). Furthermore, BA treatment did not change the onset of loss of body mass in SMNΔ7 SMA mice (Figure 1B; mean onset of body mass loss for BA = 9.5 ± 0.6 d (n = 10); for vehicle mice = 9.7 ± 0.7 d (n = 9); p = 0.925, χ2 = 0.009).
Figure 1. The effects of butyrate (BA) and 4-phenylbutyrate (4PBA) on survival of SMNΔ7 SMA mice.
SMNΔ7 SMA mice were treated daily with BA (A-B) or 4PBA (C-D) starting at PND04 and monitored for changes in lifespan (A, C) and onset of loss of body mass (B, D). Daily oral administration of BA (5 g/kg/d, t.i.d.) had no effect on the survival (A; p = 0.253) or the onset of body mass loss (B; p = 0.925) in SMNΔ7 SMA mice. The BA analogue 4PBA (500 mg/kg/d, t.i.d.) increased the average lifespan of SMNΔ7 SMA mice by 53% (C; p = 0.026) but not significantly delay the onset of loss of body mass (D; p = 0.190).
4PBA is a chemical analogue of BA that has been previously demonstrated as a neuroprotectant (Del Signore et al., 2009; Ferrante et al., 2003; Gardian et al., 2005; Petri et al., 2006; Qi et al., 2004; Ribobaraza et al., 2009; Ryu et al., 2005; Steffan et al., 2001; Ying et al., 2006). Treatment of SMNΔ7 SMA mice with 4PBA (500 mg/kg/d; t.i.d.) beginning at PND04 increases their lifespans by 53% (Figure 1C; mean survival for 4PBA mice = 18.4 ± 3.4 d (n = 11); for vehicle mice = 12.0 ± 1.1 d (n = 10); p = 0.026, χ2 = 4.988). Although oral administration of 4PBA does slightly delay the onset of body mass loss in SMNΔ7 SMA mice, the delay was not statistically significant (Figure 1D; mean onset of body mass loss for 4PBA = 15.5 ± 3.6 d (n = 11); for vehicle mice = 10.5 ± 2.0 d (n = 10); p = 0.190, χ2 = 1.718).
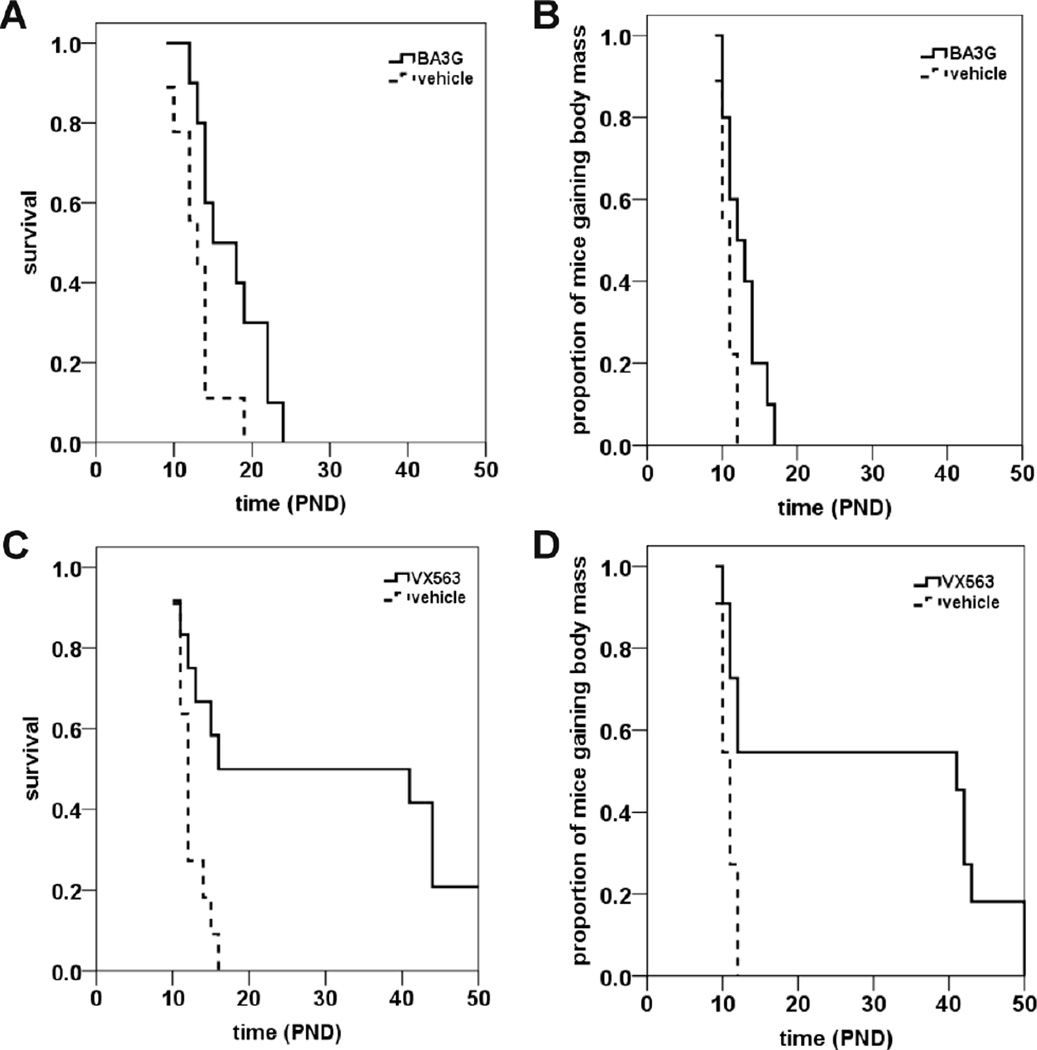
We also determined the effects of two BA ester prodrugs with chemically distinct backbones on the survival of SMNΔ7 SMA mice: BA3G and VX563. Oral administration of BA3G (5 g/kg/d, b.i.d. (bis in die, twice a day)) to SMNΔ7 SMA mice starting at PND04 increased the mean lifespan of these mice by 33% (Figure 2A; mean survival for BA3G mice = 17.3 ± 1.4 d (n = 10); for vehicle mice = 13.0 ± 1.0 d (n = 9); p = 0.024, χ2 = 5.104). BA3G slightly delays the onset of body mass loss in SMNΔ7 SMA mice (Figure 2B; mean onset of body mass loss for BA3G = 12.8 ± 0.8 d (n = 10); for vehicle mice = 10.7 ± 0.3 d (n = 9); p = 0.019, χ2 = 5.530). Remarkably, oral administration of VX563 to SMNΔ7 SMA mice beginning at PND04 (5 g/kg/d, b.i.d.) results in a 250% increase in lifespan (Figure 2C; mean survival for VX563 = 29.4 ± 4.9 d (n = 12); for vehicle mice = 12.4 ± 0.6 d (n = 11); p = 0.005, χ2 = 7.792). Moreover, the onset of loss of body mass was delayed by ~190% in SMNΔ7 SMA mice that received VX563 (Figure 2D; mean onset of body mass loss for VX563 = 29.5 ± 5.3 d (n = 11); for vehicle mice = 10.7 ± 0.3 d (n = 11); p = 0.003, χ2 = 8.815). In summary, BA prodrugs and analogues provide a greater improvement in survival of SMNΔ7 SMA mice. The drugs tested would rank as follows based on the extent of improvement in survival: VX563 > 4PBA > BA3G > BA.
Figure 2. The effects of butyrate prodrugs on survival of SMNΔ7 SMA mice.
SMNΔ7 SMA mice were treated daily with glyceryl tributyrate (BA3G; A-B) or VX563 (C-D) and monitored for changes in lifespan (A, C) and onset of loss of body mass (B, D). The drug compounds were administered orally beginning at PND04. The BA ester prodrug BA3G (5 g/kg/d, b.i.d.) increased survival by 33% (A; p = 0.024) and slightly delayed the onset of body mass loss (B; p = 0.019). Treatment of SMNΔ7 SMA mice with VX563 (6 g/kg/d, b.i.d.) beginning at PND04 strongly improved survival (C; p = 0.005) and significantly delayed the onset of body mass loss (D; p = 0.003).
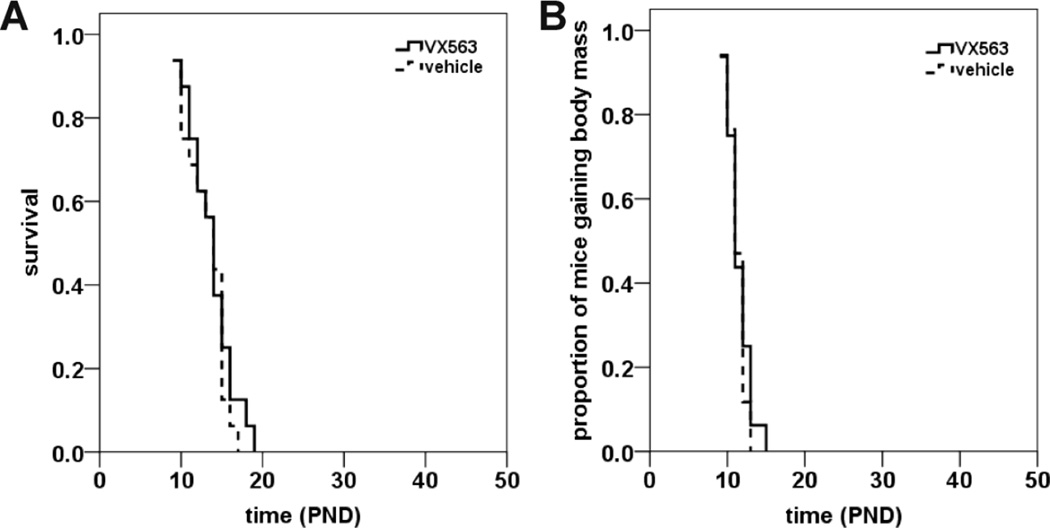
All of the drugs mentioned were given to SMNΔ7 SMA mice prior to the onset of motor neuron loss. In order to assess whether administration of the protective drug is time-dependent, SMNΔ7 SMA were treated with VX563 beginning at PND09, i.e. after the onset of motor neuron loss (Le et al., 2005). Treatment with VX563 starting at PND09 did not significantly affect the lifespan of SMNΔ7 SMA mice (Figure 3A; mean survival for VX563 = 13.7 ± 0.7 d (n = 16); for vehicle mice = 13.2 ± 0.6 d (n = 16); p = 0.507, χ2 = 0.439). Treatment of SMNΔ7 SMA mice with VX563 starting at PND09 also did not delay the onset of body mass loss (Figure 3B; mean onset of body mass loss for VX563 = 11.5 ± 0.4 d (n = 16); for vehicle mice = 11.3 ± 0.3 d (n = 17); p = 0.569, χ2 = 0.325). These results show that VX563 must be administered early, i.e. before the onset of motor neuron loss, for it to ameliorate the SMA phenotype in SMNΔ7 SMA mice.
Figure 3. The effects of administration of VX563 on survival of SMNΔ7 SMA mice beginning at PND09.
SMNΔ7 SMA mice were treated daily with VX563 beginning at PND09 and monitored for changes in lifespan (A) and onset of loss of body mass (B). There were no significant changes in the average lifespan (A; p = 0.507) or the onset of body mass loss (B; p = 0.569) between VX563-treated SMNΔ7 SMA mice treated and vehicle-treated SMNΔ7 SMA mice.
Relationship between CNS BA Levels and Protective Effect in SMNΔ7 SMA Mice
Since oral administration of BA-based prodrugs resulted in a marked amelioration of the survival of SMNΔ7 SMA mice while there was no significant improvement in lifespan of BA-treated SMNΔ7 SMA mice, we determined if the protective effects of BA prodrugs were related to tissue levels of BA. SMNΔ7 carrier mice received a single oral dose of BA, BA3G and VX563 (n =3/drug) and their forebrains were harvested for drug level determination at 1 hour after dosing. Free BA levels were determined in BA-, BA3G- and VX563-treated neonatal mice with high-performance liquid chromatography (HPLC). Free BA levels were detected in the forebrain extracts of neonatal (PND04) mice treated with BA3G or VX563 (Table 1) but were below the detection limit (12 pmol) in forebrain extracts from BA-treated mice. Thus, survival benefit is related to drug levels in the central nervous system (CNS) of SMNΔ7 SMA mice.
Table 1. Tissue drug and metabolite levels following single administration of BA compounds.
