Abstract
While prostatic adenocarcinomas are relatively indolent, some patients with advanced adenocarcinomas recur with small cell neuroendocrine carcinoma which is highly aggressive and lethal. Because glycolysis is a feature of malignancy and the degree of glycolysis generally correlates with tumor aggressiveness, we wanted to compare the metabolic differences and the molecular mechanisms involved between the two tumor types. In this study, and based on previous characterization, LNCaP and PC-3 prostate cancer cell lines were selected as models of prostatic adenocarcinoma and small cell neuroendocrine carcinoma, respectively. In addition to measuring glucose consumption, lactate secretion, and ROS levels we performed metabolic profiling in these two model systems. The role of CD44 was studied by RNA interference (RNAi) and lentivirus-mediated overexpression. Expression of key enzymes in glycolysis was studied using human tissue microarrays containing benign prostate, adenocarcinoma, and small cell neuroendocrine carcinoma. Results showed that glycolytic features of PC-3 cells were higher than that of LNCaP cells. PFKFB4 was overexpressed in human small cell carcinoma tissue vs. adenocarcinoma tissue. CD44 regulated glucose metabolism, intracellular ROS, and cell proliferation in PC-3 cells. Inhibition of CD44 also sensitized PC-3 cells to carboplatin. In conclusion, this study suggests different pathways of glucose metabolism contribute to the disparate biological behaviors of these two tumor types.
Implications
CD44 is an important regulator of glucose metabolism in small cell neuroendocrine carcinoma and may be an important therapeutic target.
Keywords: glucose consumption, prostate cancer cells, ROS, CD44, PFKFB4, glycolysis
INTRODUCTION
Prostate cancer (PCa) is the most common cancer in men in the United States with 233,000 new cases and ~30,000 deaths estimated in 20141. Hormonal therapy, to lower androgen levels and/or block androgen receptor (AR) function, is currently used to treat advanced and metastatic PCa which provides temporary symptomatic relief. However, after an average of 18 months, the cancer invariably recurs as castration resistant prostate cancer (CRPC). To treat CRPC, newer agents have been approved including enzalutamide and abiraterone which better block AR signaling and inhibit intra-tumoral androgen synthesis, respectively. Unfortunately, their effects are generally short-lived and many patients will quickly develop resistance. Importantly, although well over 90% of primary PCas are classified as adenocarcinoma (AdenoCa) with glandular formation and expression of AR and prostate specific antigen (PSA), a significant number of metastatic tumors after hormonal therapy belong to a histologic variant form of carcinoma known as small cell neuroendocrine carcinoma (SCNC)2. In an ongoing large scale study of metastatic PCa in 300 men after treatment with enzalutamide and abiraterone, we have observed that around 20% of the biopsied tumors are SCNC (unpublished data). In contrast to the relatively slow-growing and indolent AdenoCa, SCNC is highly aggressive and rapidly lethal. Histologically, SCNC is composed of neuroendocrine cells that do not form glands, are negative for AR and prostate-specific antigen (PSA), and refractory to currently available therapies3. Understanding the fundamental molecular mechanism of SCNC and discovering novel therapeutics are urgent unmet needs.
In contrast to benign cells, cancer cells generally follow the Warburg effect, displaying increased glycolysis for energy production4. This feature is accompanied by suppression of mitochondrial respiration and increased flux through the pentose phosphate pathway (PPP). The Warburg effect, initially described in 1924, has received renewed attention recently in the field of cancer metabolism due to the widespread clinical application of Fluorodeoxyglucose (FDG) – Positron Emission Tomography (PET) imaging which is based on increased glucose uptake by cancer cells. Unlike most tumors, however, FDG-PET imaging cannot detect localized, untreated PCa5, but can image SCNC6, suggesting that metabolic differences may be an underlying mechanism for the vastly different biologic behaviors of AdenoCa and SCNC. Additionally, literature has shown that prostatic AdenoCa utilizes mitochondrial respiration which is unique to the metabolism displayed in other tumor cells7.
CD44 is a cell surface protein with functions in many biological processes including cell adhesion and proliferation. In cancer cells, CD44 plays a role in tumor growth and metastasis through multiple means: angiogenesis, cell survival, cell migration, etc8–9. It has also been suggested that CD44 is a cell surface marker for cancer stem cells. A recent report showed that CD44 was involved in regulating the glycolytic pathway in colorectal cancer and lung carcinoma cell lines10. This report also found that expression of CD44 in human cancer cell lines induces a more invasive and drug-resistant phenotype11. Our lab has reported that CD44 expression is a feature of prostatic SCNC while the bulk tumor cells of AdenoCa are negative for CD4412. With reports that p53, a commonly mutated tumor suppressor in SCNC, regulates CD448 and evidence that CD44 interacts with PKM210, the glycolytic isoform of pyruvate kinase, we propose that CD44 may be modulating metabolism in SCNC. The goal of this study is to investigate the differences in glucose metabolism between the two forms of PCa and understand the underlying molecular mechanism. We hypothesize that prostatic SCNC is more glycolytically active than AdenoCa and CD44 is a key regulator of glucose metabolism that maybe a potential therapeutic target for SCNC.
MATERIALS AND METHODS
Cell lines
Prostate cancer cell lines LNCaP, VCaP, PC-3, CWRR1, DU145, and 22RV1 cells were obtained from the American Type Culture Collection (ATCC). The cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (Hyclone, Logan, UT, USA) and penicillin-streptomycin. The cells were incubated in a humidified incubator at 37°C and 5% CO2.
LNCaP and PC-3 cell lines were authenticated by DDC Medical (Fairfield, OH).
Preparation of low glucose RPMI-1640 medium
10% glucose RPMI-1640 medium was prepared with glucose free RPMI-1640 (Gibco BRL, USA) and dextrose (EMD chemicals Inc, Gibbstown, NJ, USA), and supplemented with 10% fetal bovine serum (FBS) (Hyclone) and penicillin-streptomycin.
