Abstract
OBJECTIVES
Transcatheter aortic valve replacement (TAVR) has become a treatment option for otherwise inoperable or high risk patients. Currently TAVR is performed with a combination of fluoroscopy and transesophageal echocardiography, which has visual limitations including a two dimensional view and poor soft tissue contrast. Real-time magnetic resonance imaging (rtMRI) guidance overcomes these limitations with a three dimensional view of anatomical structures, improved soft tissue contrast, and allows pinpoint accuracy of device delivery. To date a device clinically available in the US has not been used for rtMRI TAVR. We present our results using the CoreValve® device.
METHODS
The Medtronic CoreValve® delivery catheter system was minimally modified by the investigators to be MRI compatible by replacing the stainless steel components with fluoroplastic resin and high density polyethylene components. Eight swine ranging between 60 and 90 kg underwent rtMRI guided TAVR with an active guidewire via a left subclavian approach.
RESULTS
Two imaging planes (long axis view and short axis view) were utilized simultaneously for real-time imaging during implantation. Successful deployment was performed without rapid ventricular pacing or cardiopulmonary bypass. Post-deployment images were acquired to evaluate the final valve position in addition to valvular and cardiac function.
CONCLUSIONS
Our results show that the CoreValve® can be easily and effectively deployed from the left subclavian approach by utilizing rtMRI guidance, a minimally modified valve delivery catheter system, and an active guidewire. This method allows superior visualization prior to deployment, thereby allowing deployment of the valve with pinpoint accuracy. rtMRI has the added benefit of the ability to perform immediate post-procedural functional assessment, while eliminating the morbidity of radiation exposure, rapid ventricular pacing, contrast media renal toxicity, and a more invasive procedure. Use of a commercially available device brings this rtMRI guided approach closer to a clinical reality.
OBJECTIVES
Aortic stenosis is the most common type of valvular heart disease in the United States.1–4 While the disease process has a long latency period, patients rapidly decline after they become symptomatic unless they undergo valve replacement.5–7 Unfortunately, some of these patients are not suitable surgical candidates or are high risk.8,9 Without valve replacement, the mortality for these patients after onset of symptoms approaches 50 percent after two years and 80 percent after 5 years. 10,11 The advent of transcatheter aortic valve replacement (TAVR) has become a viable treatment option for otherwise inoperable or high risk aortic stenosis patients. 8,9,12 Two devices are currently available for use in the United States: Medtronic CoreValve (Medtronic Inc: Minneapolis, MN) and Edwards SAPIEN Transcatheter Heart Valve (Edward Lifesciences Inc: Irvine, CA). 13–15
While there have been multiple advances in valve design and valve delivery technology, the imaging modality employed has remained largely unchanged. TAVR is currently an intricate procedure which requires multimodality imaging including pre-procedural imaging for planning, intra-procedural imaging for guidance, and post-procedural imaging for confirmation of placement.16,17 Currently the pre-procedural evaluation usually includes echocardiography in combination with multidetector computed tomography (MDCT) or computed tomography (CT) angiogram. The TAVR procedure is most commonly performed with a combination of fluoroscopy and transesophageal echocardiography (TEE). Post-procedural imaging also routinely uses a combination of fluoroscopy and TEE to confirm valve placement and cardiac function. 16,18–20 Fluoroscopy has multiple limitations including poor soft tissue contrast, a requirement for rapid ventricular pacing, radiation exposure to the patient and surgical team, and contrast-induced nephropathy. 15,21–23 Real-time magnetic resonance image (rtMRI) guidance overcomes these limitations with improved three dimensional visualization of the anatomic structures, guidewires, delivery catheter system, and the bioprosthetic valve, while allowing delivery of the valve with pinpoint accuracy. In addition the use of MRI allows for immediate post-procedural functional assessment. 23–25
Our group has successfully performed TAVR procedures via a transapical approach using rtMRI guidance. We have proven that this is a reproducible method with high accuracy of device delivery.15,23,26 In this study, we demonstrated that the CoreValve® can be easily and effectively deployed from the left subclavian approach by utilizing rtMRI guidance, a minimally modified valve delivery catheter system, and an active guidewire. We report our results of preclinical feasibility in an acute, non-survival swine model. However, to date a device clinically available in the US has not been used for rtMRI TAVR.
METHODS
Medtronic CoreValve®
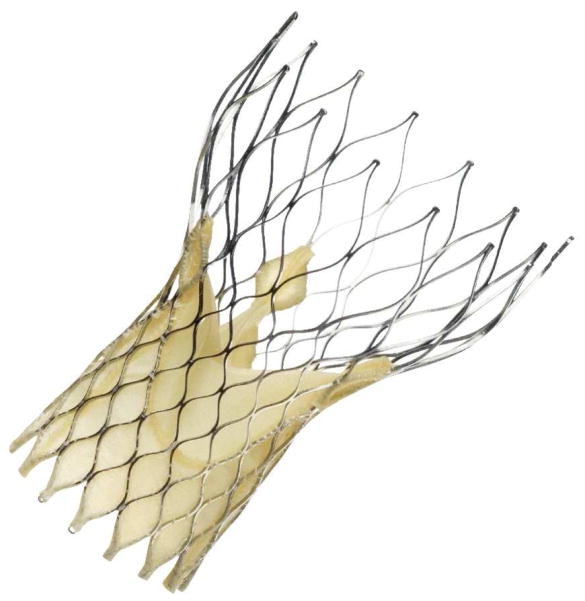
The Medtronic CoreValve® is an aortic bioprosthetic transcatheter heart valve. The valve consists of three porcine pericardial leaflets that are sutured to a self-expanding, multi-level, radiopaque, nitinol frame (Figure 1).22,27 The porcine pericardial leaflets are processed with alpha-amino oleic acid, which is an anti-mineralization treatment derived from a naturally occurring long-chain fatty acid.27 The nitinol stent is manufactured by laser cutting a nitinol tube.28 The stent is designed with three sections. The lower section of the stent has a high radial force to anchor the valve and displace the calcified leaflets. The middle section holds the three porcine pericardial leaflets and its smaller diameter in relation to the lower and upper sections to avoid occluding the coronary arteries, which largely eliminates the need for adjusting the rotational position on deployment. The upper section has the largest diameter, while exerting a lower radial force. The design of the upper portion of the stent allows it to self-center during placement.22,28 The commercially available version of the CoreValve ® is available in several different sizes (23, 26, 29, and 31mm) which correlates with annulus diameters from 18 to 29mm and ascending aorta diameters up to 43mm. The device is MR conditional and nonclinical testing to date has confirmed that it is safe to use in MR. 22,27
Figure 1.
