Abstract
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Objective
Reduced insulin sensitivity (IS) is well documented in type 1 diabetes (T1D) and may contribute to vascular complications. We examined the association of estimated IS (eIS) with incident macro- and microvascular complications in adults with T1D in the prospective CACTI study.
Methods
Participants (N=652) were 19-56 years old at baseline and re-examined 6.2±0.6 years later. Urinary albumin excretion was measured, and categorized as microalbuminuria or greater. Diabetic retinopathy (DR) was based on self-reported history, proliferative DR (PDR) as history of laser eye therapy and coronary artery calcium (CAC) was measured using electron-beam CT. Progression of CAC was defined as a change in the square root transformed CAC volume score of ≥2.5. IS was estimated (eIS) by an equation derived from clamp studies. Predictors of each complication were examined using stepwise logistic regression and subjects with complications at baseline excluded. Age, T1D duration, sex, HbA1c, SBP, LDL-C, and eIS were considered for inclusion.
Results
Greater eIS at baseline predicted lower odds of developing albuminuria (OR: 0.67, 95% CI 0.51-0.88), DR (OR 0.79, 0.64-0.97), PDR (OR: 0.76, 0.57-0.99) and CACp (OR: 0.71, 0.60-0.85) in multivariable models.
Conclusions
Greater eIS conferred protection from the development of vascular complications over 6-years in T1D.
Keywords: Insulin sensitivity, insulin resistance, type 1 diabetes, microvascular complications, macrovascular complications
Introduction
The public health burden of type 1 diabetes (T1D), a disease affecting approximately 1.4 million people in the U.S. and 30 million globally, is progressively increasing largely due to the prevalence of the associated vascular complications (1-3). Coronary artery disease (CAD) is the major cause of morbidity and mortality in patients with T1D (2-5). Annually, up to 2% of young adults with T1D develop CAD (2-5). By their mid-forties, over 70% of men and 50% of women with T1D develop coronary artery calcification (CAC) (5), a marker of subclinical atherosclerotic plaque burden. Diabetic nephropathy remains the leading cause of end-stage renal disease in the United States (6), and diabetic retinopathy is the single most common cause of new-onset blindness (7).
Despite significant improvement in conventional risk factors (e.g. hypertension, glycemic control and dyslipidemia) during the past two decades, vascular complications continue to be a major concern for health providers taking care of patients with T1D (8, 9). For that reason, there is a need for improved methods of identifying people at risk of vascular complications at an early stage, as well as additional therapeutic targets to supplement conventional risk factors in preventing development and progression of these complications.
Reduced insulin sensitivity (IS) is well documented in both adolescents and adults with T1D (10-12), and is thought to contribute both to the initiation and progression of vascular complications (13-17). Measuring insulin sensitivity by hyperinsulinemic-euglycemic clamp techniques remains invasive and too cumbersome for clinical care, but newer insulin sensitivity estimation (eIS) equations, which demonstrate strong agreement with measured glucose infusion rate, offer promise in the clinical setting (18). We recently published an eIS equation using common clinical parameters, which performed better than previous equations in estimating IS in adolescents and adults with T1D (18).
The Coronary Artery Calcification in Type 1 diabetes (CACTI) study provided the opportunity to examine the association between eIS at baseline and development of both macro-defined as progression of CAC) and microvascular (defined as albuminuria, diabetic retinopathy (DR) and/or proliferative DR (PDR)) complications in a prospective cohort of adults with T1D. We hypothesized that greater eIS at baseline would independently predict lower odds of developing both micro- and macrovascular complications over 6 years in adults with T1D.
Materials and Methods
The CACTI Study enrolled 1416 subjects 19-56 years old, 652 with and 764 without T1D, who were asymptomatic for cardiovascular disease (CVD) at the baseline visit in 2000-02 and then were re-examined 3 and 6 years later, as previously described (19). Participants (n=652) with T1D who had data available for eIS at baseline were included in this analysis. The study was approved by the Colorado Multiple Institutional Review Board and all participants provided informed consent.
We measured height and weight, and calculated BMI in kg/m2. Resting systolic (SBP) and fifth-phase diastolic blood pressure (DBP) were measured three times while the patient was seated, and the second and third measurements were averaged for subsequent analysis. After an overnight fast, blood was collected, centrifuged, and separated. Plasma was stored at 4°C until assayed. Total plasma cholesterol and triglyceride levels were measured using standard enzymatic methods, HDL cholesterol was separated using dextran sulfate and LDL cholesterol was calculated using the Friedewald formula. High performance liquid chromatography was used to measure HbA1c (HPLC, BioRad variant).
CACTI clamp cohort – estimated insulin sensitivity (eIS)
eIS was calculated using an equation developed in a subset of the study cohort (n=87, 40 with T1D and 47 normal controls, frequency matched for age, gender and weight) who underwent a 3 stage hyperinsulinemic-euglycemic clamp study to measure insulin sensitivity, as previously described in detail (10, 20). The model included waist circumference, daily insulin dose per kg body weight, triglycerides and diastolic blood pressure (DBP): exp(4.1075 – 0.01299*waist (cm) – 1.05819*insulin dose (daily units per kg) – 0.00354*triglycerides (mg/dl) – 0.00802*diastolic blood pressure (mm Hg)) (20). We have previously demonstrated that eIS, developed in the CACTI study, improved on the performance of former estimating equations in individuals with and without T1D (20).
