Abstract
Microglial activation and release of inflammatory cytokines and chemokines are crucial events in neuroinflammation. Microglial cells interact and respond to other inflammatory cells such as T cells and mast cells as well as inflammatory mediators secreted from these cells. Recent studies have shown that neuroinflammation causes and accelerates neurodegenerative disease such as Parkinson’s disease (PD) pathogenesis. 1-methyl-4-phenyl-pyridinium ion (MPP+), the active metabolite of neurotoxin 1- methyl -4- phenyl -1,2,3,6-tetrahydro pyridine (MPTP) activates glial cells and mediate neurodegeneration through release of inflammatory mediators. We have shown that glia maturation factor (GMF) activates glia and induces neuroinflammation and neurodegeneration and that MPP+ activates mast cells and release proinflammatory cytokines and chemokines. The chemokine (C-C motif) ligand 2 (CCL2) levels have been shown to be elevated and play a role in PD pathogenesis. In the present study, we analyzed if MPP+ activates mouse and human mast cells to release chemokine CCL2. Mouse bone marrow-derived mast cells (BMMCs) and human umbilical cord blood-derived cultured mast cells (hCBMCs) were incubated with MPP+ (10 μM) for 24 h and CCL2 levels were measured in the supernatant media by ELISA. MPP+-significantly-induced CCL2 release from BMMCs and hCBMCs. Additionally, GMF overexpression in BMMCs obtained from wild-type mice released significantly more CCL2, while BMMCs obtained from GMF-deficient mice showed less CCL2 release. Further, we show that MPP+-induced CCL2 release was greater in BMMCs-astrocyte co-culture conditions. Uncoupling protein 4 (UCP4) which is implicated in neurodegenerative diseases including PD was detected in BMMCs by immunocytochemistry. Our results suggest that mast cells may play role in PD pathogenesis.
Keywords: CCL2, Glia maturation factor, Mast cells, 1-methyl-4-phenyl-pyridinium, Parkinson’s disease
Introduction
Neuroinflammatory response protects the central nervous system (CNS) against injury or disease. In acute neuroinflammation, microglia become reactive and protect the tissue by phagocytosing dying cells/pathogen and also release inflammatory cytokines and chemokines [1]. However, if the duration of neuroinflammation is prolonged, activated microglia cause deleterious side-effects through various mechanisms such as inflammatory signaling pathways, increased oxidative stress, attraction and activation of inflammatory T cells and mast cells, induce neuronal dysfunction, neurodegeneration and neuronal death. Recent reports suggest that neuroinflammation causes and accelerates neurodegenerative disease mechanisms [2,3]. Neurodegeneration leads to progressive neuronal dysfunction, neuronal loss, and cognitive and motor dysfunctions. Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized by the presence of motor disturbances, Lewy bodies and dopaminergic neuronal death [4]. Neuroinflammatory responses and neuroinflammation exacerbates PD pathogenesis [5]. Dopaminergic neurotoxin 1- methyl -4- phenyl -1,2,3,6-tetrahydro pyridine (MPTP) administration induces PD in animals. 1-methyl-4-phenyl-pyridinium ion (MPP+), metabolite of MPTP has been shown to induce oxidative stress, mitochondrial damage, glial activation, inflammatory cytokine/chemokine release and dopaminergic neuronal damage [6–8].
Glia maturation factor (GMF), a brain protein previously isolated, sequenced and cloned in our laboratory [9–12] has reported to activate glial cells and induce neuroinflammation and neurodegeneration in vivo and in vitro [13–15]. Overexpression of GMF increased Experimental Autoimmune Encephalomyelitis (EAE) disease severity and induced significant releasee of proinflammatory cytokines and chemokines from astrocytes [16,17]. Mast cells play an important role in neuroinflammation and are co-localized with glial cells in neuroinflammation [1,18–21]. We have recently reported that MPP+ activates mast cells to release interleukin-1β (IL-1β), tumor necrosis factor-alpha (TNF-α) and IL-8 [22]. In the present study we examined if MPP+ activate mast cels to release CCL2. The suppression of chemokine (C-C motif) ligand 2 (CCL2) reduces attraction of immune cells to the sites of inflammation and thereby slow-down the progression of inflammation and tissue damage in neurodegeneration. Uncoupling proteins (UCPs) are endogenous neuroprotective agents and the induction of UCPs in the specific brain region has been suggested to protect neurons from oxidative stress-mediated tissue damage [23]. We have previously reported the expression of UCP2 in mast cells and that UCP2 can regulate mast cell activation [24]. UCPs are implicated in the neurodegenerative diseases including MPP+ toxicity [25,26]. Therefore, in the present study, we examined if MPP+ activate mast cells to release CCL2 and whether co-culture of mast cells and astrocytes activate each other. Additionally, we also investigated if BMMCs express UCP4.
Materials and Methods
Materials
Dulbecco’s phosphate buffered saline (DPBS), Dulbecco’s Modified Eagle Medium Nutrient Mixture F-12 (Ham) (DMEM F12), Iscove’s Modified Dulbecco’s Medium (IMDM), 2-Mercaptoethanol, GlutaMAX-1, Insulin-Transferrin-Selenium, penicillin streptomycin and fetal bovine serum (FBS) were purchased from Life Technologies (Grand Island, NY). Murine recombinant interleukin-3 (IL-3), human IL-6 and human stem cell factor (SCF) were purchased from PeproTech (Rocky Hill, NJ). Ficoll-Paque sterile solution was from GE Healthcare Bio Sciences AB (Uppsala, Sweden). AC133+ cell isolation kit was from Milltenyi Biotec (Auburn, CA). Cell culture flasks and tissue culture plates were purchased from Costar (Corning Incorporated, and Corning, NY). Enzyme-linked immunosorbent assay (ELISA) kits for mouse and human CCL2, was from R&D Systems (Minneapolis, MN). Anti-uncoupling protein-2 polyclonal antibody (Calbiochem Millipore, San Diego, CA) and Rabbit polyclonal UCP4 antibody were purchased from Novus Biologicals (Littleton, CO). ImmPRESS reagent anti-rabbit Ig peroxidase kits and ImmPACT DAB peroxidase kits were purchased from Vector Laboratories (Burlingame, CA). C57BL/6 wild-type mice were purchased from Charles River (Wilmington, MA). We have previously generated GMF-knockout (GMF-KO) mice in our laboratory and maintain a colony of these transgenic mice for our studies [27]. Adenovirus constructs were prepared at Gene Transfer Vector Core, University of Iowa as reported earlier using a replication-defective human adenovirus (serotype 5) vector. The constructs included either a full-length rat GMF cDNA (Ad5CMV GMF) or a cytoplasmic lacZ cDNA (Ad5CMVcytolacZ). A control adenovirus containing the CMV promoter but not expressing any other protein was also used. Cytospin 4 was purchased from Thermo Scientific (Runcorn, Cheshire, U.K). MPP+ and toluidine blue were purchased from Sigma (St. Louis, MO).