Medtronic CoreValve®
Medtronic CoreValve® Delivery Catheter System
The delivery catheter system is compatible with a 0.035-in guidewire. The distal end of the delivery catheter system has an atraumatic, radiopaque tip and a capsule that covers and maintains the valve in a crimped position. The handle is located on the proximal end of the delivery catheter system and is used to load and deploy the valve. The handle consists of a slider to open and close the capsule. The handle has a knob to facilitate slowly opening the slider to allow precise placement. The device also has an outer tube, which is referred to as an AccuTrak™ stability layer. This outer tube covers the catheter sheath to protect the retractable delivery catheter system, introducer sheath, and vessel walls. This layer allows the catheter to retract freely and providing a more stable platform for deployment. The first-generation of the delivery catheter system was 24 French (Fr) sheath; however, the device is currently in its third generation, and is 18 Fr. Now the outer diameter of the catheter is 15 Fr and the outer diameter of the valve capsule is 18 Fr. The delivery catheter system is approved for femoral, subclavian, axillary, or ascending aortic access sites, and it is not MRI compatible. 22,27
Delivery Catheter System Modification
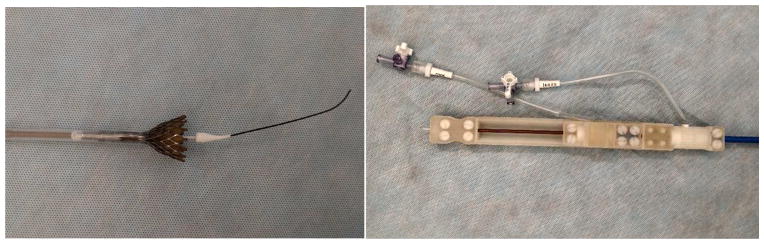
The Medtronic CoreValve® delivery catheter system was modified to be MRI compatible. The delivery catheter system sheath has an inner and outer flexible polymer tube embedded with braided stainless steel. The sheath also contains an inner shaft which has metallic components. To make the delivery catheter system MRI compatible, the inner tube and capsule were replaced with tubes made of fluoroplastic resin. The outer tube was replaced with a tube from a 14Fr Check-Flo Performer® Introducer (Cook Medical, Bloomington, IN). The metallic components in the delivery catheter system handle were replaced with corresponding parts composed of plastic materials. In addition, the handle was redesigned with a thumb slider to open and close the capsule instead of the original design which comprised of a slider and knob to facilitate opening of the capsule. The delivery catheter system modifications were implemented to maintain device performance, including rigidity and flexibility, while achieving MRI compatibility. With the replacement of the non-MRI compatible components with MRI compatible components, the profile of the device increased the outer diameter of the catheter from 15 Fr to 18 Fr and the outer diameter of the valve capsule from 18 Fr to 20 Fr. The modified delivery catheter system fits in a 20 Fr introducer (Figure 2, 3).
Figure 2.
(top and bottom right) Modified delivery catheter system; (bottom left) valve deployment with active guide wire.
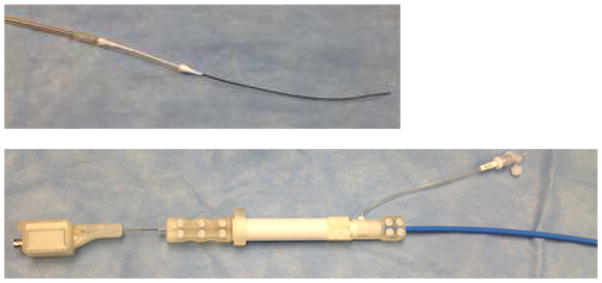
Figure 3.
The delivery system and the active guide wire. (top): The delivery system and the active guide wire. (bottom): The tip of delivery system and the active guide wire. The handle of the delivery system and the RF connector.
Active Guidewire
An active guide wire was developed to guide the delivery catheter system prior to deployment. The active guide wire includes an MRI loop coil antenna. The loop coil was manufactured using insulated 0.005″ magnet copper wire (Heraeus Medical Components Inc, St. Paul, MN) in an ISO class 7 cleanroom. The coil length was adjusted to 1.1 inches with 0.026″ outer diameter. 0.006″ profile twisted pair was used as a transmission line for the loop coil antenna. The whole structure was insulated by using medical grade polyester heat shrink tubing. The loop coil antenna was matched to 50 ohms and tuned to the Lamoure frequency of a 1.5T MRI scanner. The matching and decoupling circuit box was attached to the loop coil antenna by MMCX type RF connectors (Microstock Inc., West Point, PA)(Figure 3).14,29
Imaging Technology
All procedures and imaging were performed in a hybrid operating room suite. The imaging system used was a 1.5 Tesla Magnetom Aera (Siemens Medical Solutions, Munich, Germany). The magnet design utilizes a short 145cm long by 70cm wide bore, which is large enough for surgical access to the animal within the magnet. The imaging system has several components, which includes an interactive user interface, a large-screen display, advanced pulse sequence technology, and image reconstruction software.30–32
Interactive Front End navigation software (Siemens Corporate Research, Munich, Germany), along with an interactive real-time pulse sequence (BEAT_IRTTT), was used as real-time navigation for valve deployment. The system not only displays standard magnetic resonance images, but also has a fully interactive, real-time imaging system. The image reconstruction software takes multiple slices, which are obtained in rapid succession and can be simultaneously displayed to provide a three-dimensional rendering. These imaging planes can be quickly adjusted, which allows for real time interpretation of anatomical structures and device tracking.30–34
Animal Surgery Protocol
All experiments were performed in accordance with the protocols approved by the National Institutes of Health Animal Care and Use Committee. Induction included an intramuscular injection of Midazolam (0.5 mg/kg) and Ketamine (25mg/kg). After induction, the animals were intubated and then maintained on mechanical ventilation with Isoflurane (0.5–2.5%). The animals end tidal carbon dioxide, oxygen saturation, arterial blood pressure, and electrocardiographic telemetry were monitored throughout the entire procedure. The animal’s body temperature was maintained with a forced-air warming blanket. Prior the start of the procedure, the animals received intravenous Amiodarone (150mg–300mg) for antiarrhythmic treatment and prior to trocar insertion they were anticoagualted with heparin (300 units/kg). After completion of the experiment, the fully anesthetized animals were euthanized with an intravenous injection of phenobarbital (150mg/kg). We recorded the times and details of the procedures.
