Abstract
Objective
There are few studies evaluating whether to proceed with planned resection when a patient with non-small cell lung cancer (NSCLC) unexpectedly is found to have N2 disease at the time of thoracoscopy or thoracotomy. To help guide management of this clinical scenario, we evaluated outcomes for patients who were upstaged to pN2 after lobectomy without induction therapy using the National Cancer Database (NCDB).
Methods
Survival of NSCLC patients treated with lobectomy for clinically unsuspected mediastinal nodal disease (cT1-3N0-1, pN2 disease) from 1998-2006 in the NCDB was compared to “suspected” N2 disease patients (cT1-3N2) who were treated with chemotherapy ± radiation followed by lobectomy, using matched-analysis based on propensity scores.
Results
Unsuspected pN2 disease was found in 4.4% (2,047/46,691) of patients who underwent lobectomy as primary therapy for cT1-3N0-1 NSCLC. The 5-year survival was 42%, 36%, 21%, and 28% for patients who underwent adjuvant chemotherapy (n=385), chemoradiation (n=504), radiation (n=300), and no adjuvant therapy (n=858), respectively. Five-year survival of the entire unsuspected pN2 cohort was worse than survival of 2,302 patients were treated with lobectomy after induction therapy for cN2 disease (30% vs 40%, p < 0.001), though no significant difference in five-year survival was found in a matched-analysis of 655 patients from each group (37% vs 37%, p = 0.95).
Conclusion
This population-based analysis suggests that, in the setting of unsuspected pN2 NSCLC, proceeding with lobectomy does not appear to compromise outcomes if adjuvant chemotherapy ± radiation therapy can be administered following surgery.
Keywords: Lung cancer surgery, Unsuspected N2
Introduction
The practice of treating with induction therapy prior to potential surgery for patients with non-small cell lung cancer (NSCLC) who have pathologically confirmed N2 disease is well-supported in the literature.1 However, there are few studies evaluating whether to proceed with planned resection when a patient unexpectedly is found to have N2 disease at the time of thoracoscopy or thoracotomy. This clinical scenario has been reported to occur in 2.0% to 18.5% of patients, 2-8 and is associated with a 5-year overall survival ranging from 10% to 40%.2,4,6,9-12 Some reports,2,6,9-11,13 including those by the National Comprehensive Cancer Network and the American College of Chest Physicians (ACCP),14,15 have suggested that the proper course of action for patients with unsuspected N2 disease diagnosed at the time of surgery is to continue with resection, although this guideline is based on very limited data and noted to be a Grade 2C recommendation.15 Other authors have suggested a preference towards aborting resection in order to administer induction therapy.16
In order to improve the level of evidence available to guide treatment decisions regarding unsuspected N2 disease, we evaluated survival of patients with unsuspected N2 NSCLC who underwent lobectomy using the National Cancer Data Base (NCDB).
Methods
National Cancer Database
Data for this study was queried from the NCDB, which is jointly sponsored by the American College of Surgeons Commission on Cancer (CoC) and the American Cancer Society. With data from over 30 million patients and 1,500 cancer centers in the U.S., this database contains information on approximately 70% of all newly diagnosed cases of cancer in the United States and Puerto Rico.17 Clinical staging information was directly recorded in the NCDB using American Joint Committee on Cancer (AJCC) 6th edition TNM classifications for the years of study inclusion (1998-2006).18
Study design
This NCDB analysis was approved by the Institutional Review Board of Duke University. From a de-identified NCDB participant user file, all NSCLC patients in the NCDB who were treated with lobectomy without induction therapy for “unsuspected N2” disease (clinical T1-3N0-1M0 but pathologic N2 disease) were selected using the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) histology and topography codes. The time period starting from the beginning of 1998 to the end of 2006 was chosen because long-term survival data for patients diagnosed in this time period were available at the time of our analysis. Patients with non-malignant pathology, history of previous unrelated malignancy, and unknown survival data were excluded from analysis. In order to create a comparison group and estimate the impact of proceeding with resection for unsuspected pN2 versus aborting resection in order to deliver induction therapy, all patients treated with induction chemotherapy ± radiation followed by lobectomy for “suspected N2 disease” (clinical T1-3N2M0) were also selected. The primary outcome measured was overall survival. Hospital length of stay and 30-day readmission were secondary outcomes examined as potential surrogate markers for postoperative morbidity, which is not specifically recorded in the NCDB. Data for these secondary outcomes were only recorded for patients from 2003-2006.
Statistical analysis
Patients were grouped based on their diagnosis of N2 disease (suspected or unsuspected). Pearson's chi-square test for categorical variables and Wilcoxon rank sum test for continuous variables were used to determine differences in patient and treatment characteristics. Survival time was measured from time of diagnosis to time of death or last follow-up, and median survival and 5-year survival were estimated by the Kaplan-Meier product limit approach. The impact of adjuvant therapy on survival of patients in the unsuspected N2 group was evaluated using the Kaplan-Meier method and log-rank test.
Outcomes of patients in the suspected and unsuspected N2 groups were initially compared using the log-rank test. A matched-analysis based on propensity scores was then conducted to create a cohort of patients with unsuspected N2 NSCLC who underwent lobectomy with similar baseline characteristics to patients with suspected N2 disease who underwent induction chemotherapy ± radiation followed by lobectomy, to attempt to control for nonrandom differences in baseline characteristics between the groups, as previously described.19,20 Briefly, patients were stratified into two groups based on unsuspected pN2 disease and suspected cN2 disease and a logistic regression model was used to calculate propensity scores based on patient- and disease-related variables that were determined to most likely act as confounders. These variables were determined a priori and included age, gender, race, Charlson/Deyo comorbidity (CDCC) score, clinical T status, facility type, insurance type, and histology. Because CDCC score was not available until 2003, the matched-analysis included only patients from 2003-2006. The most appropriately-matched pairs were selected with a caliper matching algorithm (caliper distance 0.01) with controls used only once. After propensity score matching, differences between groups were assessed using Pearson's chi-square tests or Fisher's exact test for categorical variables and Student's t-test for continuous variables. The Kaplan-Meier method was used to compare overall survival from time of surgery to time of death or last follow-up across groups.