Measurement of glucose consumption and lactate secretion
LNCaP and PC-3 cells were seeded in a 96-well plate at 8,000 cells/well and incubated in low glucose RPMI-1640 medium with 10% FBS and penicillin-streptomycin for 18 hours. Glucose concentrations in the media were determined by glucose assay kit (Sigma, St. Louis, MO, USA) following manufacturer’s instructions.
Lactate concentration was determined by using an enzymatic method. Briefly, the media was collected, and hydrazine buffer, NAD, and LDH (Sigma) were added. Distilled water was used as blanks. Absorbance at 340nm was measure to determine the lactate levels.
Measurement of ROS
Intracellular ROS levels were detected using H2DCF-DA (Invitrogen, USA). Cells were cultured in a 96-well black assay plate (Corning, USA), and washed once with phosphate- buffered saline (PBS) before incubation with serum-free media containing 10µM H2DCF-DA for 30 minutes at 37°C. ROS levels were determined at excitation and emission wavelengths of 485 and 520nm, respectively, by using a Synergy H1 Hybrid multi-mode microplate reader (Bio-Tek Instruments, Winooski, VT, USA). Control media without dye was used to determine background fluorescence. Cell numbers were normalized before measurement.
Metabolic profiling
LNCaP and PC-3 cells were plated in 6 well plates (500,000 cells per well; 3 wells per condition) and incubated overnight. Cells were incubated in medium with dialyzed FBS containing labeled glucose (4.5 g/L of U-13C6 D-glucose, Cambridge Isotope Laboratories, Inc.) for 24 hours. For spent media samples, 20µL was collected from each well (in triplicate). For cell samples, cells were rinsed with cold 150 mM ammonium acetate. Then cells were scraped off with 1mL of 80% cold methanol. An internal standard of 10 nmol norvaline was added and the cell suspension was spun down at top speed for 5 minutes at 4°C. The supernatant was transferred into a glass vial and the pellet was resuspended in 200µL 80% methanol, spun again, and the supernatant was combined into the glass vial. The metabolites were dried in EZ- 2Elite evaporator at 30°C using aqueous program and stored at −80°C until mass spectrometry was performed.
Samples were resuspended in 70% acetonitrile and 5µL were injected onto a LunaNH2 (150mm × 2mm, Phenomenex) column. Analysis was performed with an UltiMate 3000RSLC (Thermo Scientific) couple to a Q Exactive mass spectrometer (Thermo Scientific). The spectrometer ran with polarity switching (+4.00kV / −4.00kV) in full scan mode containing an m/z range of 70–1050. Metabolites were separated with (1) 5mM ammonium acetate (pH 9.9) and acetonitrile. The start of the gradient was 15% to 90%(1) for 18 minutes then an isocratic step for 9 minutes and back to 15% (1) for 7 minutes. The metabolites and their isotopomers were quantified using TraceFinder 3.1 with accurate mass measurements (≤3ppm) and retention times. Correction for naturally occurring 13C was considered for isotopologue distribution measurements as described13. R programming language was used for data analysis.
Immunohistochemistry
A total of 146 cases were studied, including 73 cases of normal prostate, 60 cases of prostatic adenocarcinoma, and 13 cases of SCNC. All samples were built into tissue microarrays (TMA), and each sample was represented by 3 cores. Sections were deparaffinized with xylene and rehydrated through graded ethanol. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in methanol for 10 minutes. Heat-induced epitope retrieval (HIER) was carried out for all sections in 0.01M citrate buffer, pH 6.0 using a steamer at 95°C for 25 minutes. Rabbit anti-human polyclonal PFKFB4 primary antibody (Novus, NBPI-32528) was diluted with BSA to a concentration of 1:100 and incubated with the TMA sections for 45 minutes at room temperature. The antibody was then visualized using the NovoLink Polymer Detection System (Leica, RE7140-K). Finally, sections were rinsed with water, counterstained with hematoxylin, dehydrated through graded ethanol, cleared with xylene, and cover-slipped.
Evaluation of immunohistochemical staining
All sections were blindly analyzed by two experienced investigators (WL and ZL). The staining intensity was scored as negative (0), weakly positive (1), moderately positive (2), and strongly positive (3). Results of the cores from the same case were averaged to arrive at a score for the case, which were analyzed by a team of statisticians (GL and LD).
RNA interference
CD44 and PFKFB4 were knocked down with small interfering RNA (siRNA) using siTRAN 1.0 Transfection Reagent (OriGene, USA). PC-3 cells were transfected with 10nM of CD44 siRNA (SR300683, OriGene), PFKFB4 siRNA (SR303465, OriGene), or Universal scrambled negative control siRNA duplex (SR30004, OriGene) for 48 hours. siRNA-mediated knocking-down was confirmed by western blot analyses for CD44 (ABGENT, USA) and PFKFB4 (abcam, USA).
Infection with retrovirus vector expressing CD44
As previously described14, lentiviral vector expressing CD44 was introduced into LNCaP cells through spin infection at 1,500 rpm for 45 minutes with 8µg/ml polybrene. After 48 hours, cells were selected in puromycin and expanded. The expression of CD44 was analyzed using western blot analysis.
Western Blot
Western blot was performed as described14. Briefly, cultured cells were lysed with cell lysis buffer. The protein concentration of each sample was measured with Bio-Rad protein assay kit. Equal amounts of protein were separated on 8% SDS-polyacrylamide gels followed by transfer to polyvinylidene fluoride membrane. The membrane is blocked with non-fat milk, incubated with primary antibody at 4°C overnight, followed by secondary antibody incubation and developed with West Femto Kit (Pierce, IL, USA).
Quantitative polymerase chain reaction (qPCR) analysis
Total RNA was extracted from cultured cells with animal RNA miniprep plus kit (Bioland Scientific LLC, USA) according to manufacturer’s protocol, normalized based on the amount of β-actin mRNA. The following specific forward and reverse primers were used: PFKFB4: 5’-CAGAACAGCTGCCCTACCTC-3’ and 5’-GCTTCCTCTGGAGGTCTTGA-3’.