Valve Deployment
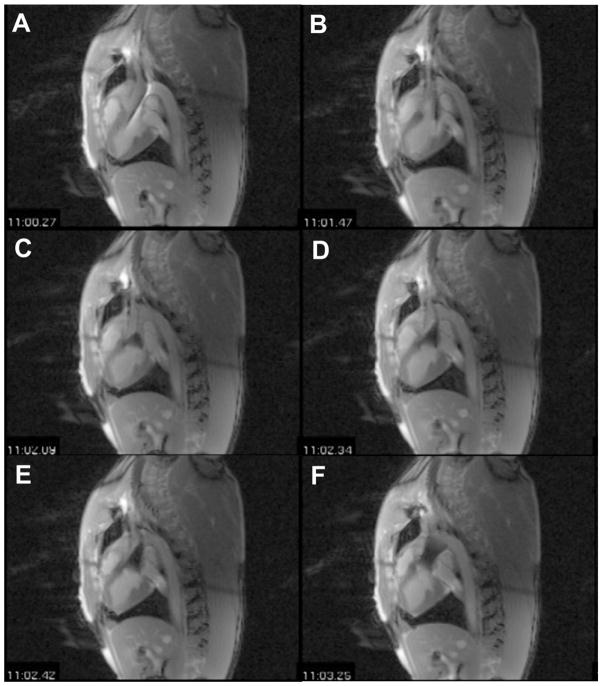
Eight swine weighing between 60 and 90 kg underwent rtMRI-guided TAVR with the Medtronic Corevalve®. The animals were sedated and intubated, then prepped and draped in a supine position, as previously described. A 4cm left subclavian cut-down was performed. Vessel loops were placed around the subclavian for proximal and distal vascular control. A purse string suture was placed in the subclavian for closure post-procedure. An incision was made in the subclavian and the 20 Fr sheath was inserted into the subclavian artery. The swine were then transferred to the MRI and a surface RF coil was secured to the anterior chest to enhance signal reception. A pre-deployment scan was performed to confirm annulus and aortic root size. The MRI compatible delivery catheter system was loaded with the appropriately sized CoreValve®. Two imaging planes (long axis view and short axis view) were used to create a virtual real time 3D reconstruction, which the surgeon could view from a projector screen in the MRI suite. The delivery catheter system was loaded over the guidewire. The guidewire was then introduced into the sheath and advanced until it was across the aortic valve (Figure 4a). The delivery catheter system was then advanced under rtMRI guidance (Figure 4b). Once the device reached the proper position, the capsule was slowly retracted (Figures 4c, 4d, 4e). Prior to deployment, repositioning of the bioprosthesis was performed if needed to ensure correct and precise placement. Once the capsule was fully retracted, the valve was deployed and the delivery catheter system was withdrawn from the sheath (Figure 4f). Throughout the procedure, the surgeon was in direct communication with the scanner operator via headphones and a microphone (Magnacoustics, Atlantic Beach, NY) to request changes in the imaging planes as needed to visualize the device and ensure proper placement.14,23,32
Figure 4.
Long axis view of rtMRI guided valve deployment.