BA drug were measured in forebrain extracts from neonatal mice receiving a single oral dose of BA, BA3G and VX563. Forebrains were dissected from PND04 pups 1 hr after dosing and drug levels were determined with HPLC. Key: BDL, below detection limit.
| drug | dose administered |
metabolite | metabolite levels (pmol/mg protein) |
|---|---|---|---|
| vehicle | N/A | BA | BDL |
| BA | 5 g/kg | BA | BDL |
| BA3G | 5 g/kg | BA | 48.50 ± 6.79 |
| VX563 | 6 g/kg | BA | 18.67 ± 2.28 |
Effect of BA Compounds on the Phenotype of SMNΔ7 SMA Mice
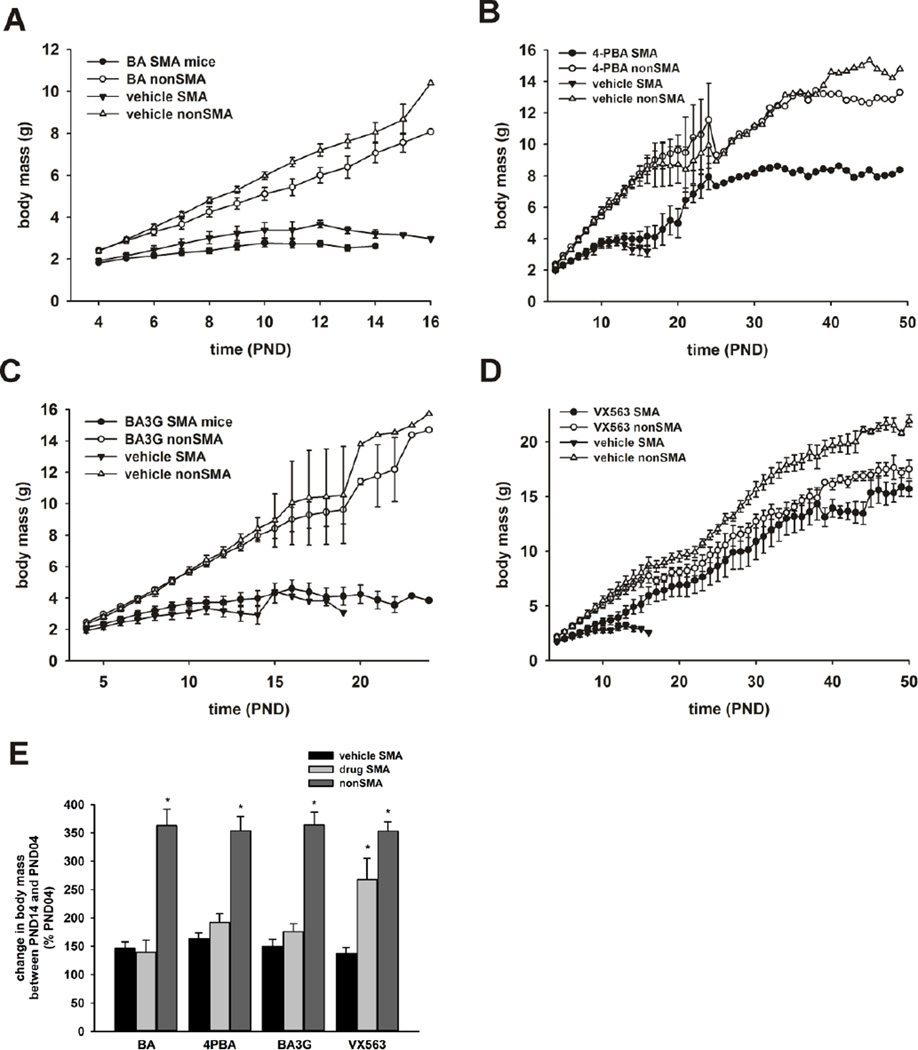
For SMNΔ7 SMA mice treated with either BA (Figure 4A), 4PBA (Figure 4B), BA3G (Figure 4C) or VX563 (Figure 4D) beginning at PND04, the body mass curve was slightly higher for drug-treated mice (solid circles) than vehicle-treated SMNΔ7 SMA mice (solid triangles) starting at PND12, but the differences were not statistically significant. During postnatal development, the body masses of SMNΔ7 SMA mice (solid circles or triangles in Figures 4A-D) were lower than age-matched non-SMA littermates (open circles or triangles) regardless of treatment. Those SMNΔ7 SMA mice treated with either 4PBA (Figure 4B) or BA3G (Figure 4C) that survived past weaning (PND18) were still markedly smaller than age-matched non-SMA littermates. Interestingly, post-weaning, VX563-treated SMNΔ7 SMA mice had similar body masses to age-matched, non-SMA littermates treated with drug (Figure 4D). It should be noted that non-SMA mice treated with VX563 were smaller than age-matched non-SMA mice receiving vehicle.
Figure 4. The effects of butyrate analogues and prodrugs on the body mass curves of SMNΔ7 SMA mice.
(A-D) Body mass curves of SMNΔ7 SMA mice (solid circles) or non-SMA littermates (either carrier or normal; open circles) treated daily with either BA (A), 4PBA (B), BA3G (C) or VX563 (D). Body mass curves for vehicle-treated SMNΔ7 SMA and non-SMA mice are shown as solid and open triangles, respectively. (E) Growth rates, which are defined by the change in body mass between PND14 and PND04, of SMNΔ7 SMA treated with either BA, 4PBA, BA3G, VX563 or their appropriate vehicle along with non-SMA littermates. The mean body masses at PND14 were expressed relative to those at PND04. The asterisk (*) denotes a statistically significant (p ≤ 0.05) difference when compared to vehicle-treated SMNΔ7 SMA mice for each drug.
Similar to previous observations (Butchbach et al., 2007a; Le et al., 2005), the change in body mass of SMNΔ7 SMA mice between PND04 and PND14—in other words, the growth rate—was less than for non-SMA littermates (Figure 4E). The growth rate was not affected by treatment of SMNΔ7 SMA mice with BA. 4PBA and BA3G did have a small but statistically insignificant effect on the growth rate of treated SMNΔ7 SMA mice. In contrast, treatment of SMNΔ7 SMA mice with VX563—the compound with the most profound effect on the survival of these mice—significantly (p = 0.034) increased the growth rate when compared against vehicle-treated SMNΔ7 SMA mice.
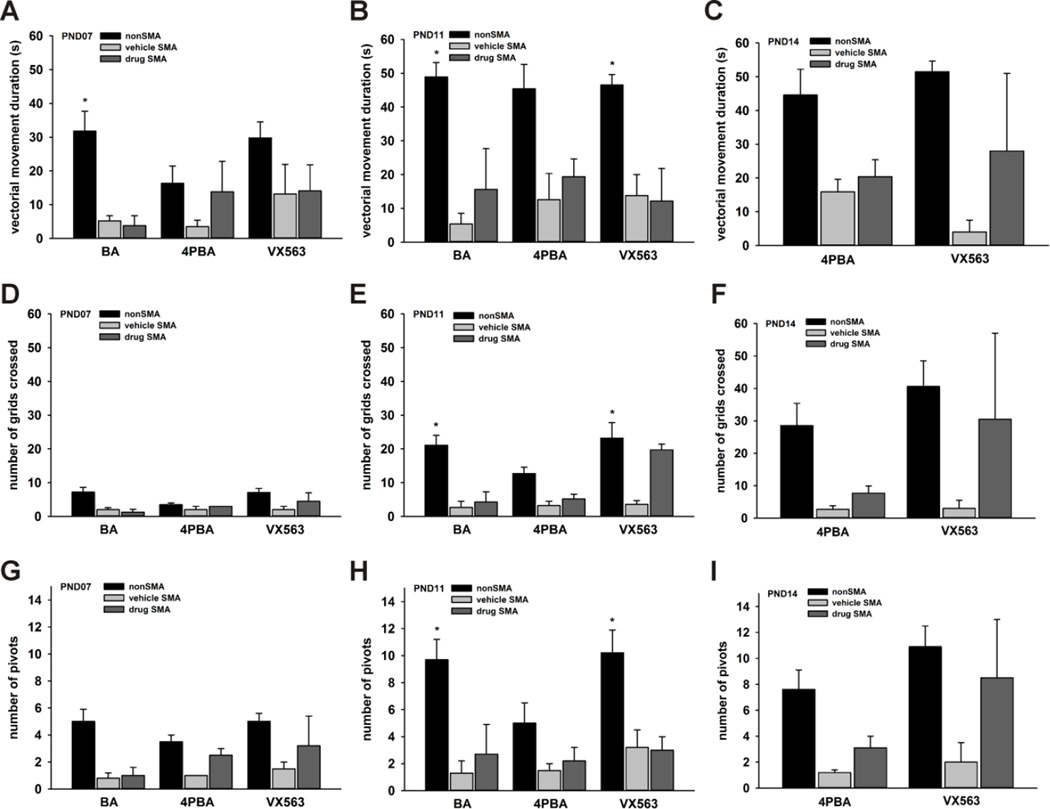
In addition to significantly improving the survival of SMNΔ7 SMA mice, 4PBA (Supplementary Movie 1) and VX563 (Supplementary Movie 2) improved the motor phenotype of SMNΔ7 SMA mice. We examined in more detail the effects of treatment with BA, 4PBA or VX563 on the motor impairment observed in SMNΔ7 SMA mice. Drug treatment of SMNΔ7 SMA mice began at PND04 and the mice were assayed for locomotor behaviors at PND07 (for BA, 4PBA and VX563 treatments), PND11 (for BA, 4PBA and VX563 treatments) and PND14 (for 4PBA and VX563 treatments only as there were an insufficient number of BA-treated SMNΔ7 SMA mice at this age). Age-matched, non-SMA (carrier as well as normal) littermates were also assayed as controls; the phenotype data for carrier and normal neonatal mice were combined as the motor phenotypes of these two groups of mice are not different (Butchbach et al., 2007a). Three indices of motor function were measured (Butchbach et al., 2007a): the duration of vectorial movement—i.e. locomotion in one direction at a distance greater than the body length, number of grids crossed (i.e. spontaneous locomotor activity) and the number of 90° pivots. In agreement with previous findings (Butchbach et al., 2007a; Butchbach et al., 2010a; Butchbach et al., 2010b), vectorial movement was reduced in vehicle-treated SMNΔ7 SMA mice at all time points assayed (Figures 5A-C). The duration of vectorial movement was not significantly altered in SMNΔ7 SMA mice treated with BA, 4PBA or VX563. While none of the butyrate compounds had a statistically significant effect on spontaneous locomotor activity (Figures 5D-F), there were trends for phenotypic improvement in SMNΔ7 SMA mice treated with either 4PBA or VX563. Pivoting tended to increase in 4PBA- and VX563-treated SMNΔ7 SMA mice at all 3 time points (Figures 5G-I) but none of the changes were statistically significant. There was a high degree of variability within each SMNΔ7 SMA mouse treatment group especially at PND14; this variability in phenotype could be due to the presence of strong and weak responders to 4PBA and VX563.
Figure 5. The effects of butyrate analogues and prodrugs on the motor phenotype of SMNΔ7 SMA mice.
SMNΔ7 SMA mice were treated with BA, 4PBA, VX563 or their appropriate vehicle daily beginning at PND04 and were assayed for locomotor behaviors at PND07 (all drugs), PND11(all drugs) and PND14 (4PBA and VX563). Age-matched, non-SMA (carrier as well as normal) littermates were also assayed as controls. Vectorial movement (A-C), or locomotion in one direction at a distance greater than the body length (Butchbach et al., 2007a), was reduced in vehicle-treated SMNΔ7 SMA mice at PND07 (A), PND11 (B) and PND14 (C). The duration of vectorial movement was not significantly altered in SMNΔ7 SMA mice treated with BA, 4PBA or VX563. Spontaneous locomotor activity (D-F)—as measured by the number of grids crossed in 60 s (Butchbach et al., 2007a)—was reduced in vehicle-treated SMNΔ7 SMA mice at PND07 (D), PND11 (E) and PND14 (F). While none of the butyrate compounds had a statistically significant effect on spontaneous locomotor activity, there were trends for phenotypic improvement in 4PBA- and VX563-treated SMNΔ7 SMA mice. The number of 90° pivots (G-I) was reduced in vehicle-treated SMNΔ7 SMA mice at PND07 (G), PND11 (H) and PND14 (I). There were no statistically significant changes in pivoting behavior in SMNΔ7 SMA mice treated with BA, 4PBA or VX563 although there were trends for increased pivoting in 4PBA- and VX563-treated SMNΔ7 SMA mice. The asterisk (*) denotes a statistically significant (p ≤ 0.05) difference when compared to vehicle-treated SMNΔ7 SMA mice for each drug.