Diabetic nephropathy
Diabetic nephropathy was defined as incident albuminuria. Albuminuria was defined as AER ≥ 20 μg/min if timed urine samples were obtained, or ACR ≥ 30 mg/g for spot samples if AER was unavailable. Two timed overnight urine samples were collected in duplicate and urine creatinine and albumin were measured (RIA, Diagnostic Products) and averaged. At both visits, urinary albumin excretion rate (AER) and albumin/creatinine ratio (ACR) were measured. Glomerular filtration rate (GFR) (ml/min/1.73m2) was determined using CKD-EPI creatinine and CKD-EPI cystatin C equations respectively (21). Serum creatinine was measured according to package insert instructions using a Roche Mira Plus II analyzer until 2006 and then an Olympus AU400e (r=0.9999 between methodologies) traceable to the National Institutes of Standards and Technology Standard Reference Material in the University of Colorado Clinical Translational Research (CTRC) Lab. Cystatin C was measured in the University of Colorado Hospital clinical lab using the commercially available Dade-Behring assay following package insert instructions on a BNII or Prospec instrument as previously described (22).
Diabetic retinopathy
Diagnosis of DR was based on self-reported history of diabetic retinopathy. Self-reported DR has been validated as both a sensitive and specific tool for determining DR (23).
Proliferative diabetic retinopathy
Diagnosis of PDR was based on self-reported history of proliferative retinopathy with laser eye treatment. Self-reported prior laser treatment has been validated as both a sensitive and specific tool for determining PDR (23, 24).
CAC progression
CAC measurements were obtained in duplicate using an ultrafast Imatron C-150XLP electron beam computed tomography (EBCT) scanner (Imatron, San Francisco, CA). The average of the two Agatston scores was used as the CAC score for that visit. Scans were repeated on follow-up, an average of 6.2±0.6 years after the baseline exam. Presence of CAC was defined as a CAC score > 0. Progression of CAC (CACp) was defined as an increase in the CAC volume score of ≥ 2.5 square root transformed units. This definition of progression has previously been shown to represent significant progression of atherosclerosis (9).
Statistical Analysis
Analyses were performed in SAS (version 9.3 for Windows; SAS Institute, Cary, NC). Differences between men and women were assessed using Chi-Square for categorical variables and t-test for continuous variables. Logistic regression was performed to evaluate the associations between variables at baseline and development of incident albuminuria, incident DR, incident PDR and CACp. We excluded subjects with albuminuria (n=129), DR (n=184) and PDR (n=145) at baseline in our analyses. For CACp we did not exclude baseline disease as we were measuring progression of disease rather than incidence.
Variables considered for inclusion in the multivariable models were based on a priori criteria: significance in previous work, significant contribution to the model (p-value of <0.1), or confounding between the main variable of interest and the outcome by >10%. The following variables were considered for inclusion in the models: eIS, HDL-C, LDL-C, systolic blood pressure, antihypertensive medications, HbA1c, T1D duration and age. Stepwise logistic regression was used to determine which variables remained in multivariable models predicting DR, PDR, albuminuria and CACp, respectively. Only variables with p-value<0.1 in stepwise selection were included in the models. Odds ratios (OR) represent the odds of developing incident DR, incident PDR and incident albuminuria, or experiencing CACp for every unit increase in the independent variable, and are reported with 95% CI. Significance was based on an α-level of 0.05.
Results
Over 6 years 9.6% of men and 6.1% of women developed incident albuminuria, 11.3% of men and 6.0% of women developed incident PDR, 23.2% of men and 14.3% of women developed incident DR and 52.5% of men and 34.0% of women experienced CACp as previously described (15). Baseline subject characteristics stratified by gender are shown in Table 1.
Table 1. Baseline Characteristics for Study Population with T1D.