Mouse Primary Mast Cell Culture
Primary mouse bone marrow-derived mast cells (BMMCs) were grown from bone marrow cells obtained from adult wild type mice and GMF-KO mice as we and others reported previously [22,24,28,29]. Briefly, bone marrow cells were removed and cultured in DMEM supplemented with IL-3 (10 ng/ml), 10% heat-inactivated FBS, 1% Penicillin-Streptomycin, 20 μM 2-mercaptoethanol, 1% L-glutamine for 4–6 weeks in a 5% CO2 incubator at 37°C. Non-adherent cells were removed twice each week with the addition of new culture medium. About 97% of the cells were mast cells after 5 weeks of culture as identified by 0.1% toluidine blue staining [24]. Bone marrows from several mice were pooled and cultured to get more mast cells. This study was conducted according to the recommendations in the guide for the care and use of laboratory animals of the National Institutes of Health (NIH). This protocol was also approved by the Committee on the Ethics of Animal Experiments of the University of Iowa (Iowa City, IA).
Overexpression of GMF and Stimulation of Mouse Mast cells with MPP+
Transient transfection to overexpress GMF in cultured mast cells was carried out as reported earlier [17]. Brie y, adenovirus constructs were prepared using a replication –defective human adenovirus (serotype 5) vector at the University of Iowa Gene Transfer Vector Core. The constructs contained either a full-length rat GMF cDNA (Ad5CMV GMF) or a cytoplasmic lacZ cDNA (Ad5CMV cytolacZ). Viral dose of 20 multiplicity of infectivity (MOI) was added to mast cells in serum-free and antibiotic-free DMEM/F12 medium for 4h. After this infection period, cells were washed once with DMEM/F12 and plated again in tissue culture plates. MPP+ (10 μM) was added and incubated for an additional 24 h at 37°C. In mock-transfected controls, the above procedure was carried out in the absence of virus. GMF was overexpressed as mentioned above in BMMCs obtained from wild type mice and GMF-KO mice. These mast cells were plated in separate 24 well culture plates at 0.5 to 1×106 cells/ml in mouse mast cell culture medium and left overnight at 37°C. The cells were then incubated with (MPP+) for 24 h. After the incubation period, the culture supernatant media was collected by centrifugation and stored at −80°C. CCL2 was assayed in these media using commercial enzyme-linked immunosorbent assay (ELISA) kit.
Mouse Primary Astrocyte Culture
Pregnant C57BL/6 mice (wild-type and GMF-KO) were sacrificed on day 16 to obtain embryos. Astrocytes were isolated and cultured as we have previously reported [30–32]. Astrocytes were grown in DMEM F12 medium supplemented with 10% FBS and 1% penicillin/streptomycin at 37°C in a 5% CO2 and 95% air atmosphere in tissue culture flasks.
Mouse Mast Cells and Mouse Astrocytes Co-Culture
BMMCs and primary mouse astrocytes from wild-type mice were co-cultured as we reported previously [22]. These cells were plated to evaluate the effect of MPP+ (10 μM) stimulation individually on either mast cells or astrocytes or in a co-culture system consisting of both mast cells as well as astrocytes in the same wells. Cells were incubated with MPP+ for 24 h and the supernatant media were collected by centrifugation. CCL2 level was measured in the supernatant media by colorimetric ELISA which was read using a micro plate reader (Molecular Devices, Sunnyvale, CA).
Human Primary Mast Cell Culture and Stimulation with MPP+
Human umbilical cord blood-derived mast cells (hCBMCs) were cultured as we have reported previously [22,33,34]. Briefly, human umbilical cord blood (20 ml or more) was collected in anti-coagulant citrate phosphate dextrose solution at the Department of Obstetrics and Gynecology (University of Iowa Hospitals and Clinics, Iowa City, IA) as approved by the Institutional Review Board of the University of Iowa (IRB#200910784) [35]. Hematopoietic stem cells (CD34+) were isolated by magnetic-associated cell sorting (MACS) procedure using an AC133+ cell isolation kit. CD34+ cells were cultured in IMDM supplemented with 100–200 ng/ml SCF, 50 ng/ml IL-6, 10% FBS, 2-mercaptoethanol and 1% penicillin-streptomycin for 12 to 14 weeks in tissue culture flasks at 37°C in a 5% CO2 incubator. Human mast cells cultured over 12 weeks with >99% purity as determined by tryptase staining were used for the experiments. These hCBMCs were incubated with MPP+ (10 μM) for 24 h at 37°C. Then the supernatants were collected and assayed CCL2 levels by ELISA.
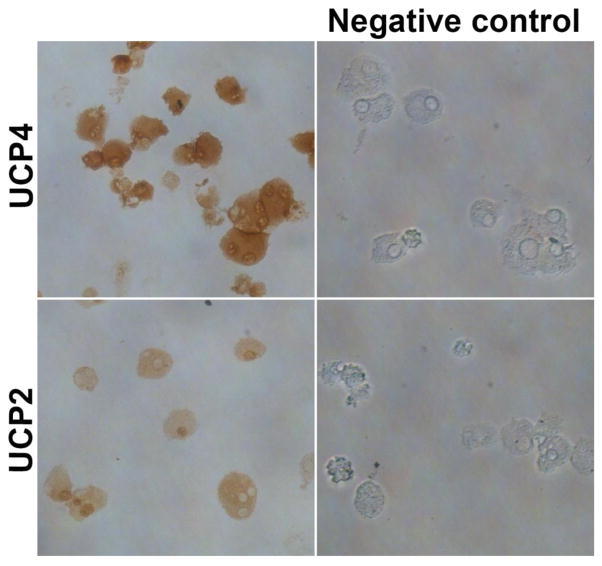
Immunocytochemistry (ICC) for UCP4 and UCP2 in Mouse Primary Mast Cells
Cytospin smears of BMMCs obtained from wild-type mice were prepared using a Cytospin 4 cytocentrifuge. These smears were immunostained for the expression of UCP4 and UCP2 using rabbit polyclonal UCP4 antibody and rabbit anti-uncoupling protein-2 polyclonal antibody, respectively. ICC was carried out using ImmPRESS reagent anti-rabbit Ig peroxidase kit and ImmPACT DAB peroxidase substrate kit as reported previously [22]. Negative staining controls were performed without primary antibodies to exclude any non-specific staining reactions. DAB peroxidase substrate produces brown color with positive reactions indicating the presence of UCPs.