Cell proliferation assay
Cell proliferation and viability was quantified using the Quick Cell Proliferation Colorimetric Assay Kit Plus (Biovision). Briefly, cells were cultured at 50,000 cells/well in a 96-well plate and incubated. At collection time, 10µL/well of WST reagent were added to each well and incubated at 37°C for 4 hours. Then the plate was shaken for 1 minute and the absorbance was measured at 420nm with the reference wavelength of 650nm on a plate reader. BCA assay was performed to normalize to total protein amount.
Clonogenic Cell Survival assay
Cells were transiently transfected with respective siRNAs. After 24 hours, cells were plated and treated (1uM for carboplatin) in 60mm dishes (500 cells/dish). After 24 hours, cultured cells were washed twice in complete growth medium and maintained for 10–14 days. The media was then discarded, washed with PBS, and stained with crystal violet. Surviving colonies were counted and imaged to determine plating efficiency.
Statistical analysis
All experiments were performed in triplicates, and the data are presented as means ± SD. Statistical significance was determined using the unpaired student t-test. A p value of <0.05 was considered significant. Data analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA) and Microsoft Excel 2007.
RESULTS
Increased glycolytic features in PC-3 cells compared to LNCaP cells
Cancer is characterized by increased glycolysis, regardless of oxygen availability, and the glycolytic rate generally correlates with tumor aggressiveness. Prostatic adenocarcinoma and SCNC are two histologically distinct tumor types with different clinical courses. The former usually pursues an indolent course while the latter is highly aggressive and invariably lethal. We therefore aimed to determine whether glucose metabolism may be different between the two tumor types. We used LNCaP cells and PC-3 cells to model adenocarcinoma and SCNC respectively, based on our previous characterization of the two cell lines15. A glycolytic phenotype is characterized by increased glucose consumption and lactate secretion along with reduced ROS levels10. Thus, we measured the glucose and lactate concentrations of media from cultured LNCaP and PC-3 cells. Consistent with our hypothesis, we found that media from cultured PC-3 cells contained lower glucose levels and higher lactate levels, suggesting that PC-3 cells consume more glucose and secrete more lactate than LNCaP cells (Figures 1A and 1B). With increased metabolic flux towards the glycolytic pathway, ROS levels decrease, due to reduced mitochondrial respiration. We measured ROS levels within LNCaP and PC-3 cells and found significantly lower ROS levels in PC-3 cells (Figure 1C).
Figure 1. Increased glycolytic features in PC-3 cells compared to LNCaP cells.
A: glucose consumption in LNCaP and PC-3 cells (*p=0.002). B: lactate secretion by LNCaP and PC-3 cells (*p=0.043). C: ROS levels in LNCaP and PC-3 cells (*p=0.001).
To further confirm these results, we performed metabolic profiling on extracted samples from both cells and media with glucose labeling to trace the metabolic pathways. With negative values from media samples indicating that the metabolite was consumed, we see that PC-3 cells consume more glucose than LNCaP cells, as expected (Figure 2A). Concordantly, PC-3 media samples showed higher lactate levels indicating increased lactate secretion by these cells compared to LNCaP cells (Figure 2B). When looking at the extracted samples from the cells with labeled glucose, we find more unlabeled (M0) tricarboxylic acid (TCA) cycle intermediates in PC-3 cells while LNCaP cells show increased glucose labeling in these intermediates (Figures 2C–2F). Decreased labeling in the TCA cycle metabolites is consistent with a shift in metabolic flux away from mitochondrial respiration and towards other glycolysis branch pathways. The increased M2 labeling in citrate levels in PC-3 cells suggests that citrate is being used to fuel other biosynthetic pathways for rapid cell proliferation (Figure 2G). All of these observations are consistent with our hypothesis that glycolysis is favored by the highly aggressive tumor cells of prostatic SCNC compared to the relatively slow-growing and indolent tumor cells of adenocarcinoma.
Figure 2. Differential metabolic profiling of LNCaP and PC-3 cells and media.
Metabolite levels were measured in the spent media for glucose (A) and lactate (B). TCA cycle metabolite isotopomer levels were measured from cell samples with uniformly-labeled glucose: alpha-ketoglutarate (C), succinate (D), fumarate (E), malate (F), and citrate (G). (*p<0.05, **p<0.01, ***p<0.001)
Expression of PFKFB4 in prostatic adenocarcinoma and SCNC
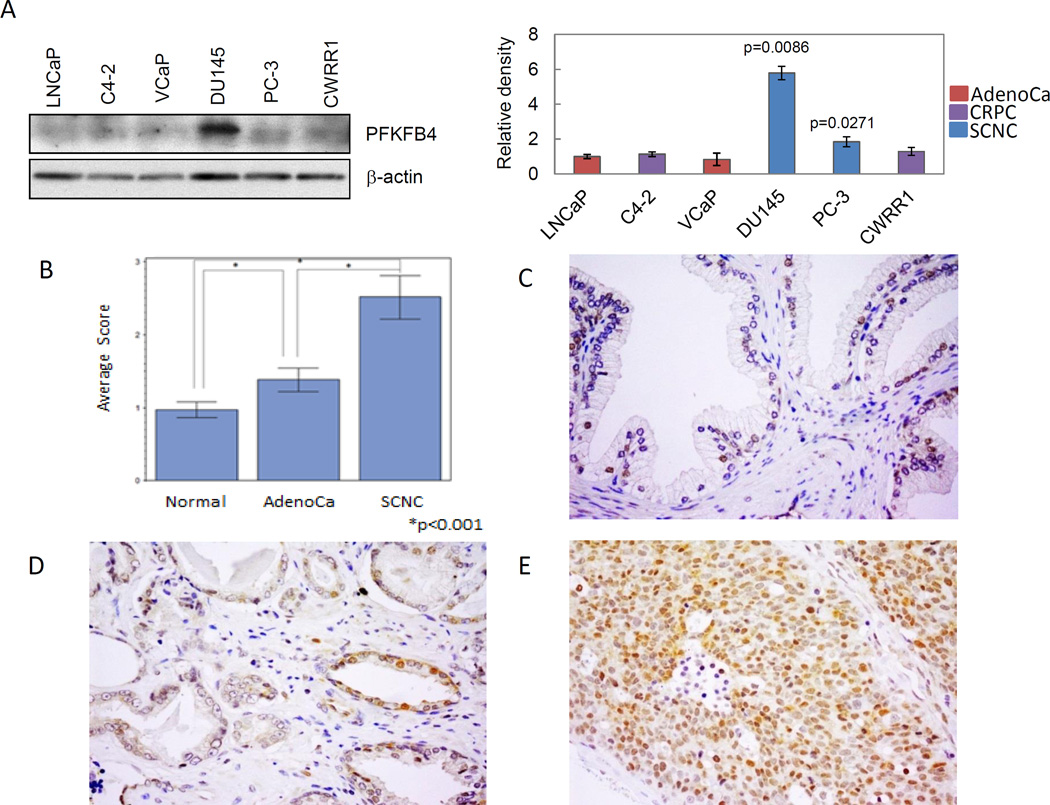
Several metabolic enzymes involved in glycolysis have shown to be potential therapeutic targets for certain cancers. One enzyme of importance is 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 (PFKFB4), an isoform of phosphofructokinase 2 (PFK2), which is required for PCa cell survival and balancing glycolytic activity and antioxidant production in PCa cells16. We thus wanted to investigate if the expression of PFKFB4 is differentially expressed in adenocarcinoma vs. SCNC.
