Abstract
Derivation of cardiomyocyte cell lines from human fibroblasts (induced pluripotent stem cells, iPSCs) has made it possible not only to investigate the electrophysiological and Ca2+ signaling properties of these cells, but also to determine the altered electrophysiological and Ca2+-signaling profiles of such cells lines derived from patients expressing mutation-inducing pathologies. This approach has the potential of generating in vitro human models of cardiovascular diseases where cellular pathology can be investigated in detail and possibly specific pharmacotherapy developed. Although this approach has been applied to a number of mutations in channel proteins that cause arrhythmias, there are only few detailed reports addressing Ca2+ signaling pathologies beyond measurements of Ca2+ transients in intact non-voltage clamped cells. Unfortunately full understanding of Ca2+ signaling pathologies remains elusive, not only because of the plethora of Ca2+ signaling proteins defects that cause arrhythmias and cardiomyopathies, but also because detailed functional properties of Ca2+ signaling proteins are difficult to obtain. Catecholaminergic polymorphic ventricular tachycardia (CPVT1) is a malignant inherited arrhythmogenic disorder predominantly caused by mutations in the cardiac ryanodine receptor (RyR2). Thus far over 150 mutations in RyR2 have been identified that appear to cause this arrhythmia, a number of which have been expressed and studied in transgenic mice or cell-line models. The development of human iPSC-technology makes it possible to create human heart cell-lines carrying these mutations, making detailed identification of Ca2+ signaling defects and its specific pharmacotherapy possible.
In this review we shall first briefly summarize the essential characteristics of the mammalian cardiac Ca2+ signaling, then compare them to Ca2+ signaling phenotypes of human induced pluripotent stem cell derived cardiomyocytes (hiPSC-CM) and to those of rat neonatal cardiomyocytes, and categorize the possible variance in Ca2+ signaling defects caused by different CPVT-inducing mutations as expressed in hiPSC-CMs.
Keywords: Catecholaminergic polymorphic ventricular tachycardia (CPVT), cardiomyocytes derived from human induced pluripotent stem cells (hiPSC-CM), Ca2+ signaling, stem-cell-derived disease models, ryanodine receptor
Graphical Abstract

1. Calcium Signaling in adult Cardiomyocytes
Following seminal experiments of Ringer some 150 years ago it became apparent that Ca2+ played a central role in initiation and regulation of cardiac contractility, but It was not until the 1960s that the advent of technologies of voltage-clamp for ventricular strips [1, 2], the development of Ca2+ sensitive dyes and the whole-cell patch-clamp technique for single cells [3,4] made it possible to explore the intricate details of cardiac Ca2+ signaling. The picture that emerged suggested that the entry of Ca2+ through the Ca2+ channel, during the action potential, triggers much larger releases of Ca2+ from the RyR2 receptors [5] located on the dyadic junctions of sarcoplasmic reticulum (SR), setting in motion the activation of contraction (Fig. 1). This process became known as Ca2+-induced Ca2+-release (CICR, [6]), a mechanism that seems to have evolved specifically in mammalian heart versus a faster voltage-gated Ca2+ release (VICR) mechanism in skeletal muscle. The advent confocal Ca2+ imaging in line-scan mode made it possible to capture focal rise of Ca2+ in the dyadic micro-domains between SR and t-tubular membranes, known as Ca2+ spark [7]. Ca2+ spark are sporadic events that occur during diastole or are triggered by activation of ICa. Cleemann et al. [8] using 2-D rapid (240 f/s) confocal Ca2+ imaging in whole cell clamped ventricular myocytes dialyzed with high concentrations of EGTA to limit the diffusion of Ca2+-bound Fluo-3, imaged simultaneously over 100s of sparks and found spark durations of ~7ms, and spark amplitudes that were invariable with voltage, but spark frequencies that were dependent on the magnitude of ICa. These studies also showed that a Ca2+ spark represented the release of ~100,000 calcium ions, resulting most likely from activation of ryanodine receptor clusters in a dyadic junction. Consistent with this idea the sparks occurred at the same cellular location with over 70% consistency from beat to beat (8).
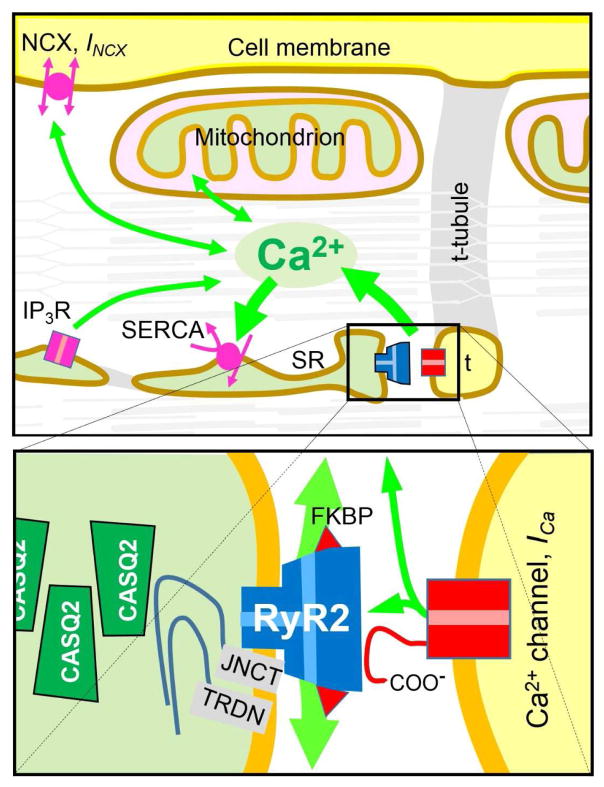
Figure 1.
Schematic of cardiac Ca2+ signaling. Top panel show Ca fluxes (green arrows) generated by dyadic junctions (box), Na+/Ca2+ exchangers (NCX, INCX), sarco- and endoplasmic reticular Ca2+ ATPase (SERCA), inositol triphosphate receptor (IP3R), and mitochondria in relation to the cell membrane, the transverse tubular system (t) and the sarcoplasmic reticulum (SR). All are shown superimposed on the contractile filaments to signify the regulation of cellular contraction and relaxation by cytosolic Ca2+. The lower diagram shows key proteins within dyadic junctions where clusters of Ca2+ release channels (ryanodine receptors, RyR2) in the SR membrane are functionally and structurally associated with Ca2+ channels (DHP receptors, L-type Ca2+ current, ICa) in t-tubules, calsequestrin (CASQ2) in SR and, triadin (TRDN), junction (JNCT), and FKBP (calstabin) in association with RyR2.
It is important to note that although CICR is a ligand-gated process, it has significant voltage-dependence [9], such that the gain (amplification factor) of the process may exceed 100 at −40 to −30 mVs, but it drops to values below 15 at voltages positive to 10 mVs, [10]. The mechanism for this unexpected property of CICR remains somewhat controversial, but experiments on rat atrial myocytes using rapid 2-D (240 f/s) confocal imaging have identified two groups of RyR2: those near the surface membrane, associated with dihydropyridine receptors (DHPRs), showing significant voltage-dependence and those centrally located, not associated with DHPRs, showing no voltage-dependence [11]. These findings imply that the voltage-dependence of gain of CICR reflects possible direct interaction of RyR2 with Ca2+ channels. In support of this possibility, cellular introduction of LA-fragments of cloned carboxyl-tail of alpha subunit of Ca2+ channel into freshly isolated rat atrial myocytes imparted voltage-dependence to the CICR of centrally located “naked” RyR2. This finding was specific to LA, but not to the K fragment of carboxyl-tail that includes the I-Q motif [11].
