Abstract
Hyperglycemia induces oxidative stress and thereby may exacerbate β-cell dysfunction in type 2 diabetes (T2DM). Notably, glutathione (GSH), synthesized from N-Acetylcysteine (NAC), neutralizes reactive oxygen species within cells and is low in individuals with diabetes.
Aim
Determine if NAC supplementation improves β-cell function and glucose tolerance by decreasing oxidative stress in T2DM.
Methods
Thirteen subjects (6M/7F) with T2DM (duration: 0-13 years, median: 2 years), treated with diet/exercise alone (n=7) or metformin (n=6), underwent a 2-hour oral glucose tolerance test (OGTT) at baseline, after 2 weeks supplementation with 600 mg NAC orally twice daily, and again after 2 weeks supplementation with 1200 mg NAC twice daily. The following measurements were made: fasting glucose and fructosamine for glycemic control, incremental AUC glucose (0-120 mins) for glucose tolerance, and Δ insulin/Δ glucose (0-30 minutes) for the early insulin response to glucose. Fasting erythrocyte GSH and GSSG (oxidized glutathione) levels, plasma thiobarbituric acid reactive substances (TBARS), and urine F2α isoprostanes were measured to assess oxidative status.
Results
Subjects were middle aged (Mean ± SEM: 53.9 ± 3.2 years), obese (BMI 37.3 ± 2.8 kg/m2), and relatively well-controlled (HbA1c 6.7 ± 0.3%, 50 mmol/mol). Glycemic control, glucose tolerance, insulin release, and oxidative markers did not change with either dose of NAC.
Conclusions
Based on the lack of any short-term benefit from NAC supplementation on markers of glucose metabolism, β-cell response, and oxidative status, it is unlikely to be a valuable therapeutic approach for treatment of type 2 diabetes.
Keywords: N-Acetylcysteine, β-cell function, antioxidant
1.0 Introduction
Hyperglycemia can exacerbate β-cell dysfunction, with oxidative stress proposed as a major mediator of this “glucotoxic” effect1,2. Oxidative stress is marked by the excessive production of reactive oxygen species (ROS) in the setting of insufficient and/or defective anti-oxidant systems. Subjects with diabetes have been shown to have elevated markers of oxidative stress3 Chronic excessive glucose causes toxic effects on various organs, and the pancreatic islets in particular contain very low levels of antioxidant enzyme activity making them especially susceptible to damage from oxidative stress4. Given that hyperglycemia increases oxidative stress markers and oxidative stress is implicated as a mediator of β-cell dysfunction and progression to glucose intolerance5, we hypothesize that anti-oxidant use may reduce oxidative stress thereby resulting in improved β-cell function and glucose tolerance.
One such anti-oxidant compound warranting further investigation is N-Acetylcysteine (NAC). NAC is a precursor of the anti-oxidant glutathione and as such, increases the available glutathione pool. Reduced glutathione (GSH) is able to neutralize reactive oxygen species by forming oxidized glutathione (GSSG) and water. GSH levels have been shown to be low in individuals with diabetes6,7 and impaired glucose tolerance8,9 Furthermore, inhibition of glutathione synthesis in human islets in vitro led to increased hydrogen peroxide, decreased insulin mRNA and decreased insulin secretion which was prevented by increasing glutathione peroxidase activity10. These data suggest that increasing GSH levels by administration of the precursor NAC could protect the islet from oxidative stress and improve insulin secretion.
Animal studies have demonstrated the beneficial effects of NAC on β-cell function. Oral NAC in diabetic C57BL/KsJ-dbdb mice helped maintain glucose-stimulated insulin secretion and lowered glucose levels11. Marked attenuation of the development of hyperglycemia in ZDF rats treated daily with NAC has also been described12. Co-infusion of NAC during a hyperglycemic clamp in non-diabetic rats prevented an increase in markers of oxidative stress and improved insulin sensitivity13.