Statistical Analysis
Results were analyzed by GraphPad InStat 3 software. Data were presented as mean ± SEM and analyzed using One-way Analysis of Variance (ANOVA) followed by Tukey-Kramer multiple comparison tests to determine statistically significant differences between the groups. Unpaired t-test was used when comparing only two groups. A p-value of <0.05 was considered statistically significant.
Results
MPP+ Activates Mouse Primary Mast Cells and Release CCL2
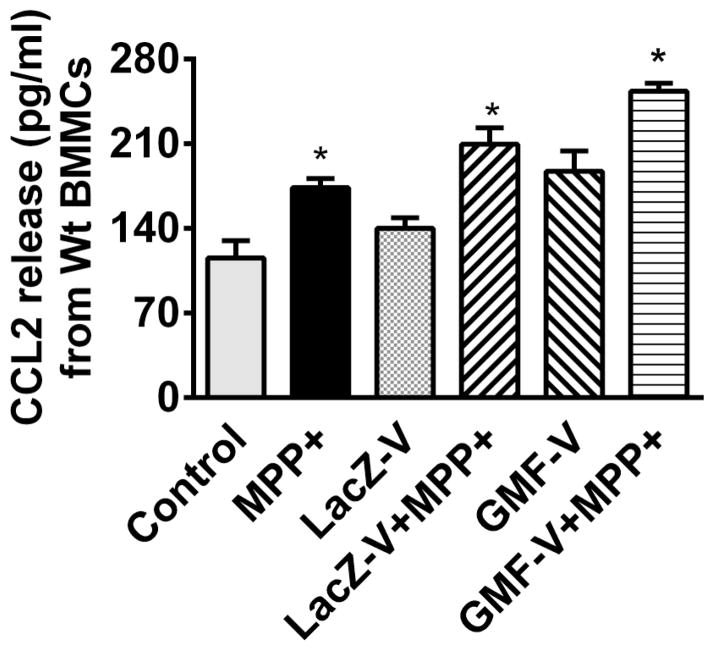
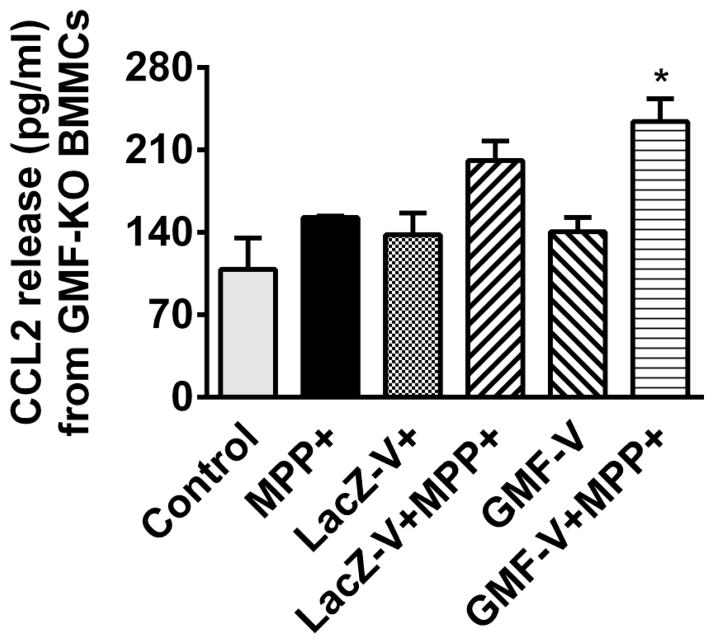
BMMCs obtained from wild type mice were incubated with or without MPP+ (10 μM) for 24 h at 37°C. The supernatant media were collected and CCL2 release was measured by ELISA (n=6). MPP+ significantly (P=<0.05) increased CCL2 release from BMMCs obtained from wild type mice (Fig. 1). Next, BMMCs obtained from wild-type mice were infected with Ad5CMV GMF to overexpress GMF or Ad5CMV cytolacZ as control. MPP+ also increased CCL2 release from LacZ V and GMF-V infected BMMCs when compared with their respective MPP+ untreated conditions (Fig. 1). BMMCs released more CCL2 after GMF overexpression with GMF-V as compared to LacZ-V treated cells (Fig. 1). Further, we also infected BMMCs obtained from GMF-KO mice with Ad5CMV GMF to overexpress GMF or Ad5CMV cytoLacZ as control. These cells were then incubated with or without MPP+ (10 μM) for an additional 24 h at 37°C. Then the supernatant media were collected and measured for CCL2 release by ELISA (n=4). MPP+ increased the release of CCL2 from control BMMCs and LacZ-V BMMCs but the increase were not significant (Fig. 2). However, MPP+ significantly (p<0.05) increased CCL2 release from GMF-V infected BMMCs when compared with GMF-V control without MPP+ treatment (Fig. 2).
Fig. 1.
Effect of MPP+ on CCL2 release from mouse primary mast cells obtained from wild-type mice. BMMCs obtained from wild-type mice were infected with Ad5CMV GMF to overexpress GMF or Ad5CMV cytoLacZ as control. These cells were then incubated with or without MPP+ (10 μM) for 24 h at 37°C. CCL2 levels were measured in the supernatant media were by ELISA (n=6). MPP+ significantly increased the release of CCL2 from BMMCs. MPP+ also increased CCL2 release from LacZ V and GMF-V infected BMMCs when compared with their respective MPP + untreated conditions. BMMCs released more CCL2 after GMF overexpression with GMF -V when compared to LacZ-V control. *=p<0.05, compared to respective unstimulated control cells, ANOVA and Tukey-Kramer.