The matched-analysis and survival analysis described above were repeated to compare the survival of patients who had surgery plus adjuvant chemotherapy vs. patients who received induction chemotherapy followed by surgery. An additional matched-analysis was performed to compare the survival of patients who had surgery plus adjuvant chemoradiation vs. induction chemoradiation with surgery.
To further evaluate the impact of adjuvant therapies on patients with unsuspected N2 disease, we performed a multivariable analysis on a subgroup of patients who had undergone complete resection and excluded patients who died within 30 days of lobectomy because these patients most likely did not receive adjuvant therapy, and were excluded from the analysis to prevent selection bias that could overestimate the benefit of adjuvant therapy benefits. For patients with unsuspected N2 disease, a Cox proportional hazards model was used to compare overall survival between groups receiving different adjuvant therapies (adjuvant chemotherapy, chemoradiation, radiation, and no adjuvant therapy), adjusting for age, gender, race, Charlson/Deyo comorbidity (CDCC) score, clinical T stage, facility type, insurance type, histology and tumor location. All covariates included in the model were determined a priori according to clinical relevance.
All statistical analyses were performed using Stata Statistical Software: Release 13.0 (StataCorp LP, College Station, TX). A 2-sided p-value of 0.05 was used to define significance.
Results
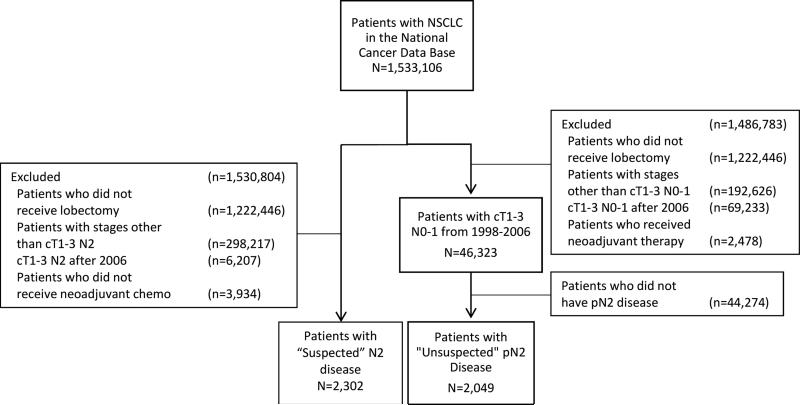
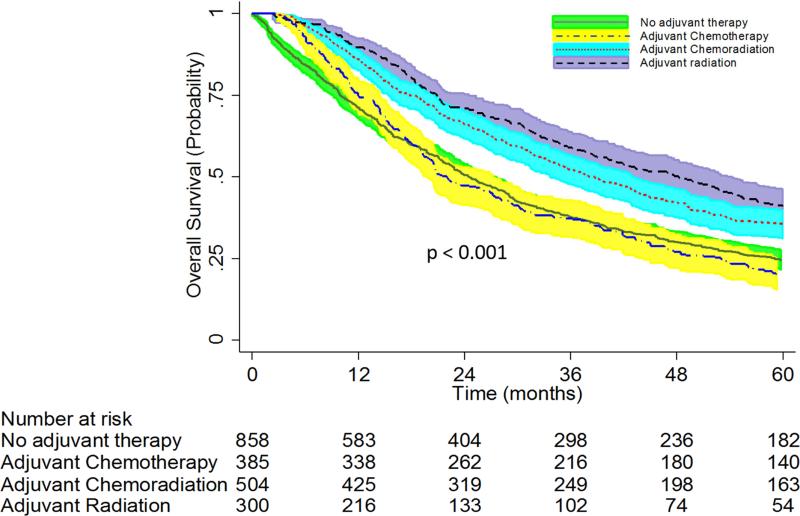
Between 1998 and 2006, 2,047 (4.4%) of 46,691 patients who underwent lobectomy as primary therapy for cT1-3 N0-1 NSCLC had “unsuspected” pN2 disease after surgical resection (Figure 1). The median survival was 31 months (95% CI: 29-33) and the overall 5-year survival was 30% (95% CI: 28-32%) for the entire unsuspected N2 cohort. In this cohort, no adjuvant therapy was administered for 858 (42%) of patients, while adjuvant chemotherapy was given to 385 (19%) patients, adjuvant chemoradiation to 504 (25%), and adjuvant radiation to 300 (15%) patients. Among patients in this unsuspected N2 group, the 5-year survival was significantly better for patients who received adjuvant chemotherapy (42% [95% CI: 36-47%]) or adjuvant chemoradiation (36% [95% CI: 31-40%]) compared to patients who received adjuvant radiation (21% [95% CI: 16-26%]) and patient who had no adjuvant therapy (28% [95% CI: 24-31%]) (Figure 2). Multivariable adjusted survival analyses demonstrated that when compared to no adjuvant therapy, adjuvant chemotherapy (HR 0.67; 95% CI: 0.55-0.82, p<0.001) and chemoradiation (HR 0.75; 95% CI: 0.58-0.92, p<0.001) were associated with improved survival, while adjuvant radiation treatment alone (HR 1.14; 95% CI: 0.84-1.56, p=0.40) was not associated with improved survival.
Figure 1.
Consort Diagram Showing Schema of Study Subject Selection
Figure 2.
Overall Survival of Patients with Unsuspected pN2 Disease Stratified by Type of Adjuvant Therapy. Patients with “unsuspected N2” disease had underwent lobectomy as primary therapy for cT1-3 N0-1 NSCLC and were found to have pN2 disease at the time of surgery. These patients then went on to receive either adjuvant chemotherapy, adjuvant chemoradiation, adjuvant radiation therapy, or no adjuvant therapy.
During the same time period, 2,302 patients underwent lobectomy after induction chemotherapy (n=898, 39%) or induction chemoradiation (n=1,404, 61%) for “suspected” N2 disease (cT1-3N2M0) NSCLC (Figure 1). Baseline characteristics and peri- and post-operative data for the “unsuspected” and “suspected” groups are detailed in Tables 1 and 2. Patients with unsuspected N2 disease were older, less likely to be male, had a higher CDCC score, and were more likely to be on Medicare or Medicaid than patients with suspected N2 disease. Patients in the unsuspected N2 group also had a lower clinical T status, were less likely to be treated in a comprehensive community cancer program, and were more likely to have a histology report of adenocarcinoma as compared to patients with suspected N2 disease. There were no significant differences between the groups regarding patients’ race.
