Abstract
Background & Aims
Sepsis is an acute systemic inflammatory response to infection associated with high patient mortality (28-40%). We hypothesized that interleukin (IL)-30, a novel cytokine protecting mice against liver injury resulted from inflammation, would generate a protective effect against systemic inflammation and sepsis-induced death.
Methods
Sepsis was induced by lipopolysaccharide (LPS) or cecal ligation and puncture (CLP). The inhibitory effects of IL-30 on septic inflammation and associated therapeutic effects were determined in wild-type, IL-30 (p28)−/−, IL10−/−, and CD1d−/− mice.
Results
Mice treated with pIL30 gene therapy or recombinant IL-30 protein (rIL30) were protected from LPS-induced septic shock or CLP-induced polymicrobial sepsis and showed markedly less liver damage and lymphocyte apoptosis than control septic mice. The resulting reduction in mortality was mediated through attenuation of the systemic pro-inflammatory response and augmentation of bacterial clearance. Mice lacking IL-30 were more sensitive to LPS-induced sepsis. Natural killer–like T cells (NKT) produced much higher levels of IL-10 and lower levels of interferon–gamma and tumor necrosis factor–alpha in IL-30–treated septic mice than in control septic mice. Likewise, deficiency in IL-10 or NKT cells abolished the protective role of IL-30 against sepsis. Furthermore, IL-30 induced IL-10 production in purified and LPS-stimulated NKT cells. Blocking IL-6R or gp130 inhibited IL-30 mediated IL-10 production.
Conclusions
IL-30 is important in modulating production of NKT cytokines and subsequent NKT cell–mediated immune regulation of other cells. Therefore, IL-30 has a role in prevention and treatment of sepsis via modulation of cytokine production by NKT.
Keywords: Sepsis, liver injury, IL-10, NF-κB, natural killer T cells, IL-6R, gp130
Introduction
Interleukin (IL)-30, a subunit of IL-27, was recently revealed to have IL-27–independent biological functions [1]. One of its identified functions is repair of liver injury induced by acute and chronic inflammation. IL-30 regulates the intrinsic ability of CD4+ T cells to produce interferon gamma (IFN-γ) in acute liver inflammation [2,3]. In chronic liver inflammation, IL-30 recruits natural-killer–like T (NKT) cells to the liver to remove activated hepatic stellate cells [4]. Another of its functions is inhibition of central nervous system autoimmunity via antagonizing Th1 and Th17 responses in experimental autoimmune uveitis [1,5]. These limited in vivo studies show that IL-30 has an anti-inflammatory role in inflammatory diseases. Expansion of this concept in other inflammatory disease models is needed to better understand this role and the mechanisms underlying it.
Sepsis, a systemic inflammatory response to infection, is the most common cause of patient mortality in intensive care units, with a global incidence of approximately 18 million cases per year and a mortality rate of 28-40% [6]. Cancer patients are nearly 10 times more susceptible to sepsis than people without cancer, and sepsis accounts for 8.5% of all deaths among cancer patients [7]. Inflammation and opposing immunosuppression occur concomitantly in sepsis. The early organ dysfunction observed in the setting of sepsis is secondary both to cellular activation by bacterial products, including lipopolysaccharide (LPS), and elaborated inflammatory cytokines. Levels of various potent cytokines, including tumor necrosis factor alpha (TNF-α) and IFN-γ, are higher in patients with sepsis than in persons without sepsis, and these higher cytokine levels are associated with higher morbidity and mortality and development of multiple system organ dysfunction with subsequent organ failure [8]. EBI3, another subunit of IL-27, has been shown to act as a negative feedback mechanism that limits protective innate immune responses in sepsis, and a high expression level of EBI3 is positively correlated with high mortality rate in children with sepsis [9,10]. The role of IL-30 in sepsis has not been studied.
We hypothesized that IL-30 would protect mice from systemic inflammation and sepsis-induced death. Using LPS-induced septic shock and cecal ligation and puncture (CLP)–induced polymicrobial septic models, we identified a novel function of IL-30 in sepsis and its associated mechanism.
Materials and Methods
Animals
Male C3H/HeJ, C57BL/6J, CD1D−/−, and B6129SF1/J mice aged 6 to 8 weeks were used in experiment (Jackson Laboratory, Bar Harbor, ME). IL30−/− mice were generated as described in Supplemental Materials and Methods. Mice were injected with cytokine-encoding (pIL30 or pIL27) or control plasmid (pCtrl) cDNA into each of its two hind limb tibialis muscles by electroporation (a total of 10 μg per mouse; 5 μg per muscle in a volume of 30 μL per muscle) [2]. Three days later, the mice were subjected to an inflammatory challenge with LPS or CLP surgery. Recombinant IL-30 protein (rIL30; 1 μg) or control vehicle (PBS) were injected twice at 24 h and 1 h before LPS challenge for preventive treatment; one dose of rIL30 were injected at 1 h after CLP challenge for therapeutic treatment [11]. Reagents and plasmids are described in detail in Supplemental Materials and Methods.
Sepsis models
For the LPS-induced septic shock model, mice were injected intraperitoneally with LPS (Escherichia coli 0111:B4; Sigma-Aldrich, St. Louis, MO) at doses varying from 20 mg/kg to 70 mg/kg in different mouse strains. For the polymicrobial sepsis model, sepsis was induced in mice by the CLP surgical technique established by Dr. Rittirsch [12]. In brief, the cecum was exposed by a midline incision and then tied off with a 3-0 silk ligature 1 cm from the distal end. The ligated portion was then subjected to one puncture with a 21-gauge needle. As a control, sham surgery mice were subjected to anesthesia and laparotomy but not ligation and puncture of the cecum. C3H/HeJ mice were orthotopically inoculated with LM8 osteosarcoma cells (1×105) to generate tumors and subjected to LPS or CLP challenge 13 days later (tumor diameter reached 5~10mm). Their survival was monitored and recorded every 8 h for 6 days. Mice were euthanized immediately if they remained moribund for three consecutive 8-h check-ups.