Fig. 2.
Effect of MPP+ on CCL2 release from mouse primary mast cells obtained from GMF-KO mice. We infected primary BMMCs obtained from GMF-KO mice with Ad5CMV GMF to overexpress GMF or Ad5CMV cytolacZ as control. These cells were incubated with or without MPP+ (10 μM) for 24 h at 37°C. Then the supernatant media were collected and measured CCL2 release by ELISA (n=4). MPP+ significantly increased CCL2 release from GMF-V infected BMMCs when compared with GMF-V control. *=p<0.05, compared to respective unstimulated cells, ANOVA and Tukey-Kramer.
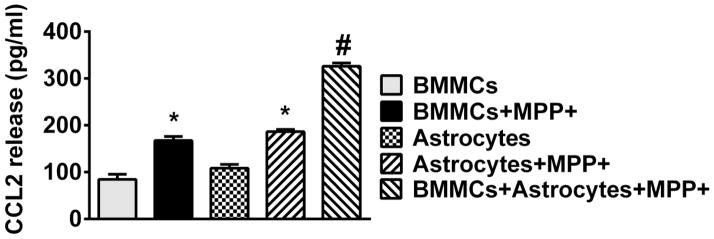
Mouse Primary Mast Cells and Mouse Primary Astrocytes Co-Culture Enhances CCL2 Release with MPP+ Stimulation
BMMCs and astrocytes were obtained from wild-type mice. The effect of MPP+ (10 μM) stimulation for 24 h on either BMMCs or astrocytes or in an astrocyte BMMCs co-culture system was determined by analyzing CCL2 release in the culture supernatant media (n=4). MPP+-induced significantly more (p<0.05) CCL2 release from BMMCs and astrocytes as compared with control cells treated with only buffer. Further, results showed more CCL2 release from astrocyte and mast cell co-culture conditions than released either from astrocytes culture alone or BMMCs culture alone after incubation with MPP+ (Fig. 3).
Fig. 3.
Effect of MPP+ on Mouse primary mast cells and mouse astrocyte co-culture. BMMCs and mouse astrocytes were co-cultured in tissue culture plates to evaluate the effect of MPP+ (10 μM) stimulation individually on either BMMCs or astrocytes or in a co-culture system consisting of both BMMCs and astrocytes in the same wells (n=4). Cells were incubated with MPP+ for 24 h and CCL2 levels were measured in the supernatant media by ELISA. More CCL2 release was observed from astrocyte and BMMCs co-culture system than released either from astrocytes culture alone or BMMCs culture alone after incubation with MPP+. #=p<0.05, compared to astrocytes+MPP+ or BMMCs+MPP+. *=p<0.05, compared to respective unstimulated control cells, ANOVA and Tukey-Kramer.
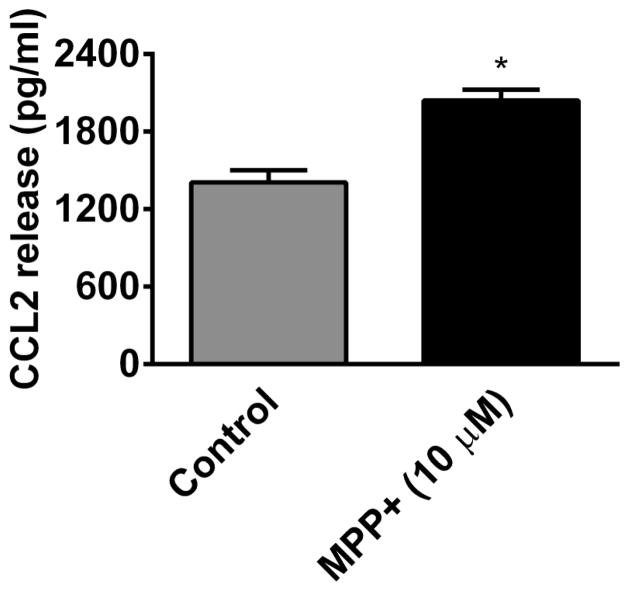
MPP+ Activates Human Mast Cells and Releases CCL2
In another set of experiments, we incubated hCBMCs with MPP+ (10 μM) for 24 h and CCL2 release was measured in the culture supernatant by ELISA (n=4). MPP+ significantly (p<0.05) increased CCL2 release from hCBMCs as compared to the release from control mast cells treated only with buffer (Fig. 4).
Fig. 4.
MPP+ activates human mast cells and release CCL2. hCBMCs were incubated with MPP+ (10 μM) for 24 h and the release of CCL2 was measured in the culture supernatants by ELISA (n=4). MPP+ significantly increased (p<0.05) CCL2 release from hCBMCs as compared to the release from control hCBMCs treated only with buffer. *=P<0.05, control Vs MPP+, unpaired ‘t’ test.
Mouse Primary Mast Cells Express UCP4 as Determined by ICC
We have analyzed the expression of UCP4 and UCP2 in the cytospin smears of BMMCs by ICC (n=3). We demonstrate that BMMCs were positive (brown color) for both UCP4 (upper panel) as well as UCP2. Negative staining controls performed without primary antibodies did not stain positive for UCPs (Fig. 5). We stained UCP2 to compare with the UCP4 staining.
Fig. 5.
UCP4 expression in mouse primary mast cells. Cytospin smears of BMMCs were prepared and immunostained for the expression of UCP4 and UCP2 (n=3). We show the expression of UCP4 (upper panel) by BMMCs in addition to UCP2 (lower panel). Positive immunoreactivity was detected by brown colour. Negative control staining without primary antibodies did not show positive reaction for UCPs. Original magnification 400x.