Table 1.
Pre-Operative Demographics and Clinical Characteristics
| Unsuspected N2 (N=2,047) | Suspected N2 (N=2,302) | p-value | |
|---|---|---|---|
| Patient Age | <0.01 | ||
| Mean (SD) | 66.4 (10.3) | 61.7 (9.7) | |
| Median, IQR | 67 (60,74) | 62 (55,69) | |
| Sex, n (%) | <0.01 | ||
| Male | 939 (45.9%) | 1,191 (51.7%) | |
| Female | 1,108 (54.1%) | 1,111 (48.3%) | |
| Race, n (%) | 0.22 | ||
| White | 1,795 (87.7%) | 2,050 (89.1%) | |
| Black | 157 (7.7%) | 168 (7.3%) | |
| Other | 95 (4.6%) | 84 (3.7%) | |
| Charlson/Deyo Score, n (%) | <0.01 | ||
| 0 | 600 (58.5%) | 1,010 (71.4%) | |
| 1 | 325 (31.7%) | 335 (23.7%) | |
| 2 | 100 (9.8%) | 70 (5.0%) | |
| Insurance Status, n (%) | <0.01 | ||
| Uninsured | 26 (1.3%) | 42 (1.9%) | |
| Private | 808 (40.3%) | 1,179 (52.5%) | |
| Medicare/aid | 1,093 (54.5%) | 907 (40.4%) | |
| Other Government | 69 (3.4%) | 92 (4.1%) | |
| Unknown | 11 (0.6%) | 24 (1.1%) | |
| Clinical T Status, n (%) | <0.01 | ||
| 1 | 988 (48.3%) | 670 (29.1%) | |
| 2 | 1,010 (49.3%) | 1,282 (55.7%) | |
| 3 | 49 (2.4%) | 350 (15.2%) | |
| Facility Type, n (%) | 0.02 | ||
| Community Cancer Program | 163 (8.0%) | 161 (7.0%) | |
| Comprehensive Community Cancer Program | 965 (47.1%) | 1,138 (49.4%) | |
| Academic/Research Program | 854 (41.7%) | 959 (41.7%) | |
| Other specified types of cancer programs | 65 (3.2%) | 44 (1.9%) | |
| Histology, n (%) | <0.01 | ||
| Adenocarcinoma | 1,194 (58.4%) | 1,015 (44.1%) | |
| Squamous | 388 (19.0%) | 602 (26.2%) | |
| Large cell | 100 (4.9%) | 139 (6.0%) | |
| Bronchioalveloar | 123 (6.0%) | 45 (2.0%) | |
| Neuroendocrine | 30 (1.5%) | 11 (0.5%) | |
| Other | 209 (10.2%) | 489 (21.3%) |
* P values provided are from Wilcoxon Rank Sum test on continuous variables and from Chi-square test on categorical variables.
Table 2.
Peri- & Post-Operative Demographics and Clinical Characteristics
| Unsuspected N2 (N=2,047) | Suspected N2 (N=2,302) | p-value | |
|---|---|---|---|
| Re-admission in 30 days, n (%) | <0.01 | ||
| Yes | 60 (2.9%) | 75 (3.3%) | |
| Unknown | 1,062 (51.9%) | 984 (42.8%) | |
| Regional Lymph Nodes (LN) Examined | |||
| No. of patients with LN examined | 2,032 (99.2%) | 2,112 (91.7%) | |
| Median (IQR) | 11 (6,19) | 8 (3,19) | <0.01 |
| Positive Lymph Nodes (LN) Examined | <0.01 | ||
| No. of patients with LN Examined | 2,033 (99.2%) | 2,100 (91.2%) | |
| Median (IQR) | 3 (1,5) | 2 (0,9) | |
| Surgical Inpatient Stay, Days from Surgery | <0.01 | ||
| No. of patients with available data | 924 | 1,176 | |
| Mean (SD) | 7.9 (9.0) | 7.4 (8.2) | |
| Tumor Location, n (%) | <0.01 | ||
| RLL | 406 (19.8%) | 331 (14.4%) | |
| LLL | 263 (12.9%) | 177 (7.7%) | |
| RML | 81 (4.0%) | 83 (3.6%) | |
| RUL | 586 (28.6%) | 1,087 (47.2%) | |
| LUL | 638 (31.2%) | 536 (23.3%) | |
| Other | 73 (3.6%) | 88 (3.8%) | |
| Surgical Margins, n (%) | <0.01 | ||
| No Residual Tumor | 1,785 (87.2%) | 1,971 (85.6%) | |
| Microscopic Residual Tumor | 116 (5.7%) | 91 (4.0%) | |
| Macroscopic Residual Tumor | 55 (2.7%) | 82 (3.6%) | |
| Unknown | 91 (4.5%) | 158 (6.9%) | |
| Pathologic T stage | <0.01 | ||
| T0 | 1 (0.1%) | 95 (4.1%) | |
| T1 | 693 (33.9%) | 596 (25.9%) | |
| T2 | 1,122 (54.8%) | 577 (25.1%) | |
| T3 | 122 (6.0%) | 108 (4.7%) | |
| T4 | 106 (5.2%) | 61 (2.7%) | |
| IS | 0 (0.0%) | 3 (0.1%) | |
| Unknown | 3 (0.2%) | 862 (37.5%) | |
| Pathologic N Stage | <0.01 | ||
| N0 | 0 (0.0%) | 630 (42.1%) | |
| N1 | 0 (0.0%) | 144 (9.6%) | |
| N2 | 2,047 (100.0%) | 718 (47.9%) | |
| N3 | 0 (0.0%) | 6 (0.4%) | |
| Pathologic M Stage | <0.01 | ||
| M1 | 0 (0.0%) | 16 (0.7%) | |
| Thirty Day Mortality | 50 (2.5%) | 56 (2.4%) | 0.97 |
* P values provided are from Wilcoxon Rank Sum test on continuous variables and from Chi-square test on categorical variables.