(a) Guide wire advanced across aortic valve; (b) delivery catheter system advanced over guide wire and into position; (c, d, e) retracting capsule to deploy valve; (f) valve deployed
Post-Deployment Valve Assessment
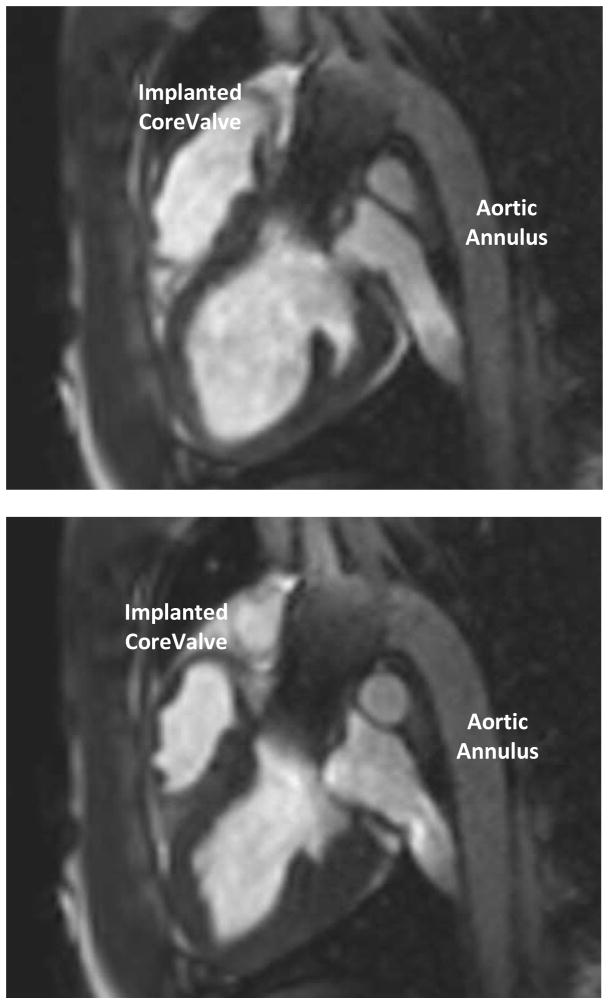
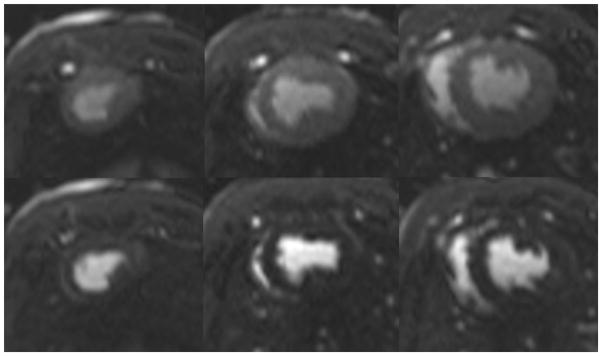
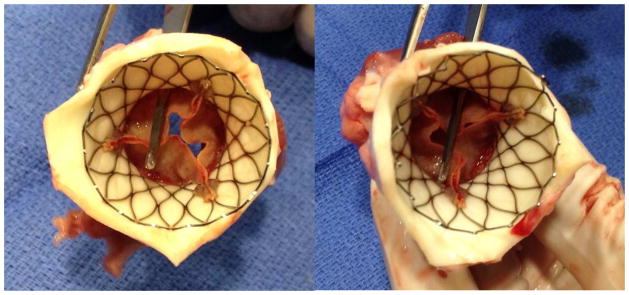
After removal of the delivery catheter system, post-deployment images were acquired to confirm the position of the prostheses and to evaluate valvular and global heart function. Gated cine-MRI was used to assess aortic valve function and left ventricular function (Figure 5). Phase contrast cine-MRI was used to assess blood flow through newly implanted bioprosthesis and to evaluate for intravalvular or paravalvular regurgitation. An MR first-pass perfusion scan was also performed in several animals with intravenous injection of gadolinium with diethylenetriamine penta-acetic acid (Gd-DTPA; Magnavist, Berlex Inc, Montville, NJ) contrast media to assess coronary blood flow (Figure 6). A necropsy was performed on all animals following valve placement and post-deployment functional scan to grossly assess valve placement (Figure 7).23,30,32,35
Figure 5.
Post-deployment CINE to confirm valve position (long axis view).
(top) diastole; (bottom) systole
Figure 6.
Post-deployment perfusion scan. (left column) apex, (middle column) mid-wall, (right column) base. (top row) arterial phase (bottom row) venous phase
Figure 7.
Necropsy results confirm the open coronary ostia.
RESULTS
Valve Deployment
All eight swine underwent successful MRI-guided TAVR with the Medtronic Corevalve®. The left subclavian cut-down with sheath placement was 21 minutes +/− 9 minutes. The pre-deployment scan was used to measure the annulus and aortic root size to determine valve selection. The sequences were measured with an oblique sagittal view which corresponded to a similar view seen with a parasternal long-axis view via transthoracic echocardiogram and a midesophageal long-axis view via TEE for valve measurement.36,37 The annulus and aortic root sizes were 26+/−2 mm and 22+/−3 mm, respectively. A 26mm CoreValve® bioprosthesis was used for all eight experiments. An active guidewire was utilized in six experiments and a passive Nitrex guidewire (EV3, Plymouth, MN) for two experiments. This passive guidewire was an 0.035-in, 180cm angled, hydrophilic guidewire. There were no issues with valve deployment. The time for placing the guidewire was 60 seconds +/− 10 seconds and the time for deploying the valve under rtMRI guidance was 25 seconds +/− 3 seconds.
Post-Deployment Valve Assessment
The post-deployment scans confirmed precise valve placement in all eight experiments as well as confirmed global and regional cardiac function (Figure 5). The time-resolved velocity of blood flow through the valve was measured using a CINE phased-contrast imaging sequence in short-access planes proximal and distal to the valve. These imaging sequences confirmed adequate systolic blood flow with optimal valve functioning. There was no evidence of turbulent blood flow, regurgitation or a paravalvular leak. An MR first-pass perfusion scan was performed in two animals with intravenous injection of Gd-DTPA, which confirmed adequate coronary perfusion (Figure 6). The necropsy results confirmed the MRI findings (Figure 7). On necropsy, all eight animals had apposition of the bioprosthesis to the annulus and ascending aorta. All valves were deployed in the correct position, with the proximal end 4–6mm below the annulus and without occluding the coronary ostia (Figure 7).