Effect of BA Compounds on Motor Neuron Loss in the SMNΔ7 SMA Mouse Spinal Cord
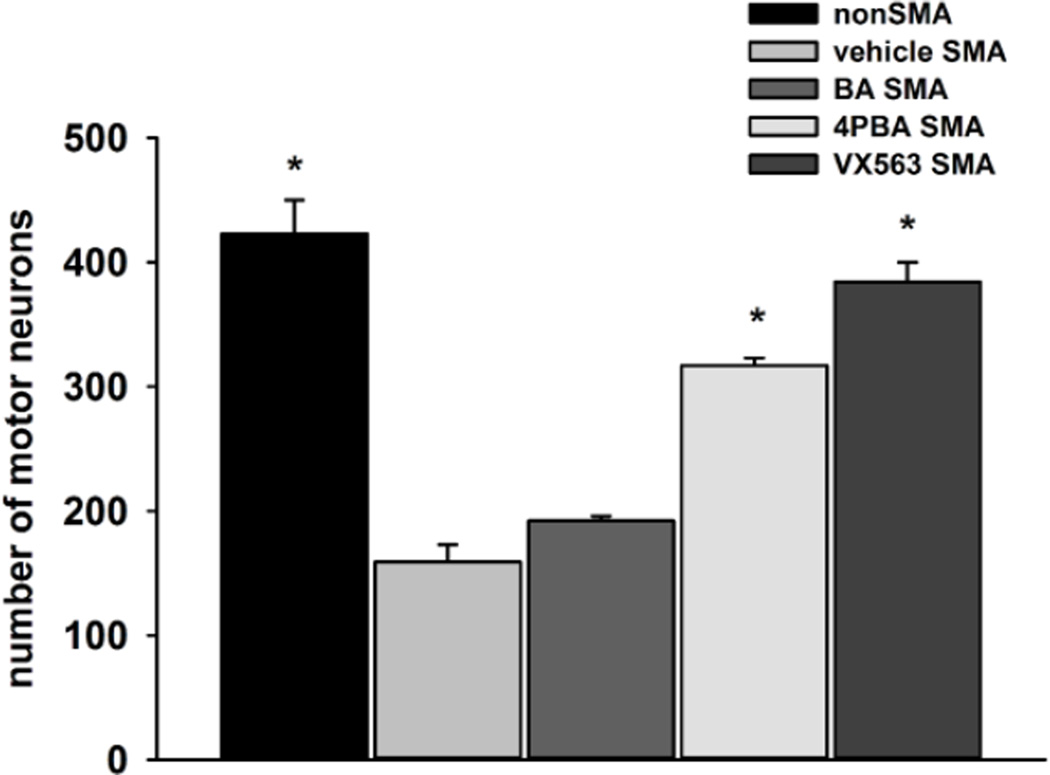
Motor neuron loss occurs in the ventral horn of the lumbar spinal cords in the SMNΔ7 SMA mouse and worsens as the phenotype progresses (Le et al., 2005; Mentis et al., 2011). The effect of BA compounds (BA, 4PBA and VX563) on motor neuron loss was examined in SMNΔ7 SMA mice at PND11 (n = 3/drug). Drug dosing began at PND04 in these experiments. As shown in Figure 6, there was a 62% loss of lumbar motor neurons in SMNΔ7 SMA when compared to age-matched carrier mice (159 ± 14 vs. 423 ± 27; p > 0.001). Administration of BA had no effect on the motor neuron loss in SMNΔ7 SMA mice (192 ± 4 for BA-treated mice; p = 1.000). 4PBA and VX563, on the other hand, resulted in a marked elevation in the number of lumbar motor neurons when compared to vehicle-treated SMNΔ7 SMA mice (Figure 6; 317 ± 6 for 4PBA (p = 0.001) and 384 ± 16 for VX563 (p > 0.001). In fact, treatment with VX563 restored motor neuron numbers at PND11 to those observed in carrier mice. The magnitude of change in motor neuron numbers is related to the magnitude of phenotypic amelioration.
Figure 6. The effects of butyrate compounds on the number of motor neurons in the lumbar spinal cord of PND11 SMNΔ7 SMA mice.
SMNΔ7 SMA mice were treated with either vehicle, BA, 4PBA or VX563 daily (n = 3/group) beginning at PND04 until PND11. Quantification of lumbar motor neurons was done in SMNΔ7 SMA mice within the aforementioned treatment groups as well age-matched, non-SMA littermates. The asterisk (*) denotes a statistically significant (p ≤ 0.05) difference when compared to vehicle-treated SMNΔ7 SMA mice for each drug.
Effect of BA Compounds on SMN2 Expression in the SMNΔ7 SMA Spinal Cord
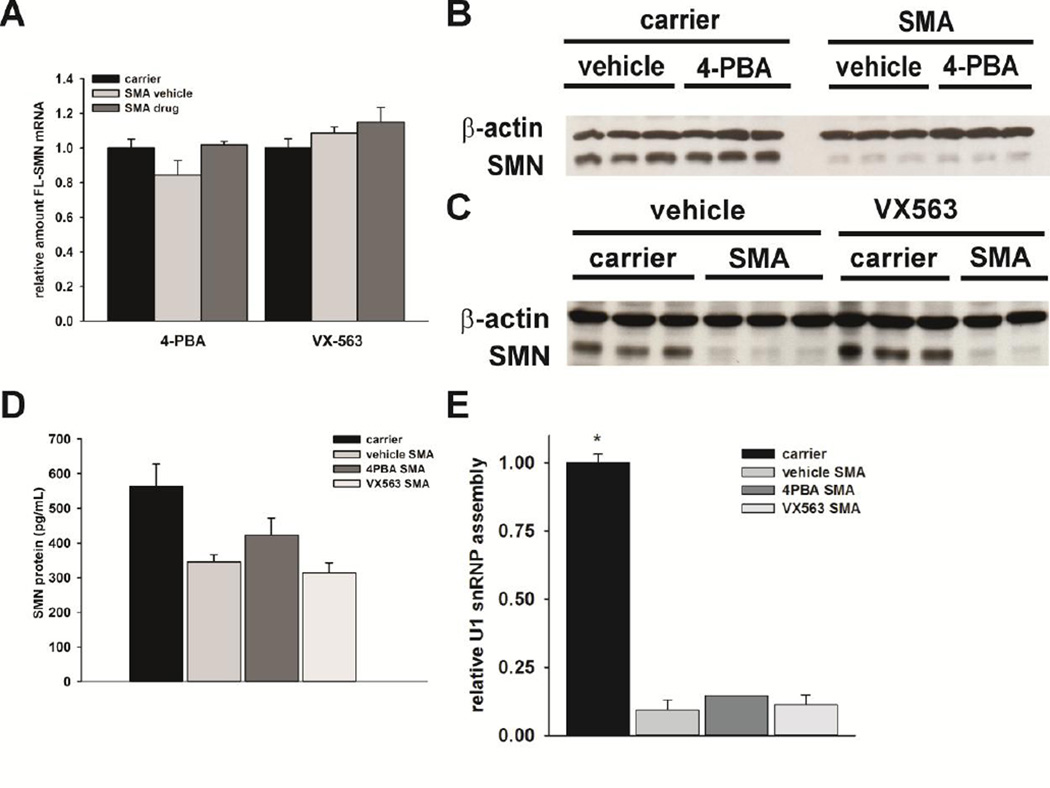
As it has been reported that BA and its analogue 4PBA increase SMN expression in cultured cells (Andreassi et al., 2004; Chang et al., 2001), we measured the effects of 4PBA and VX563 on SMN expression in the spinal cord of SMNΔ7 SMA mice. Using quantitative RT-PCR with human-specific primers, the effects of 4PBA and VX563 on SMN2 mRNA expression were examined in the spinal cords of SMNΔ7 SMA mice treated for 5 days with drug. The amounts of FL-SMN mRNA were quantified relative to the geometric mean of three transcripts (Hprt, Actb and Pol2a) so as to minimize the variability in the expression of any single housekeeping gene (Vandesompele et al., 2002). Neither compound significantly increased the amounts of FL-SMN (Figure 7A) mRNA in the SMNΔ7 SMA mouse spinal cord.
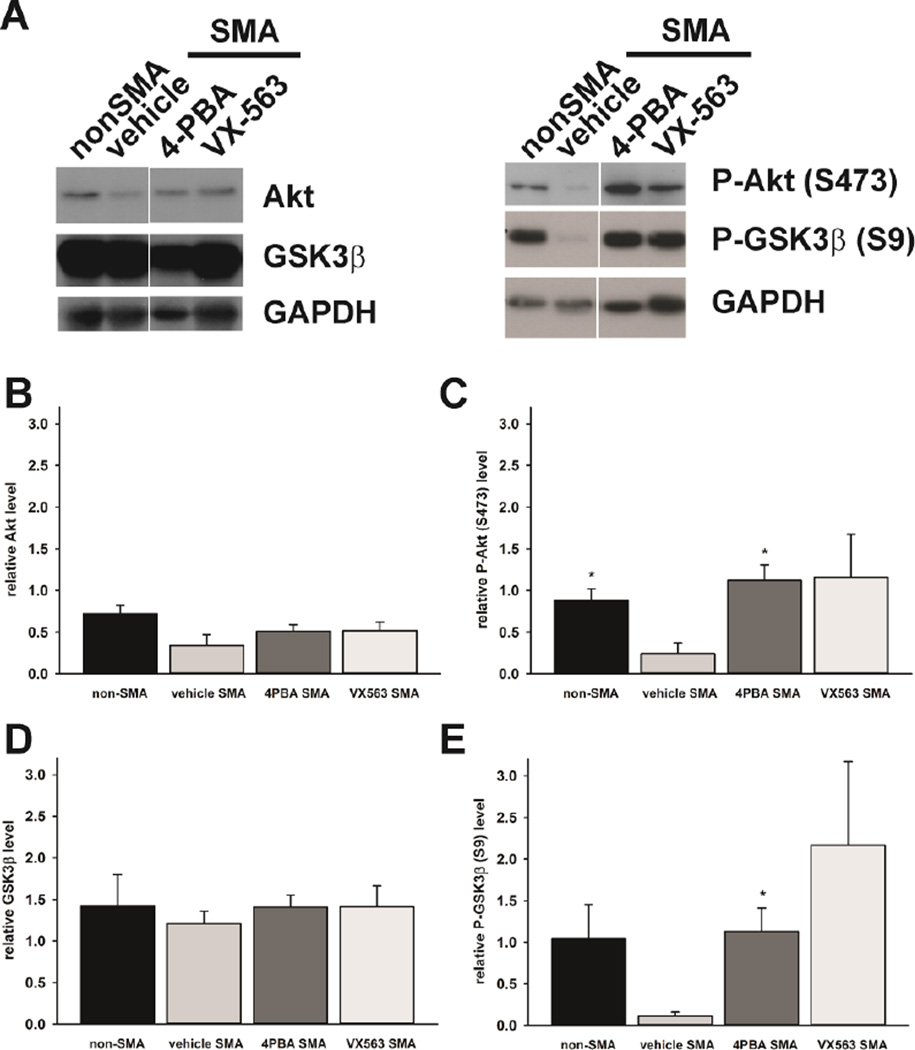
Figure 7. The effects of 4PBA and VX563 on SMN expression and function in the spinal cords of SMNΔ7 SMA mice.
SMNΔ7 SMA mice (n = 3/group) were treated with either 4PBA, VX563 or their appropriate vehicles for 5 days beginning at PND04. Age-matched carrier mice were also included as controls. Neither 4PBA nor VX563 significantly affected the levels of FLSMN (A) mRNAs in treated spinal cord samples as determined by qRT-PCR. Immunoblot analysis shows that the levels of total SMN protein in the spinal cord were not altered by treatment with either 4PBA (B) or VX563 (C). Quantification of human SMN protein levels by ELISA also shows no significant changes in response to 4PBA or VX563 treatment (D). In vitro U1 snRNP assembly was not altered in spinal cord samples from SMNΔ7 SMA mice treated with either 4PBA or VX563 relative to vehicle-treated SMNΔ7 SMA mice (E). The asterisk (*) denotes a statistically significant (p ≤ 0.05) difference when compared to vehicle-treated SMNΔ7 SMA mice for each drug.
Next, immunoblot analysis was used to determine the effects of 4PBA and VX563 on SMN protein levels in vivo. Interestingly, neither 4PBA (Figure 7B) nor VX563 (Figure 7C) led to a detectable change in the amount of SMN protein in the spinal cords of SMNΔ7 SMA mice. Furthermore, quantification of SMN protein using ELISA confirmed the immunoblot findings in that neither BA drugs tested altered SMN protein levels in SMNΔ7 SMA mouse spinal cord extracts (Figure 7D). Lastly, we measured the effects of BA drug treatment on the assembly of small nuclear ribonucleoproteins (snRNPs), which is the only molecularly established function of SMN (Li et al., 2014) that is impaired in the spinal cord of SMA mice (Gabanella et al., 2007; Zhang et al., 2008). After treatment with 4PBA or VX563 for 5 days, none of the drugs tested had any effect on in vitro snRNP assembly in SMNΔ7 SMA mouse spinal cord extracts (Figure 7E).
Collectively, these observations show that BA-based compounds ameliorate the SMA phenotype in neonatal mice independent of SMN induction in the spinal cords of SMNΔ7 SMA mice.