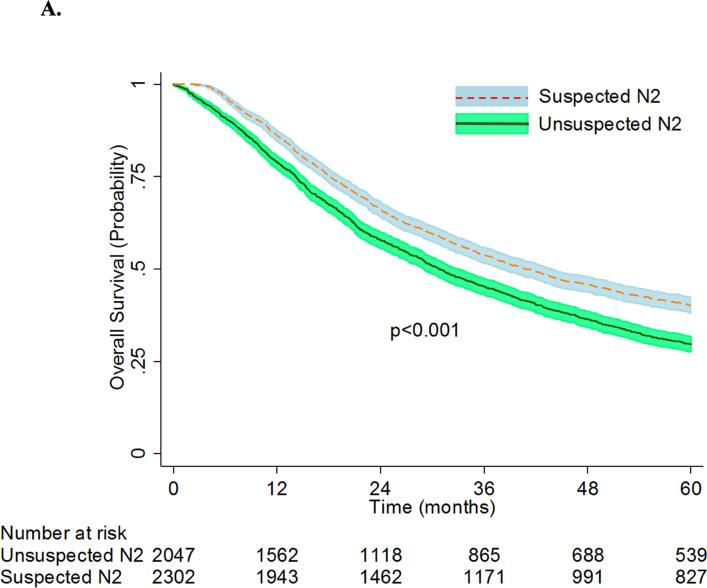
The patients in the unsuspected N2 group had a greater median number of regional lymph nodes examined, and were more likely to have a complete resection. The unsuspected N2 group had a mean (SD) pathologic tumor size of 3.8 (4.3) cm while the suspected N2 group had a mean (SD) pathologic tumor size of 4.3 (6.0) cm. Patients with unsuspected N2 disease were less likely to be readmitted within 30 days of surgery, but had a higher mean surgical inpatient stay as compared to patients with suspected N2 disease. Differences were also found in pathologic stages between the groups. More patients with unsuspected N2 disease had pathologic stage T2 tumors. All patients in the unsuspected group had pN2 disease, whereas significant down staging had occurred in the suspected group, and only 47.9% continued to have pN2 disease after surgery. No significant differences were found between the thirty-day mortality rates between the two groups. However, median survival (31 months [95% CI: 29-33] versus 41 months [95% CI: 38-44]) and overall 5-year survival (30% [95% CI: 28-32%] versus 40% [95% CI: 38%-42%], (p<0.001) was worse for the entire unsuspected N2 cohort when compared to that of the suspected N2 cohort (Figure 3A).
Figure 3A.
Overall Survival of Patients with Unsuspected vs. Suspected N2 NSCLC. Patients with “unsuspected N2” disease had underwent lobectomy as primary therapy for cT1-3 N0-1 NSCLC and were found to have pN2 disease at the time of surgery. Patients with “suspected N2” disease had clinical stage IIIA-N2 NSCLC who underwent induction chemotherapy ± radiation followed by lobectomy.
Unsuspected N2 NSCLC vs. Suspected N2 NSCLC: Matched Analysis
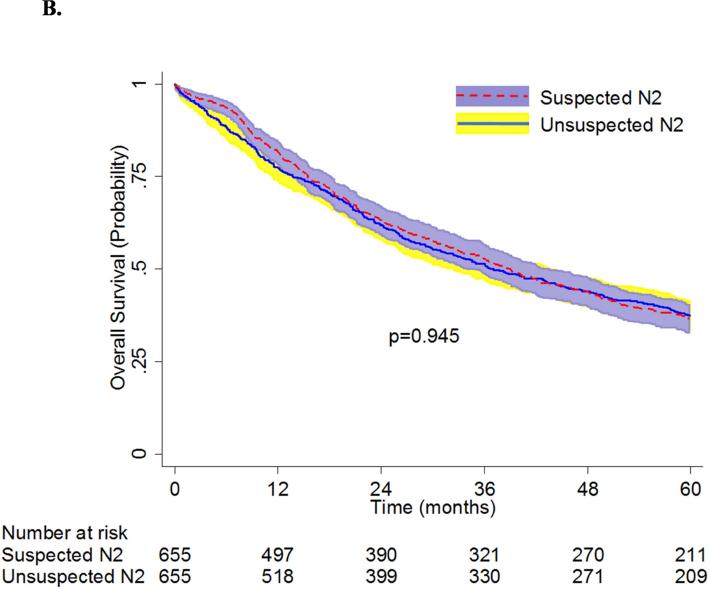
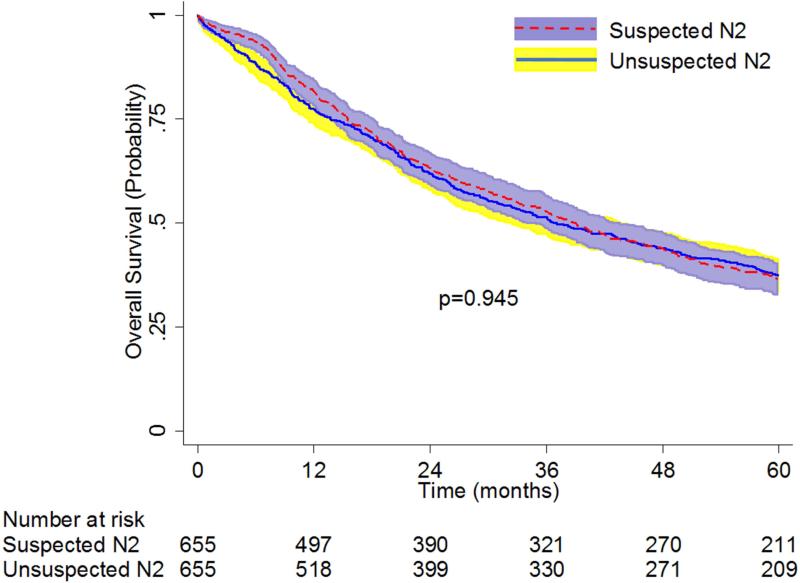
Propensity-matching created a population of 655 patients with unsuspected N2 disease who underwent lobectomy and 655 patients with suspected N2 disease who underwent induction chemotherapy ± radiation followed by lobectomy. Table 3 shows that there were no significant differences between the pre-operative characteristics for the two groups after matching. Of the 655 patients from the unsuspected N2 group, adjuvant chemotherapy was given to 203 (31%) patients, and adjuvant chemoradiation was given to 181 (27.6%) patients. The thirty-day mortality rates of the patients with unsuspected N2 disease (1.8%, n=12) and those with suspected N2 disease (2.6%, n=17) were not significantly different (p=0.35). There were also no significant differences in median survival (39 months [95% CI: 35-43] vs 37 months [95% CI: 32-44]) and overall 5-year survival (37% [95% CI: 33-40] vs 37% [95% CI: 34-41]) between patients with unsuspected N2 disease and patients with suspected N2 disease (Figure 3B).