Calcium signaling during evolution appears to have evolved from a surface-membrane (Ca2+-channel/ NCX) dominated process, as in amphibian & shark hearts [2, 12], to an intracellular regulated system, in mammalian hearts, where the Ca2+ channel serves as a trigger to release of Ca2+ from an internal Ca2+ stores of SR, and where Ca2+ is sequestered via SERCA2a/Phospholamban proteins and stored on Ca2+-binding protein calsequestrin [13]. In mammalian hearts with morphological changes that accompany growth and differentiation of the heart that includes the development of t-tubules, SR, adrenergic receptors, and myofilaments, there appears also to be a shift from Ca2+ entry across the membrane to Ca2+ release from intracellular stores.
In addition to the fast ICa-gated CICR mechanism, a slower IP3-gated Ca2+ release mechanism appears also to have evolved in the mammalian hearts [14]. This signaling pathway appears to be more dominant in immature developing heart, possibly playing a critical role in nuclear signaling during growth and development [15–18]. The role of IP3-gated signaling in EC-coupling of healthy adult hearts remains somewhat cloudy, but significant data supports over expression of IP3-gated signaling pathway in a number of cardiac pathologies [19–21].
Mitochondria also appear to contribute to Ca2+ signaling processes of cardiac myocytes. Early studies on isolated mitochondria show that mitochondria sequester and release Ca2+ from and to the cytosol [22]. The sequestered Ca2+ is thought to stimulate production of nitric oxide (NO), which in turn regulates oxygen consumption, ATP production, and ROS generation [23]. Nevertheless, it remains still unclear whether mitochondria play a critical role in beat-to-beat Ca2+ regulation of the myocyte. Since mitochondria make up some 30–40% of the cardiac cell volume, their contribution to cellular Ca2+ signaling could be significant [24]. In support of direct role for mitochondria in cellular Ca2+ signaling, shear stress pulses (pressurized flow of solution) appeared to trigger Ca2+ transients that originate from mitochondrial Ca2+ pools, by a mechanism independent of CICR, that had mitochondrial pharmacology [25]. The notion of a rapid (~500 ms) mitochondrial Ca2+ release was also confirmed in different cardiac cell types (adult ventricular cells, rat neonatal cardiomyocytes and hiPS-CM), under voltage-clamp conditions, and with genetically targeted mitochondrial probes. This is somewhat surprising, but is supported by key observations that: a) shear stress pulses induce Ca2+ release only after Ca2+-loading; b) perinuclear and peripheral mitochondrial populations often produce simultaneous Ca2+ signals with opposite polarity (uptake vs. release); c) the propensity of mitochondria to release Ca2+ increases with Ca2+ loading (e.g. by Na+ withdrawal); and d) mitochondrial Ca2+ release may escape detection in whole-cell measurements, where mitochondrial Ca2+ release maybe counter-balanced by uptake signals in different populations of mitochondria [25, 26].
Cardiac Ca2+ signaling remains an active area of research as state-of-the-art technologies ranging from molecular, genetic, electrophysiological and imaging approaches become available. Nevertheless, though ICa-gated Ca2+ release is the dominant pathway for activation of contraction in the heart, other pathways that include transport of Ca2+ across the membrane or released from ER and mitochondria may critically modulate cardiac Ca2+ signaling depending on pathophysiological or developmental stage of the heart.
2. Ca2+ signaling in human fibroblast-derived cardiomyocytes (hiPSC-CM)
It is well documented that iPSC-CMs beating clusters display mixed ventricular, atrial, and pacemaker-like cell populations based on their action potential phenotypes [27–29]. Although research is still ongoing to develop protocols that generate purer populations of a specific type of myocyte, the effort to generate homogenous cell populations of atrial, ventricular or pacemaker myocytes, remains to be fully achieved. Characterization of iPSC-CMs has revealed that while molecular, electrophysiological, metabolic, and Ca2+ signaling properties of hiPSC-CM are remarkably similar to those of adult cardiomyocytes, the genetic expression pattern, the cellular morphology of hiPSC-CM, might be more similar to the neonatal instead of adult cardiac myocytes [30, 31]. Studies with human embryonic stem cell-derived cardiomyocytes have revealed that t-tubules are absent in these developing cells [31, 32]. Table 1 compares the molecular and functional properties of a number of hiPSC derived cardiomyocyte cell-lines reported thus far. A cursory survey of the reported data, suggests significant differences between different cell lines, even though detailed analysis of Ca2+ signaling parameters that includes: the level of expression of Ca2+ channels, ICa-gated Ca release and its gain from RyR2s of SR, the magnitude of SR Ca2+ store, and the level of functional expression of NCX and SERCA/PLB and their regulation by adrenergic agonists, or the role of mitochondria in hiPSC-CM Ca2+ signaling; is often missing.
Table 1.
Calcium signaling characteristic of various hiPSC-CM cell lines.
| hiPSC-CM Cell-lines | mRNA | Immunostaining | Action Potential | ICa | SR Ca2+ content | INCX | SERCA | Spontaneous Ca2+ transients | Ca2+ sparks | IP3R |
|---|---|---|---|---|---|---|---|---|---|---|
| [69] | NKX2.5 MLC2v ACTN2 MYH6 GATA4 a |
No data | No data | No data | ++ | No data | No data | ++ | No data | No data |
| [40] | RyR2, IP3R2, SERCA2a Cav1.2 PLB; CSQ |
α-actinin & RyR2 | No data | No data | ++++ | No data | Thapsigargin suppress spontaneous and caffeine induced Ca2+ transients | suppressed by nifedipine or ryanodine | No data | 2-APB or U73122 suppress Ca2+ transients |
| [28] | NCX1, RyR2, SERCA2a, MHC | α-actinin & TPM | 45% N-CM, 38% V-CM, 17% A-CM | No data | + | No data | No data | U-shape Ca2+ wave; rise of calcium in the periphery faster than center | No data | No data |
| [41] | No data | RyR2 & CSQ | No data | No data | + | No data | No data | ++ | No data | No data |
| [35] | No data | No data | 54% V-CM, 22% N-CM, 24% A-CM | ICa, bell shape I-V | No data | No data | No data | No data | No data | No data |
| [33] [45] | No data | RyR2 | No data | ICa, bell shape I-V | ++++ | ++++ | Thapsigargin suppress spontaneous Ca-transients | Suppressed by NCX or RyR2 blockers | Sporadic and brief | 2-APB or U73122 slightly affect spontaneous beating |
| [29] | No data | α-actinin & α-MHC | 61% V-CM, 17.4% A-CM and 21.6% N-CM, | No data | No data | No data | No data | Ca2+ increase first at periphery then propagate to centre | Stochastic and repetitive | No data |
| [34] | No data | No data | No data | ICa bell shape I-V | ++++ | ++++ | SERCA Ca2+ transport are functional | Slower time to peak and decay rates | No data | No data |
CSQ: calsequestrin; PLB Phospholamban; MHC: Myosin heavy chain; RyR2: Ryanodine receptor type 2; NCX: Sodium Calcium exchanger; TPM Tropomyosin
2.1. L-type Ca2+ current expression and whole cell Ca2+ transients
L-type Ca2+ channels are critical in excitation-contraction coupling of both cardiac and skeletal muscles. Human or mouse iPSC-CMs express robust levels of L-type Ca2+ channels, the gating properties and pharmacology of which are similar to those reported for mammalian and human cardiomyocytes [33–35]. ICa is activated at potentials positive to −40 mV, reached its peak values of ~ 8 pA/pF at 0 mV, reversed direction becoming outward at potentials positive to +60 mVs (e.g. fig. 2A), and could be blocked by conventional L-type Ca2+ channel blockers. The developed Ca2+ transients [36, 37] had similar bell-shaped voltage-dependencies as that found for ICa ; i.e. ICa-gated Ca2+ release activated at −30 mV, peaked at ~ 0 mV, and triggered little or no release of Ca2+ at potentials positive to +60 mVs (Fig. 2A). Repolarization from potentials positive to 60 mVs back to the resting potentials also triggered significant release of Ca2+ consistent with the idea that the influx of Ca2+ and not the depolarization of membrane triggers the release of Ca2+, as proposed for the adult mammalian cardiomyocytes (Fig. 2A, red traces, [38]. Positive depolarizing potentials also activated significant influx of transmembrane Ca2+ on the Na+/Ca2+ exchanger (NCX), which appears to contribute significantly to a slower developing component of Ca2+ transients [33]. This possibility is supported by the finding that 1 μM nifedipine while completely blocking ICa, suppresses only the rapidly developing component of Ca2+ transients, suggestive of considerable contribution of Na+/Ca2+ exchanger to Ca2+ transients [33]. This property of hiPSC-CM is consistent with previous findings in developing and neonatal mammalian cardiomyocytes suggestive of a more significant role for Na+/Ca2+ exchanger in EC-coupling of developing cardiomyocytes [39]. It should be noted that other groups have reported that in intact, non-patch clamped single or small monolayer clusters of hiPSC-CMs, 1 μM nifedipine completely suppressed the cellular Ca2+ transients [40], suggestive of full dependence of Ca2+-transients on ICa. This finding is not necessarily contradictory to reports in whole cell clamped hiPSC-CM identifying both nifedipine-sensitive and insensitive components Ca2+ transients, as in intact cells blocking ICa would either suppress excitability fully or shorten the action potential to levels that could not support the longer depolarization periods required for the influx of Ca2+ on Na+/Ca2+ exchanger. Simultaneous recordings of Ca2+ transients and contractions from small clusters of iPS-CMs also show similar characteristic as those of adult mammalian cardiomyocytes [41]. Comparison of the Ca2+ handling of field-stimulated (0.5Hz) hiPSC-CMs to those of acutely-isolated adult primary ventricular rabbit and mouse cardiomyocytes under identical experimental conditions, also showed that their Ca2+ transient amplitudes were not significantly different than those of adult rabbit and mouse cardiomyocytes, whereas time to peak and decay rates of Ca2+ transients were significantly slower in hiPSC-CM [34].
