Abstract
Background
Withdrawing life-sustaining therapy because of perceived poor neurological prognosis (WLST-N) is a common cause of hospital death after out-of-hospital cardiac arrest (OHCA). Although current guidelines recommend against WLST-N before 72 h (WLST-N<72), this practice is common and may increase mortality. We sought to quantify these effects.
Methods
In a secondary analysis of a multicenter OHCA trial, we evaluated survival to hospital discharge and survival with favorable functional status (modified Rankin Score ≤ 3) in adults alive >1h after hospital admission. Propensity score modeling the probability of exposure to WLST-N<72 based on pre-exposure covariates was used to match unexposed subjects with those exposed to WLST-N<72. We determined the probability of survival and functionally favorable survival in the unexposed matched cohort, fit adjusted logistic regression models to predict outcomes in this group, and then used these models to predict outcomes in the exposed cohort. Combining these findings with current epidemiologic statistics we estimated mortality nationally that is associated with WLST-N<72.
Results
Of 16,875 OHCA subjects, 4,265 (25%) met inclusion criteria. WLST-N<72 occurred in one-third of subjects who died in-hospital. Adjusted analyses predicted that exposed subjects would have 26% survival and 16% functionally favorable survival if WLST-N<72 did not occur. Extrapolated nationally, WLST-N<72 may be associated with mortality in approximately 2,300 Americans each year of whom nearly 1,500 (64%) might have had functional recovery.
Conclusions
After OHCA, death following WLST-N<72 may be common and is potentially avoidable. Reducing WLST-N<72 has national public health implications and may afford an opportunity to decrease mortality after OHCA.
Introduction
Cardiac arrest is the most common cause of death in the United States, with an estimated 326,000 out-of-hospital cardiac arrest (OHCA) victims assessed by emergency medical services (EMS) annually.[1] Between 50 and 89% of OHCA patients with return of spontaneous circulation (ROSC) die in the hospital.[2, 3] Fear of survival with severe brain injury or belief that aggressive care is futile prompt some clinicians and proxies to choose withdrawal of life-sustaining therapy (WLST). WLST because of perceived neurological injury and assumed poor prognosis (WLST-N) is the most common proximate cause of death after OHCA.[4, 5]
Current evidence-based guidelines recommend delaying WLST-N for at least 72 hours after ROSC because, prior to this time, no clinical sign or test precludes a favorable neurological outcome and clinical examination is not reliable before that time point.[6–8] Even thereafter, the most accurate neurological predictors still do not have perfect specificity for predicting poor outcome,[10] and patients who remain comatose on post-arrest day 3 may still awaken and have favorable recoveries.[11] Despite this, WLST-N before 72 hours (WLST-N<72) is common.[5, 12] Premature WLST-N after OHCA may increase mortality, reduce favorable neurological outcomes and confound the results of clinical trials.
In order to estimate the mortality resulting from premature WLST-N, we conducted a secondary analysis of a large randomized controlled trial (the Resuscitation Outcomes Consortium (ROC) PRIMED trial), which enrolled OHCA subjects at 151 hospitals across North America. During the trial, published guidelines differed from current guidelines and suggested that certain combinations of prognostic signs might be sufficient to preclude favorable outcome as early as 24h after OHCA.[9] We quantified the incidence and timing of WLST-N and WLST-N<72 in the ROC PRIMED cohort, then used a propensity-matched cohort to estimate the effect of WLST-N<72 on outcome. Our primary hypothesis was that predicted survival in those exposed to WLST-N<72 is greater than nil. Our secondary hypothesis was that there is between-hospital practice variation in WLST-N after adjustment for case mix.
Methods
Study design and patients
This is a secondary analysis of the ROC PRIMED trial (clinicaltrials.gov NCT00394706), conducted June 2007 to October 2009. Results of this trial, which tested the use of an impedance threshold device compared to a sham device and early versus late rhythm analysis and defibrillation in OHCA, have been reported.[13, 14] No difference in outcomes was identified for either comparison. Subjects were ≥18 years old with EMS-treated OHCA. Excluded were OHCA arrests due to trauma or exsanguination, pregnant patients, and prisoners. In the present analysis, we included only the subgroup of subjects who had ROSC, were treated at any participating hospital and survived at least 60 minutes after hospital arrival.
Primary exposures and outcomes
Consistent with current guidelines, we defined WLST-N<72 as WLST-N occurring within 72 hours after arrest. Since the date, but not time, of WLST-N was recorded, we conservatively defined “WLST-N<72” as occurring within 2 calendar days after arrest. Therefore, exposure to WLST-N<72 ranged between 61 minutes to 72 hours after ROSC, but some patients categorized as exposed to WLST-N on or after day 3 might actually have been exposed between 49 and 72 hours after ROSC and been misclassified. Research coordinators recorded the date and cause of death for all subjects in one of the following four categories: 1) “subject is unstable and continued life support is impossible or futile (including multiple system organ failure, recurrent cardiac arrest without ROSC, and intractable shock);” 2) “subject meets criteria for brain death;” 3) “subject is stable but care is withdrawn or limited because of non-neurological considerations (including underlying terminal illness, pre-existing advanced directives or surrogate’s understanding of the subject’s wishes);” or 4) “subject is deemed to have a poor neurological prognosis and care is withdrawn or limited resulting in death” (WLST-N). Of note, to guide neurological prognostication and the decision for WLST-N, the ROC PRIMED Manual of Operations recommended at a minimum “daily neurological assessment of [the] subject” with “two complete assessments at least 24 hours apart” demonstrating “no improvement in neurological status over 3 days” and/or “ominous [electroencephalographic] or evoked potential evaluations.” These recommendations were consistent with, but less specific than, the 2006 American Academy of Neurology consensus guidelines.[9] The Manual of Operations was distributed to EMS providers participating in the ROC PRIMED trial, but was not actively distributed to inpatient providers. EMS agencies were the unit of study in ROC PRIMED, and so subjects were transported to a range of hospitals including academic and non-academic centers. The ROC PRIMED trial team had no direct oversight of inpatient care of WLST practices and inpatient providers received no formal training as part of the study.
Primary outcomes were survival to hospital discharge and survival to discharge with favorable functional status at the time of discharge (modified Rankin Score (mRS) ≤ 3). We defined survival to hospital discharge as transfer to rehabilitation, a skilled nursing facility or home residence. mRS was assigned at hospital discharge using a standard instrument.