Table 3.
Pre-Operative Demographics and Clinical Characteristics of the Matched Groups
| Unsuspected N2 (N=655) | Suspected N2 (N=655) | p-value | |
|---|---|---|---|
| Patient Age | 0.73 | ||
| Mean (SD) | 64.5 (10.8) | 64.8 (9.0) | |
| Median, IQR | 65 (56,73) | 66 (59,72) | |
| Sex, n (%) | 0.91 | ||
| Male | 290 (44.3%) | 292 (44.6%) | |
| Female | 365 (55.7%) | 363 (55.4%) | |
| Race, n (%) | 0.98 | ||
| White | 574 (87.6%) | 576 (87.9%) | |
| Black | 59 (9.0%) | 57 (8.7%) | |
| Other | 22 (3.4%) | 22 (3.4%) | |
| Charlson/Deyo Score, n (%) | 0.82 | ||
| 0 | 419 (64.0%) | 430 (65.7%) | |
| 1 | 185 (28.2%) | 177 (27.0%) | |
| 2 | 51 (7.8%) | 48 (7.3%) | |
| Insurance Status, n (%) | 0.61 | ||
| Uninsured | 14 (2.2%) | 13 (2.0%) | |
| Private | 282 (43.5%) | 271 (42.3%) | |
| Medicare | 317 (48.9%) | 326 (50.9%) | |
| Medicaid | 30 (4.6%) | 22 (3.4%) | |
| Other Government | 5 (0.8%) | 9 (1.4%) | |
| Clinical T Status, n (%) | 0.69 | ||
| 1 | 269 (41.1%) | 272 (41.5%) | |
| 2 | 369 (56.3%) | 361 (55.1%) | |
| 3 | 17 (2.6%) | 22 (3.4%) | |
| Facility Type, n (%) | 0.90 | ||
| Community Cancer Program | 54 (8.2%) | 56 (8.6%) | |
| Comprehensive Community Cancer Program | 311 (47.5%) | 299 (45.7%) | |
| Academic/Research Program | 285 (43.5%) | 296 (45.2%) | |
| Other specified types of cancer programs | 5 (0.8%) | 4 (0.6%) | |
| Histology, n (%) | 0.98 | ||
| Adenocarcinoma | 331 (50.5%) | 335 (52.2%) | |
| Squamous | 152 (23.2%) | 145 (22.1%) | |
| Large cell | 32 (4.9%) | 28 (4.3%) | |
| Bronchioalveloar | 20 (3.1%) | 22 (3.4%) | |
| Neuroendocrine | 7 (1.1%) | 6 (0.9%) | |
| Other | 113 (17.3%) | 119 (18.2%) |
* P values provided are from Wilcoxon Rank Sum test on continuous variables and from Chi-square test on categorical variables.
Figure 3B.
Overall Survival of Patients with Unsuspected vs. Suspected N2 NSCLC: Matched-Analysis Based on Propensity Scores. Patients with “unsuspected N2” disease had underwent lobectomy as primary therapy for cT1-3 N0-1 NSCLC and were found to have pN2 disease at the time of surgery. Patients with “suspected N2” disease had clinical stage IIIA-N2 NSCLC who underwent induction chemotherapy ± radiation followed by lobectomy.
Two subgroup analyses were performed. First, a matched-analysis using propensity scores comparing patients with unsuspected N2 disease who underwent lobectomy and chemotherapy to patients with suspected N2 disease who underwent induction chemotherapy and lobectomy created two cohorts of 219 patients. There were no significant differences in baseline characteristics between the two groups (data not shown). There was no significant difference in overall 5-year survival between the unsuspected and suspected N2 groups (40% [95% CI: 33-46] vs 35% [95% CI: 28-41]; p = 0.54), respectively.
In the second subgroup analysis, a matched-analysis created a population of 201 patients with unsuspected N2 disease who underwent lobectomy and adjuvant chemoradiation and a population of 201 patients with suspected N2 disease who underwent induction chemoradiation followed by lobectomy. The 5 year survival of these patients with unsuspected N2 disease (38% [95% CI: 32-45]) was significantly higher than those with suspected N2 disease (30% [95% CI 24-37]), p=0.014.
Discussion
In our population-based analysis, we found that the incidence of unsuspected pN2 disease of patients undergoing lobectomy for clinical T1-3N0-1 NSCLC was 4.4%. The overall 5-year survival of this entire unsuspected N2 cohort was 30%. Among patients in this unsuspected N2 group, patients who received adjuvant chemotherapy with or without radiation had improved survival when compared to patients who received adjuvant radiation alone or no adjuvant therapy. In univariate analysis, the overall 5-year survival of the entire unsuspected N2 cohort was worse than a cohort of patients who were treated with lobectomy after induction therapy for suspected N2 (cN2) disease, but there was no difference in the overall survival between the two groups in matched analysis.