Figure 2.
Comparison of ICa- and caffeine-induced Ca2+ signals in hiPSC-CM from a healthy control subject and a CPVT1-afflicted patient with point mutation F2483I in RyR2. Currents were recorded with a 150ms or 250 ms step depolarizations from holding potential of −40 mV in 10 mV steps to +60 mV. (A) and (B) Left part of panels shows representative ICa and fluorescence traces where depolarization to 0mV rapidly activates Ca2+ release (black traces) while clamp pulses to +60 or +70 mV only activate rises in Ca2+ on repolarization (red traces). The right panel shows the I–V curves for ICa and the corresponding Ca2+ Fluo-4 signal from control and CPVT hiPSC-CM. (C) Representative caffeine-induced NCX currents and corresponding fluorescence Ca2+ signal from control and mutant hiPSC-CM. (D) Average values of caffeine-activated Ca2+ signals (top) and INCX currents (bottom) in each group. (E) Confocal image of immunofluorescence labeled RyR2 (green) in a small cluster of control hiPS-CM with DAPI-labeled nuclei (blue). Modified from [33]
The quintessential property of the gain of CICR in cardiomyocytes is its voltage-dependence, (See above, [2, 12]). This characteristic of CICR appears also to be preserved in hiPSC-CM, suggesting that iPSC-derived cardiomyocytes have the phenotypic cardiac Ca2+ signaling characteristics, in sharp contrast to the neuronal CICR that shows no indication of voltage-dependence [42]. Thus, the available data on characterization of spontaneously- or depolarization-triggered Ca2+ transients suggests that hiPS-CMs express the cardiac-type excitation–contraction-coupling that closely resembles that found in the mammalian myocardium.
2.2. Functional SR Ca2+ store: SERCA AND NCX
Molecular studies show that Ca2+-handling proteins of the mammalian heart: RyR2, IP3R2, SERCA2a, PLN are all expressed in hiPSC-CM [28, 40, 43]. Immuno-fluorescence studies suggest punctuates clustering of RyR2s near the surface membrane in hiPSC-CM (Fig. 2E), consistent with functional findings that Ca2+ influx via the L-type Ca2+ channels triggers the release of the SR Ca2+ stores [33]. Immuno-cyto-staining studies of RyR2 and sarcomeric alpha-actinin, in small mono-layered clusters of hiPSC-CM, show that sarcomeric alpha-actinin staining has a relatively disorganized striated sarcomeric pattern [44]. In another study, RyR2 expression was also detected throughout the cytosol with some myofilaments co-localization [40].
The magnitude SR Ca2+ stores of hiPSC-CM has been quantified using rapid and short applications of caffeine pulses. Caffeine concentrations of 3–5 mM triggered large global Ca2+ transients that activated inward INCX, of about 2.5 pA/pF (Fig. 2D) as compared to ~1.0 pA/pF in rat neonatal cardiomyocytes, confirming functionally the existence of large SR Ca2+ stores in hiPSC-CM [45]. The larger density of INCX activated on application of caffeine as compared to values of ~1.0 pA/pF in adult cardiomyocytes of rat, rabbit, mouse, and human hearts suggests either larger stores of Ca2+ accessed by caffeine in hiPSC-CM or a greater density of NCX in hiPSC-derived myocytes. Comparison of caffeine triggered Ca2+ transients in hiPSC-CM with adult rabbit and mouse cardiomyocytes, in another study [34], suggests equivalent magnitudes, but diverse INCX values of only 0.6 pA/pF in hiPSC- CM and 1.3pA/pF in rabbit myocytes. This diversity between the two sets of data may have resulted from either higher Ca2+ buffering concentrations, or developmental maturity of iPSC-CMs used. Consistent with this idea the same group also reported that the sequestration rate of the thapsigargin-sensitive caffeine-triggered store in hiPSC-CM was significantly slower than that of rabbit or mouse cardiomyocytes, [34]. Yet others have reported very small caffeine induced Ca2+ release stores even when compared to human embryonic stem cell derived myocytes [28]. Given the variation in technics of creating hiPSC-CM cell-lines, the level of their maturity, the degree of Ca2+ buffering and concentrations of Ca2+-sensitive dyes used in intact or whole-cell clamped myocytes, it is likely that the quantitative variations in Ca2+ signaling parameters result from both developmental and electrophysiological approaches used.
2.3. Calcium Sparks and sporadic focal Ca2+ releases
Our experiments, using rapid (120–240 f/s) 2-D confocal imaging, show that the majority of sporadic and brief Ca2+-sparks and focal releases in hiPSC-CMs had spatiotemporal properties analogous to those of adult rat cardiomyocytes, Ca2+ spark durations rarely exceeded 20 ms in control hiPSC-CM [33]. Others using line-scan imaging, have identified stochastic and repetitive Ca2+ sparks that re-occurred at the same site in hiPSC-CM. Ca2+ sparks appear to be predominately triggered by activation of L-type Ca2+ channels, and are enhanced in frequency on elevation of extracellular Ca2+. Ca2+ diffusion from the center of Ca2+ sparks to periphery appears often to be asymmetric [29].