Pathology review
Mice that were subjected to the LPS challenge were killed 24 h later, and their spleen, liver, left lower lobe of the lung, kidney, and intestine were isolated, fixed, embedded in paraffin, and cut into 4-mm sections. The tissue sections were stained with hematoxylin and eosin (H&E). All tissue sections from each mouse were examined microscopically for pathologic signs. Two independent experts blinded to the treatment assessed the degree of inflammation and cell death and/or apoptosis. Each section was scored on a 5-point scale: grade 0 = no histologic lesion; 1 = minimal or rare lesion (lesion affected less than 10% of the tissue); 2 = mild or infrequent lesion (lesion affected 10-20% of tissue); 3 = moderate or frequent lesion (lesion affected 20-40% of tissue); 4 = marked, extensive, or severe lesion (lesion affected 40-100% of tissue). The final average scores were analyzed to determine the significance of differences between the groups of mice.
Intracellular cytokine staining and flow cytometry
Mice were injected intraperitoneally with LPS at a dose of 20 mg/kg and were sacrificed 6 h later. Their spleen, liver, and lungs were collected for analysis. Four hours before death, each mouse was injected with Brefeldin A (250 μg/mouse; Sigma) to stop cytokine secretion. The spleens were minced over a 70-μm cell strainer. The livers and lungs were cut into 1-mm3 pieces and digested with 0.025% collagenase (25 mg in 100 mL) at 37°C, then shaken at 200 shakes/min for 30 min (liver) or 60 min (lung). Single cell suspensions were gotten and red blood cells were subjected to lysis in lysis buffer (Biolegend, San Diego, CA). Liver lymphocytes were suspended in Percoll solution as described elsewhere [13]. NKT cells were purified and cultured as described in Supplemental Materials and Methods. NKT cells or splenocytes from septic mice or healthy mice were incubated with rIL30 at indicated concentrations for 3 h to induce IL-10 production in the presence of 5ug/mL BFA. Cell surfaces staining and intracellular staining were performed as described previously [14]. Stained cells were analyzed by flow cytometry and the data by FlowJo software.
Luminex cytokine assay
Mice were injected intraperitoneally with LPS at a dose of 20 mg/kg and were killed 6 or 24 h later. Their serum was collected for analysis. The expression of IFN-γ, IL-6, IL-10, IL-17A, and TNF-α in each serum specimen was quantified by Luminex kits (Millipore) according to the manufacturer's instructions.
Statistical analysis
The data are presented as mean ± standard error of the mean (SEM) for each group. Differences in the experimental means for flow cytometric and cytokine values were considered significant if P was <0.05 as determined by using the one-way analysis of variance on ranks. Differences in survival curves were analyzed by log-rank survival analysis, and differences were considered significant at P<0.05. In vivo experiments were started with 6 mice per group and performed a power analysis based on the result. Then repeat the experiment with the number of mice indicated by the power analysis. All data shown are representative of three independent experiments acquired in triplicate (in vitro) or at least two independent experiments (in vivo). All statistical analyses were conducted by using GraphPad Prism 6 (GraphPad Software, La Jolla, CA).
Results
IL-30 protects against both LPS- and CLP-induced sepsis
To understand the potential mechanisms of IL-30's regulation of inflammation and to investigate the effects of IL-30 on sepsis, two mouse models of sepsis were used. In both models, the mice received electroporation-mediated delivery of IL-30–encoding DNA (pIL30) in muscle, which led to IL-30 expression and circulation in serum. Sepsis was then induced by LPS challenge or CLP.
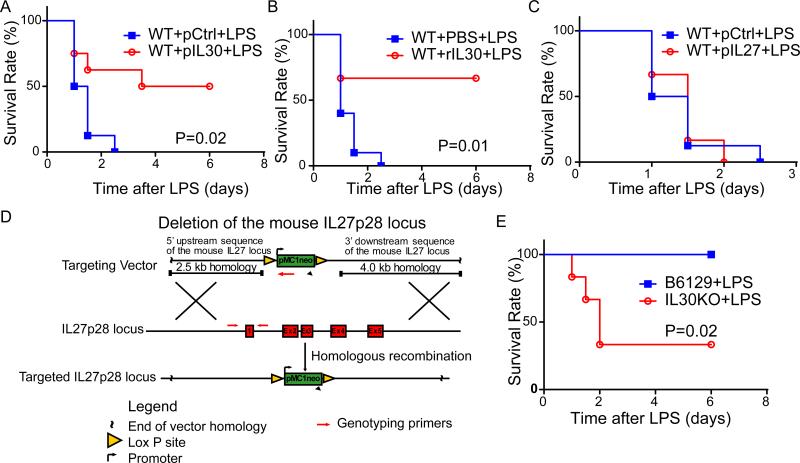
For the LPS model, pIL30 was given 72 h before LPS challenge to ensure that induced IL-30 protein expression was adequate for biological activity, because IL-30 protein production normally lags behind gene delivery [2]. The expression level of IL-30 in serum on day 3 after electroporation was 409.4±30.96 pg/mL (Fig. S1). As expected, pIL30-treated mice, unlike mice treated with control DNA (pCtrl), were resistant to the lethal LPS challenge (P<0.05). All of the pCtrl-expressing mice died within 60 h after LPS injection, while only 50% of the pIL30–treated mice died. Although the surviving mice showed signs of LPS-induced shock in the first hours, the survivors had completely recovered after 84 h after LPS stimulation, showing that pIL30 gene therapy offers significant protection against LPS-induced septic shock (Fig. 1A). Likewise, injected rIL30 protein significantly increased survival rate in LPS-challenged mice (Fig. 1B). IL-30 is one subunit of IL-27. Surprisingly, exogenous expression of IL-27 increased LPS-induced mortality in mice (Fig. 1C), indicating that the effect of IL-30 on sepsis is independent on IL-27.
Figure 1.
IL-30 attenuates lipopolysaccharide (LPS)-induced sepsis. Sepsis was induced in mice by LPS challenge (20 mg/kg for panel A-C and 70 mg/kg for panel E). (A) Survival curve of pIL30- or pCtrl-treated septic C57BL/6 mice. N=8. (B) Survival curve of rIL30- or PBS-treated septic C57BL/6 mice. N=8. (C) Survival curve of pIL27- or pCtrl-treated septic C57BL/6 mice. N=6. (D) Generate IL-30 knockout (KO) mice. The IL-30 locus (middle) was targeted by the targeting vector (top), which contained the homologous sequence of IL-30 and two LoxP site flanking exons 1, 2, 3, 4 and 5. Homologous recombination resulted in the floxed allele (bottom). Neo, neomycin resistance gene. (E) Survival curve of septic IL-30−/− and WT control mice (B6129S). N=6. Results represent at least two independent experiments.