Covariates
In the present study, we selected a priori biologically plausible covariates for adjusted analyses. Demographic factors were age, gender, race or ethnicity, and residential status prior to arrest, which we categorized as home, rehabilitation, assisted living, nursing home or unknown. Elements of past medical history abstracted from the hospital record included presence or absence of coronary artery disease, past myocardial infarction (MI), congestive heart failure, past coronary artery bypass grafting, diabetes, dialysis dependence, illicit drug or alcohol use, cancer or terminal illness. Arrest-specific covariates were presenting rhythm, categorized as shockable (ventricular tachycardia, ventricular fibrillation, or shock administered by an automatic defibrillator), pulseless electrical activity, asystole, “no shock advised” by an automatic defibrillator, and unknown; intervals from 911 call to professional cardiopulmonary resuscitation (CPR) and from CPR initiation to ROSC; bystander CPR; presence of ST-elevation MI; and arrest etiology. We also included use of cardiac catheterization and targeted temperature management.
Statistical analyses
Baseline population characteristics, outcomes, and the temporal distribution of causes of death after ROSC were summarized using descriptive statistics. At the hospital level, associations between the proportion of subjects with favorable functional survival and the proportion exposed to WLST-N or WLST-N<72 for each hospital were modeled using linear regression. To appropriately regress one ratio against another ratio with a shared denominator, we constructed a full linear model including an intercept term and interactions to avoid spurious correlations.[15] This model provides unbiased estimates of the coefficients, but a correlation coefficient (e.g. R2) cannot be reported. Regression analyses were restricted to hospitals with at least 5 treated cases. Subject characteristics between the cases exposed to WLST-N and WLST-N<72 and unexposed cases were compared using t-tests, Chi Squared, or Fisher’s Exact tests. We built adjusted linear regression models including factors with associations at the α=0.1 level to estimate hospital-level factors associated with exposure to WLST-N and WLST-N<72. To quantify practice variation between hospitals, we calculated median odds ratios across hospitals for exposure to WLST-N and WLST-N<72, both without adjustment and after adjustment for measurable hospital-level factors associated with exposure. Median odds ratios are defined as the median of the absolute values of difference odds ratios between two randomly selected centers calculated at the center level (i.e. the median change in odds of exposure moving from one randomly selected center to another randomly center), in this case representing the center-level regional variation in WLST-N and WLST-N<72.[16]
In the cohort of subjects exposed to WLST-N<72, there were no survivors because this exposure assures a fatal outcome. Therefore, it is impossible to directly test the effect of WLST-N<72 on outcome. Instead, we used two indirect methods to determine if excess mortality was associated with exposure. First, we generated a propensity score that modeled the probability of exposure to WLST-N<72 using all pre-exposure covariates listed above and hospital site of admission. We used this propensity score to match one subject unexposed to WLST-N<72 to each exposed subject based on the squared distances of the propensity scores and without regards to geographical clustering. We considered subjects with WLST-N after 72 hours from arrest to be unexposed to WLST-N<72. We used a test of equal proportions to determine whether the probabilities of survival and functionally favorable survival exceeded 5% in the propensity-matched unexposed cohort. In addition, we examined whether the 95% confidence band of the logistic regression model predicting mortality as a function of the propensity score in the entire unexposed cohort included a mortality of 100% at the highest propensity scores.
As a second estimate of the excess mortality associated with exposure to WLST-N<72, we fit adjusted logistic regression models to predict the odds of survival and functionally favorable survival in the unexposed cohort. We then used these models to predict the expected survival and functionally favorable survival in the cohort exposed to WLST-N<72, specifically asking whether the predicted proportions of survivors and functionally favorable survivors were greater than 5%.
We combined these estimates of excess mortality from WLST-N<72 with current epidemiological data to estimate the total annual excess mortality associated with exposure to WLST-N<72 in the United States and Canada.
Results
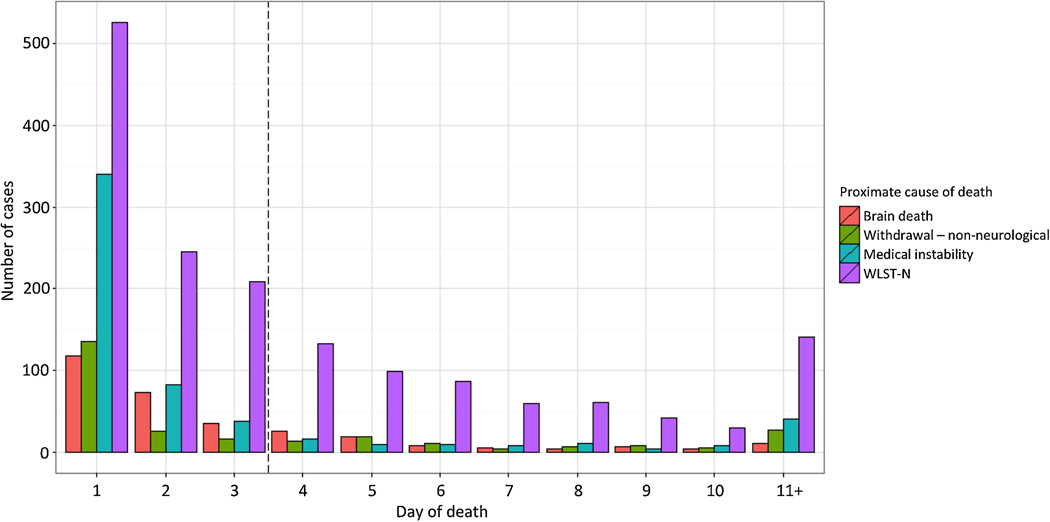
Of 16,875 subjects treated by emergency medical services (EMS) in the ROC PRIMED study, 4,265 (25%) arrived at a participating hospital and survived at least 60 minutes. Median age was 65 years (IQR 53–77) and 64% were male (Table 1). Of the 4,265 subjects who met inclusion criteria, 1,490 (35%) survived to hospital discharge, and 1,101 (26%) had a favorable functional outcome (Table 2). The most common cause of death was WLST-N, which occurred in 1626 subjects (59% of non-survivors). Among these, 919 subjects (22% of hospitalized subjects, 33% of non-survivors) were exposed to WLST-N<72. WLST-N occurred most frequently within 1 day of ROSC (Figure 1).
Table 1.