We first determined the expression levels of PFKFB4 protein in various PCa cell lines. Western blot results showed that, compared to naïve AdenoCa LNCaP and VCaP cells, or to castration-resistant AdenoCa C4-2 and CWRR1 cells, prostatic SCNC PC-3 and DU145 cells express relatively higher levels of PFKFB4. Of note, PFKFB4 protein level is significantly higher in DU145 cells (Figure 3A).
Figure 3. Elevated expression of PFKFB4 in small cell neuroendocrine carcinoma tissue.
A: Protein expression of PFKFB4 in established prostate cancer cell line. Left: western blot; Right: diagram showing the relative density of protein expression. B: The average scores of PFKFB4 staining in normal and malignant prostate tissues (*p<0.001). Immunohistochemical staining of PFKFB4 staining in benign prostate tissue (C), prostatic adenocarcinoma (D), and SCNC (E).
We then performed immunohistochemistry using multiple tissue microarrays (TMA) containing benign prostate (n=73), prostatic adenocarcinoma (n=60), and prostatic SCNC (n=13). PFKFB4 was differentially expressed in benign and malignant prostate tissues (Figure 3B). Most cases of benign prostate tissue were negative for PFKFB4, with only occasional scattered luminal secretory cells being positive (Figure 3C). The adenocarcinoma cells were weakly or moderately stained (Figure 3D). In contrast, most of the SCNC cells were strongly and diffusely positive for PFKFB4 (Figure 3E). Detailed scoring of the staining and statistical analysis showed that the expression of PFKFB4 in benign prostate tissue was lower than that of prostatic adenocarcinoma (p<0.001), and its expression was significantly higher in SCNC than in adenocarcinoma (p<0.001).
CD44 is a regulator of glucose metabolism and PFKFB4 in prostate cancer cells
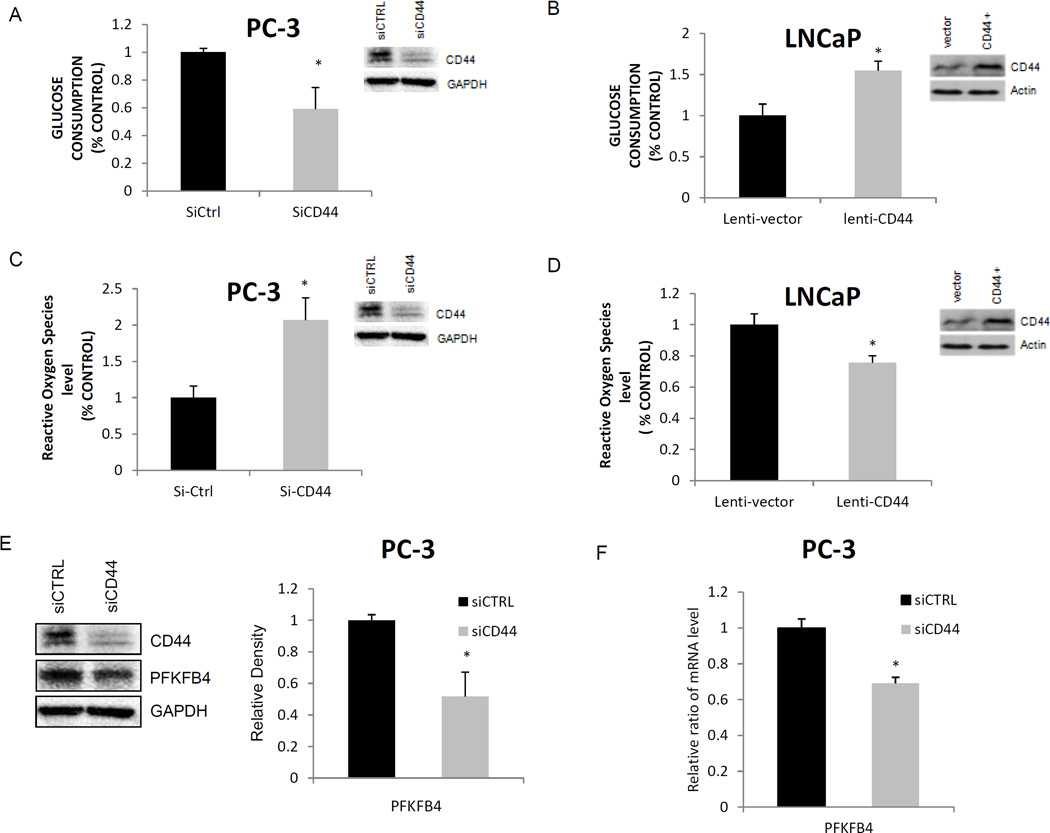
CD44 has been implicated in controlling glucose metabolism, and it is also expressed in prostatic SCNC but not adenocarcinoma12. Similarly, our lab has shown that CD44 is expressed in PC-3 cells and not in LNCaP cells15. Therefore, we further hypothesized that CD44 may be a regulator of glucose metabolism in prostatic SCNC. We performed CD44 knockdown studies in PC-3 cells and CD44 overexpression studies in LNCaP cells to study changes in glucose metabolism. When CD44 was knocked down in PC-3 cells, glucose consumption levels decreased significantly compared to that in the parental cells (p=0.003; Figure 4A). Conversely, overexpression of CD44 in LNCaP cells led to an increase in glucose consumption (p<0.001; Figure 4B). These findings suggest that CD44 expression in prostatic SCNC is involved in the tumor’s increased glycolytic activity.