To study the function of endogenous IL-30, IL-30–deficient mice were generated (Fig. 1D). IL-30 deficiency was validated by both genotyping (Fig. S2A) and assessing IL-30 expression level in splenocytes stimulated by LPS plus IFN-γ (Fig. S2B). All IL-30–deficient mice either died or became moribund within 6 h after LPS injection. Forty-eight hours after LPS injection, 66.7% of IL-30–deficient mice were dead, while all wild-type mice survived (Fig. 1E), showing that IL-30 deficiency increases susceptibility to LPS-induced death.
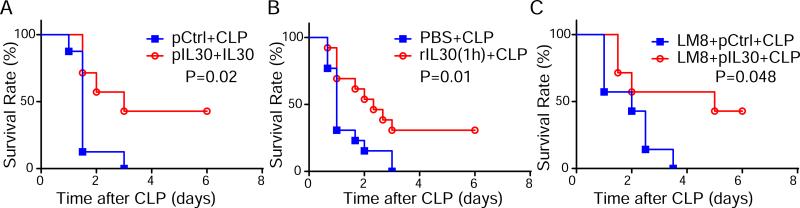
Because sepsis induced by administration of LPS in mice may not accurately replicate many important features of human sepsis, CLP model, the animal model that most closely recapitulates human clinical sepsis, were used to test the effect of IL-30. In this model, 40% of pIL30-treated mice survived and recovered from severe polymicrobial sepsis, whereas all pCtrl-treated mice died within 3 days (Fig. 2A). In consistent with reduced endotoxin level in serum of LPS-challenged mice treated with pIL30 (Fig. S3), bacterial titers were significantly lower in the liver and blood of pIL30–treated than in controls mice challenged with CLP (Fig. S4), showing the increased LPS clearance and bacterial clearance in mice overexpressing IL-30. When rIL30 was administered after onset of sepsis (1 h after CLP), 30% of the treated mice survived (Fig. 2B), indicating both preventive and therapeutic effect of IL-30 on sepsis. It is valuable in finding safe treatment of sepsis for cancer-bearing subject because cancer patients bear 10 fold higher risk in sepsis than cancer-free patients. Significantly, pIL30 decreased mortality due to sepsis in the osteosarcoma-bearing mice (Fig. 2C). Together, these data suggest that both pIL30 gene therapy and rIL30 protein have protective effects against LPS-induced and CLP-induced mortality, and these protective effects do not depend on IL-27. Our findings therefore suggest a potential new role of IL-30 against sepsis.
Figure 2.
IL-30 attenuates cecal ligation and puncture (CLP)-induced sepsis. Sepsis was induced in mice by CLP. (A) Survival data of pIL30- or pCtrl-treated septic C57BL/6 mice. N=7~8. (B) Survival curve of rIL30- or PBS-treated septic C57BL/6 mice. rIL30 (1 μg) or control vehicle (PBS) were injected at a single dose 1 h after CLP. N=12~13. (C) Survival data of pIL30- or pCtrl-treated septic mice bearing LM8 osteosarcoma. N=7. Results represent at least two independent experiments.
IL30 attenuates liver inflammation and hepatocyte damage
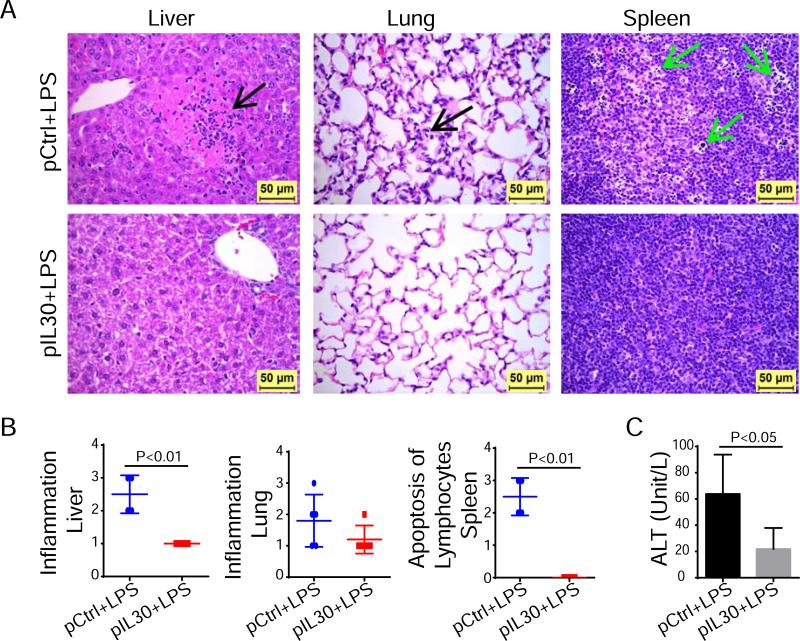
Sepsis is associated with organ injury and function failure. Mice challenged with LPS had various degrees of acute inflammation in the liver, lung, kidney, and small intestine, as well as cell death or apoptosis of individual hepatocytes, lymphocytes in the splenic lymphoid tissue, and epithelial cells lining the crypts of the small intestine (Fig. 3A and Fig. S5A). The degree of inflammatory reaction in the livers of septic mice treated with pIL30 was significantly lower than that in the livers of septic mice treated with pCtrl (grade 1.0±0.0 versus 2.5±0.29) (Fig. 3A, 3B).
Figure 3.
IL-30 attenuates liver injury and suppresses lymphocytes apoptosis of septic mice. Liver, lung, spleen and other tissues were collected 24 h after LPS challenge. (A) Histopathology in liver, lung, and spleen (magnification 400×). Black arrow in liver shows acute inflammation and hepatocellular death. Black arrow in lung shows alveolar inflammation. Green arrows in spleen show numerous lymphocytes that underwent cell death or apoptosis. (B) Histologic grades of lesions in tissues. N=4~5. (C) Alanine aminotransferase (ALT) level in serum of septic mice 24 h after LPS challenge. N=6. Results represent two independent experiments.