The 5-year overall survival of patients with unsuspected N2 disease of 30% in this study is within the range reported by other studies (10% to 40%).2,4-6,9 The incidence of unsuspected pN2 disease of 4.4% in our cohort is within but on the low side of the range previously reported by other single institution studies (2.0% to 18.5%).2-8 The NCDB does not have information on how patients were clinically staged, but presumably, given the dates of the study from 1998-2006, a significant percentage of patients were staged with positron emission tomography (PET)/computed tomography (CT) which may have contributed to the low incidence of unsuspected N2 disease. Studies that have reported higher incidences of unsuspected pN2 disease were often older9,11 and may have relied more on computed tomography (CT) or X-ray2,4,5 alone than on PET/CT. Studies reporting lower incidences of unsuspected N2 disease (2.9-7.3%) often were from centers that used a combination of CT and PET/CT imaging.3,6,7 An important limitation regarding this study is that the NCDB does not contain details regarding mediastinal lymph node evaluation; it is unknown how many lymph node stations were evaluated and how many complete mediastinal lymph node dissections were performed. The low incidence of unsuspected N2 disease could also be because not all patients received comprehensive lymph node evaluation. In addition, the true incidence of unsuspected N2 disease in cT1-3 N0-1 patients in the NCDB is likely somewhat higher than the rate of 4.4% that we observed, because we realize that our analysis has not captured patients who were cT1-3 N0-1 but had a positive mediastinal lymph node by EBUS or mediastinoscopy and then were treated with induction therapy or definitive non-surgical therapy. Although our study's results are not appropriate to comment on when pathologic mediastinal staging should be done for cT1-3 N0-1 patients, the low incidence of unsuspected N2 disease does not necessarily suggest that there needs to be increased use of mediastinoscopy or EBUS in patients with T1N0 or T2N0 NSCLC. As noted previously, the routine use of mediastinoscopy does not greatly increase the negative predictive value of PET/CT and has poor sensitivity as a screening test.21
Although the incidence of unsuspected N2 disease is relatively low, thoracic surgeons will likely find a clinically meaningful number of patients with unsuspected pN2 disease when undertaking thoracoscopy or thoracotomy considering the prevalence of lung cancer worldwide. Although induction chemotherapy prior to surgery is established as superior to primary surgery for N2 disease,22 there is uncertainty regarding the proper course of action for clinically unsuspected N2 disease. From the limited available data, previous authors have recommended continuing with resection if unsuspected N2 disease is diagnosed at the time of thoracotomy or thoracoscopy.2,6,9-11,13 The findings from this present study suggest that continuing with planned resection with lobectomy is appropriate if a surgeon encounters unsuspected pN2 disease in the operating room and deems that the patient is likely to be able to tolerate adjuvant chemotherapy. The findings also suggest that surgeons do not need to routinely perform intra-operative pathologic evaluation of mediastinal nodes once they have proceeded to VATS or thoracotomy for lobectomy.
The importance of adjuvant chemotherapy in improving survival must be stressed, as previous randomized trials have demonstrated a benefit to adjuvant chemotherapy for nodal disease.23-26 One potential drawback to primary resection for unsuspected pN2 is the possibility for surgical morbidity to impact the use of adjuvant therapy. Because use of adjuvant chemotherapy with or without radiation was associated with superior survival in the unsuspected pN2 cohort, surgical decision-making regarding unsuspected pN2 disease should incorporate consideration of the patient's comorbid conditions and performance status. When one encounters unsuspected pN2 in the operating room, surgeons should consider the likelihood that patients will tolerate resection well enough to also be able to receive adjuvant chemotherapy following lobectomy based on the patient's age, comorbidities, functional status and other relevant clinical factors.
There are several limitations to this study. First, the retrospective nature of this study allows the possibility of unobserved and therefore uncontrolled confounding or selection bias. Second, although the NCDB does contain the Charlson Deyo co-morbidity index and though we were able to match on this variable, the NCDB does not contain data on performance status, pulmonary function tests or specific co-morbidities; thus it is possible that patients in the suspected N2 group were healthier in ways we were not able to measure because some clinicians may not choose multimodality therapy that includes surgery if patients are felt to be higher risk for the treatment. Third, the lack of data on recurrence and disease-free survival, as well as performance status data of patients at different time points prior to neoadjuvant or adjuvant therapies, in the NCDB, precludes assessment of these important clinical variables. Fourth, we were not able to make a direct comparison between patients with unsuspected pN2 disease and patients who were treated with induction therapy for biopsy-proven N2 disease because the NCDB does not specifically provide pathologic staging details prior to or following induction therapy. Thus, there is certainly a possibility that patients in the suspected N2 cohort did not have invasive mediastinal staging prior to therapy and therefore may have been overstaged. Fifth, for both cohorts, the NCDB does not have data regarding details of the clinical and pathologic staging, including details on whether biopsies were submitted for immunohistochemical staining, and therefore there may be patients in the groups who were either overstaged or understaged. Sixth, the NCDB does not contain information on how many patients were suspected to have N2 disease and were started on induction therapy and ultimately did not proceed to resection. Seventh, the NCDB does not have data on different types of unsuspected N2 disease (e.g., unsuspected N2 disease that is identified on the final pathology report delivered several days after surgery versus unsuspected N2 disease that is discovered intraoperatively by frozen section).
Another important limitation is that we were not able to analyze the outcomes of patients who underwent surgery from 2007 and onwards. At the time of our analysis, long-term survival data was only available until the end of 2006. Since that time, surgical techniques, preoperative staging tests, chemotherapy and radiation have likely improved and our findings may not be as applicable to today's practice. However, the strength of analyzing an older cohort from 1998 to 2006 was that long-term survival data was available for the patients in our study.
Finally, since there is no delineation between “bulky” versus “non-bulky” N2 disease and single-station versus multi-station N2 disease in the dataset, it is not possible to determine if patients with unsuspected N2 disease were more likely to have single-station N2 disease when compared to patients in the suspected N2 group. However, given the results of a recent national survey regarding practice patterns for stage IIIA-N2 disease,1 it is likely that patients in the “suspected” group did not proceed with surgery after induction therapy in the presence of extensive nodal disease.
Based on these findings and current NSCLC guidelines, it appears reasonable to proceed with lobectomy if unsuspected single-station microscopic lymph node involvement is discovered at the time planned lobectomy. If more advanced disease is encountered, or if it appears that resection would involve pneumonectomy, the decision to proceed with resection or to conclude the procedure without resection in order to use induction therapy should be made on a case by case basis. If multi-station macroscopic disease is discovered unexpectedly in a setting of possible pneumonectomy, proceeding with surgery is less likely to produce the best outcome, although this scenario is unlikely.
Future research on unsuspected N2 disease will be important to validate and confirm the present study findings. National or multi-center prospective databases should aim to collect further details regarding perioperative complications, co-morbidities, methods of clinical and pathologic staging, and the specific type of N2 disease (microscopic vs. macroscopic, single station vs. multi-station). Further work with more granular data will help to identify factors that will predict which group of patients with unsuspected N2 disease would most benefit from proceeding with lobectomy.
In conclusion, although patients with unsuspected pN2 disease treated with lobectomy overall do worse than patients with clinical T1-T3 cN2 disease treated with induction therapy followed by lobectomy, a matched-analysis showed that there is no significant difference between the overall survival of patients with unsuspected pN2 disease and those with suspected N2 disease. In addition, patients with unsuspected N2 disease who undergo lobectomy followed by adjuvant chemotherapy with or without radiation have superior survival when compared to patients who undergo adjuvant radiation alone or no adjuvant therapy. Thus, in the setting of unsuspected pN2 disease, proceeding with lobectomy is appropriate if the patient is likely able to tolerate adjuvant chemotherapy with or without radiation therapy.