Demographic and clinical characteristics of out-of-hospital cardiac arrest patients surviving to hospital admission, stratified by exposure to withdrawal of life-sustaining therapy based on anticipated neurological prognosis (WLST-N)
| Characteristic | Total WLST-N | Early WLST-N (<72h) | Overall population (n = 4,265) |
||
|---|---|---|---|---|---|
| Exposed (n = 1,626) |
Unexposed (n = 2,639) |
Exposed (n = 919) |
Unexposed (n = 3,346) |
||
| Age (median (IQR)), years | 69 (57, 80) | 62 (52, 74) | 74 (61, 82) | 63 (52, 75) | 65 (53, 77) |
| Male, n (%) | 982 (60.4%) | 1758 (66.6%) | 534 (58.1%) | 2206 (65.9%) | 2740 (64.3%) |
| Race/ethnicity, n (%)& | |||||
| Caucasian | 475 (29.2%) | 849 (32.2%) | 260 (28.3%) | 1064 (31.8%) | 1324 (31.0%) |
| Hispanic/Latino | 21 (1.3%) | 46 (1.7%) | 11 (1.2%) | 56 (1.7%) | 67 (1.6%) |
| African American/Black | 101 (6.2%) | 194 (7.4%) | 41 (4.5%) | 254 (7.6%) | 295 (6.9%) |
| Asian | 47 (2.9%) | 78 (3.0%) | 32 (3.5%) | 93 (2.8%) | 125 (2.9%) |
| Other | 17 (1.0%) | 35 (1.3%) | 10 (1.1%) | 42 (1.3%) | 52 (1.2%) |
| Unknown/missing | 988 (60.8%) | 1494 (56.6%) | 576 (62.7%) | 1906 (57.0%) | 2482 (58.2%) |
| Medical comorbidities, n (%) | |||||
| CAD | 73 (4.5%) | 129 (4.9%) | 43 (4.7%) | 159 (4.8%) | 202 (4.7%) |
| MI | 150 (9.2%) | 268 (10.2%) | 92 (10.0%) | 326 (9.7%) | 418 (9.8%) |
| CHF | 169 (10.4%) | 202 (7.7%) | 106 (11.5%) | 265 (7.9%) | 371 (8.7%) |
| CABG | 79 (4.9%) | 117 (4.4%) | 45 (4.9%) | 151 (4.5%) | 196 (4.6%) |
| Diabetes | 350 (21.5%) | 501 (19.0%) | 202 (22.0%) | 649 (19.4%) | 851 (20.0%) |
| Dialysis | 33 (2.0%) | 26 (1.0%) | 21 (2.3%) | 38 (1.1%) | 59 (1.4%) |
| Illicit drug or alcohol use | 83 (5.1%) | 125 (4.7%) | 43 (4.7%) | 165 (4.9%) | 208 (4.9%) |
| Cancer | 115 (7.1%) | 139 (5.3%) | 79 (8.6%) | 175 (5.2%) | 254 (6.0%) |
| Terminal illness | 1 (0.1%) | 1 (0.0%) | 1 (0.1%) | 1 (0.0%) | 2 (0.0%) |
| Residential status, n (%)* | |||||
| Home | 1399 (88.2%) | 2399 (93.3%) | 765 (85.4%) | 3033 (93.0%) | 3798 (91.4%) |
| Rehabilitation | 9 (0.6%) | 10 (0.4%) | 3 (0.3%) | 16 (0.5%) | 19 (0.5%) |
| Assisted living | 40 (2.5%) | 51 (2.0%) | 28 (3.1%) | 63 (1.9%) | 91 (2.2%) |
| Nursing home | 138 (8.7%) | 111 (4.3%) | 100 (11.2%) | 149 (4.6%) | 249 (6.0%) |
| Unknown | 40 (2.5%) | 68 (2.6%) | 23 (2.5%) | 85 (2.5%) | 108 (2.5%) |
| Initial rhythm, n (%)^ | |||||
| VT/VF | 501 (31.1%) | 1334 (50.9%) | 227 (24.9%) | 1608 (48.4%) | 1835 (43.3%) |
| PEA | 483 (30.0%) | 455 (17.4%) | 297 (32.6%) | 641 (19.3%) | 938 (22.2%) |
| Asystole | 498 (30.9%) | 626 (23.9%) | 300 (33.0%) | 824 (24.8%) | 1124 (26.5%) |
| Shock not advised | 121 (7.5%) | 164 (6.3%) | 80 (8.8%) | 205 (6.2%) | 285 (6.7%) |
| Unknown | 9 (0.6%) | 43 (1.6%) | 6 (0.7%) | 46 (1.4%) | 52 (1.2%) |
| CPR intervals, mean (SD), min | |||||
| 911 call to CPR start | 9.71 (6.00) | 10.53 (8.43) | 9.80 (6.15) | 10.33 (7.95) | 10.22 (7.60) |
| CPR start to ROSC | 16.39 (8.26) | 12.54 (8.76) | 16.99 (8.73) | 13.18 (8.61) | 13.99 (8.77) |
| Arrest etiology$ | |||||
| Unknown/presumed cardiac | 1540 (94.7%) | 2526 (95.7%) | 874 (95.1%) | 3192 (95.4%) | 4066 (95.3%) |
| Respiratory | 60 (3.7%) | 66 (2.5%) | 34 (3.7%) | 92 (2.7%) | 126 (3.0%) |
| Exposure | 21 (1.3%) | 28 (1.1%) | 7 (0.8%) | 42 (1.3%) | 49 (1.1%) |
| Overdose | 19 (1.2%) | 23 (0.9%) | 5 (0.5%) | 37 (1.1%) | 42 (1.0%) |
| Trauma | 0 (0.0%) | 2 (0.1%) | 0 (0.0%) | 2 (0.1%) | 2 (0.0%) |
| Missing | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| STEMI | 183 (12.1%) | 527 (21.1%) | 84 (9.8%) | 626 (19.8%) | 710 (17.7%) |
| TTM/hypothermia | 766 (47.1%) | 1092 (41.8%) | 344 (37.4%) | 1514 (45.6%) | 1858 (43.8%) |
| Cardiac cath in first 24h | 203 (12.5%) | 720 (27.8%) | 81 (8.8%) | 842 (25.5%) | 923 (21.9%) |
Respiratory: foreign body obstruction, hanging, mechanical suffocation, respiratory, strangulation, smoke inhalation, drowning;
Exposure: chemical poisoning, drug poisoning, electrocution, excessive heat or cold, lightning, radiation exposure, venomous stings
Percentages calculated from cases with known residential status
Percentages calculated from cases with known initial rhythm
Percentages may sum to >100 because subjects may be counted as members of multiple races or ethnicities
Abbreviations: WLST-N – Withdrawal of life-sustaining therapy based on perceived poor neurological prognosis; WLST-N<72 – WLST-N before 72 hours after return of spontaneous circulation; IQR – Interquartile range; CAD – Coronary artery disease; MI – Myocardial infarction; CHF – Congestive heart failure; CABG – Coronary artery bypass grafting; VT/VF – Ventricular tachycardia or fibrillation; PEA – Pulseless electrical activity; SD – Standard deviation; CPR – Cardiopulmonary resuscitation; ROSC – Return of spontaneous circulation; STEMI – ST-elevation myocardial infarction; TTM – Targeted temperature management; Cath – catheterization.