Effects of BA Compounds on Histone Deacetylase Activity in SMNΔ7 SMA Mice
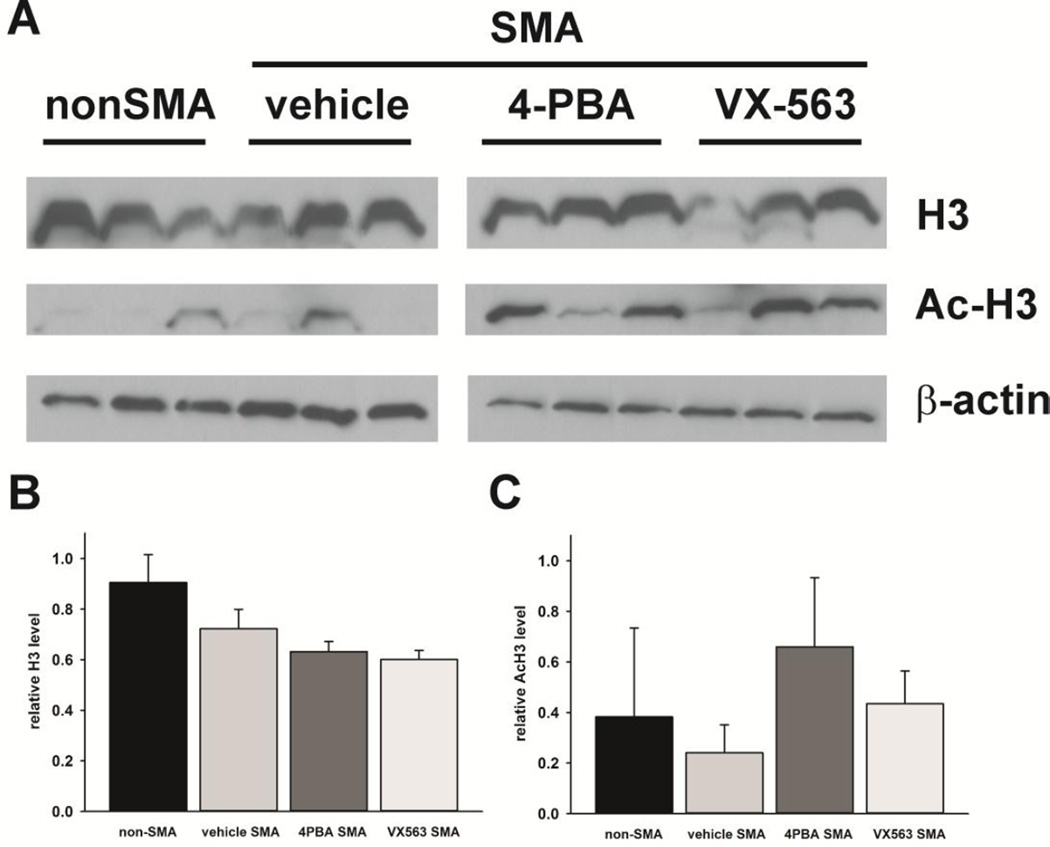
If SMN induction is not the mechanism by which BA-based compounds exert their protective effects in SMNΔ7 SMA mice, what is the mechanism for the neuroprotection mediated by these compounds? BA and 4PBA function as weak inhibitors of histone deacetylase (HDAC) activity (Boffa et al., 1978; Davis et al., 2000). SMNΔ7 SMA mice were treated with 4PBA or VX563 for 5 days beginning at PND04 (n = 3/group) and HDAC activity was measured in spinal cord extracts. HDAC activity was assessed by immunoblot measurement of the acetylation of histone H3 (Ac-H3), a primary substrate of HDACs, using an acetylation-specific antibody (Figure 8A). While the levels of H3 were not significantly different between the treatment groups, treatment of SMNΔ7 SMA mice with 4PBA or VX563 showed a tendency for elevated Ac-H3 levels when compared against vehicle-treated SMNΔ7 SMA mice. The differences, however, were not statistically significant due, most likely, to the high degree of variability within each treatment group (Figures 8B-C). These data suggest that HDAC activity may be reduced in the spinal cords of SMNΔ7 SMA mice treated with 4PBA or VX563.
Figure 8. The effects of 4PBA and VX563 on histone deacetylase (HDAC) activity in SMNΔ7 SMA mice.
HDAC activity was measured by the levels of acetylated histone H3 (H3) in spinal cord extracts. SMNΔ7 SMA mice (n = 3/group) were treated with either 4PBA, VX563 or their appropriate vehicles for 5 days beginning at PND04. Age-matched non-SMA mice were also included as controls. The effects of these compounds on both the levels of and the acetylation of H3 were determined by immunoblot (A). The band intensities for either H3 or acetylated H3 (Ac-H3) were normalized against those for the loading control β-actin. The levels of H3 protein (B) were not affected by drug but tended to be lower in SMNΔ7 SMA spinal cord samples. These differences were not statistically significant. The acetylation of H3 at lysine 9 (K9) (C) was greater in spinal cord samples from SMNΔ7 SMA mice treated with 4PBA or VX563. The differences, however, were not statistically significant due to Ac-H3 variability within treatment groups.
Effects of BA Compounds on Akt Signaling in SMNΔ7 SMA Mice
BA increases activation of Akt signaling by increasing its phosphorylation (Rahmani et al., 2003). We determined the effects of BA compounds on the phosphorylation of Akt and its substrate glycogen synthase kinase 3β (GSK3β) in the spinal cords of SMNΔ7 SMA mice. SMNΔ7 SMA mice were treated with 4PBA or VX563 for 5 days beginning at PND04 (n = 3/group). As shown in Figure 9A, the levels of Akt protein as well as the phosphorylation of Akt at serine 473 (S473) were reduced in SMNΔ7 SMA mouse spinal cord extracts when compared to age-matched, non-SMA mice. The changes in Akt protein levels were not statistically significant (Figure 9B) but the phosphorylation of Akt at S473 was significantly diminished in SMNΔ7 SMA samples (Figure 9C; p = 0.0245). 4PBA and VX563 increased the phosphorylation of Akt at S473 in SMNΔ7 SMA spinal cord samples (Figure 9C). This effect was statistically significant when comparing 4PBA-treated SMA samples to vehicle-treated samples (p = 0.0185) but not for VX563-treated samples (p = 0.160).
Figure 9. The effects of 4PBA and VX563 on Akt signaling in SMNΔ7 SMA mice.
SMNΔ7 SMA mice (n = 3/group) were treated with either 4PBA, VX563 or their appropriate vehicles for 5 days beginning at PND04. Age-matched non-SMA mice were also included as controls. The effects of these compounds on both the levels of and the phosphorylation of Akt and one of its substrates, GSK3β, were determined by immunoblot (A). The band intensities for each target protein were normalized against those for the loading control GAPDH. The levels of Akt protein (B) were not affected by disease status (compare non-SMA to vehicle SMA) or by drug treatment. The phosphorylation of Akt at serine 473 (S473) was reduced in the spinal cord of SMNΔ7 SMA mice (C). Treatment of these mice with either 4PBA or VX563 increased Akt phosphorylation. As with Akt, the levels of GSK3β protein (D) were not affected by disease status or by drug treatment. The phosphorylation of GSK3β at serine 9 (S9) was reduced in the spinal cord of SMNΔ7 SMA mice (E). Treatment of these mice with either 4PBA or VX563 increased GSK3β phosphorylation. The asterisk (*) denotes a statistically significant (p ≤ 0.05) difference when compared to vehicle-treated SMNΔ7 SMA mice.
GSK3β protein levels were not different between SMNΔ7 SMA and non-SMA samples (Figure 9D). The phosphorylation of GSK3β at serine 9 (S9), however, was reduced in SMNΔ7 SMA mouse spinal cord extracts when compared to non-SMA control samples (Figure 9E; p = 0.0841). Treatment of SMNΔ7 SMA mice with 4PBA and VX563 increased the phosphorylation of GSK3β at S9 (Figure 9E). The increase in GSK3β phosphorylation was statistically significant (p = 0.0234) following 4PBA treatment but not following VX563 treatment (p = 0.1093).
To our knowledge, we have demonstrated for the first time that Akt and GSK3β phosphorylation levels are reduced in SMNΔ7 SMA mouse spinal cords. 4PBA and VX563 restore the normal phosphorylation states of Akt and GSK3β in these tissues.
DISCUSSION
In this study, we show that oral administration of BA-based compounds like 4-PBA and BA prodrugs BA3G and VX563 improve the survival and phenotype of SMNΔ7 SMA mice. In fact, VX563 improved survival by 250%; the very strong response of SMNΔ7 SMA mice to VX563 could be due to its superior pharmacokinetics when compared against those for BA (Egorin et al., 1999; McCaffrey et al., 1996). Interestingly, none of the compounds tested increased SMN expression in the spinal cords of treated mice; however, HDAC activity was inhibited and the phosphorylation of Akt and GSK3β was restored to healthy levels in treated spinal cord extracts.
When delivered orally, BA has no effect on the SMNΔ7 mouse model of SMA. Ad libitum oral administration of BA to a different mouse model for SMA (SMN2;mSmnΔ7/Δ7) increases their survival by 4 – 5 days (Chang et al., 2001). The different genotypes between these SMA mouse models could account for the discordant drug response; however, it is possible that the mice in the Chang et al. (Chang et al., 2001) received different amounts of BA compared to the dose used in this study. Because the drug was administered to the mice through their water, we do not know how much BA each SMA mouse actually received. In this study, BA levels in the CNS were not detectable in mice dosed with 5 g/kg/d BA but were detected in the mice treated with the BA prodrugs BA3G and VX563. In order to conclusively establish drug efficacy, there is a need to effectively monitor dosing, especially in the CNS, to ensure that animals are receiving sufficient drug to have an effect.
Some SMNΔ7 SMA mice within our study cohort responded very favorably to 4PBA, BA3G and VX563 while other mice were not affected by drug treatment. The variability in responsiveness to these drugs could explain the lack of statistically significant benefit on motor function. There was no correlation between body mass at PND04 and response to drug as measured by lifespan (data not shown). Sex or litter size also did not impact responsiveness to BA compounds. Consistent with our observation in mice, studies in humans have also pointed to the possibility of inter-individual drug response (Brahe et al., 2005; Brichta et al., 2006; Swoboda et al., 2009; Weihl et al., 2006). Our ability to identify biomarkers distinguishing drug responders from non-responders will greatly affect drug study designs and analyses of drug efficacy. Identification of biomarkers in mice might also be relevant in humans.
VX563 treatment restores motor neuron counts in the ventral spinal cords of SMNΔ7 SMA mice to those observed in age-matched, non-SMA littermates; however, VX563 does not completely rescue the SMA phenotype in these mice. Restoration of SMN solely in motor neuron or reduction of SMN solely in motor neurons does not markedly alter the survival of these animals (Martinez et al., 2012; McGovern et al., 2015). Furthermore, there is rescue of both electrophysiological activity and innervation status of skeletal muscle when replacing SMN solely in motor neurons (Martinez et al., 2012; McGovern et al., 2015). Thus, rescue of SMN levels in motor neurons does not correlate with survival. Rather, elevation of SMN in all neurons and astrocytes has a major impact with some mice showing normal survival (Gavrilina et al., 2008). This could be due to restoration of SMN to the autonomic nervous system and its control of cardiac function which is abnormal in SMNΔ7 SMA mice (Bevan et al., 2010; Biondi et al., 2012; Heier et al., 2010; Shababi et al., 2012; Shababi et al., 2010). Similarly, either massive expression of SMN in the muscles of SMNΔ7 SMA mice or reduction of SMN levels does not significantly alter survival (Gavrilina et al., 2008; Iyer et al., 2015). In the current case, VX563 restores the number of ventral horn motor neurons at a specific time point and presumably does exert an influence on other cell types in (and out of) the CNS but does not exert sufficient influence on those other cell types to fully rescue survival.
4PBA and the BA prodrug VX563 markedly increased the survival of SMNΔ7 SMA mice and improved their phenotype, but neither compound increased SMN expression in the spinal cords of treated mice. These findings contrast those observed in SMA patient cell lines where BA and 4PBA increase SMN expression (Andreassi et al., 2004; Brahe et al., 2005; Chang et al., 2001). It is possible that these compounds do not increase SMN expression in vivo or in motor neurons within the spinal cord. Future studies will elucidate the reasons underlying this disconnect. Other compounds ameliorate the SMA phenotype in SMA mouse models without affecting SMN expression. The rho kinase inhibitor Y-27632 ameliorates the phenotype of SMA mice independent of SMN expression (Bowerman et al., 2010). Follistatin increases the lifespan of SMNΔ7 SMA mice (Harris and Butchbach, 2015; Rose Jr et al., 2009). This recombinant protein does not increase SMN expression in vivo suggesting that its effects are independent of SMN (Rose Jr et al., 2009). Consequently, SMN-independent neuroprotectants like 4PBA, BA prodrugs, Y-27632 and follistatin could be used in concert with compounds that increase SMN expression in SMA cells in vivo to further ameliorate the disease phenotype.
What are some of the SMN-independent, neuroprotective pathways that could be activated in SMA mice treated with BA compounds? Inhibiting HDAC activity is an important target for the development of neuroprotective therapies against neurodegenerative diseases (Chuang et al., 2009). Weak HDAC inhibitors like 4PBA and BA are neuroprotective in animal models for amyotrophic lateral sclerosis (ALS; (Del Signore et al., 2009; Petri et al., 2006; Ryu et al., 2005)), Alzheimer disease (Ribobaraza et al., 2009), Huntington disease (Ferrante et al., 2003; Gardian et al., 2005; Steffan et al., 2001), stroke (Qi et al., 2004) and dentatorubralpallidoluysian atrophy (Ying et al., 2006). Similar to our findings that 4PBA and BA prodrugs ameliorate the survival and phenotype of SMNΔ7 SMA, other HDAC inhibitors including valproic acid (VPA) (Tsai et al., 2008), trichostatin A (TSA) (Avila et al., 2007; Liu et al., 2014; Narver et al., 2008) and suberoylanilide hydroxamic acid (SAHA) (Riessland et al., 2010) have been found to improve survival in mouse models for SMA. Remarkably, while most of these studies showed increased SMN expression in vivo in response to HDAC inhibitor treatment (Avila et al., 2007; Riessland et al., 2010; Tsai et al., 2008), we did not observe any detectable changes in SMN mRNA or protein levels in SMNΔ7 SMA mice treated with 4PBA or VX563. Consistent with our findings, Liu et al. also demonstrated that the beneficial effects of TSA treatment in SMA mice are independent of SMN upregulation (Liu et al., 2014). Moreover, in a clinical trial, SMA patients treated with VPA do not show increased SMN expression even though VPA treatment results in increased histone acetylation (Renusch et al., 2015). Thus, one possibility is that the protective effects of HDAC inhibition on SMA mouse models result from activation of neuroprotective pathways that are yet to be characterized.