The inflammatory responses in the lung, small intestine, and kidney were slightly lower in pIL30-treated than in pCtrl-treated septic mice, but the differences were not statistically significant (Fig. 3A, 3B and Fig. S5A, S5B). The most severe cell death or apoptosis was detected in the lymphocytes of the splenic lymphoid tissue of pCtrl-treated septic mice, while pIL30-treated septic mice had normal lymphocytes (Fig. 3). Also, the incidence of single cell death or apoptosis of hepatocytes was significantly lower in pIL30-treated septic mice than in pCtrl-treated septic mice (incidence 0/4 vs 4/4). These results support a role for IL-30 in protecting hepatocytes and lymphocytes against septic injury. To determine the adequacy of liver function after LPS challenge, the concentration of liver enzyme alanine aminotransferase (ALT), which is released into the circulation upon injury, was investigated 24 h after LPS challenge. ALT level was lower in pIL30-treated septic mice than in pCtrl-treated septic mice (21.8±8.0 vs 63.8±15.0 U/L; Fig. 3C). Together, these results show that IL-30 protects the liver against sepsis-induced dysfunction and injury and reduces lymphocytes apoptosis.
IL-30 increases IL-10 production and decreases IFN-γ and TNF-α production
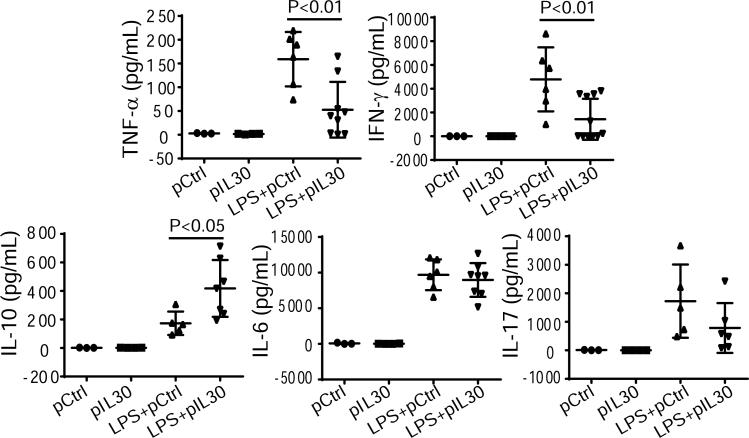
The protective effects in pIL30-treated mice challenged with LPS were accompanied by changes in the levels of specific cytokine expression when compared with pCtrl-treated mice. Six hours after LPS challenge, pIL30-treated mice had a lower plasma concentration of pro-inflammatory cytokines IFN-γ and TNF-α (1430±521 vs 4788±1100 pg/mL, 52.7±19.6 vs 159.1±23.4 pg/mL, respectively) and higher concentration of anti-inflammatory cytokine IL-10 (417.9±75.3 vs 173.2±35.6 pg/mL) than pCtrl-treated mice (Fig. 4). But no significant decrease in IL-6 and IL-17A level in serum of pIL30-treated mice. Levels of IFN-γ and TNF-α remained suppressed (253.9±146.2 vs 3442±1466 pg/mL, 21.75±11.3 vs 94.83±18.02 pg/mL, respectively) in pIL30-treated mice 24 h after LPS challenge, but the level of IL-10 was almost the same as that in pCtrl-treated mice (237.4±135.3 vs 307.4±88.91 pg/mL; Fig. S6). These findings show that IL-30 expression results in specific modulation of cytokine production induced by LPS.
Figure 4.
IL-30 increases IL-10 production and suppresses IFN-γ and TNF-α production in serum of septic mice. Sepsis was induced by LPS challenge. Levels of cytokines (IFN-γ, IL-10, TNF-α, IL-17, and IL-6) in serum of pIL30- or pCtrl– treated mice 6 h after LPS challenge. Results represent two independent experiments.
IL-30 suppresses inflammation by inducing IL-10–producing NKT cells and reducing IFN-γ–producing NKT cells
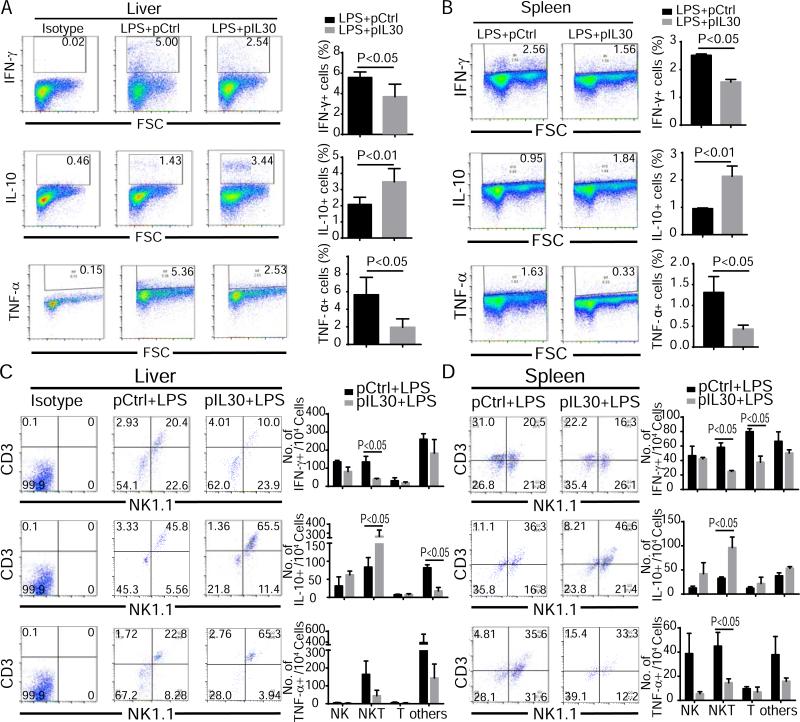
To determine which tissues were responsible for cytokine production changes in pIL30-treated mice after LPS challenge, cells representing three important organs related to sepsis (liver lymphocytes, splenocytes, and immune cells in lung) were examined in pCtrl- and pIL30-treated mice. As demonstrated in Figure 5, the pIL30-treated mice had fewer IFN-γ+ and TNF-α+ and more IL-10+ liver lymphocytes and splenocytes than pCtrl-treated mice (Fig. 5A, 5B). In contrast, the pIL30-treated mice had fewer TNF-α+ lung immune cells than pCtrl-treated mice, but no marked difference in IFN-γ+ or IL-10+ lung immune cells (Fig. S8A). This result shows that the regulatory effect of IL-30 on inflammation is more effective in liver and spleen than in lung.
Figure 5.