Discussion
In the present study, we demonstrate that MPP+-induced mouse primary mast cells and human mast cells to release CCL2. Incubation of mouse mast cells obtained from wild-type mice released significantly more CCL2 as compared to mouse mast cells obtained from GMF-KO mice. Further, we show that overexpression of GMF increases CCL2 release from BMMCs. Additionally, MPP+ released more CCL2 in BMMC plus astrocyte co-culture conditions. We also report the expression of UCP4 in mouse mast cells. Recently we have shown that MPP+-induced IL-1β, TNF-α, IL-8, CCL5 and β-hexosaminidase from BMMCs/hCBMCs and suggested that mast cells could play role in PD pathogenesis [22]. Mast cells participate in the regulation of innate and adaptive immune responses [36]. They are distributed in all the tissues including the brain [37,38] and are implicated in neuroinflammation [1]. Most of the mast cells are generally localized on the brain side of the blood vessel and communicate with microglia, astrocytes and neurons [39]. Mast cells are co-localized adjacent to glial cells in the brain indicating the presence of interactions between these cells. Mast cells contribute to both normal cognition, emotionality, sleep and fundamental neurobehavioral functions, but also promote deleterious effects in the brain [40]. The number as well as activation/degranulation status of mast cells in the brain vary with species, age, external environment and the techniques to detect [39]. In the normal human brain without stress, disease or trauma mast cells are less in number. However, even smaller number of activated mast cells can impact vascular, microvascular and perivascular structures as well as neurons, astrocytes and microglial activation [39]. Normally mast cells can move through blood-brain-barrier (BBB) and also traverse the blood-spinal cord and BBB in disease conditions [41]. Recent reports have suggested that mast cells induce neuroinflammation by releasing proinflammatory cytokines, chemokines and other inflammatory mediators including TNF-α, IL-1β, IL-6, CCL2, histamine, reactive oxygen species (ROS), reactive nitrogen species (RNS) and nitric oxide (NO) [32,38,42–44]. CCL2 is expressed in astrocytes, microglia and neurons and is an important chemokine implicated in the pathogenesis of neurodegenerative diseases including PD [45]. Although the levels of cytokines/chemokines are less in the normal brain, aberrant expression occurs in neuroinflammatory conditions. CCL2 recruits leukocytes at the site of inflammation in the brain and also cause opening of BBB [46,47]. CCL2 released from mast cells in response MPP+ could increase the infiltration of other inflammatory cells into the brain in PD pathogenesis. CCL2 released from mast cells could also contribute to the elevated levels of CCL2 observed in PD and in MPTP-induced animal model of PD [45,48]. Our present report that MPP+ activates mast cells and induces the release of CCL2 is similar to our recent demonstration that MPP+ induces CCL2 release from mouse astrocytes in vitro [8]. Increased CCL2 level in PD has been suggested as a neuroprotective mechanism since CCL2 attracts inflammatory cells to the site of inflammation. However, significant increase of CCL2 could result in over-infiltration of inflammatory cells thereby inducing neuroinflammatory pathways and neuronal death. Astrocytes can be activated by mast cells through CD40-CD40 ligand interactions in the in vitro co-culture system [1,28,41]. It is known that activated astrocytes release GMF. We have previously shown that GMF activates BMMCs to release inflammatory mediators including CCL2 [22]. GMF from mouse astrocytes could activate mouse mast cells in the in vitro co-culture system in addition to exogenous MPP+.
Our present results show reduced CCL2 release in BMMCs obtained from GMF-KO mice which is in agreement with our previous findings that GMF is involved in proinflammatory mediators release from glial cells and mediates neuroinflammation [14,49–51]. We have also previously shown reduced expression of inflammatory cytokines from astrocytes and microglial cells obtained from GMF-KO mice as compared to glial expression from wild-type mice and return of increased levels in GMF-KO cells reconstituted to overexpress GMF with an adenoviral construct [52]. Similarly, the overexpression of GMF in BMMCs in the current study showed increased CCL2 release. This increased release could be due to the activation of p38 mitogen-activated protein kinase (p38 MAPK) and nuclear factor kappa-B (NF-kB) signaling pathways as we have previously reported [14,53]. We recently showed that GMF-deficiency in astrocytes upregulates antioxidant level and reduces the production of ROS in MPP+-induced toxicity [8]. Our another study showed that absence of GMF suppresses dopaminergic neuronal loss, glial activation, and expression of inflammatory mediators in the SNpc and striatum of MPTP treated mice [15]. We also recently demonstrated MPP+-induced dopaminergic neuronal loss in primary cultures of the mouse mesencephalic neurons/glia obtained from GMF-KO and wild-type mice [54]. We found that lack of GMF in GMF-KO neurons/glia led to decreased production of ROS, TNF-α, IL-1β as compared to wild-type neurons/glia. Further, overexpression of GMF induced dopaminergic neurodegeneration [8]. These previous findings support our current findings that GMF regulates MPP+-induced neurotoxicity in mast cells.
Mitochondrial dysfunction is involved in the neurodegenerative diseases progression [55]. UCPs are inner mitochondrial proteins that protect neurons by reducing the production of free radicals. Five members (UCP1-5) of the UCP family proteins have been identified. UCPs de link ATP production from biofuel oxidation in the mitochondria and reduces oxidative stress [26]. We have previously reported the expression of UCP2 in the mast cells but the expression of UCP4s is not yet reported in these cells. Moreover, we have recently reported that mast cells could mediate neuroinflammation in PD [22]. Therefore, the present study was carried out to demonstrate the expression of UC4 in mouse primary mast cells. We also stained UCP2 to compare with the expression of UCP4. We report the expression of UCP4 in BMMCs by immunohistochemistry and in the future we will analyze the UCPs expression dynamics in mast cells after treating with MPP+. Both UCP2 and UCP4 protect neurons from mitochondrial dysfunction, oxidative damage, cell survival, preserving ATP synthesis and implicated in neurodegeneration in PD [26,56]. Previous study showed that overexpression of UCPs protected neurons after MPP+ and dopamine-induced toxicity [26]. UCP4 is primarily expressed in the brain and is neuroprotective [57]. Overexpression of UCP4 in mast cells could protect neurons in PD and other neurodegenerative diseases. UCP4 preserves mitochondrial depolarization and decreases oxidative stress against MPP+ toxicity in SH-SY5Y cells [57]. Overexpression of UCP4 was also shown to suppress apoptosis, regulate calcium homeostasis, and reduce oxidative stress [26,57]. Macrophages overexpressing UCP2 produce less ROS in response to lipopolysaccharides [58]. UCP2 is expressed in lymphocytes, dendritic cells, neutrophils and macrophages [59]. Now we report that BMMCs express UCP4. We have previously shown that UCP2−/− BMMCs have greater release of proinflammatory molecules histamine, IL-6 and prostaglandin D2 (PGD2) release and ERK phosphorylation after activation [24]. Overexpression of UCP2 in LAD2 human mast cells reduced histamine release when activated [24]. Our previous and other studies suggested that UCP2 is a novel regulator of mast cell function with implications for treatment of mast cell-mediated inflammatory diseases including neurodegenerative and autoimmune diseases [24,26] and the current results show that UCP4 expression dynamics in mast cells could also regulate PD pathogenesis. In conclusion, neurotoxin MPP+ activates mast cells to release CCL2 which is implicated in the pathogenesis of PD.