Table 2.
Subjects’ outcomes, stratified by exposure to withdrawal of life-sustaining therapy for neurological reasons (WLST-N) and WLST-N before 72 hours.
| Outcome | Total WLST-N | Early WLST-N (<72h) | Overall population (n = 4,265) |
||
|---|---|---|---|---|---|
| Exposed (n = 1,626) |
Unexposed (n = 2,639) |
Exposed (n = 919) |
Unexposed (n = 3,346) |
||
| Survival, n (%) | 0 (0.0%) | 1490 (56.7%) | 0 (0.0%) | 1490 (44.7%) | 1490 (35.0%) |
| Cause of death, n (%)* | |||||
| Instability or re-arrest | -- | 563 (49.1%) | -- | 563 (23.1%) | 563 (20.3%) |
| Brain death | -- | 305 (26.5%) | -- | 305 (12.5%) | 305 (11.0%) |
| Withdrawal for non-neurological reason | -- | 266 (23.2%) | -- | 266 (10.9%) | 266 (9.6%) |
| Withdrawal for neurological reason | 1626 (100%) | -- | 919 (100%) | 707 (29.0%) | 1626 (58.6%) |
| Modified Rankin Scale, n (%) | |||||
| 0 | -- | 359 (13.7%) | -- | 359 (10.8%) | 359 (8.5%) |
| 1 | -- | 354 (13.5%) | -- | 354 (10.7%) | 354 (8.3%) |
| 2 | -- | 90 (3.4%) | -- | 90 (2.7%) | 90 (2.1%) |
| 3 | -- | 298 (11.4%) | -- | 298 (9.0%) | 298 (7.0%) |
| 4 | -- | 210 (8.0%) | -- | 210 (6.3%) | 210 (5.0%) |
| 5 | -- | 165 (6.3%) | -- | 165 (5.0%) | 165 (3.9%) |
| 6 | 1626 (100%) | 1139 (43.6%) | 919 (100%) | 1846 (55.6%) | 2765 (65.2%) |
| Hospital length of stay, mean (SD), days | |||||
| Survivors | -- | 17.32 (19.6) | -- | 17.32 (19.6) | 17.32 (19.6) |
| Non-survivors | 4.70 (10.1) | 3.85 (10.3) | 1.91 (8.2) | 5.57 (10.8) | 4.35 (10.2) |
Percent of non-survivors
Abbreviations: WLST-N – Withdrawal of life-sustaining therapy based on perceived poor neurological prognosis; WLST-N<72 – WLST-N before 72 hours after return of spontaneous circulation; SD – Standard deviation.
Figure 1.
Daily totals of subjects who died after out-of-hospital cardiac arrest, stratified by cause of death. Dashed line indicates the 72-hour threshold before which withdrawal of life-sustaining therapy for anticipated neurological prognosis was considered to be “early”.
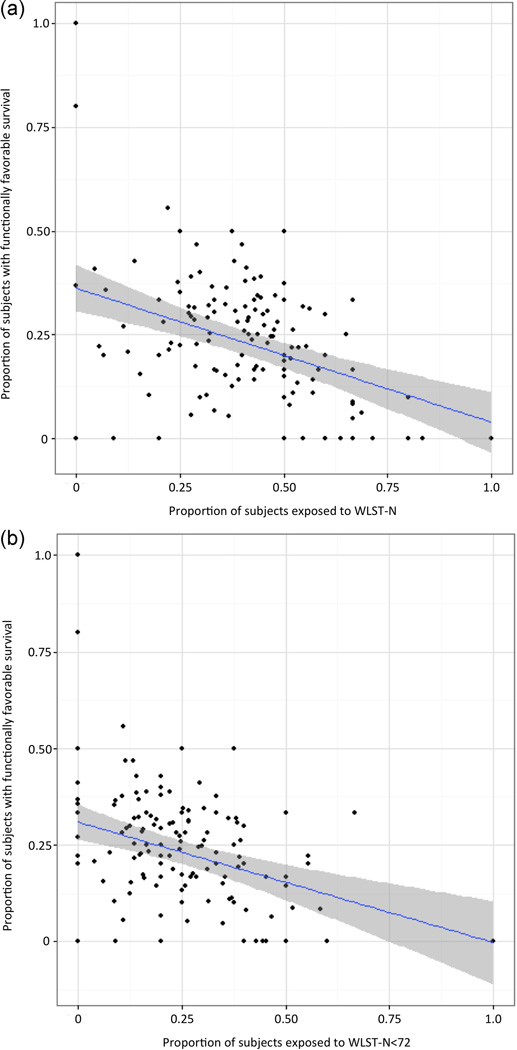
The proportion of cases exposed to WLST-N and WLST-N<72 at individual hospitals varied from 0 to 1.0, and in a full linear model, there was a negative correlation between rate of exposure and rate of functionally favorable survival at each hospital (Figure 2). The only hospital-level factor associated with the proportion of cases exposed to WLST-N and WLST-N<72 was lower proportion of cases with ST-elevation MI (Supplemental Table 1). Unadjusted median odds ratios across hospitals for exposure to WLST-N and WLST-N<72 were 1.68 and 1.61, respectively, indicating the median odds of exposure to WLST-N were 68% greater, and the odds of exposure to WLST-N<72 were 61% greater, for a patient admitted to one hospital in any randomly selected pair of hospitals. Adjusted median odds ratios were 1.63 and 1.58, respectively.
Figure 2.