2.4. IP3-gated Ca2+ pools
Although the primary Ca2+-signaling pathway in adult mammalian cardiomyocytes occurs through activation of RYR2, in some myocytes and at different stages of their development there appears to be significant contribution from the IP3-gated signaling pathway [46] [47]. IP3R-gated Ca2+ release is expressed both in endoplasmic reticulum and the nuclear envelop [48]. The functional role of IP3R in cardiomyocytes remains poorly understood. One study shows that IP3R mRNA levels were about 50-fold lower than those of RYR2 mRNA in adult cardiac myocytes [14], nevertheless there is abundant expression of this protein in embryonic, neonatal, and adult atrial myocytes [18, 49–52]. In fact, it has been reported that the spontaneous electrical activity of embryonic pacemaker and stem cell-derived cardiomyocytes depends, in part, on IP3-mediated Ca2+ release [53–55]. Since IP3-gated Ca2+ signaling is reported to trigger both sub-sarcolemmal and perinuclear Ca2+ releases in developing cardiomyocytes [18], it is more likely that the sub-sarcolemmal Ca2+ release would activate the Na+/Ca2+ exchanger to depolarize the surface membrane, while Ca2+ released at the nuclear envelop might have higher likelihood to be sequestered by SERCA2a [56]. Immuno-cyto-staining studies also suggest that IP3R is mostly distributed around the nucleus and that this pool may contribute to the modulation of Ca2+-signaling in hiPSC-CM [57] [40]. Consistent with this idea IP3R antagonist 2-APB or phospholipase C inhibitor U73122 have been reported to significantly slow the kinetics and decrease the amplitude of whole cell Ca2+ transients [40]. We have not been able to confirm this finding in our hiPSC-CM and neonatal cardiomyocytes, and in sharp contrast find that Ca2+ release from the SR and mitochondria might be the primary mechanism for the spontaneous pacing activity. In our study U73122 only slightly affected the beating frequency of hiPSC-CM, suggesting that IP3R Ca2+ signaling played a minor role in generation of Ca2+ transients and regulation of pacing [33].
2.5. Mitochondrial Ca2+ signaling
There is little or no information as yet on mitochondrial Ca2+ signaling in hiPSC-CM. The contribution of mitochondrial Ca2+ signaling to spontaneous beating activity of hiPSC-CM has been examined recently using genetically encoded mitochondrial probes targeted to mitochondrial subunit VIII of cytochrome C. These studies showed both release and uptake of Ca2+ by different mitochondrial populations in hiPSC-CM; generally, though not always, perinuclear mitochondrial population released Ca2+ while the peripheral mitochondria took-up Ca2+ (Fig. 3, traces and panels 1,2,3). FCCP, a mitochondrial uncoupler, at 50nM suppressed the spontaneous beating of hiPSC-CM and prolonged the relaxation time of caffeine-induced Ca2+ transients, suggestive of a direct role for mitochondria in the cycling of cytosolic Ca2+ and regulation of pacing, (fig 3, trace 4 and panel 4). Our results therefore support a novel role for rapid release of Ca2+ from mitochondria in generation and regulation of spontaneous pacing [33].
Figure 3.
Suppression of oscillatory mitochondrial Ca2+ signals in a spontaneously pacing control hiPSC-CM infected with the genetically engineered targeted, Ca2+ probe, myticam-E31Q [26]. (A) Tracings showing changes in regional mitochondrial Ca2+ signals (top) and INCX (bottom) before and 30 s after exposure to 50 nM FCCP. Increasing mitochondrial Ca2+ corresponds to downward deflection. (B) Images show baseline fluorescence (F0) and color-coded regions of interest (ROI, with “n” indicating nuclei) and 4 differential fluorescence images (measured at the times indicated along the traces) showing repeatable mitochondrial Ca2+ signals before FCCP application (1, 2, 3) and smaller, more localized responses after (4). Modified from [45]
3. Ca2+ signaling profiles of catecholaminergic polymorphic ventricular tachycardia (CPVT1) in hiPSC-CM model: “from mice to men”
Two genetic forms of CPVT have been thus far identified: 1) CPVT1, with point mutations in RyR2, accounting for at least 50% of all cases, resulting from abnormal intracellular Ca2+ handling caused by autosomal dominant mutations in the RYR2 gene [58] [59]; and 2) CPVT2, with an autosomal recessive mutations in cardiac calsequestrin, CASQ2, isoform also resulting in Ca2+ signaling defect [60].
CPVT1 causing mutations have been extensively studied in transgenic mice models or transformed cell lines. Evidence from a number of labs suggest that CPVT1 results from PKA-mediated phosphorylation of RyR2/FKBP complex resulting in SR diastolic Ca2+ leak that produces local depolarizations that trigger DADs via activation of NCX [61]. It has also been proposed that SR Ca2+ overload and altered Ca2+ sensitivity of SR luminal sites, not related to FKBP modulation, can cause diastolic Ca2+ release from RyR2, leading to local depolarizations and arrhythmogenesis [62] These opposing views are in part fueled by multiplicity of point mutations that cause CPVT1, and the variety of genetically engineered mice and animal cell-lines used. The iPSC technology has made it possible to create human cardiomyocyte cell-lines that express CPVT1 causing mutations. The general approach has been to obtain dermal fibroblasts from patients expressing CPVT1 arrhythmias, reprogramming and driving them into hiPSC-CM lineage [27]. To date, over 150 mutations have been identified in RyR2 gene that causes CPVT1 [63] and 11 patient-specific CPVT1 hiPSC-CM cell lines have been generated and evaluated by several labs (Table 2). Since Ca2+ signaling pathway defects play critical roles in many cardiac pathologies, accurate knowledge as to the specific details of Ca2+ signaling defects in various CPVT1 models is required to provide insights into the mechanisms underlying the particular form of the disease and help design approaches for it in vitro pharmacotherapy, before applying the therapy to patients. Table 2 summarizes the Ca2+ signaling phenotypes of CPVT1 causing mutations as expressed in hiPSC-CM cell-lines, as depicted in the cartoon of RyR2 (Fig. 4). Only few studies appear to have attempted simultaneous measurements of membrane currents and Ca2+ signaling parameters beyond measurements of Ca2+ transients. The parameters required for full analysis of Ca2+ signaling must include: the density of ICa and INCX, the characteristics of ICa-gated Ca2+ release, the gain of CICR, Ca2+ storage capacity of the SR and its fractional release, the unitary properties of Ca2+ sparks and their frequency of occurrence, in addition to frequency of spontaneously triggered DADs or EADs, the generation of aberrant Ca2+ transients, and the alterations caused by application of isoproterenol. For instance, in hiPSC-CM from a patient expressing point mutation F2483I in RyR2, the caffeine-triggered Ca2+ transients and accompanying INCX were found to be significantly smaller than in control myocytes (Fig. 2D), consistent with a smaller SR Ca2+ store, possibly due to Leaky RyR2 [33]. Interestingly the smaller store of Ca2+ appeared to have been in part compensated for by a larger fractional release of Ca2+ triggered by ICa. Thus, the gain of CICR was higher in F2483I mutant expressing hiPSC-CM. As might be expected rapid electrical pacing, beta-adrenergic stimulation, or higher intracellular Na+ exacerbated Ca2+ handling abnormalities of F2483I mutant cells and increased the diastolic Ca2+ levels, thereby triggering spontaneously occurring Ca2+ transients [33]. Confocal Ca2+ imaging also showed that the F2483I mutants exhibit larger and more recurrent and spatially wandering sparks compared to sporadic and brief sparks of control iPSC-CM, especially under Ca2+-overload conditions. Often, the Ca2+-induced Ca2+-release events continued after repolarization in CPVT1-CMs suggesting slower decay of Ca2+ transients, Fig. 2B, consistent with the continued release of Ca2+. A large fraction (59%) of CPVT-CMs also showed slower frequency of spontaneous beating and 34% developed arrhythmia and DAD after Isoproterenol stimulation [27].
Table 2.