Acknowledgments
This material is based upon work supported, in part, by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development (BX002477-01, A.Z.), and by the National Institutes of Health (NIH) National Institute of Neurological Disorders and Stroke Grant NS073670 (A.Z.), and the Reproductive Scientist Development Program HD000849, RR024980 (MS) and CSTA U54TR001013. The Maternal Fetal Tissue Bank at the University of Iowa is supported by the University of Iowa Carver College of Medicine and Department of Obstetrics & Gynecology.
References
- 1.Dong H, Zhang X, Qian Y. Mast cells and neuroinflammation. Med Sci Monit Basic Res. 2014;20:200–206. doi: 10.12659/MSMBR.893093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyman M, Lloyd DG, Ji X, Vizcaychipi MP, Ma D. Neuroinflammation: the role and consequences. Neurosci Res. 2014;79:1–12. doi: 10.1016/j.neures.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Jarrott B, Williams SJ. Chronic Brain Inflammation: The Neurochemical Basis for Drugs to Reduce Inflammation. Neurochem Res. 2015 doi: 10.1007/s11064-015-1661-7. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 4.Trudler D, Nash Y, Frenkel D. New insights on Parkinson’s disease genes: the link between mitochondria impairment and neuroinflammation. J Neural Transm. 2015;122:1409–1419. doi: 10.1007/s00702-015-1399-z. [DOI] [PubMed] [Google Scholar]
- 5.Stojkovska I, Wagner BM, Morrison BE. Parkinson’s disease and enhanced inflammatory response. Exp Biol Med (Maywood) 2015;240:1387–1395. doi: 10.1177/1535370215576313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czlonkowska A, Kohutnicka M, Kurkowska-Jastrzebska I, Czlonkowski A. Microglial reaction in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) induced Parkinson’s disease mice model. Neurodegeneration. 1996;5:137–143. doi: 10.1006/neur.1996.0020. [DOI] [PubMed] [Google Scholar]
- 7.Zhai A, Zhu X, Wang X, Chen R, Wang H. Secalonic acid A protects dopaminergic neurons from 1-methyl-4-phenylpyridinium (MPP(+))-induced cell death via the mitochondrial apoptotic pathway. Eur J Pharmacol. 2013;713:58–67. doi: 10.1016/j.ejphar.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 8.Khan MM, Kempuraj D, Zaheer S, Zaheer A. Glia Maturation Factor Deficiency Suppresses 1-Methyl-4-Phenylpyridinium-Induced Oxidative Stress in Astrocytes. J Mol Neurosci. 2014;53:590–599. doi: 10.1007/s12031-013-0225-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim R, Miller JF, Zaheer A. Purification and characterization of glia maturation factor beta: a growth regulator for neurons and glia. Proc Natl Acad Sci U S A. 1989;86:3901–3905. doi: 10.1073/pnas.86.10.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim R, Zaheer A. Structure and function of glia maturation factor beta. Adv Exp Med Biol. 1991;296:161–164. doi: 10.1007/978-1-4684-8047-4_16. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan R, Zaheer A, Jaye M, Lim R. Molecular cloning and expression of biologically active human glia maturation factor-beta. J Neurochem. 1991;57:483–490. doi: 10.1111/j.1471-4159.1991.tb03777.x. [DOI] [PubMed] [Google Scholar]
- 12.Zaheer A, Fink BD, Lim R. Expression of glia maturation factor beta mRNA and protein in rat organs and cells. J Neurochem. 1993;60:914–920. doi: 10.1111/j.1471-4159.1993.tb03237.x. [DOI] [PubMed] [Google Scholar]
- 13.Zaheer S, Wu Y, Sahu SK, Zaheer A. Suppression of neuro inflammation in experimental autoimmune encephalomyelitis by glia maturation factor antibody. Brain Res. 2011;1373:230–239. doi: 10.1016/j.brainres.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaheer A, Zaheer S, Sahu SK, Knight S, Khosravi H, Mathur SN, Lim R. A novel role of glia maturation factor: induction of granulocyte-macrophage colony-stimulating factor and pro-inflammatory cytokines. J Neurochem. 2007;101:364–376. doi: 10.1111/j.1471-4159.2006.04385.x. [DOI] [PubMed] [Google Scholar]
- 15.Khan MM, Zaheer S, Thangavel R, Patel M, Kempuraj D, Zaheer A. Absence of Glia Maturation Factor Protects Dopaminergic Neurons and Improves Motor Behavior in Mouse Model of Parkinsonism. Neurochem Res. 2015;40:980–990. doi: 10.1007/s11064-015-1553-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaheer S, Wu Y, Sahu SK, Zaheer A. Overexpression of glia maturation factor reinstates susceptibility to myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis in glia maturation factor deficient mice. Neurobiol Dis. 2010;40:593–598. doi: 10.1016/j.nbd.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaheer A, Mathur SN, Lim R. Overexpression of glia maturation factor in astrocytes leads to immune activation of microglia through secretion of granulocyte-macrophage-colony stimulating factor. Biochem Biophys Res Commun. 2002;294:238–244. doi: 10.1016/S0006-291X(02)00467-9. [DOI] [PubMed] [Google Scholar]
- 18.Skaper SD, Facci L, Giusti P. Neuroinflammation, microglia and mast cells in the pathophysiology of neurocognitive disorders: a review. CNS Neurol Disord Drug Targets. 2014;13:1654–1666. doi: 10.2174/1871527313666141130224206. [DOI] [PubMed] [Google Scholar]
- 19.Nelissen S, Lemmens E, Geurts N, Kramer P, Maurer M, Hendriks J, Hendrix S. The role of mast cells in neuroinflammation. Acta Neuropathol. 2013;125:637–650. doi: 10.1007/s00401-013-1092-y. [DOI] [PubMed] [Google Scholar]