A) The proportion of cases exposed to WLST-N versus rates of functionally favorable survival within each hospital. Exposure to WLST-N was negatively associated with functionally favorable survival (coefficient −0.23; P = 0.01) B) The proportion of cases exposed to WLST-N<72 versus rates of functionally favorable survival within each hospital. Exposure to WLST-N<72 was negatively associated with survival (coefficient −0.32; P < 0.01)
Multiple characteristics differed between the cohorts exposed to WLST-N<72 and those unexposed (Table 3). In comparison with patients not exposed to WLST-N<72, exposed patients were older, were more likely to be female, had a significantly higher rate of congestive heart failure and cancer, had less likely to live at home, had much lower rates of shockable rhythm, had longer response times and arrest was less commonly witnessed. After excluding 3 exposed subjects because of a large proportion of missing data, we were able to match all exposed subjects to an unexposed control based on propensity score. No differences on measured variables between the exposed and unexposed cohorts persisted after matching (Table 3). In the matched unexposed cohort, there were 226 subjects (25% [95%CI 22–28%]) who survived and 149 subjects (16% [95%CI 14–19%]) with functionally favorable survival, exceeding our clinically important threshold of 5%. Among those with the highest propensity scores, mortality in the unexposed cohort was significantly lower than 100% (Figure 3). The logistic regression model derived in the unexposed cohort (Supplemental Table 1) predicted that 237 (26% [95%CI 23–100%]) of 919 subjects exposed to WLST-N<72 would have survived had they not been exposed and 146 (16% [95%CI 14–100%]) would have had functionally favorable survival.
Table 3.
Cohort characteristics, stratified by exposure to withdrawal of life-sustaining therapy before 72 hours after out-of-hospital cardiac arrest, before and after propensity score matching.
| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| Characteristic | Exposed (n = 916) |
Unexposed (n = 3,149) |
P value | Exposed (n = 916) |
Unexposed (n = 916) |
P value |
| Age (median) | 70.5 | 62.4 | < 0.001 | 70.5 | 70.9 | 0.64 |
| Male sex (%) | 58.1 | 65.9 | < 0.001 | 58.1% | 57.5% | 0.81 |
| Race or ethnicity (%) | ||||||
| Caucasian | 28.4% | 32.3% | 0.02 | 28.4% | 28.8% | 0.84 |
| Hispanic/Latino | 1.2% | 1.7% | 0.23 | 1.2% | 1.2% | 1.00 |
| African American/Black | 4.5% | 7.4% | < 0.001 | 4.5% | 4.7% | 0.82 |
| Asian | 3.5% | 2.9% | 0.37 | 3.5% | 3.2% | 0.70 |
| Other | 1.1% | 1.3% | 0.60 | 1.1% | 1.2% | 0.83 |
| Unknown/missing | 62.6% | 56.6% | <0.01 | 62.6% | 62.7% | 0.96 |
| Medical comorbidities, (%) | ||||||
| CAD | 4.7% | 4.9% | 0.81 | 4.7% | 4.4% | 0.74 |
| MI | 10.0% | 9.9% | 0.88 | 10.0% | 9.4% | 0.64 |
| CHF | 11.6% | 7.8% | <0.01 | 11.6% | 12.1% | 0.72 |
| CABG | 4.9% | 4.5% | 0.62 | 4.9% | 5.3% | 0.67 |
| Diabetes | 22.1% | 19.7% | 0.13 | 22.1% | 23.4% | 0.50 |
| Dialysis | 2.3% | 1.2% | 0.04 | 2.3% | 2.3% | 1.00 |
| Illicit drug or alcohol use | 4.7% | 4.9% | 0.78 | 4.7% | 3.8% | 0.36 |
| Cancer | 8.6% | 5.3% | <0.01 | 8.6% | 7.9% | 0.55 |
| Terminal illness | 0.1% | 0.0% | 0.50 | 0.1% | 0.0% | 0.32 |
| Residential status, (%) | ||||||
| Home | 83.3% | 90.8% | < 0.001 | 83.3% | 84.4% | 0.95 |
| Rehabilitation | 0.2% | 0.5% | 0.2% | 0.3% | ||
| Assisted living | 3.1% | 1.9% | 3.1% | 2.9% | ||
| Nursing home | 10.9% | 4.6% | 10.9% | 10.0% | ||
| Unknown | 2.5% | 2.3% | 2.5% | 2.3% | ||
| Initial rhythm shockable (%) | ||||||
| No | 74.5% | 51.3% | 74.5% | 74.1% | ||
| Yes | 24.7% | 48.0% | < 0.001 | 24.7% | 24.9% | 0.96 |
| Missing | 0.9% | 0.6% | 0.9% | 1.0% | ||
| 911 to CPR time (min) | 9.8 | 10.3 | 0.07 | 9.8 | 9.8 | 0.92 |
| CPR to ROSC Time (min) | 17.0 | 13.3 | < 0.001 | 17.0 | 16.9 | 0.91 |
| Witnessed arrest (%) | 59.2% | 72.0% | < 0.001 | 59.2% | 60.3% | 0.63 |
| STEMI (%) | ||||||
| No | 84.2% | 75.9% | 84.2% | 84.0% | ||
| Yes | 9.2% | 18.6% | <0.001 | 9.2% | 10.2% | 0.64 |
| Missing | 6.7% | 5.4% | 6.7% | 5.9% | ||
| TTM/hypothermia (%) | 37.3% | 44.8% | < 0.001 | 37.3% | 36.7% | 0.77 |
| Cardiac cath within 24h (%) | 8.8% | 25.0% | < 0.001 | 8.8% | 8.0% | 0.50 |
| Outcomes n (%) | ||||||
| Overall survival to discharge | 0 (0) | 1432 (45) | <0.001 | 0 (0) | 226 (25) | <0.001 |
| Functionally favorable survival | 0 (0) | 1068 (34) | <0.001 | 0 (0) | 149 (16) | <0.001 |
Abbreviations: CAD – Coronary artery disease; MI – Myocardial infarction; CHF – Congestive heart failure; CABG – Coronary artery bypass grafting; CPR – Cardiopulmonary resuscitation; ROSC – Return of spontaneous circulation; STEMI – ST-elevation myocardial infarction; TTM – Targeted temperature management; Cath – catheterization
Figure 3.
The association of between each unexposed subject’s propensity for exposure to WLST-N<72 based on baseline clinical characteristics and in-hospital mortality. The 95% confidence band of the spline curve at the highest propensity scores does not include a 1.0 probability of in-hospital mortality, indicating excess mortality associated with exposure after adjustment for baseline clinical characteristics.