IL-30 attenuates IFN-γ and TNF-α production and augments IL-10 production in NKT cells of septic mice. Liver and spleen tissues were collected 6 h after LPS challenge. Production of cytokines in immune cells were analyzed by flow cytometry. (A, B) IFN-γ+, IL-10+, and TNF-α+ lymphocytes were quantified in liver (A) and spleen (B). (C, D) Cytokine-positive liver lymphocytes (C) and splenocytes (D) cells were stained with anti-CD3 and anti-NK1.1 to show the cell source of cytokine production. N=6. Results represent three independent experiments.
Zhang et al. found that CD4+ cells were the main target of IL-30 in ConA-induced liver injury [3]. In our previous study, NKT cells were the population mainly regulated by IL-30 in liver fibrosis [4]. To elucidate the cellular source for the protective effects of IL-30 in sepsis, we analyzed the T cell, NK cell, and NKT cell populations among the cytokine-producing cells of mice. The results showed that about 23.8% of IFN-γ–producing cells, 44.5% of IL-10–producing cells, and 29% of TNF-α–producing cells in the liver and 23.2% of IFN-γ–producing cells, 34.3% of IL-10–producing cells, and 36.2% of TNF-α–producing cells in the spleen were NKT cells, indicating that NKT cells produce significant amounts of IFN-γ, TNF-α, and IL-10 in sepsis conditions. Compared to pCtrl-treated mice, pIL30-treated mice showed markedly lower numbers of NKT cells producing IFN-γ and higher numbers of NKT cells producing IL-10 in the liver and spleen, as well as significantly lower numbers of TNF-α–producing NKT cells in the spleen but not in the liver (Fig. 5C, 5D). In lung tissues, the only difference was fewer TNF-α–producing cells in pIL30-treated mice than in pCtrl-treated mice (Fig. S8). These data indicate that IL-30 regulates the nature of cytokine responses to exposure to LPS in NKT cells.
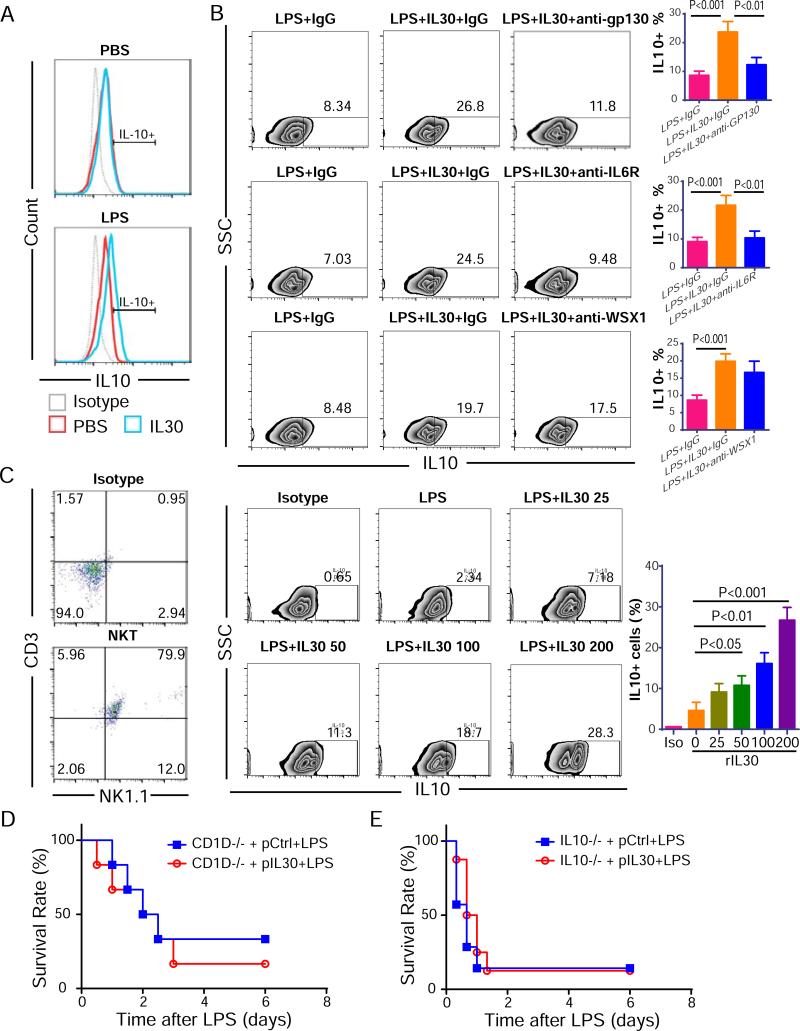
In in vitro, stimulating normal splenocytes with a high dose of rIL30 did not increase the IL10+ cell population; but in splenocytes from septic mice, stimulated with a high dose of rIL30 increased the IL10+ cell population from 9.3±2.0% to 23.7±3.6% (Fig 6A). The induction in IL-10 was blocked by anti-gp130 or anti-IL-6Rα, but not anti-WSX1 neutralization antibody, establishing that this IL-30–mediated IL-10 induction is dependent on signaling through gp130 and IL-6R but not WSX1 (Fig. 6B). We confirmed IL-10 inducing effect of IL-30 in isolated NKT cells. rIL-30 increased IL-10 expression in a dose-dependent manner, with significant effect at a concentration ≥50 ng/mL (Fig. 6C). To validate the observation that the protective effect of IL-30 is mediated through IL-10 production in NKT cells, NKT-deficient CD1D−/− mice received pIL30 therapy were challenged with LPS as already described. pIL30 had no beneficial effect on survival of CD1D−/− mice (Fig. 6D), suggesting that NKT cells are required for the effect of IL-30 and are critical for the protective effect of IL-30 against LPS-caused sepsis. Also, the protective effect of pIL30 against LPS-induced sepsis was lost in the absence of IL-10 in IL-10 KO mice (Fig. 6E), which agrees with the observation that pIL30 elevated the number of IL-10–producing cells (mainly IL-10+ NKT cells) after LPS challenge. Therefore, NKT cells, especially IL-10–producing NKT cells, are the key effector cells responding to IL-30 treatment during sepsis.
Figure 6.