- 20.Skaper SD, Giusti P, Facci L. Microglia and mast cells: two tracks on the road to neuroinflammation. FASEB J. 2012;26:3103–3117. doi: 10.1096/fj.11-197194. [DOI] [PubMed] [Google Scholar]
- 21.Kim DY, Jeoung D, Ro JY. Signaling pathways in the activation of mast cells cocultured with astrocytes and colocalization of both cells in experimental allergic encephalomyelitis. J Immunol. 2010;185:273–283. doi: 10.4049/jimmunol.1000991. [DOI] [PubMed] [Google Scholar]
- 22.Kempuraj D, Thangavel R, Yang E, Pattani S, Zaheer S, Santillan DA, Santillan MK, Zaheer A. Dopaminergic Toxin 1-Methyl-4-Phenylpyridinium, Proteins alpha-Synuclein and Glia Maturation Factor Activate Mast Cells and Release Inflammatory Mediators. PLoS One. 2015;10:e0135776. doi: 10.1371/journal.pone.0135776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conti B, Sugama S, Lucero J, Winsky-Sommerer R, Wirz SA, Maher P, Andrews Z, Barr AM, Morale MC, Paneda C, Pemberton J, Gaidarova S, Behrens MM, Beal F, Sanna PP, Horvath T, Bartfai T. Uncoupling protein 2 protects dopaminergic neurons from acute 1,2,3,6-methyl-phenyl-tetrahydropyridine toxicity. J Neurochem. 2005;93:493–501. doi: 10.1111/j.1471-4159.2005.03052.x. [DOI] [PubMed] [Google Scholar]
- 24.Tagen M, Elorza A, Kempuraj D, Boucher W, Kepley CL, Shirihai OS, Theoharides TC. Mitochondrial uncoupling protein 2 inhibits mast cell activation and reduces histamine content. J Immunol. 2009;183:6313–6319. doi: 10.4049/jimmunol.0803422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu AC, Ho PW, Kwok KH, Ho JW, Chan KH, Liu HF, Kung MH, Ramsden DB, Ho SL. Mitochondrial UCP4 attenuates MPP+ - and dopamine-induced oxidative stress, mitochondrial depolarization, and ATP deficiency in neurons and is interlinked with UCP2 expression. Free Radic Biol Med. 2009;46:810–820. doi: 10.1016/j.freeradbiomed.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 26.Ho PW, Ho JW, Liu HF, So DH, Tse ZH, Chan KH, Ramsden DB, Ho SL. Mitochondrial neuronal uncoupling proteins: a target for potential disease-modification in Parkinson’s disease. Transl Neurodegener. 2012;1:3. doi: 10.1186/2047-9158-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim R, Zaheer A, Khosravi H, Freeman JH, Jr, Halverson HE, Wemmie JA, Yang B. Impaired motor performance and learning in glia maturation factor-knockout mice. Brain Res. 2004;1024:225–232. doi: 10.1016/j.brainres.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Kim DY, Hong GU, Ro JY. Signal pathways in astrocytes activated by cross-talk between of astrocytes and mast cells through CD40-CD40L. J Neuroinflammation. 2011;8:25. doi: 10.1186/1742-2094-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sayed BA, Walker ME, Brown MA. Cutting edge: mast cells regulate disease severity in a relapsing-remitting model of multiple sclerosis. J Immunol. 2011;186:3294–3298. doi: 10.4049/jimmunol.1003574. [DOI] [PubMed] [Google Scholar]
- 30.Zaheer A, Yorek MA, Lim R. Effects of glia maturation factor overexpression in primary astrocytes on MAP kinase activation, transcription factor activation, and neurotrophin secretion. Neurochemical research. 2001;26:1293–1299. doi: 10.1023/a:1014241300179. [DOI] [PubMed] [Google Scholar]
- 31.Zaheer A, Mathur SN, Lim R. Overexpression of glia maturation factor in astrocytes leads to immune activation of microglia through secretion of granulocyte-macrophage-colony stimulating factor. Biochemical and biophysical research communications. 2002;294:238–244. doi: 10.1016/S0006-291X(02)00467-9. [DOI] [PubMed] [Google Scholar]
- 32.Kempuraj D, Khan MM, Thangavel R, Xiong Z, Yang E, Zaheer A. Glia maturation factor induces interleukin-33 release from astrocytes: implications for neurodegenerative diseases. J Neuroimmune Pharmacol. 2013;8:643–650. doi: 10.1007/s11481-013-9439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kempuraj D, Saito H, Kaneko A, Fukagawa K, Nakayama M, Toru H, Tomikawa M, Tachimoto H, Ebisawa M, Akasawa A, Miyagi T, Kimura H, Nakajima T, Tsuji K, Nakahata T. Characterization of mast cell-committed progenitors present in human umbilical cord blood. Blood. 1999;93:3338–3346. [PubMed] [Google Scholar]
- 34.Kempuraj D, Asadi S, Zhang B, Manola A, Hogan J, Peterson E, Theoharides TC. Mercury induces inflammatory mediator release from human mast cells. J Neuroinflammation. 2010;7:20. doi: 10.1186/1742-2094-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santillan MK, Leslie KK, Hamilton WS, Boese BJ, Ahuja M, Hunter SK, Santillan DA. Collection of a lifetime: a practical approach to developing a longitudinal collection of women’s healthcare biological samples. Eur J Obstet Gynecol Reprod Biol. 2014;179:94–99. doi: 10.1016/j.ejogrb.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bulfone-Paus S, Bahri R. Mast Cells as Regulators of T Cell Responses. Front Immunol. 2015;6:394. doi: 10.3389/fimmu.2015.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Theoharides TC, Valent P, Akin C. Mast Cells, Mastocytosis, and Related Disorders. N Engl J Med. 2015;373:163–172. doi: 10.1056/NEJMra1409760. [DOI] [PubMed] [Google Scholar]