In our cohort, 25% of EMS-treated OHCA patients were transported to the hospital and survived at least 1 hour after hospital arrival, which is consistent with previous studies.[17] Nationally, there are approximately 52.1 per 100,000 treated by EMS after OHCA, or approximately 167,000 patients.[18] Therefore, we estimated that 41,750 OHCA victims are successfully resuscitated and at risk for exposure to WLST-N<72 each year. Our results suggest an estimated 2,296 to 2,338 who otherwise would have survived to discharge will instead die after WLST-N<72 (Table 4). Approximately 1,470 of these may have had a functionally favorable recovery, while 826 to 868 might survive with an unfavorable neurological outcome.
Table 4.
Extrapolation of key results to national epidemiological data
| If WLST-N<72 were eliminated: | |||||||
|---|---|---|---|---|---|---|---|
| EMS-treated out-of-hospital cardiac arrests |
→ | Surviving to hospital |
→ | Exposed to WLST-N<72 |
Predicted outcome without exposure | Lives annually | % Improvement in outcomes |
| 321 mil × 52.1/100K[18] = 167,000 | × | 0.25[17] = 41,750 | × | 0.22 = 9,185 | Survival | ||
| Propensity match: 0.25 (0.22 –0.28) | 2,296 (2,021– 2,572) | 5.5% (4.8% – 6.2%) | |||||
| Logistic regression model: 0.26 (0.23 – 1.0) | 2,388 (2,113 – 9,185) | 5.7% (5.1% – 100%) | |||||
| Favorable outcome | |||||||
| Propensity match: 0.16 (0.14 – 0.19) | 1,470 (1,286 – 1,745) | 3.5% (3.1% – 4.2%) | |||||
| Logistic regression model: 0.16 (0.14 – 1.0) | 1,470 (1,286 – 9,185) | 3.5% (3.1% – 100%) | |||||
Abbreviations: EMS – Emergency medical services; WLST-N<72 - Withdrawal of life-sustaining therapy because of perceived neurological injury and assumed poor prognosis before 72 hours of return of spontaneous circulation
Discussion
Following initial resuscitation from OHCA, withdrawal of life-sustaining therapy because of perceived poor neurological prognosis before 72 hours after arrest (WLST-N<72) was common, occurring in one third of hospitalized subjects, despite the absence of reliable prognostic findings during this time period. All subjects exposed to WLST-N<72 died. To our knowledge, this is the first estimate of the impact of WLST-N<72 on outcomes. Based on two prognostic models, our results indicate that 26% of the patients who had early WLST-N might have survived had life-sustaining therapy not been withdrawn, and 64% of these might have had functionally favorable survival. Nationally, eliminating WLST-N<72 and its attributable mortality might save as many as 2,300 lives after OHCA, of which a majority might have functionally favorable recovery.
Similar point estimates of preventable mortality were obtained using two different approaches to the data, strengthening the validity of our findings. If our findings are consistent with national practice, eliminating any preventable mortality from WLST-N<72 by delaying neurological prognostication, as recommended in international guidelines,[6, 7, 9] might increase survival to hospital discharge by 5.5 to 5.7% and increase the rate of functionally favorable survival by 3.5%. These gains could be widely accomplished without investment in new technologies or resources and are comparable to or exceed the survival benefit from introduction of advanced life support,[19] bystander CPR[20] or use of an automated external defibrillator,[21] each of which has been the appropriate focus of considerable research and public health efforts.
A potential adverse effect of eliminating WLST-N<72 might be an increase in the number of patients discharged with severe functional impairment (mRS >3) by as much as 2.0 to 2.2% per year. Our data are insufficient to quantify the social or economic costs of this result. Several factors would be expected to mitigate this potential consequence. First, cardiac arrest survivors continue to improve functionally after hospital discharge, and many patients who are not functionally independent at hospital discharge achieving independence by 6 months after discharge.[22] Second, eliminating WLST-N<72 does not preclude delayed WLST-N after 72h from ROSC. Surrogate decision makers who currently chose WLST-N<72 could still choose WLST-N at a later time if a patient’s neurological function did not improve over that time. Finally, patients with severe neurological dysfunction who do not improve neurologically after discharge generally die within 3–6 months, limiting the duration of long-term survival with severe disability.[23]
Inappropriately pessimistic prognostication and therapeutic nihilism have been recognized as a source of excess mortality after stroke and traumatic brain injury.[24] In patients with intracerebral hemorrhage, WLST-N is the single most important factor in determining patient outcomes, regardless of disease severity.[25] Importantly, WLST-N in clinical practice can bias both trials and observational studies, obscuring real relationships between clinical interventions and outcomes, a problem that has significantly impacted cardiac arrest care and research. This self-fulfilling prophecy of prematurely predicting a poor neurological outcome, and then using this prognostication to guide early WLST-N, is one of the single most important limitations in research of brain injured patients, including survivors of OHCA. These results underline the high percentage of patients who are prone to selection and treatment indication biases from clinicians early after admission.
A strength of our study is that we prospectively distinguished brain death, WLST for non-neurological reasons, and WLST-N. There are many appropriate reasons for WLST prior to 72 hours after OHCA, including pre-existing advanced directives, surrogate representations of a patient’s wishes with regard to medical care, progression to brain death, and overt hemodynamic or other instability that makes ongoing medical care futile in the assessment of the treating clinician. Our classification of cause of death distinguished between care limitations for these reasons and WLST-N. To our knowledge, few other large OHCA databases have collected information on the proximate cause for WLST. Because there appears to be the possibility of preventable mortality attributable to WLST-N<72, future studies and trials should specifically measure WLST-N in order to assess the potential for bias from this practice.
Use of therapeutic hypothermia or cardiac catheterization was associated with reduced exposure to WLST-N<72. The recent Targeted Temperature Management (TTM) trial did not find particular temperatures to be superior,[26] though prior trials indicated that post-arrest temperature management was superior to no temperature management.[27] Some of the protective effect of temperature management may result from avoiding WLST-N<72 in hypothermia-treated patients because of concern that prognostication may be confounded. The strict standardization and blinding of neurological prognostication the TTM trial may have mitigated this effect.[28, 29] Similarly, clinicians may have reduced proclivity to withdraw life-sustaining therapy in patients whom they have subjected to cardiac catheterization. This reduced risk of WLST-N<72 may contribute to the improved patient outcomes with cardiac catheterization after OHCA in risk-adjusted analyses.[4] Alternatively, this association may reflect selection bias whereby patients who appear to have a more favorable neurological prognosis are more likely to be provided aggressive treatment.