Figure.
Survival of Unsuspected pN2 vs Clinical Stage IIIA-N2 NSCLC Patients: Matched-analysis
Table 4.
Peri- & Post-Operative Demographics and Clinical Characteristics of the Matched Groups
| Unsuspected N2 (N=655) | Suspected N2 (N=655) | p-value | |
|---|---|---|---|
| Re-admission in 30 days, n (%) | 0.03 | ||
| Yes | 34 (5.2%) | 43 (6.6%) | |
| Unknown | 28 (4.3%) | 42 (6.4%) | |
| Regional Lymph Nodes (LN) Examined | <0.01 | ||
| No. of patients with LN examined | 655 | 651 | |
| Median (IQR) | 11 (7,19) | 9 (4,20) | |
| Positive Lymph Nodes (LN) Examined | <0.01 | ||
| No. of patients with LN Examined | 650 (100.0%) | 655 (100.0%) | |
| Median (IQR) | 3 (1,5) | 2 (0,6) | |
| Surgical Inpatient Stay, Days from Surgery | 0.06 | ||
| No. of patients with available data | 598 | 550 | |
| Median (IQR) | 6 (4,9) | 6 (4,8) | |
| Tumor Location, n (%) | 0.89 | ||
| RLL | 130 (19.9%) | 129 (19.7%) | |
| LLL | 75 (11.5%) | 73 (11.2%) | |
| RML | 19 (2.9%) | 19 (2.9%) | |
| RUL | 234 (35.7%) | 218 (33.3%) | |
| LUL | 173 (26.4%) | 186 (28.4%) | |
| Other | 24 (3.7%) | 30 (4.6%) | |
| Surgical Margins, n (%) | 0.01 | ||
| No Residual Tumor | 582 (88.9%) | 573 (87.5%) | |
| Microscopic Residual Tumor | 42 (6.4%) | 26 (4.0%) | |
| Macroscopic Residual Tumor | 16 (2.4%) | 25 (3.8%) | |
| Other | 15 (2.3%) | 31 (4.7%) | |
| Pathologic T stage | <0.01 | ||
| T0 | 0 (0.0%) | 27 (4.1%) | |
| T1 | 190 (29.0%) | 218 (33.3%) | |
| T2 | 387 (59.1%) | 174 (26.6%) | |
| T3 | 38 (5.8%) | 16 (2.4%) | |
| T4 | 40 (6.1%) | 18 (2.8%) | |
| IS | 0 (0.0%) | 0 (0.0%) | |
| Other | 0 (0.0% | 202 (30.8%) | |
| Pathologic N Stage | <0.01 | ||
| N0 | 0 (0.0%) | 191 (40.4%) | |
| N1 | 0 (0.0%) | 55 (11.6%) | |
| N2 | 655 (100.0%) | 226 (47.8%) | |
| N3 | 0 (0.0%) | 1 (0.2%) | |
| Pathologic M Stage | 0 (0.0%) | 7 (1.1%) | <0.01 |
| Thirty Day Mortality | 12 (1.8%) | 17 (2.6%) | 0.35 |
* P values provided are from Wilcoxon Rank Sum test on continuous variables and from Chi-square test on categorical variables.
Perspective Statement.
Outcomes of patients with clinical T1-3N0-1 non-small cell lung cancer (NSCLC) found to have pN2 disease during resection are well-characterized. A matched-analysis based on propensity scores of the National Cancer Data Base, showed no significant difference in survival between patients with unsuspected pN2 who received lobectomy and those with cN2 NSCLC who received induction therapy before lobectomy.
Acknowledgements
The data used in this study are derived from a de-identified National Cancer Data Base file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigators.
This work was supported by the NIH funded Cardiothoracic Surgery Trials Network (M.G.H), 5U01HL088953-05 and by the American College of Surgeons Resident Research Scholarship (C.J.Y.).
Glossary of Abbreviations
- NSCLC
Non-small cell lung cancer
- CDCC
Charlson/Deyo comorbidity score
- PET/CT
Positron Emission Tomography/Computed Tomography
- LN
Lymph Nodes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Central Message: Aborting planned lobectomy to administer chemotherapy before resection is not mandatory in the setting of unsuspected of pN2 NSCLC.
None of the authors have any conflict of interest in regards to the subject of the manuscript.
Disclosures:
A.K., B.C.G., M.S.M., X.W., have no disclosures to report. One of the authors (T.A.D.) serves as a consultant for Scanlan International, Inc.
Author contributions
C.J.Y. contributed in data acquisition, study design, data analysis and interpretation, writing, and revisions. A.K. contributed in study design, data analysis and interpretation, writing, and revisions. X.W. contributed to analyzing and interpreting the data as well as revisions. M.G.H., B.C.G., M.S.M, T.A.D., and M.F.B. contributed for study design, data analysis and interpretation and revisions.