Characteristics of 11 hiPSC-CM cell lines expressing CPVT-causing mutations.
| CPVT Cell-lines | Content Ca2+ store | Ca2+ sparks | L-type ICa | Beating frequency | Diastolic Ca2+ | Aberrant Ca2+ transients & oscillations | β-Adrenergic responses |
|---|---|---|---|---|---|---|---|
| Exon3-del[66] | No data | No data | No data | +++++ | Beating frequency Diastolic Ca2+ |
||
| S406L [64] | No change | Bigger and longer | No data | No data | Comparable to control, with Iso | +++++ | Spark frequency & decay time |
| R420Q [44] | No data | No data | with Iso | ++ Iso: ++++++ |
59% insensitive to; 16% arrhythmogenic; 25% [Ca2+]i rise associated with Ca2+ transients | ||
| E2311D [65] | No data | No data | No data | No data | No data | +++++ Triggered from multiple foci |
DAD frequency |
| P2328S [43] [66] | No data | No data | Comparable to control | +++++ | Diastolic Ca2+ | ||
| F2483I [27] [33] | Longer & wandering | Comparable to control | with Iso | with Iso | +++++ | Beating frequency DAD |
|
| T2538R[66] | No data | No data | No data | No change | +++++ | Beating frequency | |
| M4109R [67] | No data | No data | No data | No data | No data | +++++ | DAD Frequency |
| L4115F [66] | No data | No data | No data | +++++ | Beating frequency | ||
| Q4201R [66] | No data | No data | No data | 26 | +++++ | Beating frequency | |
| V4653F [66] | No data | No data | No data | +++++ | Beating frequency Diastolic Ca2+ |
Figure 4.
Diagram of RyR2 receptor showing key functional domains in relation to 11 CPVT1-causing mutations that are located from N-terminal to central domain to C-terminal (red circles), which have been studied in hiPSC-CM. Functional domains: Trans-membrane α-helices (M#), phosphorylation sites (P), and binding sites for FKBP, calmodulin (CaM) and protein kinase A (PKA/MKAP) are also indicated.
In the N-terminal segment, two CPVT1 causing point mutations have been reported in human cell lines, fig. 4. Novak et al. [44] reported that R420Q mutation exhibited immature ultra-structural features including poorly developed SR, poorly organized myofibrillar sarcomeric pattern, and higher mitochondrial expression. They also report 60% slower spontaneous beating rates as compared to control hiPSC-CM. Caffeine induced Ca2+ transients were smaller and shorter, consistent with smaller SR Ca2+ stores but with faster reuptake of intracellular Ca2+. Even though isoproterenol increased the amplitude and maximal rate of rise and fall of Ca2+ transients in control cells, three types of responses were found in R420Q mutant cells: 1) 59% of CPVT1-CM were irresponsive to isoproterenol; 2) 16% CPVT1-CM showed arrhythmogenic responses; and 3) in 25% of CPVT-CMs isoproterenol decreased Ca2+ transients and elevated diastolic Ca2+ levels, Table 2. In another N-terminal CPTV1-causing mutation, S406L [64], although cardiomyocytes appeared to have similar diastolic and systolic Ca2+ levels and comparable SR Ca2+ content under basal condition as control myocytes, in the presence of isoproterenol the diastolic Ca2+, the frequency of Ca2+ sparks, and the susceptibility to DADs and arrhythmia increased, even though systolic Ca2+ and SR Ca2+ content remained unaltered [64]. Interestingly, high frequency electrical pacing also increased the percentage of cells with abnormal Ca2+ handling. These authors report that dantrolene restored Ca2+ sparks frequency in CPVT mutant cells both under basal and adrenergic stimulated conditions, and abolished DAD and triggered arrhythmias [64]. Comparison of Ca2+ content of these two point mutations in the N-terminal domain suggests a decrease in R420Q, consistent with RyR2 leak hypothesis, and no change in S406L, possibly supporting increased luminal Ca2+ sensitivity of SR, Table 2.
Four hiPSC-CM cell lines have been thus far created carrying CPVT point mutations flanking FKBP domain in the cytosolic region of RyR2, Fig 4. In E2311D cell-line Ca2+ transients appeared to initiate from multiple loci and isoproterenol increased the number of sites triggering the spontaneous Ca2+ transients. Under basal conditions, 43% CPVT-CMs developed DAD during the diastolic depolarization phase. 12% of the cells also developed DADs even when paced at low frequencies of 0.5Hz, and the DAD frequencies increased significantly after β-adrenergic stimulation. Interestingly, CaMKII inhibitor KN-93 stabilized the Ca2+ activity and suppressed isoproterenol-induced DAD in this model [65]. The P2328S CPVT1 causing mutation cell-line [43] also displays aberrant Ca2+ cycling, decreased Ca2+ content, and a fractional Ca2+ release that was significantly higher both in control and isoproterenol-treated myocytes. In this cell-line both EAD and DAD were consistently recorded and their frequency was increased on adrenergic stimulation.
Penttinen et al. [66] also developed 6 CPVT cell lines with different mutations located in different parts of the RyR2 protein from N-terminal, to central, to cytoplasmic, to C-terminal domains (Fig. 4) that included: Exon-3 deletion and point mutations T2538R, P2328S, Q420I, L4115F and V4653F. All cell lines showed abnormalities in Ca2+ transients and spontaneous beating frequency both under basal conditions and adrenergic stimulation, but diastolic Ca2+ was only increased in P2328S CPVT-CMs. Unfortunately there is no data on Ca2+ content of SR or its fractional release in five of six mutant cell-lines, providing no mechanistic support for either of the two CPVT1 hypothesis, Table 2. Nevertheless, pharmacotherapy with dantrolene was effective in suppressing Ca2+ cycling abnormalities only in cells having the RyR2 mutations in the N-terminal and central regions, but not in transmembrane domains, consistent with the antiarrhythmic effect of this drug in patients with RyR2 mutations in the N-terminal or central regions of RyR2 protein [66].
Itzhaki et al. also generated a hiPSC-CM heterozygous cell line carrying point mutation at M4109R from a CPVT patient [67]. These myocytes also show abnormal Ca2+ transients and appeared to develop store-overload induced Ca2+ release (SOICR) at much lower Ca2+ concentrations than the healthy control cells. In this cell-line, DADs developed in most cells even when paced at 0.5 to 1 Hz. Adrenergic stimulation significantly increased the frequency and magnitude of DADs and flecainide and thapsigargin significantly suppressed the incidence of DAD [67].
4. Conclusion and Perspectives
Although there are many descriptive electrophysiological studies and measurements of Ca2+ transients in hiPSC-CM cell-lines, there are only few reports that have quantified the Ca2+ signaling parameters ad their regulation in whole-cell patch clamped myocytes. In few studies where hiPSC-CM cell-lines, created either from normal subjects or CPVT patients, were studied in greater detail using confocal and TIRF imaging in voltage-clamped cells, the data suggest that all the parameters of mammalian cardiac signaling (i.e., phenotypic bell-shaped voltage-dependence of ICa-gated Ca2+ release and its voltage-dependent gain, spontaneously triggered Ca2+ sparks, functional levels of NCX gene, β-adrenergic regulation of ICa, and SERCA2a/PLB complex) are expressed in human fibroblast-derived cardiomyocytes. It is, therefore, fair to conclude that hiPSC-CM can serve as a reliable model for human cardiomyocytes, but care must be exercised to characterize fully the various electrophysiological and Ca2+ signaling parameters of each new hiPSC-CM cell line before pharmacological and pathophysiological studies are undertaken.
Clearly, there appears to be significant variations on the degree of maturity of cell-lines generated by different labs that may underlie some of the observed differences in the reported Ca2+ content of SR, their beta-adrenergic modulation, the systolic and diastolic Ca2+ levels, and their spontaneous beating rates. It is not as yet clear whether such variations arise solely from developmental stages of created hiPSC-CM or are related to various cellular biological approaches and in vitro experimental conditions modifying the physiological signaling pathways.