Figure 4. CD44 modulates glucose metabolism, intracellular ROS levels, and PFKFB4 expression.
A: PC-3 cells were transfected with siRNA targeting CD44, and glucose consumption was measured. Glucose consumption decreased with siCD44 (*p=0.003). B: lentivirus mediated overexpression of CD44 protein or control in LNCaP cells led to increased glucose consumption (*p<0.001). C: Intracellular ROS increased after siRNA mediated CD44 ablation in PC-3 cells (*p<0.001). D: Intracellular ROS decreased after lentivirus mediated CD44 overexpression in LNCaP cells (*p<0.001). E: CD44 knockdown in PC-3 cells results in reduced PFKFB4 protein levels (*p<0.001). F: CD44 knockdown in PC-3 cells results in reduced expression of PFKFB4 mRNA (*p<0.001).
As mentioned previously, suppressing mitochondrial respiration in malignant tumor cells reduces the production of ROS and leads to tumor cells’ resistance to various therapies17. We therefore investigated the relationship between CD44 expression and intracellular ROS levels in prostate cancer cells. After CD44 knockdown, we observed significantly increased ROS levels in PC-3 cells (Figure 4C). Similarly, overexpression of CD44 decreased ROS production in LNCaP cells (Figure 4D). As a glycolytic regulator, we examined whether CD44 affected PFKFB4 expression since PFKFB4 levels showed a positive correlation with SCNC. CD44 ablation in PC-3 cells showed a reduction in both protein and mRNA levels of PFKFB4 (Figure 4E–4F). Overall, the data shows that CD44 plays a key role in altering glucose metabolism and affecting glycolytic enzymatic activity in prostatic SCNC.
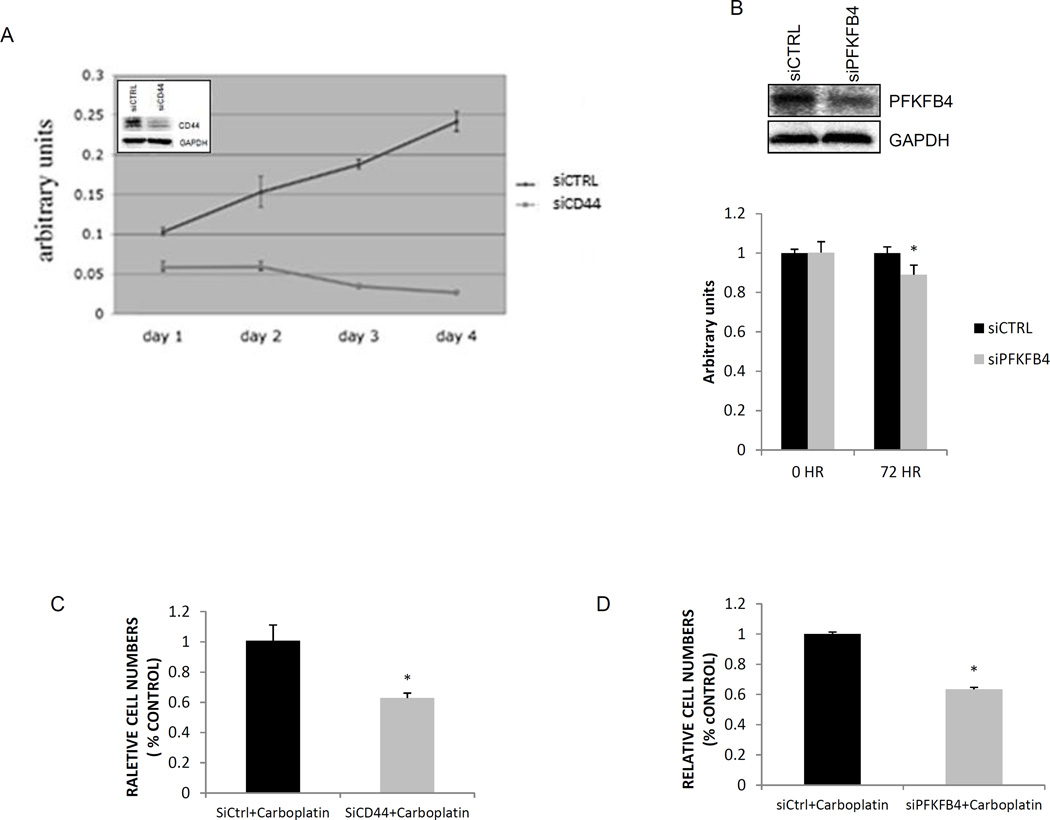
CD44 knockdown inhibits cell proliferation and sensitizes SCNC tumor cells to carboplatin
Since glycolytic activity often correlates with cell proliferation, we examined the role of CD44 in the regulation of cell proliferation. Our study showed that the proliferation of PC-3 cells was significantly inhibited after CD44 knockdown (Figure 5A). Given that glucose consumption is necessary for the survival and proliferation of cancer cells, and CD44 knockdown results in a decrease in glucose consumption and proliferation of PC-3 cells as described above, we hypothesized that the expression of CD44 may have therapeutic implications in SCNC. This hypothesis would be consistent with a previous report that the expression of CD44 in human cancer cell lines induces a more invasive and drug-resistant phenotype11.