There is a lack of concrete data in humans on the effects of NAC on insulin secretion, particularly in subjects with diabetes. Studies in humans including individuals with non-insulin dependent diabetes, have shown that oral NAC is able to decrease markers of oxidative stress14-16 and increase GSH levels14,17. NAC also decreased oxidative stress markers induced by a high glucose content meal in subjects with type 2 diabetes (T2DM)18. One small pilot study found that arginine-induced insulin secretion was improved in 4 subjects with T2DM after 28 days of oral NAC vs. placebo19 However, given the scarcity of available data, additional human studies are needed to determine if treatment with NAC can improve β-cell function20.
2.0 Research Design and Methods
2.1 Study Design
This was an open-label pilot study in subjects with type 2 diabetes that compared the effect of 1200 mg daily and 2400 mg daily of NAC to baseline. Eligible subjects underwent study procedures at three time points: at baseline, after 2 weeks on NAC 600 mg BID and again after an additional 2 weeks on NAC 1200 mg BID.
2.2 Subjects
Subjects were recruited through local newspaper and flyer advertisements in the Seattle, WA area. Subjects underwent a screening visit to determine eligibility which consisted of providing written informed consent, undergoing a medical history and physical exam, a fasting blood draw, and a screening 75 gm 2 hour OGTT. The study enrolled men and women between the ages of 18-75 years who had a diagnosis of T2DM based on: 1) fasting plasma glucose values ≥ 126 mg/dl, 2) two-hour post oral glucose challenge values ≥ 200 mg/dl or 3) HbA1 C value ≥ 6.5% (48 mmol/mol) and who were either treated with diet and exercise alone or with diet, exercise and metformin. T2DM subjects taking metformin were required to be on a stable dose of metformin for at least 3 months. Individuals had to be in otherwise good health as determined by medical history, physical examination, and laboratory tests. Exclusion criteria included: pregnant or lactating females; uncontrolled diabetes mellitus with severe hyperglycemia (hemoglobin A1C ≥ 9%); chronic oral or parenteral corticosteroid treatment (> 7 consecutive days of treatment) within 8 weeks prior to screening; use of HIV protease inhibitors, diabetes medications besides metformin or use of niacin; chronic inflammatory diseases or use of anti-inflammatory drugs; thyroid abnormalities (TSH < 0.5 or > 5 μU/ml); creatinine > 1.5 in men and > 1.3 mg/dl in women; individuals with clinical hepatic disease or ALT greater than ≥ 1.5 times upper limit of normal within 60 days preceding the first dose of the study drug; weight loss of >5% body weight within the last 6 months or starting an intensive exercise program within 4 weeks of study initiation; tobacco use; excessive alcohol consumption (self-reported > 2 drinks a day); use of any investigational drug in the last 30 days; or anemia (hematocrit < 33%).
The study was approved by the Institutional Review Boards of the Veterans Affairs Puget Sound Health Care System and the University of Washington in accordance with ethical standards on human experimentation. All subjects signed a written informed consent.
2.3 NAC Supplementation
Subjects were studied at baseline after which they were given a 30-day supply of NAC capsules. They were instructed to take one capsule (600 mg) orally twice daily for the first 2 weeks followed by 2 capsules (1200 mg) twice daily for the second 2 weeks. The NAC was generously donated by TwinLab (American Fork, UT). Dose and duration of NAC were based on prior human studies reporting significant effect on GSH and the GSH:GSSG ratio with NAC administration (1200 mg/day)17. Subjects were instructed to bring their NAC with them for study visits after 2 and 4 weeks at which time the pills were counted to assess adherence.