DISCUSSION
Our group as well as other groups, such as Kahlert et al, have demonstrated that rtMRI can be utilized as the only imaging modality for pre-deployment imaging, imaging guidance for valve deployment, and post-deployment scanning in TAVR procedures with a reproducible method and high accuracy of device delivery.14,23,26,31,32,38,39 rtMRI guided TAVR has come closer to a clinical reality over the past decade. We have modified our methodology with the knowledge we have gained from multiple experiments to what we believe is a pre-clinical model that can be translated to a clinical trial. Our first experiment utilized a commercially available stentless valve mounted on a custom-designed, balloon-expandable platinum stent. The valves were implanted via a transapical approach in eight swine with a custom-designed delivery device. The results confirmed the feasibility of transapical TAVR with rtMRI guidance.31 The next experiment in our series again utilized a commercially available stentless valve mounted on a custom-designed, balloon-expandable platinum stent. The valves were also implanted via an apical approach in 28 swine with a custom-designed delivery device. Ten of the swine were allowed to survive to 1, 3 and 6 months with intermittent imaging. Long term results were able to demonstrate stability of the implants.23 We also compared balloon-expandable with self-expandable stents mounted in commercially available valves. These valves were implanted via a transapical approach in 22 swine. The swine were allowed to survive to 1, 3 and 6 months with intermittent imaging. We concluded that self-expanding stents were easier to deploy under rtMRI guidance and had fewer complications.26 As the clinically approved technology developed, our group then performed an experiment using a commercially available balloon-expandable valve and minimally modified valve delivery device. We implanted the valve in eight swine via a transapical approach. The results of this experiment confirmed the feasibility of deploying a commercially available balloon-expandable stent with a minimally modified delivery device via a transapical approach.14 While these experiments showed the feasibility of an apical approach, we wanted a commercially available, self-expanding device that would be amenable to delivery via a transcatheter approach. We then performed a feasibility experiment with our active guidewire for transapical deployment of a self-expandable stents mounted in commercially available valves in 16 swine. Six of the swine were allowed to survive to 90 days. The results of the experiment confirmed the benefit of an active guidewire as well as the stability of our custom-designed stent over time.15 This led us to our current experiment, which demonstrates the feasibility of utilizing rtMRI guidance with a minimally modified valve delivery catheter system, commercially available bioprosthetic valve, and an active guidewire for TAVR.
While the CoreValve® nitinol stent is MRI compatible, the Medtronic delivery device required some modification to be MRI compatible. Our delivery catheter system modifications were carefully implemented to maintain device performance, including rigidity and flexibility, while achieving MRI compatibility. As part of the design modifications, the substituted inner tube and capsule were slightly thicker than the original components, since the fluoroplastic resin we used didn’t have braided stainless to aid in rigidity. As a result, the outer diameter of the valve capsule was increased from fitting in an 18 Fr introducer sheath to a 20 Fr introducer sheath. The increased sheath size wasn’t a limitation for our surgical subclavian cut down. Since our experiment was an acute, non-survival model, surgical repair of the subclavian was not performed. For a transition to a clinical model, we do not anticipate the increased profile to be a limitation, as there are percutaneous closure devices, such as the Perclose ProGlide device, which are approved to close 20Fr access sites.
We also found that an active guidewire significantly improved its visualization while advancing across the aortic valve and reduced the time required to advance the guidewire. The thin profile of a guidewire makes it difficult to visualize on MRI. The active guidewire overcomes this limitation by being visualized even if it is moved outside the imaging slice. 34,40 We used an active guidewire with a loop coil antenna. The guidewire was then assigned a unique image signature, so the user can pick a color with the imaging software to match the image signature. We chose neon green since it was easily visible when imposed on the darker rtMRI images. This greatly aided in visualizing guidewire advancement across the aortic valve. During one of our experiments the connector between the guidewire and the RF connector was damaged, so we utilized a passive Nitrex guidewire (EV3, Plymouth, MN) for two experiments. The time required to advance the passive guidewire across the aortic valve was approximately two minutes in both experiments while our average time of advancing the passive and active guidewires in all eight experiments was 60 seconds +/− 10 seconds. It took extra time to adjust the long axis imaging plane to visualize the tip of the catheter in the imaging slice. As the passive guidewire was advanced, the long axis plan had to be continually readjusted. In addition, visualization of the passive guidewire was limited and artifact obscured the two imaging planes. As seen in our experiments, passive guidewires have multiple limitations including poor contrast and excessive artifacts.30 Once the delivery device was advanced over the guidewire, the active guidewire again aided in visualization of the delivery device.
While CT is the most accepted imaging modality for pre-procedural imaging, MRI provides comparable information about cardiac and aortic anatomy and geometry.41–43 We found that in our experiments the MRI pre-deployment scan was helpful in confirming annulus and aortic root sizes prior to starting the procedure. Because of the superior soft tissue contrast and improved visualization, rtMRI has the added benefit of allowed prompt recognition of periprocedural complications such as dissection, perforation in the aorta or left ventricle from the tip of the delivery device or dislocation of the prosthetic valve into the ascending aorta. Our experiment also included a post-deployment scans not only to confirm valve placement, but also to confirm global and regional cardiac function. We used a CINE phased-contrast imaging sequence to measure the time-resolved velocity of blood flow through the valve. All eight of our experiments had adequate systolic blood flow with optimal valve functioning. There was no evidence of turbulent blood flow, regurgitation or a paravalvular leak. We were also performed an MR first-pass perfusion scan in two animals with intravenous injection of Gd-DTPA, which confirmed adequate coronary perfusion. We also performed a necropsy on all animals, as this was an acute, non-survival model. Necropsy was able to confirm the MRI findings with apposition of the bioprosthesis to the annulus and ascending aorta. In addition, all valves were precisely deployed with the proximal end 4–6mm below the annulus and without occluding the coronary ostia.
rtMRI has multiple benefits over the current imaging modality of echocardiography and fluoroscopy. X-ray fluoroscopy exposes the patients and medical staff to significant doses of ionizing radiation. Conversely, rtMRI uses non-ionizing radiation with no known genetic effects or changes in chemical binding and no known deleterious effects to health.44 rtMRI guided TAVR using the CoreValve® doesn’t require the use of rapid ventricular pacing to aid with ventricular unloading, as the valve design doesn’t occlude trans-aortic blood flow during deployment. While balloon aortic pre-dilation with rapid ventricular pacing is used prior to CoreValve® deployment, several authors have found similar outcomes without pre-dilation.45–47 Also, rtMRI guidance does not necessitate the use of contrast media for a TAVR; however, fluoroscopy usually requires the use of contrast media to aid in visualization.21–23 Contrast media has been associated with multiple complications, such as contrast-induced nephropathy and anaphylaxis.48 While gadolinium can be used with the first-pass perfusion scan during post-deployment scanning to evaluate myocardial perfusion, it is not required to complete the procedure, especially if the patient has risk factors for contrast-induced nephropathy.41 Utilizing rtMRI guidance may allow the use of TAVR in patients with renal insufficiency, which is a contraindication to the procedure.15,49
Although rtMRI has multiple benefits over conventional imaging modalities for TAVR, it also has several hurdles that would need to be overcome prior to a clinical model. Some of the limitations include non-MRI compatible implants and the cost of a hybrid MRI setup. Unlike fluoroscopy and CT, the continuous interaction between an MRI’s magnetic field with ferromagnetic materials lead to the potential cause of movement and heating of metallic objects and damage to electronic circuits. These phenomena require special attention for screening patients prior to rtMRI procedures. Imaging of patients with non-MRI compatible implants such as some implanted cardiac devices is contraindicated. Prior to rtMRI guided TAVR, patients would need to follow routine MRI screening protocols including a standardized questionnaire to ensure that there are no contraindications to imaging.50–52 Patients with non-MRI compatible implants will be excluded from rtMRI guidance.