Figure 5. CD44 and PFKFB4 regulate cell proliferation and sensitize PC-3 cells to carboplatin.
A: CD44 knockdown inhibits the proliferation of PC-3 cells. B: PFKFB4 knockdown inhibits PC-3 cell proliferation (*p=0.015). C: CD44 knockdown sensitizes PC-3 cells to carboplatin (*p<0.001). D: PFKFB4 knockdown sensitizes PC-3 cells to carboplatin (*p=0.02).
Conventional and newer therapeutic PCa drugs targeting the AR signaling pathway are ineffective for SCNC as these tumor cells do not express AR. Because of this, many clinicians treat SCNC with carboplatin, a cisplatin analog, because pulmonary small cell neuroendocrine carcinoma is sensitive to this drug. However, the therapeutic benefit of carboplatin for prostatic SCNC is usually limited18. Our evidence suggests that CD44 and PFKFB4 may be involved in the drug resistant phenotype observed in SCNC. This phenotype is associated with glycolysis and low ROS levels. Reports have shown that manipulating the metabolic flux within tumor cells, by shifting to mitochondrial respiration, increasing ROS levels, or inactivating the PPP, can contribute to increased drug sensitivity10. Additionally, CD44-expressing cancer cells have shown chemoresistance in previous studies. We therefore investigated whether CD44 or PFKFB4 ablation would sensitize PC-3 cells to carboplatin. First, we found that PC-3 cells with PFKFB4 knockdown also significantly inhibited cell proliferation (Figure 5B).We then discovered that sensitivity of PC-3 cells to carboplatin treatment was significantly enhanced with knockdown of either CD44 or PFKFB4 (Figures 5C and 5D) implying that inhibition of these key glycolytic proteins may alter the metabolic flux and sensitize SCNC tumor cells to carboplatin.
DISCUSSION
The key finding in this study is that PC-3 cells, characteristic of SCNC, have increased glycolytic activity compared to LNCaP cells which is of clinical relevance. Most PCa patients are initially diagnosed with adenocarcinoma with tumor cells expressing luminal differentiation markers AR and PSA. These tumors are highly curable when they are low grade and localized. Advanced adenocarcinomas are treated with androgen ablation therapy (e.g., Lupron). Novel drugs inhibiting intratumoral androgen synthesis (abiraterone) or better inhibit AR (enzalutamide) can be used after conventional hormonal therapy has failed. Importantly, a significant proportion of patients will recur in the form of SCNC after the above therapies2. SCNC is extremely aggressive, rapidly fatal, and does not respond to the above therapies.
We have previously shown that LNCaP cells possess important features of adenocarcinoma with cells expressing AR and PSA and dependent on androgen for proliferation15. Similar to adenocarcinoma, LNCaP cells do not express CD44 and have wild type p53. In contrast, PC3 cells are characteristic of SCNC with tumor cells being negative for AR and PSA but expressing high levels of neuroendocrine (NE) markers. Similar to SCNC, PC-3 cells express CD44 and contain mutant p5315. These findings validate the efficacy of performing the studies mentioned in LNCaP and PC-3 cell lines.
In this study, we demonstrate that glucose metabolism may be an important difference between the two tumor types that could potentially be targeted for the treatment of prostatic SCNC. This conclusion is based on biochemical studies in cell line models as well as immunohistochemical studies in human cancer tissues. Our study provides a metabolic basis for the difference of biological behavior of the two tumors, revealing novel strategies to target the metabolic pathway in SCNC. Furthermore, we showed that CD44, a cell-surface molecule expressed in SCNC but not adenocarcinoma, appears to be a critical regulator of glucose metabolism specifically in SCNC which displayed a glycolytic phenotype. CD44 ablation in PC-3 cells inhibits glucose consumption and increases ROS production, possibly through changes in activity and expression of important glycolytic enzymes. In addition, knocking down CD44 inhibits cell proliferation and increases sensitivity of PC-3 cells to carboplatin, a chemotherapeutic agent currently used to treat aggressive prostatic SCNC. Overall, our evidence suggests that CD44 may be an important and selective molecular target for prostatic SCNC.
These findings are consistent with previous reports. For example, Singh et al showed that HIV-1 120-kDa glycoprotein variant IIIB (gp120-IIIB) causes apoptosis of PC-3 and DU145 cells through its interaction with CXCR4, and the inhibitory function is associated with downregulation of CD4419. Another report showed that antitumor activity of hyaluronic acid synthesis inhibitor 4-methylumbelliferone in PCa cells is also associated with a downregulation of CD4420.
The function of CD44 in tumor invasion and metastases, especially in bone metastases, has been reported21–23. Recent studies revealed that CD44 may be involved in metabolic regulation in colorectal cancer and breast cancer cells. Our studies demonstrates that in PCa, CD44 is involved in the aggressive behavior of the tumor cells when there is a phenotypic switch from adenocarcinoma to SCNC which likely occurs through regulation of glucose metabolism.
PFKFB4 is an important enzyme in regulating glucose metabolism that catalyzes the synthesis and degradation of fructose-2,6-biphosphate24, 25. Silencing of PFKFB4 should divert glucose-6-phosphate towards the glycolytic pathway, thereby depleting the pentose phosphate pathway16. Knockdown of CD44 reduces PFKFB4 expression which can lead to decreased levels of NADPH. NADPH is required to maintain cellular stores of glutathione, an antioxidant preventing ROS accumulation. This may provide an explanation for the increased ROS levels observed after CD44 ablation in PC-3 cells (Figure 6). The above studies involving PFKFB4 manipulation are consistent with results obtained in a previous study16.
Figure 6. A potential pathway for CD44 molecularly regulating cell proliferation and survival through PFKFB4 in human prostatic SCNC.