Stimulation of prosurvival pathways—like the Akt pathway—may be neuroprotective in motor neuron diseases like ALS (Peviani et al., 2014) and SMA. 4PBA has been shown to alter Akt phosphorylation in other models of stressful or toxic conditions (Özcan et al., 2006; Qin et al., 2010). We found that 4PBA and VX563 treatment of SMNΔ7 SMA mice restored the phosphorylation levels of Akt at S473 to those observed in non-SMA mice. Increasing AKT phosphorylation by reduction of the upstream antagonist phosphatase and tensin homolog (PTEN) positively modulates axonal growth and survival in primary motor neurons (Ning et al., 2010). Reduction of Smn causes a decrease in phosphorylation of Akt at S473 in cultured cortical neurons. An inherent dysregulation of mTOR activity, a substrate for Akt, in cortical and motor neurons has been attributed to SMN depletion (Kye et al., 2015). Akt phosphorylation is increased in the spinal cords of SMA-like mice treated with the ionotropic glutamate receptor agonist NMDA (N-methyl-D-aspartate) (Biondi et al., 2010). Increasing exercise in the SMA-like mice reduces the expression of the insulin-like growth factor-1 (IGF-1) receptor and increases Akt phosphorylation (Biondi et al., 2015). Taken together, these studies show the importance of Akt phosphorylation on neuroprotection from SMN deficiency in SMA motor neurons. Akt has been implicated as a focal point for cell survival pathways (Rodgers and Theibert, 2002). GSK3β, which is in its active state under basal conditions, is inhibited through phosphorylation by Akt on S9 (Woodgett, 1990). When GSK3β activity is repressed, many of its downstream targets—primarily transcription factors—transition from inactive to active states. GSK3s play a part in multiple steps of neuronal differentiation and development due to its ability to regulate many transcription factors (Hur and Zhou, 2010). Treating the Tg2576 transgenic mouse model for Alzheimer disease with 4PBA increases phosphorylation of GSK3β at S9 in the hippocampus (Ribobaraza et al., 2009). We found that 4PBA and VX563 increased GSK3β phosphorylation at S9 in SMNΔ7 SMA mice. Inhibition of GSK3β by maleimide-based compound BiP-135 increases the lifespan of SMNΔ7 SMA mice by about 2 days (Chen et al., 2012). Our results suggest that modulation of the AKT/GSK3β pathway by 4PBA and VX563 may contribute to the neuroprotective effects observed the SMNΔ7 SMA mouse model. Future studies will investigate if BA analogues and prodrugs exert their neuroprotective effects in an AKT/GSK3β-dependent manner and how AKT phosphorylation exerts these effects in SMA neurons, possibly through crosstalk between MAP kinase and CREB (cAMP responsive element-binding protein) pathways (Branchu et al., 2013).
4PBA has already been used in clinical trials with SMA patients. SMA patients that were treated with 4PBA (500 mg/kg/day) in a pilot study for 7 days showed improvements in motor function as compared to their baseline (before treatment) measurements (Mercuri et al., 2004). However, in a randomized, double-blind, placebo-controlled study, 4PBA did not improve the disease severity of type II SMA patients (Mercuri et al., 2007). Administration of 4PBA to SMA patients occurred after the onset of disease in these clinical trials. We show here that administration of BA-based compounds beginning at PND04 has an effect on SMNΔ7 SMA mice but treatment of these mice after disease onset, i.e. PND09, does not yield the same effect. These observations underscore the importance of a window of therapeutic opportunity for SMA. In addition, observing drug effects is challenged by inter-animal and inter-individual variability in responsiveness.
In summary, BA-based compounds with favorable bioavailability and pharmacokinetics significantly improve the phenotype and survival in a mouse model of SMA. Contrary to observations in cultured SMN-deficient cells, these compounds do not increase SMN2 expression in the spinal cords of treated mice. However, 4PBA and VX563 do restore the phosphorylation states of Akt and GSK3β that are reduced in untreated SMNΔ7 SMA mouse spinal cord samples. Chemically stable, BA-compounds can be used for the development of neuroprotective therapeutics for SMA either on their own or in combination with SMN2 inducing agents.
Supplementary Material
Supplementary Figure. Representative high-performance liquid chromatograms for BA.
Supplementary Movie 1. Phenotype of SMNΔ7 SMA mice treated with 4PBA at PND14.
Supplementary Movie 2. Phenotype of SMNΔ7 SMA mice treated with VX563 at PND14.
HIGHLIGHTS.
4-phenylbutyrate (4PBA), tributyrin and VX-563 have moderate effects on the phenotype and survival of some of the treated SMNΔ7 SMA mice
4PBA and VX563 act independently of SMN induction in SMNΔ7 SMA mouse spinal cords
4PBA and VX563 restore the normal phosphorylation states of Akt and GSK3β in SMNΔ7 SMA mouse spinal cords
Acknowledgments
We would like to thank Vertex Pharmaceuticals for generously providing VX563. The SMN EIA kits were generously provided by Assay Designs, now Enzo Life Sciences, through their Kits for Charity program. Additionally, we would like to thank Dr. Glenn Morris for kindly providing the SMN monoclonal antibodies, Elzbieta Slominski for her excellent technical assistance with the qRT-PCR, Warren Erdahl for his excellent assistance with the HPLC, Dr. Douglas Pfeiffer for providing access to HPLC equipment, the staff at NeuroScience Associates for assistance with spinal cord sectioning, Dr. Thanh T. Le for providing the initial SMNΔ7 breeder mice used to establish our testing colony and Kristie Schussler for her assistance in the initial phases of this project.
This study was supported by grants from Cure SMA (M.E.R.B. and A.H.M.B.), Miracles for Madison Fund (A.H.M.B.), the Nemours Foundation (M.E.R.B.), National Institute of Neurological Disorders and Stroke of the National Institutes of Health (R01NS38650 to A.H.M.B. and R01NS069601 to L.P.) and an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health (P20GM103464; M.E.R.B.). The sponsors had no role in study design, data collection/interpretation, writing of this article and the decision to publish this article.
ABBREVIATIONS
- Ac-H3
acetylated histone H3
- Actb
β-actin
- ALS
amyotrophic lateral sclerosis
- BA
butyric acid
- BA3G
glyceryl tributyrate
- b.i.d.
bis in die
- CNS
central nervous system
- CREB
cAMP responsive element-binding protein
- FL-SMN
full-length survival motor neuron
- GSK3β
glycogen synthase kinase 3β
- HDAC
histone deacetylase
- HPLC
high performance liquid chromatography
- Hprt
hypoxanthine phosphorylribosyltransferase
- IGF-1
insulin-like growth factor-1
- mSmn
mouse survival motor neuron
- NMDA
N-methyl-D-aspartate
- 4PBA
4-phenylbutyric acid
- PND
postnatal day
- Pol2a
RNA polymerase IIA
- PTEN
phosphatase and tensin homolog
- SAHA
suberoylanilide hydroxamic acid
- SMA
spinal muscular atrophy
- SMN
survival motor neuron
- SMNΔ7
survival motor neuron lacking exon 7
- snRNP
small nuclear ribonucleoprotein
- t.i.d.
ter in die
- TSA
trichostatin A
- VPA
valproic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
M.E.R.B., L.R.S., L.P. and A.H.M.B. conceived and designed the experiments; M.E.R.B., C.J.L., A.W. H., L.S., J.D.E. and E.W. performed the experiments; M.E.R.B., C.J. L., L.R.S., L.P. and A.H.M.B. analyzed the data; M.E.R.B. and A.H.M.B. wrote the paper.
All authors have read and approved submission of this work.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no significant conflicts of interest.
REFERENCES
- Andreassi C, Angelozzi C, Tiziano FD, Vitali T, De Vincenzi E, Boninsegna A, Villanova M, Bertini E, Pini A, Neri G, Brahe C. Phenylbutyrate increases SMN expression in vitro: relevance for treatment of spinal muscular atrophy. Eur. J. Hum. Genet. 2004;12:59–65. doi: 10.1038/sj.ejhg.5201102. [DOI] [PubMed] [Google Scholar]
- Avila AM, Burnett BG, Taye AA, Gabanella F, Knight MA, Hartenstein P, Cizman Z, DiProspero NA, Pellizzoni L, Fischbeck KH, Sumner CJ. Trichostatin A increases SMN expression and survival in a mouse model of spinal muscular atrophy. J. Clin. Invest. 2007;117:659–671. doi: 10.1172/JCI29562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg S, Serabe B, Aleksic A, Bomgaars L, McGuffey L, Dauser R, Durfee J, Nuchtern J, Blaney S. Pharmacokinetics and cerebrospinal fluid penetration of phenylacetate and phenylbutyrate in the nonhuman primate. Cancer Chemother. Pharmacol. 2001;47:385–390. doi: 10.1007/s002800000256. [DOI] [PubMed] [Google Scholar]
- Bevan AK, Hutchinson KR, Foust KD, Braun L, McGovern VL, Schmelzer L, Ward JG, Petruska JC, Lucchesi PA, Burghes AHM, Kaspar BK. Early heart failure in the SMNΔ7 model of spinal muscular atrophy and correction by postnatal scAAV9-SMN delivery. Hum. Mol. Genet. 2010;19:3895–3905. doi: 10.1093/hmg/ddq300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi O, Branchu J, Ben Salah A, Houdebine L, Bertin L, Chali F, Desseille C, Weill L, Sanchez G, Lancelin C, Aïd S, Lopes P, Pariset C, Lécolle S, Côté J, Holzenberger M, Chanoine G, Massaad C, Charbonnier F. IGF-1R reduction triggers neuroprotective signaling pathways in spinal muscular atrophy mice. J. Neurosci. 2015;35:12063–12079. doi: 10.1523/JNEUROSCI.0608-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi O, Branchu J, Sanchez G, Lancelin C, Deforges S, Lopes P, Pariset C, Lécolle S, Côté J, Chanoine C, Charbonnier F. In vivo NMDA receptor activation accelerates motor unit maturation, protects spinal motor neurons and enhances SMN2 gene expression in severe spinal muscular atrophy mice. J. Neurosci. 2010;30:11288–11299. doi: 10.1523/JNEUROSCI.1764-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi O, Lopes P, Desseille C, Branchu J, Chali F, Ben Salah A, Pariset C, Chanoine C, Charbonnier F. Physical exercise reduces cardiac defects in type 2 spinal muscular atrophy-like mice. J. Physiol. 2012;590:5907–5925. doi: 10.1113/jphysiol.2012.238196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boffa LC, Vidali G, Mann RS, Allfrey VG. Suppression of histone deacetylation in vivo and in vitro by sodium butyrate. J. Biol. Chem. 1978;253:3364–3366. [PubMed] [Google Scholar]
- Bowerman M, Beauvais A, Anderson CL, Kothary R. Rho-kinase inactivation prolongs survival of an intermediate SMA mouse model. Hum. Mol. Genet. 2010;19:1468–1478. doi: 10.1093/hmg/ddq021. [DOI] [PubMed] [Google Scholar]
- Brahe C, Vitali T, Tiziano FD, Angelozzi C, Pinto AM, Borgo F, Moscato U, Bertini E, Mercuri E, Neri G. Phenylbutyrate increases SMN gene expression in spinal muscular atrophy patients. Eur. J. Hum. Genet. 2005;13:256–259. doi: 10.1038/sj.ejhg.5201320. [DOI] [PubMed] [Google Scholar]
- Branchu J, Biondi O, Chali F, Collin T, Leroy F, Mamchaoui K, Makoukji J, Pariset C, Lopes P, Massaad C, Chanoine C, Charbonnier F. Shift from extracellular signalrelated kinase to AKT/cAMP response element-binding protein pathway increases survivalmotor-neuron expression in spinal-muscular-atrophy-like mice and patient cells. J. Neurosci. 2013;33:4280–4294. doi: 10.1523/JNEUROSCI.2728-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brichta L, Hofmann Y, Hahmen E, Siebzehnrubl FA, Raschke H, Blumcke I, Eyupoglu IY, Wirth B. Valproic acid increases the SMN2 protein level: a well-known drug as potential therapy for spinal muscular atrophy. Hum. Mol. Genet. 2003;12:2481–2489. doi: 10.1093/hmg/ddg256. [DOI] [PubMed] [Google Scholar]