2.4 Study Procedures
OGTT: After at least a 10 hour overnight fast during which subjects were only permitted to drink water, subjects reported to the VA Puget Sound Clinical Research Unit. Study staff recorded any adverse events or concomitant medication taken. A fasting urine sample was collected for measurement of urinary isoprostanes. The timing of blood sampling is given relative to the time of administration of the oral glucose load (at time 0). An IV was placed for blood sampling and after 15 minutes of rest, the first basal sample was drawn (-30 minutes). At the 2 and 4 week study procedure days, subjects took their dose of NAC immediately after the -30 minute blood draw. OGTT baseline samples were then drawn at -10, -5, and -1 minute time points. At time 0 the subject was asked to drink a solution containing 75 g of glucose over 5 minutes. After the glucose ingestion blood samples were drawn at 10, 20, 30, 60, 90, and 120 minutes. Glucose, insulin and c-peptide were measured at all time points. Erythrocyte GSH and GSSG levels along with plasma and urinary markers of lipid peroxidation were measured at -30 minutes. Blood samples for GSSG were aliquoted into a vial containing the inhibitor 6% metaphosphoric acid and immediately flash frozen.
2.5 Assays
The following assays were performed: glucose by glucose oxidase; insulin by automated electrochemiluminescence immunoassay (Cobas e601, Indianapolis, IN); GSH and GSSG by colorimetric assay (Bioxytech, Percipio Bio Inc., Portland, OR); TBARS by fluorometric assay of malondialdehyde (MDA) levels (Caymen Chemicals, Ann Arbor, MI); Urine isoprostanes by mass spectrometry21. GSH and GSSG samples were assayed within 4 days of collection and TBARS assays were performed within 30 days of collection. All samples were stored at -80 degrees Celsius until assayed.
2.6 Statistical Analysis
Two subjects successfully completed only two out of three OGTTs with all data included in the analysis. Data were expressed as mean ± standard error of the mean (SEM). Changes from baseline OGTT were compared to the low dose NAC (600 mg bid) and high dose NAC (1200 mg bid) using Generalized Estimating Equations using an intent to treat approach. An analysis of covariance was also performed adjusting for metformin given its reported anti-oxidant properties22. A secondary analysis was performed which included subjects with ≥ 80% compliance with NAC use (see Compliance, discussed below). A p < 0.05 was considered significant. Statistical analyses were performed using SPSS software (V19.0, SPSS Inc, Chicago, IL).
2.7 Sample Size Analysis
A priori calculation of sample size required to see a clinically meaningful effect, > 20% change from baseline, was performed using STATA software (V 13.0, StataCorp LP, College Station, TX). These calculations ensured that a missed effect is unlikely despite the small sample size in the study. For the incAUC glucose, GSH, and TBARS variables with β=0.8 and α=0.05, the calculated sample sizes for > 20% effect ranged from 6-11 subjects.
3.0 Results
3.1 Subject Characteristics
A total of 13 subjects (6 males, 7 females), age 53.9 ± 3.2 (mean ± SEM) years, with BMI 37.3 ± 2.8 kg/m2 and average HbA1c of 6.7 ± 0.3% (50 mmol/mol) were enrolled. Six subjects were taking metformin. The median duration of diabetes was 2 years with a range of 0-13 years.