NKT cells and IL-10 are required for the anti-inflammatory effect of IL-30 in septic mice. (A) The induction of IL-10 by rIL-30 in PBS/lipopolysaccharide (LPS)-stimulated splenocytes. (B) rIL-30–induced production of IL-10 was blocked by anti-gp130 or anti-IL-6Rα, but not anti-WSX1 neutralizing antibodies. (C) rIL-30 induced IL-10 production in purified and LPS-stimulated NKT cells. The purity of NKT cells was detected after culturing for 4 days. NKT cells were stimulated as listed in panel and IL-10 production measured. (D) Survival curve of pIL30- or pCtrl– treated CD1D−/− mice challenged with LPS. N=6. (E) Survival curve of pIL30- or pCtrl– treated IL10−/− mice challenged with LPS. N=6. All data shown are representative of three independent experiments acquired in triplicate in vitro or two independent experiments in vivo.
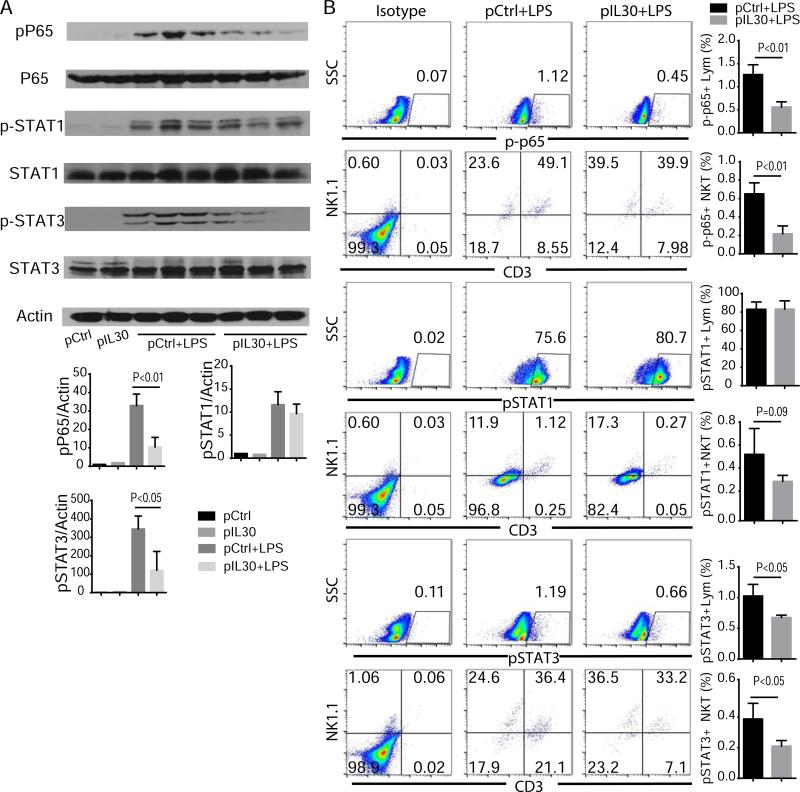
IL-30 suppresses LPS-induced NF-κB and STAT3 activation
To understand the signaling pathway by which IL-30 suppresses LPS-induced inflammation, we investigated the activation of NF-κB, STAT1, and STAT3 in septic mice, because LPS is known to stimulate activation of these transcription factors and induce cytokine expression [15]. Indeed, western blotting analysis showed that LPS challenge increased the phosphorylation of NF-κB p65, STAT1, and STAT3 in liver tissues, while pIL30 treatment decreased the LPS-induced phosphorylation of these transcription factors (Fig. 7A). Similar results were observed in liver lymphocytes on flow cytometry analysis, suggesting that IL-30 preferentially suppresses LPS-induced NF-κB, STAT1, and STAT3 activation in livers. Furthermore, these results also show that NKT cells are the primary cell population in which NF-κB p65 and STAT3 are activated 6 h after LPS challenge. STAT1 phosphorylation occurred mainly in NK cells and CD3−NK1.1− cells. pIL30 treatment significantly reduced the numbers of p-p65+ or p-STAT3+ NKTs in liver lymphocytes 6 h after LPS challenge, indicating that IL-30 signals primarily through NF-κB and STAT3 but not through STAT1 in NKT cells (Fig. 7B). Taken together, these results support the idea that IL-30 mediates its biological (immune regulatory) activities in NKT cells through the NF-κB and STAT3 signaling pathways.
Figure 7.
IL-30 suppresses sepsis-induced NF-κB and STAT3 activation in NKT cells. Liver tissues were collected 6 h after LPS challenge. (A) Immunoblotting data of p-p65, pSTAT1, and pSTAT3 in liver tissues of septic mice treated with pIL30 or pCtrl. N=3. (B) Flow cytometry data of p-p65, pSTAT1, and pSTAT3 in liver lymphocytes cells from septic mice. N=6. Results represent at least two independent experiments.
Discussion
In this study we showed that IL-30 protects mice from both LPS-induced sepsis and CLP-induced bacterial sepsis by suppressing inflammation and decreasing LPS and bacterial loading. IL-30 exerted its anti-inflammatory effect in an NKT- and IL-10–dependent manner, which suppressed IFN-γ and TNF-α production in NKT cells and other immune cells in the liver and spleen. The effect of IL-30 against inflammation in LPS-induced sepsis was mediated via suppression of NF-κB and STAT3 activation via IL-6R and gp130.
IL-27 is composed of IL-30 and EBI3 subunits. It has been shown that IL27 is a diagnosis biomarker of sepsis in critical ill patients and its expression level (especially EBI3 level) is negative associated with the outcome of the patients [10]. In animal experiments, neutralization but not supplement of IL-27 protects mice from septic death, and EBI3 deficiency protected mice from lethal sepsis [9]. There are functions of IL30 and IL30 associated molecular, but no function of IL27, EBI3 itself, and EBI3 associated molecular (such as IL-35 which is composed by EBI3 and IL12p40) in EBI3−/− mice. So EBI3−/− mice were protected from sepsis in the presence of IL-30 and absence of IL-27. However, IL30 only protected mice from septic death, and IL30 deficiency augmented septic death. IL-30−/− mice were susceptible to lethal sepsis in the presence of EBI3 and absence of IL-27. It seems opposite effects of IL-27/EBI3 and IL-30 in sepsis.