- Brichta L, Holker I, Haug K, Klockgether T, Wirth B. In vivo activation of SMN in spinal muscular atrophy carriers and patients treated with valproate. Ann. Neurol. 2006;59:970–975. doi: 10.1002/ana.20836. [DOI] [PubMed] [Google Scholar]
- Butchbach MER, Edwards JD, Burghes AHM. Abnormal motor phenotype in the SMNΔ7 mouse model of spinal muscular atrophy. Neurobiol. Dis. 2007a;27:207–219. doi: 10.1016/j.nbd.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butchbach MER, Edwards JD, Schussler KR, Burghes AHM. A novel method for oral delivery of compounds to the neonatal SMNΔ7 model of spinal muscular atrophy. J. Neurosci. Methods. 2007b;161:285–290. doi: 10.1016/j.jneumeth.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butchbach MER, Rose FF, Jr, Rhoades S, Marston J, McCrone JT, Sinnott R, Lorson CL. Effect of diet on the survival and phenotype of a mouse model for spinal muscular atrophy. Biochem. Biophys. Res. Commun. 2010a;391:835–840. doi: 10.1016/j.bbrc.2009.11.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butchbach MER, Singh J, Gurney ME, Burghes AHM. The effect of diet on the protective action of D156844 observed in spinal muscular atrophy mice. Exp. Neurol. 2014;256:1–6. doi: 10.1016/j.expneurol.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butchbach MER, Singh J, Þorsteindóttir M, Saieva L, Slominski E, Thurmond J, Andrésson T, Zhang J, Edwards JD, Simard LR, Pellizzoni L, Jarecki J, Burghes AHM, Gurney ME. Effects of 2,4-diaminoquinazoline derivatives on SMN expression and phenotype in a mouse model for spinal muscular atrophy. Hum. Mol. Genet. 2010b;19:454–467. doi: 10.1093/hmg/ddp510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JG, Hsieh-Li HM, Jong YJ, Wang NM, Tsai CH, Li H. Treatment of spinal muscular atrophy by sodium butyrate. Proc. Natl. Acad. Sci. U. S. A. 2001;98:9808–9813. doi: 10.1073/pnas.171105098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PC, Gasisina IN, El-Khodor BF, Ramboz S, Makhortova NR, Rubin LL, Kozikowski AP. Identification of a maleimide-based glycogen synthase kinase-3 (GSK-3) inhibitor, BIP-135, that prolongs the median survival time of Δ7 SMA KO mouse model of spinal muscular atrophy. ACS Chem. Neurosci. 2012;3:5–11. doi: 10.1021/cn200085z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry JJ, Osman EY, Evans MC, Choi S, Xing X, Cuny GD, Glicksman MA, Lorson CL, Androphy EJ. Enhancement of SMN protein levels in a mouse model of spinal muscular atrophy using novel drug-like compounds. EMBO Mol. Med. 2013;5:1103–1118. doi: 10.1002/emmm.201202305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang DM, Leng Y, Marinova Z, Kim HJ, Chiu CT. Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci. 2009;32:591–601. doi: 10.1016/j.tins.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifuentes-Diaz C, Nicole S, Velasco ME, Borra-Cebrian C, Panozzo C, Frugier T, Millet G, Roblot N, Joshi V, Melki J. Neurofilament accumulation at the motor endplate and lack of axonal sprouting in a spinal muscular atrophy mouse model. Hum. Mol. Genet. 2002;11:1439–1447. doi: 10.1093/hmg/11.12.1439. [DOI] [PubMed] [Google Scholar]
- Conley BA, Egorin MJ, Tait N, Rosen M, Sausville EA, Dover G, Fram RJ, Van Echo DA. Phase I study of the orally administered butyrate prodrug, tributyrin, in patients with solid tumors. Clin. Cancer Res. 1998;4:629–634. [PubMed] [Google Scholar]
- Coovert DD, Le TT, McAndrew PE, Strasswimmer J, Crawford TO, Mendell JR, Coulson SE, Androphy EJ, Prior TW, Burghes AHM. The survival motor neuron protein in spinal muscular atrophy. Hum. Mol. Genet. 1997;6:1205–1214. doi: 10.1093/hmg/6.8.1205. [DOI] [PubMed] [Google Scholar]
- Davis T, Kennedy C, Chiew YE, Clarke CL, deFazio A. Histone deacetylase inhibitors decrease proliferation and modulate cell cycle gene expression in normal mammary epithelial cells. Clin. Cancer Res. 2000;6:4334–4342. [PubMed] [Google Scholar]
- Del Signore SJ, Amante DJ, Kim J, Stack EC, Goodrich S, Cormier K, Smith K, Cudkowicz ME, Ferrante RJ. Combined riluzole and sodium phenylbutyrate therapy in transgenic amyotrophic lateral sclerosis mice. Amyotroph. Lateral Scler. 2009;10:85–94. doi: 10.1080/17482960802226148. [DOI] [PubMed] [Google Scholar]
- DiDonato CJ, Chen XN, Noya D, Korenberg JR, Nadeau JH, Simard LR. Cloning, characterization and copy number of the murine survival motor neuron gene: homolog of the spinal muscular atrophy-determining gene. Genome Res. 1997;7:339–352. doi: 10.1101/gr.7.4.339. [DOI] [PubMed] [Google Scholar]
- Dominguez E, Marais T, Chatauret N, Benkhelifa-Ziyyat S, Duque S, Ravassard P, Carcenac R, Astord S, Pereira de Moura A, Voit T, Barkats M. Intravenous scAAV9 delivery of a codon-optimized SMN1 sequence rescues SMA mice. Hum. Mol. Genet. 2011;20:681–693. doi: 10.1093/hmg/ddq514. [DOI] [PubMed] [Google Scholar]
- Edelman MJ, Bauer K, Khanwani S, Tait N, Trepel J, Karp J, Nemieboka M, Chung EJ, Van Echo D. Clinical and pharmacologic study of tributyrin: an oral butyrate prodrug. Cancer Chemother. Pharmacol. 2003;51:439–444. doi: 10.1007/s00280-003-0580-5. [DOI] [PubMed] [Google Scholar]
- Egorin MJ, Yuan ZM, Sentz DL, Plaisance K, Eiseman JL. Plasma pharmacokinetics of butyrate after intravenous administration of sodium butyrate or oral administration of tributyrin or sodium butyrate to mice and rats. Cancer Chemother. Pharmacol. 1999;43:445–453. doi: 10.1007/s002800050922. [DOI] [PubMed] [Google Scholar]
- Elsheikh B, Prior T, Zhang X, Miller R, Kolb SJ, Moore D, Bradley W, Barohn R, Bryan W, Gelinas D, Iannaccone S, Leshner R, Mendell JR, Mendoza M, Russman B, Smith S, King W, Kissel JT. An analysis of disease severity based on SMN2 copy number in adults with spinal muscular atrophy. Muscle Nerve. 2009;40:652–656. doi: 10.1002/mus.21350. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, Kubilus JK, Lee J, Ryu H, Beesen A, Zucker B, Smith K, Kowall NW, Ratan RR, Luthi-Carter R, Hersch SM. Histone deacetylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington's disease mice. J. Neurosci. 2003;23:9418–9427. doi: 10.1523/JNEUROSCI.23-28-09418.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust KD, Wang X, McGovern VL, Braun L, Bevan AK, Haidet AM, Le TT, Morales PR, Rich MM, Burghes AHM, Kaspar BK. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat. Biotechnol. 2010;28:271–274. doi: 10.1038/nbt.1610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gabanella F, Butchbach MER, Saieva L, Carissimi C, Burghes AHM, Pellizzoni L. Ribonucleoprotein assembly defects correlate with spinal muscular atrophy severity and preferentially affect a subset of spliceosomal snRNPs. PLoS ONE. 2007;9:e921. doi: 10.1371/journal.pone.0000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabanella F, Carissimi C, Usiello A, Pellizzoni L. The activity of the spinal muscular atrophy protein is regulated during development and cellular differentiation. Hum. Mol. Genet. 2005;14:3629–3642. doi: 10.1093/hmg/ddi390. [DOI] [PubMed] [Google Scholar]
- Garbes L, Riessland M, Hölker I, Heller R, Hauke J, Tränkle C, Coras R, Blümcke I, Hahnen E, Wirth B. LBH589 induces up to 10-fold SMN protein levels by several independent mechanisms and is effective even in cells from SMA patients non-responsive to valproate. Hum. Mol. Genet. 2009;18:3645–3658. doi: 10.1093/hmg/ddp313. [DOI] [PubMed] [Google Scholar]
- Gardian G, Browne SE, Choi DK, Klivenyi P, Gregorio J, Kubilus JK, Ryu H, Langley B, Ratan RR, Ferrante RJ, Beal MF. Neuroprotective effects of phenylbutyrate in the N171-82Q transgenic mouse model of Huntington's disease. J. Biol. Chem. 2005;280:556–563. doi: 10.1074/jbc.M410210200. [DOI] [PubMed] [Google Scholar]
- Gavrilina TO, McGovern VL, Workman E, Crawford TO, Gogliotti RG, DiDonato CJ, Monani UR, Morris GE, Burghes AHM. Neuronal SMN expression corrects spinal muscular atrophy in severe SMA mice while muscle specific SMN expression has no phenotypic effect. Hum. Mol. Genet. 2008;17:1063–1075. doi: 10.1093/hmg/ddm379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogliotti RG, Cardona H, Singh J, Bail S, Emery C, Kuntz N, Jorgensen M, Durens M, Xia B, Barlow C, Heier C, Plasterer HL, Jacques V, Kiledjian M, Jarecki J, Rusche J, DiDonato CJ. The DcpS inhibitor RG3039 improves survival, function and motor unit pathologies in two SMA mouse models. Hum. Mol. Genet. 2013;22:4084–4101. doi: 10.1093/hmg/ddt258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahnen E, Eyüpoglu IY, Brichta L, Haastert K, Tränkle C, Siebzehnrübl FA, Riessland M, Hölker I, Claus P, Romstöck J, Buslei R, Wirth B, Blümcke I. In vitro and ex vivo evaluation of second-generation histone deacetylase inhibitors for the treatment of spinal muscular atrophy. J. Neurochem. 2006;98:193–202. doi: 10.1111/j.1471-4159.2006.03868.x. [DOI] [PubMed] [Google Scholar]
- Harahap ISK, Saito T, San LP, Sasaki N, Gunadi, Nurputra DKP, Yusoff S, Yamamoto T, Morikawa S, Nishimura N, Lee MJ, Takeshima Y, Matsuo M, Nishio H. Valproic acid increases SMN2 expression and modulates SF2/ASF and hnRNPA1 expression in SMA fibroblast cell lines. Brain Dev. 2012;34:213–222. doi: 10.1016/j.braindev.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Harris AW, Butchbach MER. The effect of the DcpS inhibitor D156844 on the protective action of follistatin in mice with spinal muscular atrophy. Neuromuscul. Disord. 2015;25:699–705. doi: 10.1016/j.nmd.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heier CR, DiDonato CJ. Translational readthrough by the aminoglycoside geneticin (G418) modulates SMN stability in vitro and improves motor function of SMA mice in vivo. Hum. Mol. Genet. 2009;18:1310–1322. doi: 10.1093/hmg/ddp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heier CR, Satta R, Lutz C, DiDonato CJ. Arrhythmia and cardiac defects are a feature of spinal muscular atrophy model mice. Hum. Mol. Genet. 2010;19:3906–3918. doi: 10.1093/hmg/ddq330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh-Li HM, Chang JG, Jong YJ, Wu MH, Wang NM, Tsai CH, Li H. A mouse model for spinal muscular atrophy. Nat. Genet. 2000;24:66–70. doi: 10.1038/71709. [DOI] [PubMed] [Google Scholar]
- Hua Y, Sahashi K, Hung G, Rigo F, Passini MA, Bennett CF, Krainer AR. Antisense correction of SMN2 splicing in the CNS rescues necrosis in a type III SMA mouse model. Genes Dev. 2010;24:1634–1644. doi: 10.1101/gad.1941310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Y, Sahashi K, Rigo F, Hung G, Horev G, Bennett CF, Krainer AR. Peripheral SMN restoration is essential for long-term rescue of a severe spinal muscular atrophy mouse model. Nature. 2011;478:123–126. doi: 10.1038/nature10485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur EM, Zhou FQ. GSK3 signalling in neural development. Nat. Rev. Neurosci. 2010;11:539–551. doi: 10.1038/nrn2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer CC, McGovern VL, Murray JD, Gombash SE, Zaworski PG, Foust KD, Janssen PML, Burghes AHM. Low levels of survival motor neuron protein are sufficient for normal muscle function in the SMNΔ7 mouse model of SMA. Hum. Mol. Genet. 2015;24:6160–6173. doi: 10.1093/hmg/ddv332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernochan LE, Russo ML, Woodling NS, Huynh TN, Avila AM, Fischbeck KH, Sumner CJ. The role of histone acetylation in SMN gene expression. Hum. Mol. Genet. 2005;14:1171–1182. doi: 10.1093/hmg/ddi130. [DOI] [PubMed] [Google Scholar]