At a hospital level, the proportion of subjects exposed to WLST-N<72 and WLST-N varies considerably, even after adjusting for patient-level factors such as age and medical comorbidities. Median odds of exposure to WLST-N<72 were 61% greater in one hospital of any randomly selected pair. Such between-hospital variation in the use of guideline-based care practices and associated difference in patient outcomes has been described in many conditions such as severe sepsis and acute coronary syndrome.[30, 31] Our findings highlight how the application of evidence-based care is an opportunity to improve patient outcomes immediately and widely without investment in new clinical treatments, personnel, or resources.
Our study has several important limitations. First, we report associ care, and exposure invariably leads to mortality, a randomized trial would be unethical. Large, multicenter observational data with advanced observational methods such as propensity-adjusted analyses are likely the highest level of evidence possible to estimate the effects of exposure. Confidence in these findings will increase if replicated in other cohorts. Second, we cannot control for unobserved confounders. Particularly, detailed data regarding subject neurological status before or after hospital admission, associated clinical findings, and results of other diagnostic testing were not available. Moreover, adjudicating the proximate cause of death is an imperfect process, and multiple competing factors that are not fully captured by our categorical measure may contribute to the final decision for WLST. Although our propensity-matched cohorts do not differ on any measured characteristic, unmeasured factors may still account for differences in outcome. Propensity score methods minimize but do not eliminate this risk of bias due to unobserved confounders. Third, the care rendered to subjects in randomized controlled trials regardless of treatment arm is generally superior to usual care, perhaps leading us to underestimate the prevalence of exposure to WLST-N<72.[32] This bias would lead our results to underestimate the true magnitude of the effect of exposure. Finally, since only the date but not the time of WLST-N was available, some subjects were likely misclassified as being unexposed to WLST-N<72 because their exposure occurred 3 calendar days but less than 72 hours after ROSC. This classification bias would also lead us to underestimate the magnitude of the effect of exposure to WLST-N<72.
In conclusion, WLST-N<72 is common after OHCA andassociated with one-third of in-hospital mortality. Patients exposed to WLST-N<72 resemble unexposed patients with 26% chance of survival. Of these, nearly two-thirds are predicted to have had survival with a favorable functional status. Awareness of this impact on outcomes may help guide providers and families away from early limitations in care based on perceived neurological prognosis. Failure to control for the effects of WLST-N may significantly bias the results of studies of OHCA or other severe acquired brain injuries. Reducing WLST-N<72 has important public health implications and may be an opportunity to decrease mortality after OHCA.
Supplementary Material
Acknowledgments
Funding sources: The Resuscitation Outcomes Consortium is supported by a series of cooperative agreements to nine regional clinical centers and one Data Coordinating Center (5U01 HL077863 – University of Washington Data Coordinating Center, HL077866 – Medical College of Wisconsin, HL077867 – University of Washington, HL077871 – University of Pittsburgh, HL077872 – St. Michael's Hospital, HL077873 – Oregon Health and Science University, HL077881 – University of Alabama at Birmingham, HL077885 – Ottawa Hospital Research Institute, HL077887 – University of Texas SW Medical Ctr/Dallas, HL077908 – University of California San Diego) from the National Heart, Lung and Blood Institute in partnership with the National Institute of Neurological Disorders and Stroke, US Army Medical Research and Material Command, The Canadian Institutes of Health Research (CIHR) – Institute of Circulatory and Respiratory Health, Defence Research and Development Canada and the Heart, Stroke Foundation of Canada and the American Heart Association.
Dr. Elmer’s research time is supported by the NHLBI 5K12HL109068. Mr. Torres is supported by the NCI T32CA09168. Dr. Scales holds a Fellowship in Translational Health Research from the Physicians Services Incorporated Foundation. Dr. Stub is both supported by a co-funded NHMRC/NHF early career fellowship (#1090302/100516).
The sponsors had no role in the design or conduct of the present analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors declare no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; and, no other relationships or activities that could appear to have influenced the submitted work. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Data sharing: The entire ROC PRIMED data set, including data for all subjects included in this analysis, is public record and can be requested by visiting: https://biolincc.nhlbi.nih.gov/studies/rocprimed/
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fugate JE, Brinjikji W, Mandrekar JN, Cloft HJ, White RD, Wijdicks EF, et al. Post-cardiac arrest mortality is declining: a study of the US National Inpatient Sample 2001 to 2009. Circulation. 2012;126:546–550. doi: 10.1161/CIRCULATIONAHA.111.088807. [DOI] [PubMed] [Google Scholar]
- 4.Callaway CW, Schmicker RH, Brown SP, Albrich JM, Andrusiek DL, Aufderheide TP, et al. Early coronary angiography and induced hypothermia are associated with survival and functional recovery after out-of-hospital cardiac arrest. Resuscitation. 2014;85:657–663. doi: 10.1016/j.resuscitation.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemiale V, Dumas F, Mongardon N, Giovanetti O, Charpentier J, Chiche JD, et al. Intensive care unit mortality after cardiac arrest: the relative contribution of shock and brain injury in a large cohort. Intensive care medicine. 2013;39:1972–1980. doi: 10.1007/s00134-013-3043-4. [DOI] [PubMed] [Google Scholar]
- 6.Sandroni C, Cariou A, Cavallaro F, Cronberg T, Friberg H, Hoedemaekers C, et al. Prognostication in comatose survivors of cardiac arrest: an advisory statement from the European Resuscitation Council and the European Society of Intensive Care Medicine. Intensive care medicine. 2014;40:1816–1831. doi: 10.1007/s00134-014-3470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cronberg T, Brizzi M, Liedholm LJ, Rosen I, Rubertsson S, Rylander C, et al. Neurological prognostication after cardiac arrest--recommendations from the Swedish Resuscitation Council. Resuscitation. 2013;84:867–872. doi: 10.1016/j.resuscitation.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Callaway CW, Donnino MW, Fink EL, Geocadin RG, Golan E, Kern KB, et al. Part 8: Post-Cardiac Arrest Care: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132:S465–S482. doi: 10.1161/CIR.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wijdicks EF, Hijdra A, Young GB, Bassetti CL, Wiebe S Quality Standards Subcommittee of the American Academy of N. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67:203–210. doi: 10.1212/01.wnl.0000227183.21314.cd. [DOI] [PubMed] [Google Scholar]