Another hurdle is the technology and resources required to establish an rtMRI TAVR program, which can be a costly and time consuming endeavor. Our setup includes a hybrid MRI catheterization suite. We have an MRI compatible table in a procedure portion of the room, where the introducer sheath can be sterilely placed. The table is then remotely moved into the adjacent MRI bore for imaging and placement under rtMRI guidance. In case of a surgical emergency, the MRI table can be retracted from the magnet and the patient can be immediately rolled into an adjacent fully equipped operating room. The MRI catheterization suite and operating room both have full angiographic capabilities. While we used general anesthesia for our animal experiments, the MRI catheterization suite has the appropriate equipment to perform the procedure under sedation or general anesthesia. The components include an MRI compatible anesthetic equipment as well as MRI compatible telemetry, infusion pumps, and invasive hemodynamic monitoring. The operating room, adjacent to the MRI catheterization suite, has capabilities for cardiopulmonary bypass. This setup is designed for a clinical model where intraprocedural complications may dictate aborting the procedure for immediate cardiopulmonary bypass and operative repair.
CONCLUSION
Our results demonstrate that the Medtronic CoreValve® can be easily and effectively deployed from the left subclavian approach utilizing rtMRI, an active guidewire, and a modified valve delivery catheter system in swine. rtMRI guidance is unique in that this single imaging modality was successfully used for pre-deployment imaging, imaging guidance for valve deployment, and post-deployment scanning. Pre-deployment imaging allowed for precise and efficient measurement of the aortic annulus and aortic root to confirm their size. The TAVR procedure with rtMRI guidance allowed for adequate visualization of anatomic structures as well as the active guidewire, delivery device, and stent. Post-deployment imaging allowed immediate evaluation of valve function as well as assessment for regurgitation and myocardial perfusion. While rtMRI guidance has several limitations, including the cost and technical challenges of establishing a hybrid MRI suite with the necessary imaging equipment and trained medical staff, we believe that the benefits of this imaging modality far outweigh its hindrances. We have been able to successfully demonstrate in several pre-clinical experiments the feasibility of rtMRI guidance in performing a TAVR. We also have demonstrated the benefits of a single imaging modality for pre-deployment imaging, imaging guidance for valve deployment, and post-deployment scanning. This method eliminates the morbidity of radiation exposure, rapid ventricular pacing, contrast media renal toxicity, and a more invasive procedure. While we don’t envision rtMRI to supplant the current modalities for TAVR, we do envision a significant role for this modality in the near future. The success of this pre-clinical model with the use of a commercially available TAVR bioprosthetic valve brings the rtMRI guided approach closer to a clinical reality.
Perspective Statement.
Real-time MRI guided TAVR overcomes the limitations of the current imaging modalities. This method provides superior visualization and deployment with pinpoint accuracy while eliminating the morbidity of radiation exposure, rapid ventricular pacing, and contrast media renal toxicity.
Acknowledgments
Funding: This research received funding from intramural NHLBI
The authors would like to thank Medtronic for generously providing the CoreValve® bioprosthetic valves for these experiments. The authors had full freedom of investigation.
Glossary of Abbreviations
- CT
Computed tomography
- Fr
French
- MDCT
Multidetector computed tomography
- rtMRI
Real-time magnetic resonance image
- TAVR
Transcatheter aortic valve replacement
- TEE
Transesophageal echocardiography
Footnotes
Conflicts of Interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coffey S, Cox B, Williams MJ. The prevalence, incidence, progression, and risks of aortic valve sclerosis: a systematic review and meta-analysis. Journal of the American College of Cardiology. 2014;63(25 Pt A):2852–2861. doi: 10.1016/j.jacc.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 2.Maganti K, Rigolin VH, Sarano ME, Bonow RO. Valvular heart disease: diagnosis and management. Mayo Clinic proceedings. 2010;85(5):483–500. doi: 10.4065/mcp.2009.0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. Journal of the American College of Cardiology. 1997;29(3):630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 4.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368(9540):1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 5.Ross J, Jr, Braunwald E. Aortic stenosis. Circulation. 1968;38(1 Suppl):61–67. doi: 10.1161/01.cir.38.1s5.v-61. [DOI] [PubMed] [Google Scholar]
- 6.Carabello BA. Introduction to aortic stenosis. Circulation research. 2013;113(2):179–185. doi: 10.1161/CIRCRESAHA.113.300156. [DOI] [PubMed] [Google Scholar]
- 7.Turina J, Hess OM, Krayenbuhl HP. Spontaneous course of aortic valve disease and indications for aortic valve replacement. Schweizerische medizinische Wochenschrift. 1988;118(14):508–516. [PubMed] [Google Scholar]