Platinum-based chemotherapy is the first-line therapy for small cell lung cancer26, 27, however it has limited efficacy in advanced PCa18, 28 and CD44-expressing cancer cells have shown chemoresistance10.Certain anticancer drugs including cisplatin kill cancer cells by inducing ROS generation which causes apoptosis29. With evidence that CD44 depletion altered ROS levels, these measured increases in ROS in PC-3 cells should enhance sensitivity to carboplatin. We demonstrated that CD44 knockdown increased sensitivity of PC-3 cells to carboplatin, likely through a metabolic shift towards mitochondrial respiration and ROS production. This finding has important clinical implications. CD44 is considered a marker of cancer stem cells including prostate cancer stem cells30. We have demonstrated that the bulk tumor cells in prostatic adenocarcinoma are negative for CD44 while the tumor cells of SCNC are positive12. Interestingly, PC-3 cells but not LNCaP cells, express CD4415, 31, and the PC-3 human prostate carcinoma cell holoclones contain self-renewing tumor initiating cells with high levels of CD4432. Our studies further support the concept that CD44 may be a therapeutic target for aggressive SCNC, due at least in part to its modulation of glucose metabolism. In conclusion, SCNC, the more aggressive form of PCa, shows increased glycolytic activity in comparison to adenocarcinoma, and CD44 may be playing a role in regulating this altered metabolic phenotype.
Acknowledgments
Grant Support
This research was supported by a Stand Up to Cancer–Prostate Cancer Foundation–Prostate Dream Team Translational Cancer Research Grant (SU2C-AACR-DT0812). This research grant was made possible by the generous support of the Movember Foundation. Stand Up To Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research. J. Huang is supported by UCLA SPORE in Prostate Cancer (PI: Reiter), Department of Defense Prostate Cancer Research Program W81XWH-11-1-0227 (PI: Huang), W81XWH-12-1-0206 (PI: L. Wu), NIH 1R01CA158627-01 (PI: Marks), 1R01CA172603-01A1 (PI: Jiaoti Huang), Prostate Cancer Foundation Honorable A. David Mazzone Special Challenge Award (PI: Robert Reiter), and Stand-up-to-Cancer Dream Team Award (PI: Small and Witte). This work was also partially supported by National Natural Science Foundation of China, grant 81460387 (to W. Li).
REFERENCES
- 1.Siegal R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014 Jan;64(10):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Beltran H, Tagawa ST, Park K, MacDonald T, Milowsky MI, Mosquera JM, et al. Challenges in recognizing treatment-related neuroendocrine prostate cancer. J ClinOncol. 2012 Dec 20;30(36):e386–e389. doi: 10.1200/JCO.2011.41.5166. [DOI] [PubMed] [Google Scholar]
- 3.Lipianskaya J, Cohen A, Chen CJ, Hsia E, Squires J, Li Z, et al. Androgen-deprivation therapy-induced aggressive prostate cancer with neuroendocrine differentiation. Asian J Androl. 2014;16:541–544. doi: 10.4103/1008-682X.123669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Effert PJ, Bares R, Handt S, Wolff JM, Bull U, Jakse G. Metabolic imaging of untreated prostate cancer by positron emission tomography with 18fluorinelabeled deoxyglucose. J Urol. 1996;155:994–998. [PubMed] [Google Scholar]
- 6.de Carvalho Flamini R, Yamaga L, Mello ME, Wagner J, Livorsi da Cunha M, Osawa A, et al. F-18 FDG PET/CT imaging in small cell prostate cancer. Clin Nucl Med. 2010;35(6):452–453. doi: 10.1097/RLU.0b013e3181db4ce9. [DOI] [PubMed] [Google Scholar]
- 7.Costello LC, Franklin RB. The intermediary metabolism of the prostate: A key to understanding the pathogenesis and progression of prostate malignancy. Oncology. 2000 Nov;59(4):269–282. doi: 10.1159/000012183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godar S, Ince TA, Bell GW, Feldser D, Donaher JL, Bergh J, et al. Growth-inhibitory and tumor- suppressive functions of p53 depend on its repression of CD44 expression. Cell. 2008;134:62–73. doi: 10.1016/j.cell.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miletti-González KE, Chen S, Muthukumaran N, Saglimbeni GN, Wu X, Yanj J, et al. The CD44 receptor interacts with P-glycoprotein to promote cell migration and invasion in cancer. Cancer Res. 2005 Aug 1;65(15):6660–6667. doi: 10.1158/0008-5472.CAN-04-3478. [DOI] [PubMed] [Google Scholar]
- 10.Tamada M, Nagano O, Tateyama S, Ohmura M, Yae T, Ishimoto T, et al. Modulation of glucose metabolism by CD44 contributes to antioxidant status and drug resistance in cancer cells. Cancer Res. 2012 Mar 15;72(6):1438–1448. doi: 10.1158/0008-5472.CAN-11-3024. [DOI] [PubMed] [Google Scholar]