- 10.Sandroni C, Cavallaro F, Callaway CW, D'Arrigo S, Sanna T, Kuiper MA, et al. Predictors of poor neurological outcome in adult comatose survivors of cardiac arrest: a systematic review and meta-analysis. Part 2: Patients treated with therapeutic hypothermia. Resuscitation. 2013;84:1324–1338. doi: 10.1016/j.resuscitation.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Gold B, Puertas L, Davis SP, Metzger A, Yannopoulos D, Oakes DA, et al. Awakening after cardiac arrest and post resuscitation hypothermia: Are we pulling the plug too early? Resuscitation. 2013 doi: 10.1016/j.resuscitation.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 12.Perman SM, Kirkpatrick JN, Reitsma AM, Gaieski DF, Lau B, Smith TM, et al. Timing of neuroprognostication in postcardiac arrest therapeutic hypothermia*. Critical care medicine. 2012;40:719–724. doi: 10.1097/CCM.0b013e3182372f93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stiell IG, Nichol G, Leroux BG, Rea TD, Ornato JP, Powell J, et al. Early versus later rhythm analysis in patients with out-of-hospital cardiac arrest. The New England journal of medicine. 2011;365:787–797. doi: 10.1056/NEJMoa1010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aufderheide TP, Nichol G, Rea TD, Brown SP, Leroux BG, Pepe PE, et al. A trial of an impedance threshold device in out-of-hospital cardiac arrest. The New England journal of medicine. 2011;365:798–806. doi: 10.1056/NEJMoa1010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kronmal RA. Spurious Correlation and the Fallacy of the Ratio Standard Revisited. J Roy Stat Soc a Sta. 1993;156:379–392. [Google Scholar]
- 16.Merlo J, Chaix B, Ohlsson H, Beckman A, Johnell K, Hjerpe P, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health. 2006;60:290–297. doi: 10.1136/jech.2004.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong MK, Morrison LJ, Qiu F, Austin PC, Cheskes S, Dorian P, et al. Trends in short- and long-term survival among out-of-hospital cardiac arrest patients alive at hospital arrival. Circulation. 2014;130:1883–1890. doi: 10.1161/CIRCULATIONAHA.114.010633. [DOI] [PubMed] [Google Scholar]
- 18.Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. Jama. 2008;300:1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanghavi P, Jena AB, Newhouse JP, Zaslavsky AM. Outcomes After Out-of-Hospital Cardiac Arrest Treated by Basic vs Advanced Life Support. JAMA internal medicine. 2015;175:196–204. doi: 10.1001/jamainternmed.2014.5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sasson C, Rogers MA, Dahl J, Kellermann AL. Predictors of survival from out-of-hospital cardiac arrest: a systematic review and meta-analysis. Circulation Cardiovascular quality and outcomes. 2010;3:63–81. doi: 10.1161/CIRCOUTCOMES.109.889576. [DOI] [PubMed] [Google Scholar]
- 21.Sanna T, La Torre G, de Waure C, Scapigliati A, Ricciardi W, Dello Russo A, et al. Cardiopulmonary resuscitation alone vs. cardiopulmonary resuscitation plus automated external defibrillator use by non-healthcare professionals: a meta-analysis on 1583 cases of out-of-hospital cardiac arrest. Resuscitation. 2008;76:226–232. doi: 10.1016/j.resuscitation.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Bouwes A, Binnekade JM, Kuiper MA, Bosch FH, Zandstra DF, Toornvliet AC, et al. Prognosis of coma after therapeutic hypothermia: a prospective cohort study. Annals of neurology. 2012;71:206–212. doi: 10.1002/ana.22632. [DOI] [PubMed] [Google Scholar]
- 23.Aufderheide TP, Frascone RJ, Wayne MA, Mahoney BD, Swor RA, Domeier RM, et al. Standard cardiopulmonary resuscitation versus active compression-decompression cardiopulmonary resuscitation with augmentation of negative intrathoracic pressure for out-of-hospital cardiac arrest: a randomised trial. Lancet. 2011;377:301–311. doi: 10.1016/S0140-6736(10)62103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hemphill JC, 3rd, White DB. Clinical nihilism in neuroemergencies. Emerg Med Clin North Am. 2009;27:27–37. vii–viii. doi: 10.1016/j.emc.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zahuranec DB, Brown DL, Lisabeth LD, Gonzales NR, Longwell PJ, Smith MA, et al. Early care limitations independently predict mortality after intracerebral hemorrhage. Neurology. 2007;68:1651–1657. doi: 10.1212/01.wnl.0000261906.93238.72. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, et al. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. The New England journal of medicine. 2013;369:2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 27.Hypothermia after Cardiac Arrest Study G. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. The New England journal of medicine. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 28.Cronberg T, Horn J, Kuiper MA, Friberg H, Nielsen N. A structured approach to neurologic prognostication in clinical cardiac arrest trials. Scandinavian journal of trauma, resuscitation and emergency medicine. 2013;21:45. doi: 10.1186/1757-7241-21-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dragancea I, Horn J, Kuiper M, Friberg H, Ullen S, Wetterslev J, et al. Neurological prognostication after cardiac arrest and targeted temperature management 33 degrees C versus 36 degrees C: Results from a randomised controlled clinical trial. Resuscitation. 2015;93:164–170. doi: 10.1016/j.resuscitation.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Peterson ED, Roe MT, Mulgund J, DeLong ER, Lytle BL, Brindis RG, et al. Association between hospital process performance and outcomes among patients with acute coronary syndromes. Jama. 2006;295:1912–1920. doi: 10.1001/jama.295.16.1912. [DOI] [PubMed] [Google Scholar]
- 31.Yu DT, Black E, Sands KE, Schwartz JS, Hibberd PL, Graman PS, et al. Severe sepsis: variation in resource and therapeutic modality use among academic centers. Critical care. 2003;7:R24–R34. doi: 10.1186/cc2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braunholtz DA, Edwards SJ, Lilford RJ. Are randomized clinical trials good for us (in the short term)? Evidence for a "trial effect". J Clin Epidemiol. 2001;54:217–224. doi: 10.1016/s0895-4356(00)00305-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.