This review also compares the Ca2+ signaling parameters of hiPSC-CMs of control subjects to those derived from patients expressing CPVT1, Table 2. The mutations expressed in various hiPSC-CM cell lines are located in the well-known CPVT hotspots of the RyR2 gene. All mutations appear to cause aberrant Ca2+ transients and arrhythmias. Ca2+ transient abnormalities were somewhat more common in mutations located in transmembrane domain than in central and N-terminal regions, and were preferentially suppressed by dantrolene [64, 66]. Exon 3 deleted hiPSC-CMs had both lower beating frequencies and diastolic Ca2+ levels when compared to other mutations, possibly resulting from altered secondary structures critical to folding of the N-terminal domain and its conformational changes [68]. There also appears to be significant variability in myocyte responsiveness to β-adrenergic stimulation in various cell lines expressing CPVT-inducing mutations. For instance, β-adrenergic agonists failed to increase the diastolic Ca2+ in Exon-3 deleted cells, as well as in cell lines expressing T2538R, L4115F, Q4201R and V4653F, as compared to S406L, R420Q, P2328S, F2483I, which in sharp contrast have increased diastolic Ca2+ levels on exposure to Isoproterenol. Given the inter cellular variability of hiPSC-CMs’ responsiveness to beta agonists it becomes difficult to develop a comprehensive hypothesis as to the mechanisms responsible for development of CPVT on the global whole-heart levels, but this variability may underlie the intriguing finding that the occurrence of CPVT in patients are generally episodic and often unpredictable on exertion or adrenergic stimulation.
CPVT cell lines expressing mutations of different RyR2 loci also show some diversity in the SR Ca2+ content. In 4 of the 11 mutant CPVT1 human cell lines, thus far reported, the Ca2+ content of SR was smaller, consistent with “leaky” RyR2s, Table 2, but at least in F2483I cell line (located in the vicinity of FKBP binding site) the decreased Ca2+ content is accompanied by higher ICa-gated fractional Ca2+ release. In this respect, it appears to be a common finding that the rate of spontaneous beating is lower in CPVT1 mutant cell lines as compared to control lines, possibly because of the lower SR Ca2+ content. This finding is consistent with the hypothesis that Ca2+ signaling mechanism, rather than the activation of If regulates spontaneous pacing activity in hiPSC-CMs [45].
Given the inter-cellular variability in adrenergic responsiveness and diversity in Ca2+ content of SR in different CPVT cell lines, perhaps more sophisticated approaches of simultaneous mapping of both electrical and calcium signals in monolayers of CPVT-expressing cell lines should be combined with single cell studies in identifying and quantifying the possible defects of Ca2+ signaling pathways. These are difficult issues to sort out, but they must be done if iPSC-derived human cardiac cell lines are to be used reliably, especially in pharmacotherapeutic studies. Clearly there are differences in the cellular phenotype, the functional pathology, and the pharmacology of different CPVT1 cell-lines. This, we believe, bodes well for the future of this field especially as more detailed characterizations of these cell-lines and their sensitivity to various pharmacotherapies are undertaken.
Highlights.
Specific carboxyl-tail fragments of L-type Ca2+ channel maybe responsible for the voltage-dependence of cardiac CICR.
Ca2+ signaling in human fibroblast-derived cardiomyocytes shows similar voltage-dependence of CICR and Ca2+ spark properties as those of adult mammalian cardiomyocytes.
CPVT mutants myocytes of N-terminal and cytoplasmic-domains show smaller Ca2+ stores but longer, wandering, and recurrent Ca2+ sparks.
Myocytes expressing different point mutations express varied Ca2+ signaling phenotypes and pharmacological sensitivity.
Acknowledgments
We thank Drs. Naohiro Yamaguchi and Lars Cleemann for helpful suggestions. Supported by NIH R01 HL16152.
Footnotes
Conflict of interest
None of the authors have any actual or potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morad M, Trautwein W. The effect of the duration of the action potential on contraction in the mammalian heart muscle. Pflugers Archiv fur die gesamte Physiologie des Menschen und der Tiere. 1968;299:66–82. doi: 10.1007/BF00362542. [DOI] [PubMed] [Google Scholar]
- 2.Morad M, Orkand RK. Excitation-concentration coupling in frog ventricle: evidence from voltage clamp studies. The Journal of physiology. 1971;219:167–189. doi: 10.1113/jphysiol.1971.sp009656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. The Journal of biological chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 4.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Archiv : European journal of physiology. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 5.Nabauer M, Callewaert G, Cleemann L, Morad M. Regulation of calcium release is gated by calcium current, not gating charge, in cardiac myocytes. Science. 1989;244:800–803. doi: 10.1126/science.2543067. [DOI] [PubMed] [Google Scholar]
- 6.Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. The American journal of physiology. 1983;245:C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- 7.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 8.Cleemann L, Wang W, Morad M. Two-dimensional confocal images of organization, density, and gating of focal Ca2+ release sites in rat cardiac myocytes. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:10984–10989. doi: 10.1073/pnas.95.18.10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barcenas-Ruiz L, Wier WG. Voltage dependence of intracellular [Ca2+]i transients in guinea pig ventricular myocytes. Circulation research. 1987;61:148–154. doi: 10.1161/01.res.61.1.148. [DOI] [PubMed] [Google Scholar]
- 10.Adachi-Akahane S, Cleemann L, Morad M. Cross-signaling between L-type Ca2+ channels and ryanodine receptors in rat ventricular myocytes. The Journal of general physiology. 1996;108:435–454. doi: 10.1085/jgp.108.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woo SH, Cleemann L, Morad M. Spatiotemporal characteristics of junctional and nonjunctional focal Ca2+ release in rat atrial myocytes. Circulation research. 2003;92:e1–11. doi: 10.1161/01.res.0000051887.97625.07. [DOI] [PubMed] [Google Scholar]
- 12.Morad M, Cleemann L. Tunicate heart as a possible model for the vertebrate heart. Federation proceedings. 1980;39:3188–3194. [PubMed] [Google Scholar]
- 13.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annual review of physiology. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 14.Moschella MC, Marks AR. Inositol 1,4,5-trisphosphate receptor expression in cardiac myocytes. The Journal of cell biology. 1993;120:1137–1146. doi: 10.1083/jcb.120.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puceat M, Jaconi M. Ca2+ signalling in cardiogenesis. Cell calcium. 2005;38:383–389. doi: 10.1016/j.ceca.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Webb SE, Miller AL. Calcium signalling during embryonic development. Nature reviews Molecular cell biology. 2003;4:539–551. doi: 10.1038/nrm1149. [DOI] [PubMed] [Google Scholar]
- 17.Kume S, Yamamoto A, Inoue T, Muto A, Okano H, Mikoshiba K. Developmental expression of the inositol 1,4,5-trisphosphate receptor and structural changes in the endoplasmic reticulum during oogenesis and meiotic maturation of Xenopus laevis. Developmental biology. 1997;182:228–239. doi: 10.1006/dbio.1996.8479. [DOI] [PubMed] [Google Scholar]