3.2 Glucose Tolerance, β-cell Function and Oxidative Stress Markers
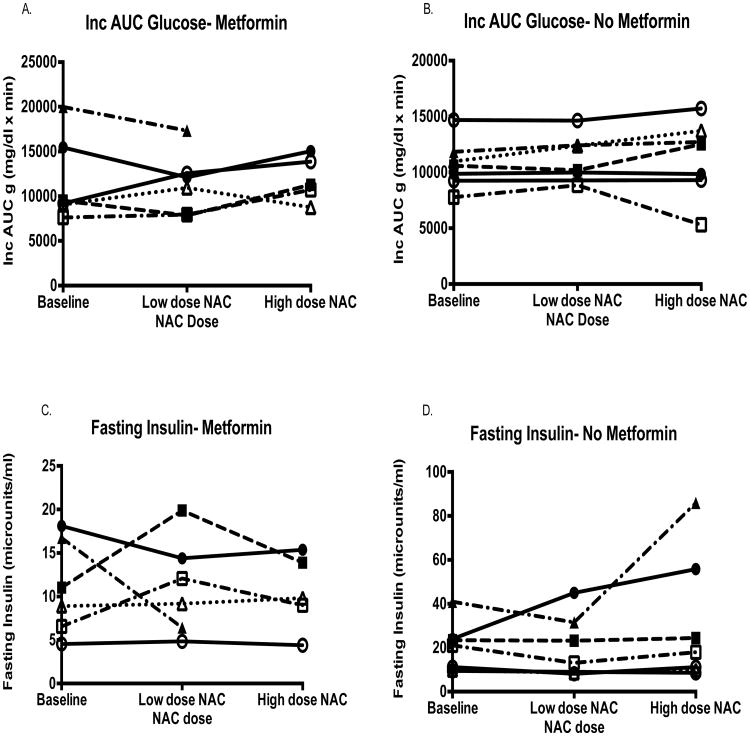
Compared to baseline, markers of glycemic control, glucose tolerance, and insulin response did not significantly change on either dose of NAC (Table 1). Markers of oxidative stress analyzed including GSH, GSH/GSSG ratio, TBARS, and urine F2α isoprostanes did not change with either dose of NAC (Table 2). No significant differences in the response to NAC were observed in the incremental AUC glucose or fasting insulin concentrations for subjects on Metformin vs. those not on Metformin (Figure 1). Metformin use did not have an impact on the response to NAC for any other variable (data not shown).
Table 1. Effect of NAC on glucose metabolism.
| Variables | Baseline | Low dose NAC % change | High dose NAC % change | P-value: baseline vs. low dose | P-value: baseline vs. high dose |
|---|---|---|---|---|---|
| Fasting glucose (mg/dl) | 121±5 | 3.76±4 | 4.34±3 | 0.94 | 1 |
| Inc AUC glucose (0-120 min) (mg/dl × min) | 11,215±942 | 3.15±5 | 10.87±6 | 1 | 0.15 |
| Fasting insulin (microU/ml) | 16±2.6 | 6.43±15 | 23.15±14 | 1 | 1 |
| Fructosamine (uMol/L) | 242±12 | -172±2 | 1.30±1 | 0.77 | 1 |
| Δ insulin/Δ glucose (0-30 min) (microU/ml)/(mg/dl) | 1.7±0.6 | -0.03±12 | -4.60±12 | 0.99 | 0.50 |
| Average ± SEM | |||||
Inc AUC= incremental area under the curve
Table 2. Effect of NAC on oxidative stress markers.
| Variables | Baseline | Low dose NAC % change | High dose NAC % change | P-value: baseline vs. low dose | P-value: baseline vs. high dose |
|---|---|---|---|---|---|
| GSH (uMol) | 762±60 | 0.39±4 | 10.31±7 | 1 | 0.44 |
| GSH/GSSG | 1,446±179 | 12.48±34 | -2.28±29 | 1 | 1 |
| TBARS (uMol) | 1.1±0.1 | -1.47±6 | 3.91±8 | 1 | 1 |
| Urine isoprostane/ Urine creatinine (ng/mg) | 1.6±0.3 | 16.68±15 | -7.79±20 | 0.74 | 0.7 |
| Average ± SEM | |||||
GSH = glutathione; GSGG = oxidized glutathione; TBARS = thiobarbituric acid reactive substances
Figure 1.
No significant differences in the incremental AUC glucose or fasting insulin were seen for subjects on Metformin (A and C) or not on Metformin (B and D) with use of low dose or high dose NAC.