References
- 1.Ettinger DS, et al. Non-small cell lung cancer. Journal of the National Comprehensive Cancer Network : JNCCN. 2012;10:1236–1271. doi: 10.6004/jnccn.2012.0130. [DOI] [PubMed] [Google Scholar]
- 2.De Leyn P, et al. Surgery for non-small cell lung cancer with unsuspected metastasis to ipsilateral mediastinal or subcarinal nodes (N2 disease). European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 1996;10:649–654. doi: 10.1016/s1010-7940(96)80380-0. discussion 654-645. [DOI] [PubMed] [Google Scholar]
- 3.Kim HK, Choi YS, Kim J, Shim YM, Kim K. Outcomes of unexpected pathologic N1 and N2 disease after video-assisted thoracic surgery lobectomy for clinical stage I non-small cell lung cancer. The Journal of thoracic and cardiovascular surgery. 2010;140:1288–1293. doi: 10.1016/j.jtcvs.2010.06.011. doi:10.1016/j.jtcvs.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Lee DH, Kim JB, Keum DY, Hwang I, Park CK. Long term survival of patients with unsuspected n2 disease in non-small cell lung cancer. The Korean journal of thoracic and cardiovascular surgery. 2013;46:49–55. doi: 10.5090/kjtcs.2013.46.1.49. doi:10.5090/kjtcs.2013.46.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takizawa T, et al. Mediastinal lymph node metastasis in patients with clinical stage I peripheral non-small-cell lung cancer. The Journal of thoracic and cardiovascular surgery. 1997;113:248–252. doi: 10.1016/S0022-5223(97)70320-9. [DOI] [PubMed] [Google Scholar]
- 6.Obiols C, et al. Survival of patients with unsuspected pN2 non-small cell lung cancer after an accurate preoperative mediastinal staging. The Annals of thoracic surgery. 2014;97:957–964. doi: 10.1016/j.athoracsur.2013.09.101. doi:10.1016/j.athoracsur.2013.09.101. [DOI] [PubMed] [Google Scholar]
- 7.Cerfolio RJ, Bryant AS, Eloubeidi MA. Routine mediastinoscopy and esophageal ultrasound fine-needle aspiration in patients with non-small cell lung cancer who are clinically N2 negative: a prospective study. Chest. 2006;130:1791–1795. doi: 10.1378/chest.130.6.1791. doi:10.1378/chest.130.6.1791. [DOI] [PubMed] [Google Scholar]
- 8.Allen MS, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. The Annals of thoracic surgery. 2006;81:1013–1019. doi: 10.1016/j.athoracsur.2005.06.066. discussion 1019-1020, doi:10.1016/j.athoracsur.2005.06.066. [DOI] [PubMed] [Google Scholar]
- 9.Nakanishi R, et al. Treatment strategy for patients with surgically discovered N2 stage IIIA non-small cell lung cancer. The Annals of thoracic surgery. 1997;64:342–348. doi: 10.1016/S0003-4975(97)00535-3. doi:10.1016/S0003-4975(97)00535-3. [DOI] [PubMed] [Google Scholar]
- 10.van Klaveren RJ, et al. Prognosis of unsuspected but completely resectable N2 non-small cell lung cancer. The Annals of thoracic surgery. 1993;56:300–304. doi: 10.1016/0003-4975(93)91164-i. [DOI] [PubMed] [Google Scholar]
- 11.Cerfolio RJ, Bryant AS. Survival of patients with unsuspected N2 (stage IIIA) nonsmall-cell lung cancer. The Annals of thoracic surgery. 2008;86:362–366. doi: 10.1016/j.athoracsur.2008.04.042. discussion 366-367, doi:10.1016/j.athoracsur.2008.04.042. [DOI] [PubMed] [Google Scholar]
- 12.Goldstraw P, Mannam GC, Kaplan DK, Michail P. Surgical management of non-small-cell lung cancer with ipsilateral mediastinal node metastasis (N2 disease). The Journal of thoracic and cardiovascular surgery. 1994;107:19–27. discussion 27-18. [PubMed] [Google Scholar]
- 13.Detterbeck F. What to do with “Surprise” N2?: intraoperative management of patients with non-small cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2008;3:289–302. doi: 10.1097/JTO.0b013e3181630ebd. doi:10.1097/JTO.0b013e3181630ebd. [DOI] [PubMed] [Google Scholar]
- 14.Ettinger DS, et al. Non-small cell lung cancer, version 6.2015. Journal of the National Comprehensive Cancer Network : JNCCN. 2015;13:515–524. doi: 10.6004/jnccn.2015.0071. [DOI] [PubMed] [Google Scholar]
- 15.Ramnath N, et al. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e314S–340S. doi: 10.1378/chest.12-2360. doi:10.1378/chest.12-2360. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson MK. Optimal management when unsuspected N2 nodal disease is identified during thoracotomy for lung cancer: cost-effectiveness analysis. The Journal of thoracic and cardiovascular surgery. 2003;126:1935–1942. doi: 10.1016/j.jtcvs.2003.07.031. doi:10.1016/j.jtcvs.2003.07.031. [DOI] [PubMed] [Google Scholar]
- 17.Sher DJ, Liptay MJ, Fidler MJ. Prevalence and predictors of neoadjuvant therapy for stage IIIA non-small cell lung cancer in the National Cancer Database: importance of socioeconomic status and treating institution. International journal of radiation oncology, biology, physics. 2014;89:303–312. doi: 10.1016/j.ijrobp.2014.01.033. doi:10.1016/j.ijrobp.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 18.Greene FL. American Joint Committee on Cancer. AJCC cancer staging manual. 6th edn American Cancer Society.; Springer; 2002. [Google Scholar]
- 19.Speicher PJ, et al. Survival in the elderly after pneumonectomy for early-stage non-small cell lung cancer: a comparison with nonoperative management. J Am Coll Surg. 2014;218:439–449. doi: 10.1016/j.jamcollsurg.2013.12.005. doi:10.1016/j.jamcollsurg.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Licht PB, Jorgensen OD, Ladegaard L, Jakobsen E. A national study of nodal upstaging after thoracoscopic versus open lobectomy for clinical stage I lung cancer. Ann Thorac Surg. 2013;96:943–949. doi: 10.1016/j.athoracsur.2013.04.011. discussion 949-950, doi:10.1016/j.athoracsur.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez FG, et al. Utility of mediastinoscopy in clinical stage I lung cancers at risk for occult mediastinal nodal metastases. The Journal of thoracic and cardiovascular surgery. 2015;149:35–41. 42, e31. doi: 10.1016/j.jtcvs.2014.08.075. doi:10.1016/j.jtcvs.2014.08.075. [DOI] [PubMed] [Google Scholar]
- 22.Rosell R, et al. Preresectional chemotherapy in stage IIIA non-small-cell lung cancer: a 7-year assessment of a randomized controlled trial. Lung Cancer. 1999;26:7–14. doi: 10.1016/s0169-5002(99)00045-8. [DOI] [PubMed] [Google Scholar]
- 23.Douillard JY, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. The Lancet. Oncology. 2006;7:719–727. doi: 10.1016/S1470-2045(06)70804-X. doi:10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 24.Arriagada R, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. The New England journal of medicine. 2004;350:351–360. doi: 10.1056/NEJMoa031644. doi:10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 25.Winton T, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. The New England journal of medicine. 2005;352:2589–2597. doi: 10.1056/NEJMoa043623. doi:10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 26.Pignon JP, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–3559. doi: 10.1200/JCO.2007.13.9030. doi:10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]