IL-30 plays a critical role in sepsis through regulation of the cytokine profiles of NKT cells. NK, NKT, T cells, and macrophages/dendritic cells are all involved in LPS-induced inflammation [16]. NKT cells act as an early amplification step in the innate immune response by producing IFN-γ during sepsis [17]. Either knockout of NKT cells regulated by gene CD1D or blockade of NKT cell signaling with anti-CD1D antibody leads to obliteration of the systemic inflammatory response, resulting in reduced production of TNF and IFN-γ and protecting mice from septic death [18,19]. Our observation that NKT deficiency abolished the protective effect of IL-30 against LPS-induced mortality, showing that the protective effect of IL-30 against sepsis is dependent on NKT cells, is supported by a recent report showing that IL-30–mediated liver protection depends on liver NKT cells in a chronic liver injury model. This observation is also supported by the observation that NKT cells comprise 10-20% of the total T cell population in the liver, a much higher percentage than in other organs [19]. This result is consistent with the pathologic data showing that IL-30 has a protective effect against LPS-induced liver damage but little effect on lung and intestine damage.
IL-27 can promote IL-10 production in Th1, Th2, Th17, Treg, and Tr1 cells and human NK cells [20,21]. The lack of NKT induction data suggests that IL-27 may not induce IL-10 in NKT cells. Here we show that IL-30 induced IL-10 production in the NKT cells of septic mice in vivo and LPS-stimulated NKT cells in vitro. Our published data show that rIL30 boosted IL-10 levels in a dose-dependent manner in CD3/CD28/CpG-stimulated splenocytes, demonstrating that IL-30 can induce IL-10 production [22]. IL-10–producing NKT cells are a distinct NKT cell subset with regulatory potential [23]. Oh and Chung found that IL-10–producing NKT cells inhibit the Th1 cell response but not the Th17 cell response. Our results also show that IL-30 increased the IL-10+ NKT population and decreased the IFN-γ+ NKT cell and IFN-γ+ T cell populations in LPS-challenged mice but had no effect on IL-17A induction. In IL-10−/− mice, the protective effect of IL-30 against sepsis was abolished. These results support a potential therapeutic application of IL-30 in modulating and shifting the NKT cell response to a more anti-inflammatory phenotype. Therefore, our data reveal new aspects of IL-30 anti-inflammatory function.
IL-30 has previously been shown to inhibit Th1 and Th17 differentiation or to antagonize pro-inflammatory cytokines by blocking gp130 signaling to exert its anti-inflammatory effects [1]. In addition to functioning as an antagonist against the gp130 signal pathway, IL-30 can also act as an agonistic cytokine via gp130 signal [24]. IL-30 by itself, or bound to other molecules such as IL-12p40, cytokine-like factor 1 (CLF1), IL-6R, or sIL-6R, activates signal transduction via IL-30/IL-6R classic signaling or IL-30/sIL-6R trans-signaling. And IL30 increase IL6Rα expression in serum of septic mice which may help IL30 signal transduction in different cell types (Fig S7). At high concentration IL30 is able to signal independently of IL-6R. IL-10 production induced by IL-30 was inhibited by blocking IL-6R or gp130, but not by blocking WSX1. This is consistent with a previous finding that splenocytes from wild type or WSX1−/− mice infected with Trypanosoma cruzi produced similar amounts of IL-10 after stimulation [25]. These findings indicate that IL-30 may act as an agonist via sIL-6R/gp130 or IL-6R/gp130 signal to induce IL-10 production in sepsis.
IL-30 signaling regulates phosphorylation of downstream STAT1 and STAT3, which are involved in signal transduction for Toll-like receptor signals and pro-inflammatory cytokine signals [24,26,27]. But these findings were from in vitro studies, and very little is known about the biological significance of these signaling pathways in vivo. The findings of our study reveal that IL-30 can suppress LPS-induced NF-κB and STAT3 activation in septic mice. Furthermore, for the first time we know of, NF-κB activation has been shown to be regulated by the IL-30 signaling pathway during sepsis. NF-κB activation is important for IFN-γ and TNF-α production after LPS challenge, and IL-30–suppressed NF-κB activation may lead to reduction of IFN-γ and TNF-α production during sepsis.
Despite the progress made in the clinical management of sepsis, sepsis morbidity and mortality rates remain high. As demonstrated by Bone et al., no single intervention, except for anti-infective drugs, has improved the survival benefit to patients with sepsis in clinical trials since 1992 [28]. There is an urgent need for novel therapeutics to more effectively treat sepsis. The results presented here demonstrate that IL-30 is an immune-regulatory cytokine inducing NKT cells to produce IL-10, lowering sepsis mortality rates by attenuating liver injury and suppressing lymphocyte apoptosis. As a novel NKT-targeted sepsis treatment, IL-30 could be combined with other molecular modulators that function via other critical organs and different mechanisms, such as IL-33, IL-37, IL-15, and IL-21 [11,29,30], to further enhance the therapeutic effect against sepsis. We propose evaluating combinations of these cytokines with IL-30 as the next step of the investigation into antisepsis therapy.
Supplementary Material
Acknowledgments
This work was supported by NIH grant DK102767. The University of Texas MD Anderson Cancer Center Multidisciplinary Research Program provided supplemental financial support. We thank Dr. Laura Pageon for pathologic analysis and Ms. Kathryn Hale for editorial assistance.
Abbreviations
- ALT
alanine aminotransferase
- CLP
cecal ligation and puncture
- H&E
hematoxylin and eosin
- IFN
interferon
- IL
interleukin
- KO
knockout
- LPS
lipopolysaccharide
- NKT cells
natural killer like T cells
- PBS
phosphate-buffered saline solution
- PCR
polymerase chain reaction
- TNF
tumor necrosis factor
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
None of the authors have an affiliation with or involvement in any organization or entity with any financial interest in this work.
Author's contributions:
JY and SL designed the study; JY, AM, JH, JJC, XX and MG performed the experiments; TD contributed the knockout mice; JY and SL analyzed the data; and JY, LM, and SL wrote the paper.