3.3 Compliance
All subjects were > 80% compliant with the low dose of NAC. However, six subjects on the high dose NAC were less than 80% compliant. Additional analyses were performed excluding subjects who were < 80% compliant on the high dose NAC. Seven subjects were included in this secondary analysis, with urine F2α isoprostanes available for a smaller sample size of four subjects (see Supplemental table). Findings showed a significant increase in fasting glucose (p=0.03) and fructosamine (p=0.02) on the high NAC dose. Urine isoprostanes also increased significantly on the high dose of NAC, but this finding is limited by the small sample size (n=4). These data are therefore suggestive that NAC, particularly at the higher dose, not only is not beneficial, but may in fact be harmful to overall glucose metabolism.
4.0 Discussion
NAC supplementation did not result in any beneficial effect at either dose. Specifically, there was no effect on fasting glucose, glycemic control, glucose tolerance, insulin secretion or oxidative stress markers in subjects with T2DM. Furthermore, when data from noncompliant subjects were removed, there was a suggestion that the higher dose of NAC may in fact have a detrimental effect on glucose levels.
This study is novel in its assessment of the effect of NAC supplementation on β-cell function and glucose tolerance in addition to oxidative stress markers in T2DM human subjects. Previous studies in subjects with T2DM, have demonstrated benefits of NAC including improved endothelial function and decreased systolic blood pressure6,15 but did not assess effects on glucose metabolism. The lack of effect of NAC on oxidant markers is unanticipated given previous animal and human studies that reported positive anti-oxidant effects with this particular supplement10-18 The finding that NAC did not improve glucose tolerance or β-cell function in this study does not eliminate the possibility that oxidative stress may still be an important contributor to the chronic deterioration of β-cell function leading to T2DM.
Reactive oxygen species inherently have extremely short half-lives and are difficult to measure directly, and thus surrogate measures of oxidative stress are employed that quantify the byproducts of oxidative stress. In this study, oxidative stress was assessed by the endogenous cellular non-enzymatic antioxidant system, glutathione, as well as the byproducts of lipid peroxidation, TBARS and urine F2α isoprostanes. However, ROS can induce a variety of damaging cellular processes besides those evaluated in this study, such as DNA oxidative lesions and damage to cell membrane integrity through induction of protein and carbohydrate structural changes. NAC may selectively target some, but not all of these pathways, potentially explaining the lack of effect in this study. Furthermore, assessment of anti-oxidant function was limited to subjects who met criteria for T2DM based on fasting plasma glucose, two-hour post oral glucose challenge, or HbA1C values. We specifically studied this population as they have established hyperglycemia and also have been shown to have decreased antioxidant capacity. NAC may have limited applicability as a therapeutic anti-oxidant in already established disease where the oxidative burden is high, but our results do not suggest that subjects with worse glycemic control, based on HbA1C or glucose levels, had improved response to NAC treatment. While our results do not exclude use of NAC as a preventative drug that might ameliorate oxidative stress in the setting of pre-diabetes, the finding that glucose tolerance and fasting insulin levels tended to increase at the higher NAC dose dampen enthusiasm. Furthermore, one study in 140 non-diabetic subjects taking NAC 600 mg daily for 8 weeks found that NAC increased HOMA-IR in smokers and obese subjects, but not in non-obese smokers, and also decreased glucose tolerance in obese subjects, suggesting this is not an isolated effect23.
We also considered that the timing of sample draw relative to NAC administration on the day of OGTT may limit the effect seen. However, analysis performed on a subset of samples comparing GSH and GSH/GSSG values before and 30 minutes after supplementation with NAC found no differences (unpublished observations). This is consistent with the GSH half-life of 4-5 days, despite a half-life of 6 hours for NAC24,25.
In summary, the lack of any short-term benefit from NAC on markers of glucose metabolism, β-cell response, and oxidative status argues that it is unlikely to be a valuable therapeutic approach for treatment of type 2 diabetes. However, future studies investigating a combination of different anti-oxidants with NAC or use in subjects with IFG and/or IGT are needed to fully evaluate the utility of antioxidant use in various stages of islet dysfunction.