- Kuefer R, Hofer MD, Altug V, Zorn C, Genze F, Kunzi-Rapp K, Hautmann RE, Gschwend JE. Sodium butyrate and tributyrin induce in vivo growth inhibition and apoptosis in human prostate cancer. Br. J. Cancer. 2004;90:535–541. doi: 10.1038/sj.bjc.6601510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kye MJ, Niederst ED, Wertz MH, Gonçalves IdoCG, Akten B, Dover KZ, Peters M, Riessland M, Neveu P, Wirth B, Kosik KS, Sardi SP, Monani UR, Passini MA, Sahin M. SMN regulates axonal local translation via miR-183/mTOR pathway. Hum. Mol. Genet. 2015;23:6318–6331. doi: 10.1093/hmg/ddu350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TT, Pham LT, Butchbach MER, Zhang HL, Monani UR, Coovert DD, Gavrilina TO, Xing L, Bassell GJ, Burghes AHM. SMNΔ7, the major product of the centromeric survival motor neuron gene (SMN2), extends survival in mice with spinal muscular atrophy and associates with full-length SMN. Hum. Mol. Genet. 2005;14:845–857. doi: 10.1093/hmg/ddi078. [DOI] [PubMed] [Google Scholar]
- Lefebvre S, Bürglen L, Reboullet S, Clermont O, Burlet P, Viollet L, Benichou B, Cruaud C, Millasseau P, Zeviani M, Le Paslier D, Frézal J, Cohen D, Weissenbach J, Munnich A, Melki J. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- Lefebvre S, Burlet P, Liu Q, Bertrandy S, Clermont O, Munnich A, Dreyfuss G, Melki J. Correlation between severity and SMN protein level in spinal muscular atrophy. Nat. Genet. 1997;16:265–269. doi: 10.1038/ng0797-265. [DOI] [PubMed] [Google Scholar]
- Li DK, Tisdale S, Lotti F, Pellizzoni L. SMN control of RNP assembly: from posttranscriptional gene regulation to motor neuron disease. Semin. Cell Dev. Biol. 2014;32:22–29. doi: 10.1016/j.semcdb.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Yazdani A, Murray LM, Beauvais A, Kothary R. The Smn-independent beneficial effects of trichostatin A on an intermediate mouse model of spinal muscular atrophy. PLoS ONE. 2014;9:e101225. doi: 10.1371/journal.pone.0101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorson CL, Hahnen E, Androphy EJ, Wirth B. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc. Natl. Acad. Sci. U. S. A. 1999;96:6307–6311. doi: 10.1073/pnas.96.11.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunke S, El-Osta A. The emerging role of epigenetic modifications and chromatin remodeling in spinal muscular atrophy. J. Neurochem. 2009;109:1557–1569. doi: 10.1111/j.1471-4159.2009.06084.x. [DOI] [PubMed] [Google Scholar]
- Martinez TL, Kong L, Wang X, Osborne MA, Crowder ME, Van Meerbeke JP, Xu X, Davis C, Wooley J, Goldhamer DJ, Lutz CM, Rich MM, Sumner CJ. Survival motor neuron protein in motor neurons determines synaptic integrity in spinal muscular atrophy. J. Neurosci. 2012;32:8703–8715. doi: 10.1523/JNEUROSCI.0204-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattis VB, Chang CWT, Lorson CL. Analysis of a read-through promoting compound in a severe mouse model of spinal muscular atrophy. Neurosci. Lett. 2012;525:72–75. doi: 10.1016/j.neulet.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattis VB, Ebert AD, Fosso MY, Chang CW, Lorson CL. Delivery of a read-through inducing compound, TC007, lessens the severity of a SMA animal model. Hum. Mol. Genet. 2009a;18:3906–3913. doi: 10.1093/hmg/ddp333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattis VB, Fosso MY, Chang CW, Lorson CL. Sucutaneous administration of TC007 reduces disease severity in an animal model of SMA. BMC Neurosci. 2009b;10:142. doi: 10.1186/1471-2202-10-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAndrew PE, Parsons DW, Simard LR, Rochette C, Ray PN, Mendell JR, Prior TW, Burghes AHM. Identification of proximal spinal muscular atrophy carriers and patients by analysis of SMNT and SMNC gene copy number. Am. J. Hum. Genet. 1997;60:1411–1422. doi: 10.1086/515465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey PG, Wang YC, Newsome DA, Leon EJ, Li B, Kim E, Tung R, Chaturvedi P, Su MSS. Pharmacokinetics and efficacy studies of a new butyrate prodrug for induction of fetal hemoglobin in anemic rhesus monkeys. Blood. 1996;88:1232. [Google Scholar]
- McGovern VL, Iyer CC, Arnold WD, Gombash SE, Zaworski PG, Blatnik AJ, III, Foust KD, Burghes AHM. SMN expression is required in motor neurons to rescue electrophysiological deficits in the SMNΔ7 mouse model of SMA. Hum. Mol. Genet. 2015;24:5524–5541. doi: 10.1093/hmg/ddv283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentis GZ, Blivis D, Liu W, Drobac E, Crowder ME, Kong L, Alvarez FJ, Sumner CJ, O'Donovan MJ. Early functional impairment of sensory-motor connectivity in a mouse model of spinal muscular atrophy. Neuron. 2011;69:453–467. doi: 10.1016/j.neuron.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercuri E, Bertini E, Messina S, Pelliccioni M, D'Amico A, Colitto F, Mirabella M, Tiziano FD, Vitali T, Angelozzi C, Kinali M, Main M, Brahe C. Pilot trial of phenylbutyrate in spinal muscular atrophy. Neuromuscul. Disord. 2004;14:130–135. doi: 10.1016/j.nmd.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Mercuri E, Bertini E, Messina S, Solari A, D'Amico A, Angelozzi C, Battini R, Berardinelli A, Boffi P, Bruno C, Cini C, Colitto F, Kinali M, Minetti C, Mongini T, Morandi L, Neri G, Orcesi S, Pane M, Pelliccioni M, Pini A, Tiziano FD, Villanova M, Vita G, Brahe C. Randomized, double-blind, placebo-controlled trial of phenylbutyrate in spinal muscular atrophy. Neurology. 2007;68:51–55. doi: 10.1212/01.wnl.0000249142.82285.d6. [DOI] [PubMed] [Google Scholar]
- Michaud M, Arnoux T, Bielli S, Durand E, Rotrou Y, Jablonka S, Robert F, Giraudon-Paoli M, Riessland M, Mattei MG, Andriambeloson E, Wirth B, Sendtner M, Gallego J, Pruss RM, Bordet T. Neuromuscular defects and breathing disorders in a new mouse model of spinal muscular atrophy. Neurobiol. Dis. 2010;38:125–135. doi: 10.1016/j.nbd.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Mitrpant C, Porensky P, Zhou H, Price L, Muntoni F, Fletcher S, Wilton SD, Burghes AHM. Improved antisense oligonucleotide design to suppress aberrant SMN2 gene transcript processing: towards a treatment for spinal muscular atrophy. PLoS ONE. 2013;8:e62114. doi: 10.1371/journal.pone.0062114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa H, Yamamoto M. High-performance liquid chromatographic analysis of serum short-chain fatty acids by direct derivatization. J. Chromatog. 1987;421:33–41. doi: 10.1016/0378-4347(87)80376-6. [DOI] [PubMed] [Google Scholar]
- Monani UR, Lorson CL, Parsons DW, Prior TW, Androphy EJ, Burghes AHM, McPherson JD. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum. Mol. Genet. 1999;8:1177–1183. doi: 10.1093/hmg/8.7.1177. [DOI] [PubMed] [Google Scholar]
- Monani UR, Sendtner M, Coovert DD, Parsons DW, Andreassi C, Le TT, Jablonka S, Schrank B, Rossoll W, Prior TW, Morris GE, Burghes AHM. The human centromeric survival motor neuron gene (SMN2) rescues embryonic lethality in Smn−/− mice and results in a mouse with spinal muscular atrophy. Hum. Mol. Genet. 2000;9:333–339. doi: 10.1093/hmg/9.3.333. [DOI] [PubMed] [Google Scholar]
- Narver HL, Kong L, Burnett BG, Choe DK, Bosch-Marcé M, Taye AA, Eckhaus MA, Sumner CJ. Sustained improvement of spinal muscular atrophy mice treated with trichostatin A plus nutrition. Ann. Neurol. 2008;64:465–470. doi: 10.1002/ana.21449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naryshkin NA, Weetall M, Dakka A, Narasimhan J, Zhao X, Feng Z, Ling KKY, Karp GM, Qi H, Woll MG, Chen G, Zhang N, Gabbeta V, Vazirani P, Bhattacharyya A, Furia B, Risher N, Sheedy J, Kong R, Ma J, Turpoff A, Lee CS, Zhang X, Moon YC, Trifillis P, Welch EM, Colacino JM, Babiak J, Almstead NG, Peltz SW, Eng LA, Chen KS, Mull JL, Lynes MS, Rubin LL, Fontoura P, Santarelli L, Haenke D, McCarthy KD, Schmucki R, Ebeling M, Sivaramakrishnan M, Ko CP, Paushkin SV, Ratni H, Gerlach I, Ghosh A, Metzger F. SMN2 splicing modifiers improve motor function and longevity in mice with spinal muscular atrophy. Science. 2014;345:688–693. doi: 10.1126/science.1250127. [DOI] [PubMed] [Google Scholar]
- Nguyen thi Man, Humphrey E, Lam LT, Fuller JR, Lynch TA, Sewry CA, Goodwin PR, MacKenzie AE, Morris GE. A two-site ELISA can quantify upregulation of SMN protein by drugs for spinal muscular atrophy. Neurology. 2008;71:1757–1763. doi: 10.1212/01.wnl.0000313038.34337.b1. [DOI] [PubMed] [Google Scholar]
- Nicole S, Desforges B, Millet G, Lesbordes J, Cifuentes-Diaz C, Vertes D, Cao ML, De Backer F, Languille L, Roblot N, Joshi V, Gillis JM, Melki J. Intact satellite cells lead to remarkable protection against Smn gene defect in differentiation skeletal muscle. J. Cell Biol. 2003;161:571–582. doi: 10.1083/jcb.200210117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning K, Drepper C, Valori CF, Ahsan M, Wyles M, Higginbottom A, Herrmann T, Shaw P, Azzouz M, Sendtner M. PTEN depletion rescues axonal growth defect and improves survival in SMN-deficient motor neurons. Hum. Mol. Genet. 2010;19:3159–3168. doi: 10.1093/hmg/ddq226. [DOI] [PubMed] [Google Scholar]
- Osman EY, Miller MR, Robbins KL, Lombardi AM, Atkinson AK, Brehm AJ, Lorson CL. Morpholino antisense oligonucleotides targeting intronic repressor Element1 improve phenotype in SMA mouse models. Human Molecular Genetics. 2014;23:4832–4845. doi: 10.1093/hmg/ddu198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özcan U, Yilmaz E, Özcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacino J, Swalley SE, Song C, Cheung AK, Shu L, Zhang X, Van Hoosear M, Shin Y, Chin DN, Gubser Keller C, Beibel M, Renaud NA, Smith TM, Salcius M, Shi X, Hild M, Servais R, Jain M, Deng L, Bullock C, McLellan M, Schuierer S, Murphy L, Blommers MJJ, Blaustein C, Berenshteyn F, Lacoste A, Thomas JR, Roma G, Michaud GA, Tseng BS, Porter JA, Myer VE, Tallarico JA, Hamann LG, Curtis D, Fishman MC, Dietrich WF, Dales NA, Sivasankaran R. SMN2 splice modulators enhance U1-pre-mRNA association and rescue SMA mice. Nat. Chem. Biol. 2015;11:511–517. doi: 10.1038/nchembio.1837. [DOI] [PubMed] [Google Scholar]
- Passini MA, Bu J, Richards AM, Kinnecom C, Sardi SP, Stanek LM, Hua Y, Rigo F, Matson J, Hung G, Kaye EM, Shihabuddin LS, Krainer AR, Bennett CF, Cheng SH. Antisense oligonucleotides delivered to the mouse CNS ameliorate symptoms of severe spinal muscular atrophy. Sci. Transl. Med. 2011;3:72ra18. doi: 10.1126/scitranslmed.3001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passini MA, Bu J, Roskelley EM, Richards AM, Sardi SP, O'Riordan CR, Klinger KW, Shihabuddin LS, Cheng SH. CNS-targeted gene therapy improves survival and motor function in a mouse model of spinal muscular atrophy. J. Clin. Invest. 2010;120:1253–1264. doi: 10.1172/JCI41615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri S, Kiaei M, Kipiani K, Chen J, Calingasan NY, Crow JP, Beal MF. Additive neuroprotective effects of a histone deacetylase inhibitor and a catalytic antioxidant in a transgenic mouse model of amyotrophic lateral sclerosis. Neurobiol. Dis. 2006;22:40–49. doi: 10.1016/j.nbd.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Peviani M, Tortarolo M, Battaglia E, Piva R, Bendotti C. Specific induction of Akt3 in spinal cord motor neurons is neuroprotective in a mouse model of familial amyotrophic lateral sclerosis. Mol. Neurobiol. 2014;49:136–148. doi: 10.1007/s12035-013-8507-6. [DOI] [PubMed] [Google Scholar]
- Porensky PN, Mitrpant C, McGovern VL, Bevan AK, Foust KD, Kaspar BK, Wilton SD, Burghes AHM. A single administration of morpholino antisense oligomer rescues spinal muscular atrophy in mouse. Hum. Mol. Genet. 2012;21:1625–1638. doi: 10.1093/hmg/ddr600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior TW, Swoboda KJ, Scott HD, Hejmanowski AQ. Homozygous SMN1 deletions in unaffected family members and modification of the phenotype by SMN2. Am. J. Med. Genet. 2005;130A:307–310. doi: 10.1002/ajmg.a.30251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Hosoi T, Okuma Y, Kaneko M, Nomura Y. Sodium 4-phenylbutyrate protects against cerebral ischemic injury. Mol. Pharmacol. 2004;66:899–908. doi: 10.1124/mol.104.001339. [DOI] [PubMed] [Google Scholar]