- 8.Leon MB, Smith CR, Mack M, et al. Transcatheter Aortic-Valve Implantation for Aortic Stenosis in Patients Who Cannot Undergo Surgery. New England Journal of Medicine. 2010;363(17):1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 9.Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. The New England journal of medicine. 2010;363(17):1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 10.Otto CM. Timing of aortic valve surgery. Heart (British Cardiac Society) 2000;84(2):211–218. doi: 10.1136/heart.84.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly TA, Rothbart RM, Cooper CM, Kaiser DL, Smucker ML, Gibson RS. Comparison of outcome of asymptomatic to symptomatic patients older than 20 years of age with valvular aortic stenosis. The American journal of cardiology. 1988;61(1):123–130. doi: 10.1016/0002-9149(88)91317-3. [DOI] [PubMed] [Google Scholar]
- 12.Saeedi M, Thomas A, Shellock FG. Evaluation of MRI issues at 3-Tesla for a transcatheter aortic valve replacement (TAVR) bioprosthesis. Magnetic resonance imaging. 2015 doi: 10.1016/j.mri.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Kindzelski BA, Zhou Y, Horvath KA. Transmyocardial revascularization devices: technology update. Med Devices (Auckl) 2015;8:11–19. doi: 10.2147/MDER.S51591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kindzelski BA, Li M, Mazilu D, Hunt T, Horvath KA. Real-time magnetic resonance-guided aortic valve replacement using Engager valve. Ann Thorac Surg. 2014;98(6):2194–2199. doi: 10.1016/j.athoracsur.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horvath KA, Mazilu D, Cai J, Kindzelski B, Li M. Transapical sutureless aortic valve implantation under magnetic resonance imaging guidance: Acute and short-term results. J Thorac Cardiovasc Surg. 2015;149(4):1067–1072. doi: 10.1016/j.jtcvs.2014.10.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hahn RT. Use of imaging for procedural guidance during transcatheter aortic valve replacement. Curr Opin Cardiol. 2013;28(5):512–517. doi: 10.1097/HCO.0b013e3283632b5e. [DOI] [PubMed] [Google Scholar]
- 17.Bloomfield GS, Gillam LD, Hahn RT, et al. A practical guide to multimodality imaging of transcatheter aortic valve replacement. JACC Cardiovasc Imaging. 2012;5(4):441–455. doi: 10.1016/j.jcmg.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Jilaihawi H, Kashif M, Fontana G, et al. Cross-sectional computed tomographic assessment improves accuracy of aortic annular sizing for transcatheter aortic valve replacement and reduces the incidence of paravalvular aortic regurgitation. J Am Coll Cardiol. 2012;59(14):1275–1286. doi: 10.1016/j.jacc.2011.11.045. [DOI] [PubMed] [Google Scholar]
- 19.Husser O, Holzamer A, Resch M, et al. Prosthesis sizing for transcatheter aortic valve implantation--comparison of three dimensional transesophageal echocardiography with multislice computed tomography. Int J Cardiol. 2013;168(4):3431–3438. doi: 10.1016/j.ijcard.2013.04.182. [DOI] [PubMed] [Google Scholar]
- 20.Smith LA, Dworakowski R, Bhan A, et al. Real-time three-dimensional transesophageal echocardiography adds value to transcatheter aortic valve implantation. J Am Soc Echocardiogr. 2013;26(4):359–369. doi: 10.1016/j.echo.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 21.McCullough PA, Wolyn R, Rocher LL, Levin RN, O’Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. The American journal of medicine. 1997;103(5):368–375. doi: 10.1016/s0002-9343(97)00150-2. [DOI] [PubMed] [Google Scholar]
- 22.Bruschi G, De Marco F, Martinelli L, Klugmann S. CoreValve(R) transcatheter self-expandable aortic bioprosthesis. Expert review of medical devices. 2013;10(1):15–26. doi: 10.1586/erd.12.64. [DOI] [PubMed] [Google Scholar]
- 23.Horvath KA, Mazilu D, Guttman M, Zetts A, Hunt T, Li M. Midterm results of transapical aortic valve replacement via real-time magnetic resonance imaging guidance. J Thorac Cardiovasc Surg. 2010;139(2):424–430. doi: 10.1016/j.jtcvs.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saeed M, Hetts SW, English J, Wilson M. MR fluoroscopy in vascular and cardiac interventions (review) The international journal of cardiovascular imaging. 2012;28(1):117–137. doi: 10.1007/s10554-010-9774-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts TP, Hassenzahl WV, Hetts SW, Arenson RL. Remote control of catheter tip deflection: an opportunity for interventional MRI. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2002;48(6):1091–1095. doi: 10.1002/mrm.10325. [DOI] [PubMed] [Google Scholar]
- 26.Horvath KA, Mazilu D, Kocaturk O, Li M. Transapical aortic valve replacement under real-time magnetic resonance imaging guidance: experimental results with balloon-expandable and self-expanding stents. Eur J Cardiothorac Surg. 2011;39(6):822–828. doi: 10.1016/j.ejcts.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.CoreValve™ System [Package Insert] Minneapolis, MN: Medtronic, Inc; 2014. [Google Scholar]
- 28.Grube E, Laborde JC, Gerckens U, et al. Percutaneous implantation of the CoreValve self-expanding valve prosthesis in high-risk patients with aortic valve disease: the Siegburg first-in-man study. Circulation. 2006;114(15):1616–1624. doi: 10.1161/CIRCULATIONAHA.106.639450. [DOI] [PubMed] [Google Scholar]
- 29.Kocaturk O, Kim AH, Saikus CE, et al. Active two-channel 0.035″ guidewire for interventional cardiovascular MRI. J Magn Reson Imaging. 2009;30(2):461–465. doi: 10.1002/jmri.21844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guttman MA, Kellman P, Dick AJ, Lederman RJ, McVeigh ER. Real-time accelerated interactive MRI with adaptive TSENSE and UNFOLD. Magn Reson Med. 2003;50(2):315–321. doi: 10.1002/mrm.10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McVeigh ER, Guttman MA, Lederman RJ, et al. Real-time interactive MRI-guided cardiac surgery: aortic valve replacement using a direct apical approach. Magn Reson Med. 2006;56(5):958–964. doi: 10.1002/mrm.21044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horvath KA, Guttman M, Li M, et al. Beating heart aortic valve replacement using real-time MRI guidance. Innovations (Phila) 2007;2(2):51–55. doi: 10.1097/IMI.0b013e31805b8280. [DOI] [PubMed] [Google Scholar]