- 18.Janowski E, Berrios M, Cleemann L, Morad M. Developmental aspects of cardiac Ca(2+) signaling: interplay between RyR- and IP(3)R-gated Ca(2+) stores. American journal of physiology Heart and circulatory physiology. 2010;298:H1939–1950. doi: 10.1152/ajpheart.00607.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakayama H, Bodi I, Maillet M, DeSantiago J, Domeier TL, Mikoshiba K, Lorenz JN, Blatter LA, Bers DM, Molkentin JD. The IP3 receptor regulates cardiac hypertrophy in response to select stimuli. Circulation research. 2010;107:659–666. doi: 10.1161/CIRCRESAHA.110.220038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Go LO, Moschella MC, Watras J, Handa KK, Fyfe BS, Marks AR. Differential regulation of two types of intracellular calcium release channels during end-stage heart failure. The Journal of clinical investigation. 1995;95:888–894. doi: 10.1172/JCI117739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barac YD, Zeevi-Levin N, Yaniv G, Reiter I, Milman F, Shilkrut M, Coleman R, Abassi Z, Binah O. The 1,4,5-inositol trisphosphate pathway is a key component in Fas-mediated hypertrophy in neonatal rat ventricular myocytes. Cardiovascular research. 2005;68:75–86. doi: 10.1016/j.cardiores.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 22.Carafoli E, Lehninger AL. Binding of adenine nucleotides by mitochondria during active uptake of CA++ Biochemical and biophysical research communications. 1964;16:66–70. doi: 10.1016/0006-291x(64)90212-8. [DOI] [PubMed] [Google Scholar]
- 23.Dedkova EN, Blatter LA. Mitochondrial Ca2+ and the heart. Cell calcium. 2008;44:77–91. doi: 10.1016/j.ceca.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Barth E, Stammler G, Speiser B, Schaper J. Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. Journal of molecular and cellular cardiology. 1992;24:669–681. doi: 10.1016/0022-2828(92)93381-s. [DOI] [PubMed] [Google Scholar]
- 25.Belmonte S, Morad M. ‘Pressure-flow’-triggered intracellular Ca2+ transients in rat cardiac myocytes: possible mechanisms and role of mitochondria. The Journal of physiology. 2008;586:1379–1397. doi: 10.1113/jphysiol.2007.149294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haviland S, Cleemann L, Kettlewell S, Smith GL, Morad M. Diversity of mitochondrial Ca(2)(+) signaling in rat neonatal cardiomyocytes: evidence from a genetically directed Ca(2)(+) probe, mitycam-E31Q. Cell calcium. 2014;56:133–146. doi: 10.1016/j.ceca.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fatima A, Xu G, Shao K, Papadopoulos S, Lehmann M, Arnaiz-Cot JJ, Rosa AO, Nguemo F, Matzkies M, Dittmann S, Stone SL, Linke M, Zechner U, Beyer V, Hennies HC, Rosenkranz S, Klauke B, Parwani AS, Haverkamp W, Pfitzer G, Farr M, Cleemann L, Morad M, Milting H, Hescheler J, Saric T. In vitro modeling of ryanodine receptor 2 dysfunction using human induced pluripotent stem cells. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2011;28:579–592. doi: 10.1159/000335753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee YK, Ng KM, Lai WH, Chan YC, Lau YM, Lian Q, Tse HF, Siu CW. Calcium homeostasis in human induced pluripotent stem cell-derived cardiomyocytes. Stem cell reviews. 2011;7:976–986. doi: 10.1007/s12015-011-9273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang GQ, Wei H, Lu J, Wong P, Shim W. Identification and characterization of calcium sparks in cardiomyocytes derived from human induced pluripotent stem cells. PloS one. 2013;8:e55266. doi: 10.1371/journal.pone.0055266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lundy SD, Zhu WZ, Regnier M, Laflamme MA. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem cells and development. 2013;22:1991–2002. doi: 10.1089/scd.2012.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gherghiceanu M, Barad L, Novak A, Reiter I, Itskovitz-Eldor J, Binah O, Popescu LM. Cardiomyocytes derived from human embryonic and induced pluripotent stem cells: comparative ultrastructure. Journal of cellular and molecular medicine. 2011;15:2539–2551. doi: 10.1111/j.1582-4934.2011.01417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lieu DK, Liu J, Siu CW, McNerney GP, Tse HF, Abu-Khalil A, Huser T, Li RA. Absence of transverse tubules contributes to non-uniform Ca(2+) wavefronts in mouse and human embryonic stem cell-derived cardiomyocytes. Stem cells and development. 2009;18:1493–1500. doi: 10.1089/scd.2009.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang XH, Haviland S, Wei H, Saric T, Fatima A, Hescheler J, Cleemann L, Morad M. Ca2+ signaling in human induced pluripotent stem cell-derived cardiomyocytes (iPS-CM) from normal and catecholaminergic polymorphic ventricular tachycardia (CPVT)-afflicted subjects. Cell calcium. 2013;54:57–70. doi: 10.1016/j.ceca.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hwang HS, Kryshtal DO, Feaster TK, Sanchez-Freire V, Zhang J, Kamp TJ, Hong CC, Wu JC, Knollmann BC. Comparable calcium handling of human iPSC-derived cardiomyocytes generated by multiple laboratories. Journal of molecular and cellular cardiology. 2015;85:79–88. doi: 10.1016/j.yjmcc.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma J, Guo L, Fiene SJ, Anson BD, Thomson JA, Kamp TJ, Kolaja KL, Swanson BJ, January CT. High purity human-induced pluripotent stem cell-derived cardiomyocytes: electrophysiological properties of action potentials and ionic currents. American journal of physiology Heart and circulatory physiology. 2011;301:H2006–2017. doi: 10.1152/ajpheart.00694.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morad M, Goldman Y. Excitation-contraction coupling in heart muscle: Membrane control of development of tension Progress in biophysics and molecular biology. 1973;27:257–313. [Google Scholar]
- 37.Morad M, Cleemann L. Role of Ca2+ channel in development of tension in heart muscle. Journal of molecular and cellular cardiology. 1987;19:527–553. doi: 10.1016/s0022-2828(87)80360-7. [DOI] [PubMed] [Google Scholar]
- 38.Cleemann L, Morad M. Role of Ca2+ channel in cardiac excitation-contraction coupling in the rat: evidence from Ca2+ transients and contraction. The Journal of physiology. 1991;432:283–312. doi: 10.1113/jphysiol.1991.sp018385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen F, Mottino G, Klitzner TS, Philipson KD, Frank JS. Distribution of the Na+/Ca2+ exchange protein in developing rabbit myocytes. The American journal of physiology. 1995;268:C1126–1132. doi: 10.1152/ajpcell.1995.268.5.C1126. [DOI] [PubMed] [Google Scholar]
- 40.Itzhaki I, Rapoport S, Huber I, Mizrahi I, Zwi-Dantsis L, Arbel G, Schiller J, Gepstein L. Calcium handling in human induced pluripotent stem cell derived cardiomyocytes. PloS one. 2011;6:e18037. doi: 10.1371/journal.pone.0018037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Germanguz I, Sedan O, Zeevi-Levin N, Shtrichman R, Barak E, Ziskind A, Eliyahu S, Meiry G, Amit M, Itskovitz-Eldor J, Binah O. Molecular characterization and functional properties of cardiomyocytes derived from human inducible pluripotent stem cells. Journal of cellular and molecular medicine. 2011;15:38–51. doi: 10.1111/j.1582-4934.2009.00996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shmigol A, Verkhratsky A, Isenberg G. Calcium-induced calcium release in rat sensory neurons. The Journal of physiology. 1995;489(Pt 3):627–636. doi: 10.1113/jphysiol.1995.sp021078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kujala K, Paavola J, Lahti A, Larsson K, Pekkanen-Mattila M, Viitasalo M, Lahtinen AM, Toivonen L, Kontula K, Swan H, Laine M, Silvennoinen O, Aalto-Setala K. Cell model of catecholaminergic polymorphic ventricular tachycardia reveals early and delayed afterdepolarizations. PloS one. 2012;7:e44660. doi: 10.1371/journal.pone.0044660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Novak A, Barad L, Lorber A, Gherghiceanu M, Reiter I, Eisen B, Eldor L, Itskovitz-Eldor J, Eldar M, Arad M, Binah O. Functional abnormalities in iPSC-derived cardiomyocytes generated from CPVT1 and CPVT2 patients carrying ryanodine or calsequestrin mutations. Journal of cellular and molecular medicine. 2015;19:2006–2018. doi: 10.1111/jcmm.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang XH, Wei H, Saric T, Hescheler J, Cleemann L, Morad M. Regionally diverse mitochondrial calcium signaling regulates spontaneous pacing in developing cardiomyocytes. Cell calcium. 2015;57:321–336. doi: 10.1016/j.ceca.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosemblit N, Moschella MC, Ondriasa E, Gutstein DE, Ondrias K, Marks AR. Intracellular calcium release channel expression during embryogenesis. Developmental biology. 1999;206:163–177. doi: 10.1006/dbio.1998.9120. [DOI] [PubMed] [Google Scholar]