Supplementary Material
Acknowledgments
We are grateful to the study participants for their contribution and time. This study was supported by funding and resources from the Department of Veteran Affairs and the Diabetes Research Center (P30DK017047). The NAC supplement was generously provided free of charge from Twin Labs. The authors have no conflicts of interest to disclose.
The authors' responsibilities were as follows: MS assisted with data analysis, interpretation and contributed to the writing of the manuscript; AF assisted with data interpretation; KU designed the study and was involved in all aspects of the study including data analysis and manuscript preparation. All authors read and approved the final manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Magdalena A. Szkudlinska, Email: magda3@u.washington.edu.
Anize D. von Frankenberg, Email: anize.frankenberg@gmail.com.
Kristina M. Utzschneider, Email: kutzschn@u.washington.edu.
References
- 1.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351–66. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boden G, Ruiz J, Kim CJ, Chen X. Effects of prolonged glucose infusion on insulin secretion, clearance, and action in normal subjects. The American journal of physiology. 1996;270:E251–8. doi: 10.1152/ajpendo.1996.270.2.E251. [DOI] [PubMed] [Google Scholar]
- 3.Robertson RP, Harmon JS. Diabetes, glucose toxicity, and oxidative stress: A case of double jeopardy for the pancreatic islet beta cell. Free Radic Biol Med. 2006;41:177–84. doi: 10.1016/j.freeradbiomed.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 4.Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. The Journal of biological chemistry. 2004;279:42351–4. doi: 10.1074/jbc.R400019200. [DOI] [PubMed] [Google Scholar]
- 5.Tiwari B, Pandey K, Abidi A, Rizvi S. Markers of Oxidative Stress during Diabetes Mellitus. Journal of Biomarkers. 2013;2013:8. doi: 10.1155/2013/378790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Mattia G, Bravi MC, Laurenti O, et al. Endothelial dysfunction and oxidative stress in type 1 and type 2 diabetic patients without clinical macrovascular complications. Diabetes research and clinical practice. 2008;79:337–42. doi: 10.1016/j.diabres.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Aaseth J, Stoa-Birketvedt G. Glutathione in Overweight Patients with Poorly Controlled Type 2 Diabetes. The Journal of Trace Elements in Experimental Medicine. 2000;13:105–11. [Google Scholar]
- 8.Konukoglu D, Hatemi H, Ozer EM, Gonen S, Akcay T. The erythrocyte glutathione levels during oral glucose tolerance test. J Endocrinol Invest. 1997;20:471–5. doi: 10.1007/BF03348003. [DOI] [PubMed] [Google Scholar]
- 9.Vijayalingam S, Parthiban A, Shanmugasundaram KR, Mohan V. Abnormal antioxidant status in impaired glucose tolerance and non-insulin-dependent diabetes mellitus. Diabetic medicine : a journal of the British Diabetic Association. 1996;13:715–9. doi: 10.1002/(SICI)1096-9136(199608)13:8<715::AID-DIA172>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka Y, Tran PO, Harmon J, Robertson RP. A role for glutathione peroxidase in protecting pancreatic beta cells against oxidative stress in a model of glucose toxicity. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:12363–8. doi: 10.1073/pnas.192445199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaneto H, Kajimoto Y, Miyagawa J, et al. Beneficial effects of antioxidants in diabetes: possible protection of pancreatic beta-cells against glucose toxicity. Diabetes. 1999;48:2398–406. doi: 10.2337/diabetes.48.12.2398. [DOI] [PubMed] [Google Scholar]