- 38.Theoharides TC, Alysandratos KD, Angelidou A, Delivanis DA, Sismanopoulos N, Zhang B, Asadi S, Vasiadi M, Weng Z, Miniati A, Kalogeromitros D. Mast cells and inflammation. Biochim Biophys Acta. 2012;1822:21–33. doi: 10.1016/j.bbadis.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silver R, Curley JP. Mast cells on the mind: new insights and opportunities. Trends Neurosci. 2013;36:513–521. doi: 10.1016/j.tins.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Chikahisa S, Kodama T, Soya A, Sagawa Y, Ishimaru Y, Sei H, Nishino S. Histamine from brain resident MAST cells promotes wakefulness and modulates behavioral states. PLoS One. 2013;8:e78434. doi: 10.1371/journal.pone.0078434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skaper SD, Facci L, Barbierato M, Zusso M, Bruschetta G, Impellizzeri D, Cuzzocrea S, Giusti P. N-Palmitoylethanolamine and Neuroinflammation: a Novel Therapeutic Strategy of Resolution. Mol Neurobiol. 2015;52:1034–1042. doi: 10.1007/s12035-015-9253-8. [DOI] [PubMed] [Google Scholar]
- 42.Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008;9:1215–1223. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mekori YA, Metcalfe DD. Mast cells in innate immunity. Immunol Rev. 2000;173:131–140. doi: 10.1034/j.1600-065x.2000.917305.x. [DOI] [PubMed] [Google Scholar]
- 44.Sismanopoulos N, Delivanis DA, Alysandratos KD, Angelidou A, Therianou A, Kalogeromitros D, Theoharides TC. Mast cells in allergic and inflammatory diseases. Curr Pharm Des. 2012;18:2261–2277. doi: 10.2174/138161212800165997. [DOI] [PubMed] [Google Scholar]
- 45.Madrigal JL, Caso JR. The chemokine (C-C motif) ligand 2 in neuroinflammation and neurodegeneration. Adv Exp Med Biol. 2014;824:209–219. doi: 10.1007/978-3-319-07320-0_15. [DOI] [PubMed] [Google Scholar]
- 46.Stamatovic SM, Shakui P, Keep RF, Moore BB, Kunkel SL, Van Rooijen N, Andjelkovic AV. Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J Cereb Blood Flow Metab. 2005;25:593–606. doi: 10.1038/sj.jcbfm.9600055. [DOI] [PubMed] [Google Scholar]
- 47.Yao Y, Tsirka SE. Monocyte chemoattractant protein-1 and the blood-brain barrier. Cell Mol Life Sci. 2014;71:683–697. doi: 10.1007/s00018-013-1459-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reale M, Iarlori C, Thomas A, Gambi D, Perfetti B, Di Nicola M, Onofrj M. Peripheral cytokines profile in Parkinson’s disease. Brain Behav Immun. 2009;23:55–63. doi: 10.1016/j.bbi.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 49.Thangavel R, Stolmeier D, Yang X, Anantharam P, Zaheer A. Expression of glia maturation factor in neuropathological lesions of Alzheimer’s disease. Neuropathol Appl Neurobiol. 2012;38:572–581. doi: 10.1111/j.1365-2990.2011.01232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zaheer S, Thangavel R, Sahu SK, Zaheer A. Augmented expression of glia maturation factor in Alzheimer’s disease. Neuroscience. 2011;194:227–233. doi: 10.1016/j.neuroscience.2011.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaheer A, Knight S, Zaheer A, Ahrens M, Sahu SK, Yang B. Glia maturation factor overexpression in neuroblastoma cells activates glycogen synthase kinase-3beta and caspase-3. Brain Res. 2008;1190:206–214. doi: 10.1016/j.brainres.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zaheer A, Zaheer S, Thangavel R, Wu Y, Sahu SK, Yang B. Glia maturation factor modulates beta-amyloid-induced glial activation, inflammatory cytokine/chemokine production and neuronal damage. Brain Res. 2008;1208:192–203. doi: 10.1016/j.brainres.2008.02.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zaheer A, Yorek MA, Lim R. Effects of glia maturation factor overexpression in primary astrocytes on MAP kinase activation, transcription factor activation, and neurotrophin secretion. Neurochem Res. 2001;26:1293–1299. doi: 10.1023/a:1014241300179. [DOI] [PubMed] [Google Scholar]
- 54.Khan MM, Zaheer S, Nehman J, Zaheer A. Suppression of glia maturation factor expression prevents 1-methyl-4-phenylpyridinium (MPP(+))-induced loss of mesencephalic dopaminergic neurons. Neuroscience. 2014;277:196–205. doi: 10.1016/j.neuroscience.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reddy PH, Reddy TP. Mitochondria as a therapeutic target for aging and neurodegenerative diseases. Curr Alzheimer Res. 2011;8:393–409. doi: 10.2174/156720511795745401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andrews ZB, Horvath B, Barnstable CJ, Elsworth J, Yang L, Beal MF, Roth RH, Matthews RT, Horvath TL. Uncoupling protein-2 is critical for nigral dopamine cell survival in a mouse model of Parkinson’s disease. J Neurosci. 2005;25:184–191. doi: 10.1523/JNEUROSCI.4269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramsden DB, Ho PW, Ho JW, Liu HF, So DH, Tse HM, Chan KH, Ho SL. Human neuronal uncoupling proteins 4 and 5 (UCP4 and UCP5): structural properties, regulation, and physiological role in protection against oxidative stress and mitochondrial dysfunction. Brain Behav. 2012;2:468–478. doi: 10.1002/brb3.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Negre-Salvayre A, Hirtz C, Carrera G, Cazenave R, Troly M, Salvayre R, Penicaud L, Casteilla L. A role for uncoupling protein-2 as a regulator of mitochondrial hydrogen peroxide generation. FASEB J. 1997;11:809–815. [PubMed] [Google Scholar]
- 59.Rousset S, Emre Y, Join-Lambert O, Hurtaud C, Ricquier D, Cassard-Doulcier AM. The uncoupling protein 2 modulates the cytokine balance in innate immunity. Cytokine. 2006;35:135–142. doi: 10.1016/j.cyto.2006.07.012. [DOI] [PubMed] [Google Scholar]