- 33.Horvath KA, Li M, Mazilu D, Guttman MA, McVeigh ER. Real-time magnetic resonance imaging guidance for cardiovascular procedures. Seminars in thoracic and cardiovascular surgery. 2007;19(4):330–335. doi: 10.1053/j.semtcvs.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lederman RJ. Cardiovascular interventional magnetic resonance imaging. Circulation. 2005;112(19):3009–3017. doi: 10.1161/CIRCULATIONAHA.104.531368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kellman P, Derbyshire JA, Agyeman KO, McVeigh ER, Arai AE. Extended coverage first-pass perfusion imaging using slice-interleaved TSENSE. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2004;51(1):200–204. doi: 10.1002/mrm.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shanewise JS, Cheung AT, Aronson S, et al. ASE/SCA guidelines for performing a comprehensive intraoperative multiplane transesophageal echocardiography examination: recommendations of the American Society of Echocardiography Council for Intraoperative Echocardiography and the Society of Cardiovascular Anesthesiologists Task Force for Certification in Perioperative Transesophageal Echocardiography. Anesthesia and analgesia. 1999;89(4):870–884. doi: 10.1097/00000539-199910000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Moss RR, Ivens E, Pasupati S, et al. Role of echocardiography in percutaneous aortic valve implantation. JACC. Cardiovascular imaging. 2008;1(1):15–24. doi: 10.1016/j.jcmg.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Kahlert P, Parohl N, Albert J, et al. Towards real-time cardiovascular magnetic resonance guided transarterial CoreValve implantation: in vivo evaluation in swine. J Cardiovasc Magn Reson. 2012;14:21. doi: 10.1186/1532-429X-14-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kahlert P, Eggebrecht H, Plicht B, et al. Towards real-time cardiovascular magnetic resonance-guided transarterial aortic valve implantation: in vitro evaluation and modification of existing devices. J Cardiovasc Magn Reson. 2010;12:58. doi: 10.1186/1532-429X-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dick AJ, Guttman MA, Raman VK, et al. Magnetic resonance fluoroscopy allows targeted delivery of mesenchymal stem cells to infarct borders in Swine. Circulation. 2003;108(23):2899–2904. doi: 10.1161/01.CIR.0000095790.28368.F9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quail MA, Nordmeyer J, Schievano S, Reinthaler M, Mullen MJ, Taylor AM. Use of cardiovascular magnetic resonance imaging for TAVR assessment in patients with bioprosthetic aortic valves: comparison with computed tomography. European journal of radiology. 2012;81(12):3912–3917. doi: 10.1016/j.ejrad.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 42.Tsang W, Bateman MG, Weinert L, et al. Accuracy of aortic annular measurements obtained from three-dimensional echocardiography, CT and MRI: human in vitro and in vivo studies. Heart (British Cardiac Society) 2012;98(15):1146–1152. doi: 10.1136/heartjnl-2012-302074. [DOI] [PubMed] [Google Scholar]
- 43.Koos R, Altiok E, Mahnken AH, et al. Evaluation of aortic root for definition of prosthesis size by magnetic resonance imaging and cardiac computed tomography: implications for transcatheter aortic valve implantation. International journal of cardiology. 2012;158(3):353–358. doi: 10.1016/j.ijcard.2011.01.044. [DOI] [PubMed] [Google Scholar]
- 44.Tsekos NV, Khanicheh A, Christoforou E, Mavroidis C. Magnetic resonance-compatible robotic and mechatronics systems for image-guided interventions and rehabilitation: a review study. Annual review of biomedical engineering. 2007;9:351–387. doi: 10.1146/annurev.bioeng.9.121806.160642. [DOI] [PubMed] [Google Scholar]
- 45.Grube E, Naber C, Abizaid A, et al. Feasibility of transcatheter aortic valve implantation without balloon pre-dilation: a pilot study. JACC. Cardiovascular interventions. 2011;4(7):751–757. doi: 10.1016/j.jcin.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 46.Mendiz OA, Fraguas H, Lev GA, Valdivieso LR, Favaloro RR. Transcatheter aortic valve implantation without balloon predilation: a single-center pilot experience. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2013;82(2):292–297. doi: 10.1002/ccd.24805. [DOI] [PubMed] [Google Scholar]
- 47.Fiorina C, Maffeo D, Curello S, et al. Direct transcatheter aortic valve implantation with self-expandable bioprosthesis: feasibility and safety. Cardiovascular revascularization medicine : including molecular interventions. 2014;15(4):200–203. doi: 10.1016/j.carrev.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 48.Hong SJ, Wong JT, Bloch KJ. Reactions to radiocontrast media. Allergy and asthma proceedings : the official journal of regional and state allergy societies. 2002;23(5):347–351. [PubMed] [Google Scholar]
- 49.Al-Lamee R, Godino C, Colombo A. Transcatheter aortic valve implantation: current principles of patient and technique selection and future perspectives. Circ Cardiovasc Interv. 2011;4(4):387–395. doi: 10.1161/CIRCINTERVENTIONS.111.961128. [DOI] [PubMed] [Google Scholar]
- 50.Kanal E, Borgstede JP, Barkovich AJ, et al. American College of Radiology White Paper on MR Safety: 2004 update and revisions. AJR. American journal of roentgenology. 2004;182(5):1111–1114. doi: 10.2214/ajr.182.5.1821111. [DOI] [PubMed] [Google Scholar]
- 51.Pohost GM. Editor’s page: is CMR safe? J Cardiovasc Magn Reson. 2001;3(3):ix. doi: 10.1081/jcmr-100107464. [DOI] [PubMed] [Google Scholar]
- 52.Sierra M, Machado C. Magnetic resonance imaging in patients with implantable cardiac devices. Reviews in cardiovascular medicine. 2008;9(4):232–238. [PubMed] [Google Scholar]