- 12.Robertson RP, Harmon J, Tran PO, Tanaka Y, Takahashi H. Glucose toxicity in beta-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes. 2003;52:581–7. doi: 10.2337/diabetes.52.3.581. [DOI] [PubMed] [Google Scholar]
- 13.Haber CA, Lam TK, Yu Z, et al. N-acetylcysteine and taurine prevent hyperglycemia-induced insulin resistance in vivo: possible role of oxidative stress. American journal of physiology Endocrinology and metabolism. 2003;285:E744–53. doi: 10.1152/ajpendo.00355.2002. [DOI] [PubMed] [Google Scholar]
- 14.Zembron-Lacny A, Slowinska-Lisowska M, Szygula Z, Witkowski K, Szyszka K. The comparison of antioxidant and hematological properties of N-acetylcysteine and alpha-lipoic acid in physically active males. Physiol Res. 2009;58:855–61. doi: 10.33549/physiolres.931590. [DOI] [PubMed] [Google Scholar]
- 15.Martina V, Masha A, Gigliardi VR, et al. Long-term N-acetylcysteine and L-arginine administration reduces endothelial activation and systolic blood pressure in hypertensive patients with type 2 diabetes. Diabetes care. 2008;31:940–4. doi: 10.2337/dc07-2251. [DOI] [PubMed] [Google Scholar]
- 16.Sen CK, Rankinen T, Vaisanen S, Rauramaa R. Oxidative stress after human exercise: effect of N-acetylcysteine supplementation. J Appl Physiol. 1994;76:2570–7. doi: 10.1152/jappl.1994.76.6.2570. [DOI] [PubMed] [Google Scholar]
- 17.De Mattia G, Bravi MC, Laurenti O, et al. Reduction of oxidative stress by oral N-acetyl-L-cysteine treatment decreases plasma soluble vascular cell adhesion molecule-1 concentrations in non-obese, non-dyslipidaemic, normotensive, patients with non-insulin-dependent diabetes. Diabetologia. 1998;41:1392–6. doi: 10.1007/s001250051082. [DOI] [PubMed] [Google Scholar]
- 18.Masha A, Brocato L, Dinatale S, Mascia C, Biasi F, Martina V. N-acetylcysteine is able to reduce the oxidation status and the endothelial activation after a high-glucose content meal in patients with Type 2 diabetes mellitus. J Endocrinol Invest. 2009;32:352–6. doi: 10.1007/BF03345726. [DOI] [PubMed] [Google Scholar]
- 19.Robertson R, Zhou H, Zhang T, Harmon JS. Chronic oxidative stress as a mechanism for glucose toxicity of the beta cell in type 2 diabetes. Cell Biochem Biophys. 2007;48:139–46. doi: 10.1007/s12013-007-0026-5. [DOI] [PubMed] [Google Scholar]
- 20.Corcoran GB, Wong BK. Role of glutathione in prevention of acetaminophen-induced hepatotoxicity by N-acetyl-L-cysteine in vivo: studies with N-acetyl-D-cysteine in mice. The Journal of pharmacology and experimental therapeutics. 1986;238:54–61. [PubMed] [Google Scholar]
- 21.Morrow JD. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:279–86. doi: 10.1161/01.ATV.0000152605.64964.c0. [DOI] [PubMed] [Google Scholar]
- 22.Esteghamati A, Eskandari D, Mirmiranpour H, et al. Effects of metformin on markers of oxidative stress and antioxidant reserve in patients with newly diagnosed type 2 diabetes: a randomized clinical trial. Clinical nutrition. 2013;32:179–85. doi: 10.1016/j.clnu.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Hildebrandt W, Hamann A, Krakowski-Roosen H, et al. Effect of thiol antioxidant on body fat and insulin reactivity. Journal of molecular medicine. 2004;82:336–44. doi: 10.1007/s00109-004-0532-5. [DOI] [PubMed] [Google Scholar]
- 24.Sansone RA, S L. Getting a Knack for NAC: N-Acetyl-Cysteine. Innovations in clinical neuroscience. 2011;8:10–4. [PMC free article] [PubMed] [Google Scholar]
- 25.Holdiness MR. Clinical pharmacokinetics of N-acetylcysteine. Clin Pharmacokinet. 1991;20:123–34. doi: 10.2165/00003088-199120020-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.