- 47.Marks AR. Intracellular calcium-release channels: regulators of cell life and death. The American journal of physiology. 1997;272:H597–605. doi: 10.1152/ajpheart.1997.272.2.H597. [DOI] [PubMed] [Google Scholar]
- 48.Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiological reviews. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- 49.Luo D, Yang D, Lan X, Li K, Li X, Chen J, Zhang Y, Xiao RP, Han Q, Cheng H. Nuclear Ca2+ sparks and waves mediated by inositol 1,4,5-trisphosphate receptors in neonatal rat cardiomyocytes. Cell calcium. 2008;43:165–174. doi: 10.1016/j.ceca.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sedan O, Dolnikov K, Zeevi-Levin N, Leibovich N, Amit M, Itskovitz-Eldor J, Binah O. 1,4,5-Inositol trisphosphate-operated intracellular Ca(2+) stores and angiotensin-II/endothelin-1 signaling pathway are functional in human embryonic stem cell-derived cardiomyocytes. Stem cells. 2008;26:3130–3138. doi: 10.1634/stemcells.2008-0777. [DOI] [PubMed] [Google Scholar]
- 51.Satin J, Itzhaki I, Rapoport S, Schroder EA, Izu L, Arbel G, Beyar R, Balke CW, Schiller J, Gepstein L. Calcium handling in human embryonic stem cell-derived cardiomyocytes. Stem cells. 2008;26:1961–1972. doi: 10.1634/stemcells.2007-0591. [DOI] [PubMed] [Google Scholar]
- 52.Kim JC, Son MJ, Subedi KP, Li Y, Ahn JR, Woo SH. Atrial local Ca2+ signaling and inositol 1,4,5-trisphosphate receptors. Progress in biophysics and molecular biology. 2010;103:59–70. doi: 10.1016/j.pbiomolbio.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 53.Sasse P, Zhang J, Cleemann L, Morad M, Hescheler J, Fleischmann BK. Intracellular Ca2+ oscillations, a potential pacemaking mechanism in early embryonic heart cells. The Journal of general physiology. 2007;130:133–144. doi: 10.1085/jgp.200609575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kapur N, Banach K. Inositol-1,4,5-trisphosphate-mediated spontaneous activity in mouse embryonic stem cell-derived cardiomyocytes. The Journal of physiology. 2007;581:1113–1127. doi: 10.1113/jphysiol.2006.125955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mery A, Aimond F, Menard C, Mikoshiba K, Michalak M, Puceat M. Initiation of embryonic cardiac pacemaker activity by inositol 1,4,5-trisphosphate-dependent calcium signaling. Molecular biology of the cell. 2005;16:2414–2423. doi: 10.1091/mbc.E04-10-0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kapoor N, Maxwell JT, Mignery GA, Will D, Blatter LA, Banach K. Spatially defined InsP3-mediated signaling in embryonic stem cell-derived cardiomyocytes. PloS one. 2014;9:e83715. doi: 10.1371/journal.pone.0083715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garcia KD, Shah T, Garcia J. Immunolocalization of type 2 inositol 1,4,5-trisphosphate receptors in cardiac myocytes from newborn mice. American journal of physiology Cell physiology. 2004;287:C1048–1057. doi: 10.1152/ajpcell.00004.2004. [DOI] [PubMed] [Google Scholar]
- 58.Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, Sorrentino V, Danieli GA. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- 59.Novak A, Lorber A, Itskovitz-Eldor J, Binah O. Modeling Catecholaminergic Polymorphic Ventricular Tachycardia using Induced Pluripotent Stem Cell-derived Cardiomyocytes. Rambam Maimonides medical journal. 2012;3:e0015. doi: 10.5041/RMMJ.10086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Postma AV, Denjoy I, Hoorntje TM, Lupoglazoff JM, Da Costa A, Sebillon P, Mannens MM, Wilde AA, Guicheney P. Absence of calsequestrin 2 causes severe forms of catecholaminergic polymorphic ventricular tachycardia. Circulation research. 2002;91:e21–26. doi: 10.1161/01.res.0000038886.18992.6b. [DOI] [PubMed] [Google Scholar]
- 61.Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, Sun J, Guatimosim S, Song LS, Rosemblit N, D’Armiento JM, Napolitano C, Memmi M, Priori SG, Lederer WJ, Marks AR. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–840. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 62.Jiang D, Wang R, Xiao B, Kong H, Hunt DJ, Choi P, Zhang L, Chen SR. Enhanced store overload-induced Ca2+ release and channel sensitivity to luminal Ca2+ activation are common defects of RyR2 mutations linked to ventricular tachycardia and sudden death. Circulation research. 2005;97:1173–1181. doi: 10.1161/01.RES.0000192146.85173.4b. [DOI] [PubMed] [Google Scholar]
- 63.Lehnart SE, Mongillo M, Bellinger A, Lindegger N, Chen BX, Hsueh W, Reiken S, Wronska A, Drew LJ, Ward CW, Lederer WJ, Kass RS, Morley G, Marks AR. Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. The Journal of clinical investigation. 2008;118:2230–2245. doi: 10.1172/JCI35346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jung CB, Moretti A, Mederos y Schnitzler M, Iop L, Storch U, Bellin M, Dorn T, Ruppenthal S, Pfeiffer S, Goedel A, Dirschinger RJ, Seyfarth M, Lam JT, Sinnecker D, Gudermann T, Lipp P, Laugwitz KL. Dantrolene rescues arrhythmogenic RYR2 defect in a patient-specific stem cell model of catecholaminergic polymorphic ventricular tachycardia. EMBO molecular medicine. 2012;4:180–191. doi: 10.1002/emmm.201100194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Di Pasquale E, Lodola F, Miragoli M, Denegri M, Avelino-Cruz JE, Buonocore M, Nakahama H, Portararo P, Bloise R, Napolitano C, Condorelli G, Priori SG. CaMKII inhibition rectifies arrhythmic phenotype in a patient-specific model of catecholaminergic polymorphic ventricular tachycardia. Cell death & disease. 2013;4:e843. doi: 10.1038/cddis.2013.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Penttinen K, Swan H, Vanninen S, Paavola J, Lahtinen AM, Kontula K, Aalto-Setala K. Antiarrhythmic Effects of Dantrolene in Patients with Catecholaminergic Polymorphic Ventricular Tachycardia and Replication of the Responses Using iPSC Models. PloS one. 2015;10:e0125366. doi: 10.1371/journal.pone.0125366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Itzhaki I, Maizels L, Huber I, Gepstein A, Arbel G, Caspi O, Miller L, Belhassen B, Nof E, Glikson M, Gepstein L. Modeling of catecholaminergic polymorphic ventricular tachycardia with patient-specific human-induced pluripotent stem cells. Journal of the American College of Cardiology. 2012;60:990–1000. doi: 10.1016/j.jacc.2012.02.066. [DOI] [PubMed] [Google Scholar]
- 68.Lobo PA, Kimlicka L, Tung CC, Van Petegem F. The deletion of exon 3 in the cardiac ryanodine receptor is rescued by beta strand switching. Structure. 2011;19:790–798. doi: 10.1016/j.str.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 69.Gupta MK, Illich DJ, Gaarz A, Matzkies M, Nguemo F, Pfannkuche K, Liang H, Classen S, Reppel M, Schultze JL, Hescheler J, Saric T. Global transcriptional profiles of beating clusters derived from human induced pluripotent stem cells and embryonic stem cells are highly similar. BMC developmental biology. 2010;10:98. doi: 10.1186/1471-213X-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]