- 11.Shah V, Taratula O, Garbuzenko OB, Taratula OR, Rodriguez-Rodriguez L, Minko T. Targeted Nanomedicine for Suppression of CD44 and Simultaneous Cell Death Induction in Ovarian Cancer: An Optimal Delivery of siRNA and Anticancer Drug. Clin Cancer Res. 2013 Nov 15;19(22):6193–6204. doi: 10.1158/1078-0432.CCR-13-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simon RA, di Sant'Agnese PA, Huang LS, Xu H, Yao JL, Yang Q, et al. CD44 expression is a feature of prostatic small cell carcinoma and distinguishes it from its mimickers. Hum Pathol. 2009 Feb;40(2):252–258. doi: 10.1016/j.humpath.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Moseley HN. Correcting for the effects of natural abundance in stable isotope resolved metabolomics experiments involving ultra-high resolution mass-spectrometry. BMC Bioinformatics. 2010 Mar 17;11(1):139. doi: 10.1186/1471-2105-11-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Pham P, Vu NB, Duong TT, Nguyen TT, Truong NH, Phan NL, et al. Suppression of human breast tumors in NOD/SCID mice by CD44 shRNA gene therapy combined with doxorubicin. Onco Targets Ther. 2012;5:77–84. doi: 10.2147/OTT.S30609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tai S, Sun Y, Squires JM, Zhang H, Oh WK, Liang CZ, et al. PC3 is a cell line characteristic of prostatic small cell carcinoma. Prostate. 2011 Nov;71(15):1668–1679. doi: 10.1002/pros.21383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ros S, Santos CR, Moco S, Baenke F, Kelly G, Howell M, et al. Functional metabolic screen identifies 6- phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 as an important regulator of prostate cancer cell survival. Cancer Discov. 2012 Apr;2(4):328043. doi: 10.1158/2159-8290.CD-11-0234. [DOI] [PubMed] [Google Scholar]
- 17.Cao B, Li M, Zha W, Zhao Q, Gu R, Liu L, et al. Metabolomic approach to evaluating adriamycin pharmacodynamics and resistance in breast cancer cells. Metabolomics. 2013;9:960–973. doi: 10.1007/s11306-013-0517-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jungi WF, Bernhard J, Hürny C, Schmitz SH, Hanselmann S, Gusset H, et al. Effect of carboplatin on response and palliation in hormone-refractory prostate cancer. Swiss Group for Clinical Cancer Research (SAKK) Support Care Cancer. 1998 Sep;6(5):462–468. doi: 10.1007/s005200050195. [DOI] [PubMed] [Google Scholar]
- 19.Singh S, Bond VC, Powell M, Singh UP, Bumpers HL, Grizzle WE, et al. CXCR4-gp120-IIIB interactions induce caspase- mediated apoptosis of prostate cancer cells and inhibit tumor growth. Mol Cancer Ther. 2009 Jan;8(1):178–184. doi: 10.1158/1535-7163.MCT-08-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lokeshwar VB, Lopez LE, Munoz D, Chi A, Shirodkar SP, Lokeshwar SD, et al. Antitumor activity of hyaluronic acid synthesis inhibitor 4-methylumbelliferone in prostate cancer cells. Cancer Res. 2010 Apr 1;70(7):2613–2623. doi: 10.1158/0008-5472.CAN-09-3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan W, Chen Y, Yao Y, Zhang H, Wang T. Increased invasion and tumorigenicity capacity of CD44+/CD24- breast cancer MCF7 cells in vitro and in nude mice. Cancer Cell Int. 2013 Jun 24;13(1):62. doi: 10.1186/1475-2867-13-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gvozdenovic A, Arlt MJ, Campanile C, Brennecke P, Husmann K, Li Y, et al. CD44 enhances tumor formation and lung metastasis in experimental osteosarcoma and is an additional predictor for poor patient outcome. J Bone Miner Res. 2013 Apr;28(4):838–847. doi: 10.1002/jbmr.1817. [DOI] [PubMed] [Google Scholar]
- 23.Hiraga T, Ito S, Nakamura H. Cancer stem-like cell marker CD44 promotes bone metastases by enhancing tumorigenicity, cell motility, and hyaluronan production. Cancer Res. 2013 Jul 1;73(13):4112–4122. doi: 10.1158/0008-5472.CAN-12-3801. [DOI] [PubMed] [Google Scholar]
- 24.Miletti-González KE, Murphy K, Kumaran MN, Ravindranath AK, Wernyj RP, Kaur S, et al. Identification if function for CD44 intracytoplasmic domain (CD44-ICD): modulation of matrix metalloproteinase 9 (MMP-9) transcription via novel promoter response element. J Biol Chem. 2012 Jun 1;287(23):18995–19007. doi: 10.1074/jbc.M111.318774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minchenko OH, Ochiai A, Opentanova IL, Ogura T, Minchenko DO, Caro J, et al. Overexpression of 6-phosphofructo-2- kinase/fructose-2,6-bisphosphatase-4 in the human breast and colon malignant tumors. Biochimie. 2005 Nov;87(11):1005–1010. doi: 10.1016/j.biochi.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Simon GR, Turrisi A. American College of Chest Physicians. Management of small cell lung cancer: ACCP evidence-based clinical practice guidelines. Chest. (2nd) 2007 Sep;132(3 Suppl):324S–339S. doi: 10.1378/chest.07-1385. [DOI] [PubMed] [Google Scholar]
- 27.Stahel R, Thatcher N, Früh M, Le Péchoux C, Postmus PE, Sorensen JB, et al. 1st ESMO Consensus Conference in lung cancer; Lugano 2010: small-cell lung cancer. Ann Oncol. 2011 Sep;22(9):1973–1980. doi: 10.1093/annonc/mdr313. [DOI] [PubMed] [Google Scholar]
- 28.Canobbio L, Guarneri D, Miglietta L, Decensi A, Oneto F, Boccardo F. Carboplatin in advanced hormone refractory prostatic cancer patients. Eur J Cancer. 1993;29A(15):2094–2096. doi: 10.1016/0959-8049(93)90040-m. [DOI] [PubMed] [Google Scholar]
- 29.Chen J. Reactive oxygen species and drug resistance in cancer chemotherapy. Austin J ClinPathol. 2014;1(4):1017. [Google Scholar]
- 30.Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006 Mar 16;25(12):1696–1708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 31.Palaputtu GS, Wu C, Silvers CR, Martin HB, Williams K, Salamone L, et al. Selective expression of CD44, a putative prostate cancer stem cell marker, in neuroendocrine tumor cells of human prostate cancer. Prostate. 2009 May 15;69(7):787–798. doi: 10.1002/pros.20928. [DOI] [PubMed] [Google Scholar]
- 32.Li H, Chen X, Calhoun-Davis T, Claypool K, Tang DG. PC3 human prostate carcinoma cell holoclones contain self-renewing tumor initiating cells. Cancer Res. 2008 Mar 15;68(6):1820–1825. doi: 10.1158/0008-5472.CAN-07-5878. [DOI] [PubMed] [Google Scholar]