References
- 1.Stumhofer JS, Tait ED, Quinn III WJ, Hosken N, Spudy B, Goenka R, et al. A role for IL-27p28 as an antagonist of gp130-mediated signaling. Nat Immunol. 2010;11:1119–26. doi: 10.1038/ni.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dibra D, Cutrera J, Xia X, Kallakury B, Mishra L, Li S. Interleukin-30: a novel antiinflammatory cytokine candidate for prevention and treatment of inflammatory cytokine-induced liver injury. Hepatology. 2012;55:1204–14. doi: 10.1002/hep.24814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang S, Liang R, Luo W, Liu C, Wu X, Gao Y, et al. High susceptibility to liver injury in IL-27 p28 conditional knockout mice involves intrinsic interferon-gamma dysregulation of CD4+ T cells. Hepatology. 2013;57:1620–31. doi: 10.1002/hep.26166. [DOI] [PubMed] [Google Scholar]
- 4.Mitra A, Satelli A, Yan J, Xueqing X, Gagea M, Hunter CA, et al. IL-30 (IL27p28) attenuates liver fibrosis through inducing NKG2D-rae1 interaction between NKT and activated hepatic stellate cells in mice. Hepatology. 2014;60:2027–39. doi: 10.1002/hep.27392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chong WP, Horai R, Mattapallil MJ, Silver PB, Chen J, Zhou R, et al. IL-27p28 inhibits central nervous system autoimmunity by concurrently antagonizing Th1 and Th17 responses. J Autoimmun. 2014;50:12–22. doi: 10.1016/j.jaut.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6:813–22. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 7.Danai PA, Moss M, Mannino DM, Martin GS. The epidemiology of sepsis in patients with malignancy. Chest. 2006;129:1432–40. doi: 10.1378/chest.129.6.1432. [DOI] [PubMed] [Google Scholar]
- 8.Schulte W, Bernhagen J, Bucala R. Cytokines in sepsis: potent immunoregulators and potential therapeutic targets--an updated view. Mediat Inflamm. 2013;2013:165974. doi: 10.1155/2013/165974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wirtz S, Tubbe I, Galle PR, Schild HJ, Birkenbach M, Blumberg RS, et al. Protection from lethal septic peritonitis by neutralizing the biological function of interleukin 27. J Exp Med. 2006;203:1875–81. doi: 10.1084/jem.20060471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong HR, Cvijanovich NZ, Hall M, Allen GL, Thomas NJ, Freishtat RJ, et al. Interleukin-27 is a novel candidate diagnostic biomarker for bacterial infection in critically ill children. Crit Care. 2012;16:R213. doi: 10.1186/cc11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alves-Filho JC, Sonego F, Souto FO, Freitas A, Verri WA, Jr, Auxiliadora-Martins M, et al. Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nat Med. 2010;16:708–12. doi: 10.1038/nm.2156. [DOI] [PubMed] [Google Scholar]
- 12.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–6. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu ZX, Govindarajan S, Okamoto S, Dennert G. NK cells cause liver injury and facilitate the induction of T cell-mediated immunity to a viral liver infection. J Immunol. 2000;164:6480–6. doi: 10.4049/jimmunol.164.12.6480. [DOI] [PubMed] [Google Scholar]
- 14.Yan J, Kong LY, Hu J, Gabrusiewicz K, Dibra D, Xia X, et al. FGL2 as a Multimodality Regulator of Tumor-Mediated Immune Suppression and Therapeutic Target in Gliomas. J Natl Cancer Inst. 2015:107. doi: 10.1093/jnci/djv137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–51. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Varma TK, Lin CY, Toliver-Kinsky TE, Sherwood ER. Endotoxin-induced gamma interferon production: contributing cell types and key regulatory factors. Clin Diagn Lab Immunol. 2002;9:530–43. doi: 10.1128/CDLI.9.3.530-543.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leung B, Harris HW. NKT Cells: The Culprits of Sepsis? Journal of Surgical Research. 2011;167(1):87–95. doi: 10.1016/j.jss.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 18.Nagarajan NA, Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J Immunol. 2007;178:2706–13. doi: 10.4049/jimmunol.178.5.2706. [DOI] [PubMed] [Google Scholar]
- 19.Hu CK, Venet F, Heffernan DS, Wang YL, Horner B, Huang X, et al. The role of hepatic invariant NKT cells in systemic/local inflammation and mortality during polymicrobial septic shock. J Immunol. 2009;182:2467–75. doi: 10.4049/jimmunol.0801463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshida H, Hunter CA. The immunobiology of interleukin-27. Annu Rev Immunol. 2015;33:417–43. doi: 10.1146/annurev-immunol-032414-112134. [DOI] [PubMed] [Google Scholar]
- 21.Laroni A, Gandhi R, Beynon V, Weiner HL. IL-27 imparts immunoregulatory function to human NK cell subsets. PLoS One. 2011;6:e26173. doi: 10.1371/journal.pone.0026173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dibra D, Li S. The cell-to-cell coordination between activated T cells and CpG-stimulated macrophages synergistically induce elevated levels of IL-10 via NF-kappaB1, STAT3, and CD40/CD154. Cell Commun Signal. 2013;11:95. doi: 10.1186/1478-811X-11-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sag D, Krause P, Hedrick CC, Kronenberg M, Wingender G. IL-10-producing NKT10 cells are a distinct regulatory invariant NKT cell subset. J Clin Invest. 2014;124:3725–40. doi: 10.1172/JCI72308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garbers C, Spudy B, Aparicio-Siegmund S, Waetzig GH, Sommer J, Holscher C, et al. An interleukin-6 receptor-dependent molecular switch mediates signal transduction of the IL-27 cytokine subunit p28 (IL-30) via a gp130 protein receptor homodimer. J Biol Chem. 2013;288:4346–54. doi: 10.1074/jbc.M112.432955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, et al. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645–55. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 26.Wang RX, Yu CR, Mahdi RM, Egwuagu CE. Novel IL27p28/IL12p40 cytokine suppressed experimental autoimmune uveitis by inhibiting autoreactive Th1/Th17 cells and promoting expansion of regulatory T cells. J Biol Chem. 2012;287:36012–21. doi: 10.1074/jbc.M112.390625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crabe S, Guay-Giroux A, Tormo AJ, Duluc D, Lissilaa R, Guilhot F, et al. The IL-27 p28 subunit binds cytokine-like factor 1 to form a cytokine regulating NK and T cell activities requiring IL-6R for signaling. J Immunol. 2009;183:7692–702. doi: 10.4049/jimmunol.0901464. [DOI] [PubMed] [Google Scholar]
- 28.Dobson GP. Addressing the global burden of sepsis: importance of a systems-based approach. Crit Care Med. 2014;42:e797–8. doi: 10.1097/CCM.0000000000000595. [DOI] [PubMed] [Google Scholar]
- 29.Nold MF, Nold-Petry CA, Zepp JA, Palmer BE, Bufler P, Dinarello CA. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010;11:1014–22. doi: 10.1038/ni.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hotchkiss RS, Opal S. Immunotherapy for sepsis--a new approach against an ancient foe. N Engl J Med. 2010;363:87–9. doi: 10.1056/NEJMcibr1004371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.