- Qin L, Wang Z, Tao L, Wang Y. ER stress negatively regulates AKT/TSC/mTOR pathway to enhance autophagy. Autophagy. 2010;6:239–247. doi: 10.4161/auto.6.2.11062. [DOI] [PubMed] [Google Scholar]
- Rahmani M, Yu C, Reese E, Ahmed W, Hirsch K, Dent P, Grant S. Inhibition of PI-3 kinase sensitizes human leukemic cells to histone deacetylase inhibitor-mediated apoptosis through p44/42 MAP kinase inactivation and abrogation of p21CIP1/WAF1 induction rather than AKT inhibition. Oncogene. 2003;22:6231–6242. doi: 10.1038/sj.onc.1206646. [DOI] [PubMed] [Google Scholar]
- Renusch SR, Harshman S, Pi H, Workman E, Wehr A, Li X, Prior TW, Elsheikh B, Swoboda KJ, Simard LR, Kissel JT, Battle D, Parthun MR, Freitas MA, Kolb SJ. Spinal muscular atrophy biomarker measurements from blood samples in a clinical trial of valproic acid in ambulatory adults. J. Neuromuscul. Dis. 2015;2:119–130. doi: 10.3233/JND-150081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribobaraza A, Cuadrado-Tejedor M, Pérez-Mediavilla A, Frechilla D, Del Río J, García-Osta A. Phenylbutyrate ameliorates cognitive deficit and reduces tau pathology in an Alzheimer's disease mouse model. Neuropsychopharmacology. 2009;34:1721–1732. doi: 10.1038/npp.2008.229. [DOI] [PubMed] [Google Scholar]
- Riessland M, Ackermann B, Förster A, Jakubik M, Hauke J, Garbes L, Fritzsche I, Mende Y, Blumcke M, Hahnen E, Wirth B. SAHA ameliorates the SMA phenotype in two mouse models for spinal muscular atrophy. Hum. Mol. Genet. 2010;19:1492–1506. doi: 10.1093/hmg/ddq023. [DOI] [PubMed] [Google Scholar]
- Riessland M, Brichta L, Hahnen E, Wirth B. The benzamide M344, a novel histone deacetylase inhibitor, significantly increases SMN2 RNA/protein levels in spinal muscular atrophy cells. Hum. Genet. 2006;120:101–110. doi: 10.1007/s00439-006-0186-1. [DOI] [PubMed] [Google Scholar]
- Rodgers EE, Theibert AB. Functions of PI 3-kinase in development of the nervous system. Int. J. Dev. Neurosci. 2002;20:187–197. doi: 10.1016/s0736-5748(02)00047-3. [DOI] [PubMed] [Google Scholar]
- Rose FF, Jr, Mattis VB, Rindt H, Lorson CL. Delivery of recombinant follistatin lessens disease severity in a mouse model of spinal muscular atrophy. Hum. Mol. Genet. 2009;18:997–1005. doi: 10.1093/hmg/ddn426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu H, Smith K, Camelo SI, Carreras I, Lee J, Iglesias AH, Dangood F, Cormier KA, Cudkowicz ME, Brown RH, Jr, Ferrante RJ. Sodium phenylbutyrate prolongs survival and regulates expression of anti-apoptotic genes in transgenic amyotrophic lateral sclerosis mice. J. Neurochem. 2005;93:1087–1098. doi: 10.1111/j.1471-4159.2005.03077.x. [DOI] [PubMed] [Google Scholar]
- Sahashi K, Ling KKY, Hua Y, Wilkinson JE, Nomakuchi T, Rigo F, Hung G, Xu D, Jiang YP, Lin RZ, Ko CP, Bennett CF, Krainer AR. Pathological impact of SMN2 mis-splicing in adult SMA mice. EMBO Mol. Med. 2013;5:1586–1601. doi: 10.1002/emmm.201302567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrank B, Götz R, Gunnersen JM, Ure JM, Toyka KV, Smith AG, Sendtner M. Inactivation of the survival motor neuron gene, a candidate gene for human spinal muscular atrophy, leads to massive cell death in early mouse embryos. Proc. Natl. Acad. Sci. U. S. A. 1997;94:9920–9925. doi: 10.1073/pnas.94.18.9920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shababi M, Habibi J, Ma L, Glascock JJ, Sowers JR, Lorson CL. Partial restoration of cardio-vascular defects in a rescued severe model of spinal muscular atrophy. J. Mol. Cell. Cardiol. 2012;52:1074–1082. doi: 10.1016/j.yjmcc.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shababi M, Habibi J, Yang HT, Vale SM, Sewell WA, Lorson CL. Cardiac defects contribute to the pathology of spinal muscular atrophy models. Hum. Mol. Genet. 2010;19:4059–4071. doi: 10.1093/hmg/ddq329. [DOI] [PubMed] [Google Scholar]
- Simard LR, Bélanger MC, Morissette S, Wride M, Prior TW, Swoboda KJ. Preclinical validation of a multiplex real-time assay to quantify SMN mRNA in patients with SMA. Neurology. 2007;68:451–456. doi: 10.1212/01.wnl.0000252934.70676.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabley DL, Harris AW, Holbrook J, Chubbs NJ, Lozo KW, Crawford TO, Swoboda KJ, Funanage VL, Wang W, Mackenzie W, Scavina M, Sol-Church K, Butchbach MER. SMN1 and SMN2 copy numbers in cell lines derived from patients with spinal muscular atrophy as measured by array digital PCR. Mol. Genet. Genomic Med. 2015;3:248–257. doi: 10.1002/mgg3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staropoli JF, Li H, Chun SJ, Allaire N, Cullen P, Thai A, Fleet CM, Hua Y, Bennett CF, Krainer AR, Kerr D, McCampbell A, Rigo F, Carulli JP. Rescue of gene-expression changes in an induced mouse model of spinal muscular atrophy by an antisense oligonucleotide that promotes inclusion of SMN2 exon 7. Genomics. 2015;105:220–228. doi: 10.1016/j.ygeno.2015.01.007. [DOI] [PubMed] [Google Scholar]
- Steffan JS, Bodai L, Pallos J, Poelman M, McCampbell A, Apostol BL, Kazantsev A, Schmidt E, Zhu YZ, Greenwald M, Kurokawa R, Housman DE, Jackson GR, Marsh JL, Thompson LM. Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature. 2001;413:739–743. doi: 10.1038/35099568. [DOI] [PubMed] [Google Scholar]
- Sumner CJ, Huynh TN, Markowitz JA, Perhac JS, Hill B, Coovert DD, Schussler K, Chen X, Jarecki J, Burghes AHM, Taylor JP, Fischbeck KH. Valproic acid increases SMN levels in spinal muscular atrophy patient cells. Ann. Neurol. 2003;54:647–654. doi: 10.1002/ana.10743. [DOI] [PubMed] [Google Scholar]
- Swoboda KJ, Prior TW, Scott CB, McNaught TP, Wride MC, Reyna SP, Bromberg MB. Natural history of denervation in SMA: relation to age, SMN2 copy number and function. Ann. Neurol. 2005;57:704–712. doi: 10.1002/ana.20473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swoboda KJ, Scott CB, Reyna SP, Prior TW, LaSalle B, Sorenson SL, Wood J, Acsadi G, Crawford TO, Kissel JT, Krosschell KJ, D'Anjou G, Bromberg MB, Schroth MK, Chan GM, Elsheikh B, Simard LR. Phase II open label study of valproic acid in spinal muscular atrophy. PLoS ONE. 2009;4:e5268. doi: 10.1371/journal.pone.0005268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurmond J, Butchbach MER, Palomo M, Pease B, Rao M, Bedell L, Keyvan M, Pai G, Mishra R, Haraldsson M, Andresson T, Bragason G, Thosteinsdottir M, Bjornsson JM, Coovert DD, Burghes AHM, Gurney ME, Singh J. Synthesis and biological evaluation of novel 2,4-diaminoquinazoline derivatives as SMN2 promoter activators for the potential treatment of spinal muscular atrophy. J. Med. Chem. 2008;51:449–469. doi: 10.1021/jm061475p. [DOI] [PubMed] [Google Scholar]
- Tisdale S, Pellizzoni L. Disease mechanisms and therapeutic approaches in spinal muscular atrophy. J. Neurosci. 2015;35:8691–8700. doi: 10.1523/JNEUROSCI.0417-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiziano FD, Bertini E, Messina S, Angelozzi C, Pane M, D'Amico A, Alfieri P, Fiori S, Battini R, Berardinelli A, Boffi P, Bruno C, Cini C, Minetti C, Mongini T, Morandi L, Orcesi S, Pelliccioni M, Pini A, Villanova M, Vita G, Locatelli M, Mercuri E, Brahe C. The Hammersmith functional score correlates with the SMN2 copy number: a multicentric study. Neuromuscul. Disord. 2007;17:400–403. doi: 10.1016/j.nmd.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Tsai LK, Tsai MS, Ting CH, Li H. Multiple therapeutic effects of valproic acid in spinal muscular atrophy model mice. J. Mol. Med. 2008;86:1243–1254. doi: 10.1007/s00109-008-0388-1. [DOI] [PubMed] [Google Scholar]
- Valori CF, Ning K, Wyles M, Mead RJ, Grierson AJ, Shaw PJ, Azzouz M. Systemic delivery of scAAV9 expressin SMN prolongs survival in a model of spinal muscular atrophy. Sci. Transl. Med. 2010;2:35ra42. doi: 10.1126/scitranslmed.3000830. [DOI] [PubMed] [Google Scholar]
- Van Meerbeke JP, Gibbs RM, Plasterer HL, Miao W, Feng Z, Lin MY, Rucki AA, Wee CD, Xia B, Sharma S, Jacques V, Li DK, Pellizzoni L, Rusche JR, Ko CP, Sumner CJ. The DcpS inhibitor RG3039 improves motor function in SMA mice. Hum. Mol. Genet. 2013;22:4074–4083. doi: 10.1093/hmg/ddt257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. research0034.1-research0034.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viollet L, Bertrandy S, Beuno Brunialti AL, Lefebvre S, Burlet P, Clermont O, Cruaud C, Guénet JL, Munnich A, Melki J. cDNA isolation, expression and chromosomal localization of the mouse survival motor neuron gene (Smn) Genomics. 1997;40:185–188. doi: 10.1006/geno.1996.4551. [DOI] [PubMed] [Google Scholar]
- Vitte JM, Davoult B, Roblot N, Mayer M, Joshi V, Courageot S, Tronche F, Vadrot J, Moreau MH, Kemeny F, Melki J. Deletion of murine Smn exon 7 directed to liver leads to severe defect of liver development associated with iron overload. Am. J. Pathol. 2004;165:1731–1741. doi: 10.1016/S0002-9440(10)63428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihl CC, Connolly AM, Pestronk A. Valproate may improve strength and function in patients with type III/IV spinal muscular atrophy. Neurology. 2006;67:500–501. doi: 10.1212/01.wnl.0000231139.26253.d0. [DOI] [PubMed] [Google Scholar]
- Williams JH, Schray RC, Patterson CA, Ayitey SO, Tallent MK, Lutz GJ. Oligonucleotide-mediated survival of motor neuron protein expression in CNS improves phenotype in a mouse model of spinal muscular atrophy. J. Neurosci. 2009;29:7633–7638. doi: 10.1523/JNEUROSCI.0950-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth B, Brichta L, Schrank B, Lochmüller H, Blick S, Baasner A, Heller R. Mildly affected patients with spinal muscular atrophy are partially protected by an increased SMN2 copy number. Hum. Genet. 2006;119:422–428. doi: 10.1007/s00439-006-0156-7. [DOI] [PubMed] [Google Scholar]
- Woodgett JR. Molecular cloning and expression glycogen synthase kinase-3/factor A. EMBO J. 1990;9:2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying M, Xu R, Wu X, Zhu H, Zhuang Y, Han M, Xu T. Sodium butyrate ameliorates histone hypoacetylation and neurodegenerative phenotypes in a mouse model for DRPLA. J. Biol. Chem. 2006;281:12580–12586. doi: 10.1074/jbc.M511677200. [DOI] [PubMed] [Google Scholar]
- Young PJ, Le TT, Nguyen thi Man, Burghes AHM, Morris GE. The relationship between SMN, the spinal muscular atrophy protein, and nuclear coiled bodies in differentiated tissues and cultured cells. Exp. Cell Res. 2000;256:365–374. doi: 10.1006/excr.2000.4858. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Lotti F, Dittmar K, Younis I, Wan L, Kasim M, Dreyfuss G. SMN deficiency causes tissue-specific perturbations in the repertoire of snRNAs and widespread defects in splicing. Cell. 2008;133:585–600. doi: 10.1016/j.cell.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Janghra N, Mitrpant C, Dickinson RL, Anthony K, Price L, Eperon IC, Wilton SD, Morgan J, Muntoni F. A novel morpholino oligomer targeting ISS-N1 improves rescue of severe spinal muscular atrophy transgenic mice. Hum. Gene Ther. 2013;24:331–342. doi: 10.1089/hum.2012.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure. Representative high-performance liquid chromatograms for BA.
Supplementary Movie 1. Phenotype of SMNΔ7 SMA mice treated with 4PBA at PND14.
Supplementary Movie 2. Phenotype of SMNΔ7 SMA mice treated with VX563 at PND14.