| Men (n=298) | Women (n=354) | P-value | |
|---|---|---|---|
| Age (years) | 37 ± 9 | 36 ± 9 | 0.07 |
| Diabetes duration (years) | 24 ± 9 | 23 ± 9 | 0.29 |
| HbA1c (%) | 8.0 ± 1.2 | 8.0 ± 1.3 | 0.88 |
| HbA1c (mmol/mol) | 64 ± 13 | 64 ± 14 | 0.88 |
| LDL-C (mg/dl) | 104 ± 30 | 98 ± 28 | 0.006 |
| HDL-C (mg/dl) | 51 ± 14 | 60 ± 17 | <0.0001 |
| Triglycerides (mg/dl) | 80 (61-113) | 77 (61-104) | 0.23 |
| Systolic BP (mm Hg) | 121 ± 14 | 114 ± 14 | <0.0001 |
| Diastolic BP (mm Hg) | 80 ± 9 | 75 ± 8 | <0.0001 |
| BMI (kg/m2) | 26.5 ± 3.9 | 26.0 ± 4.7 | 0.09 |
| Waist circumference (cm) | 90.3 ± 11.7 | 80.8 ± 12.0 | <0.0001 |
| AER (μg/min) | 7.4 (4.5-23.8) | 5.8 (3.8-11.8) | <0.0001 |
| eGFRCREASTININE at baseline (mL/min/1.73m2) | 100 ± 27 | 105 ± 28 | 0.02 |
| eGFRCREATININE at year 6 (mL/min/1.73m2) | 97 ± 22 | 99 ± 21 | 0.36 |
| eGFRCYSTATIN C at baseline (mL/min/1.73m2) | 107 ± 23 | 106 ± 20 | 0.68 |
| eGFRCYSTATIN C at year 6 (mL/min/1.73m2) | 102 ± 23 | 102 ± 20 | 0.96 |
| eIS (mg/kg-1 min-1) | 3.8 ± 1.4 | 4.8 ± 1.6 | <0.0001 |
| On antihypertensive medications (%) | 41% | 35% | 0.08 |
| Ever smoker (% yes) | 18% | 22% | 0.20 |
| Albuminuria (% yes) | 27% | 17% | 0.005 |
| PDR (% yes) | 27% | 19% | 0.02 |
| Retinopathy (% yes) | 33% | 26% | 0.07 |
Data are means ± SD, % or median (25th – 75th %)
Each standard deviation (SD) increase in eIS at baseline was associated with lower odds of incident albuminuria, DR, PDR and CACp (Figure 1). Furthermore, in stepwise multivariable logistic regression models examining shared risk factors of vascular complications, each SD increase in eIS at baseline was independently associated with lower odds of incident albuminuria (OR: 0.52, 95% CI 0.35-0.83, p=0.005), incident DR (OR: 0.69, 95% CI 0.50-0.95, p=0.02), PDR (OR: 0.65, 95% CI 0.42-0.99, p=0.049) and CACp (OR: 0.59, 95% CI 0.46-0.77, p<0.0001), Table 2). HbA1c predicted increased odds of developing DR, PDR and CACp in multivariable models (Table 2). Diabetes duration and male sex predicted DR, PDR and CACp (Table 2). Moreover, age predicted CACp (Table 2).
Figure 1. Forest plot for incident micro- and macrovascular complications.
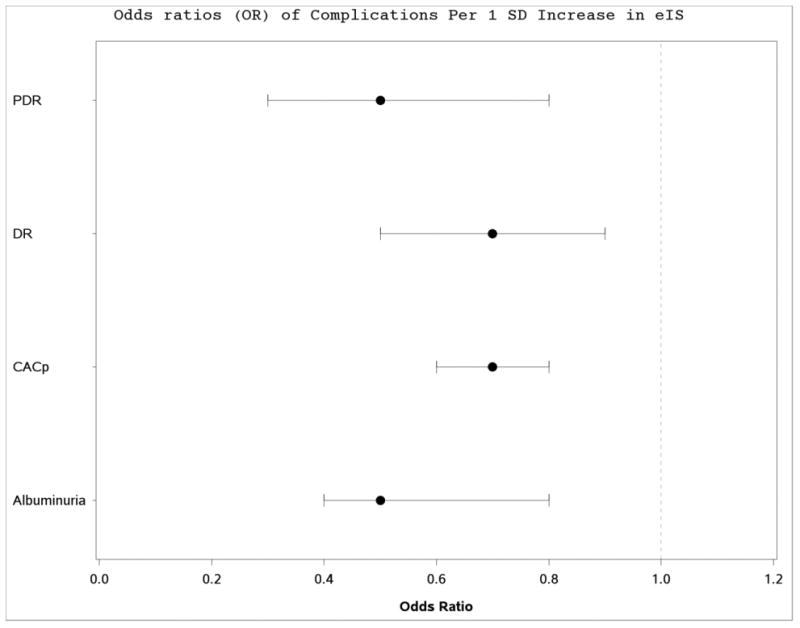
Data is presented as OR and 95% CI. OR represent the increase in odds of developing albuminuria, DR, PDR and CACp for every 1 SD increase in the eIS (1.58 mg/kg-1 min-1)
Table 2. Multivariable models predicting incident micro- and macrovascular complications.
| Albuminuria (n=26) | DR (n=62) | PDR (n=31) | CACp (n=185) | |
|---|---|---|---|---|
| Age (per 10 years) | – | – | – | 1.99 (1.43-2.75) P<0.0001 |
| Diabetes duration (per 10 years) | – | 2.04 (1.43-2.91) P<0.0001 | 1.85 (1.15-3.00) P=0.01 | 2.24 (1.62-3.10) P<0.0001 |
| Male sex | – | 2.08 (1.13-3.84) P=0.02 | 2.40 (1.04-5.52) P=0.04 | 1.73 (1.08-2.77) P=0.02 |
| HbA1c (per 1%) | – | 1.41 (1.10-1.79) P=0.006 | 1.71 (1.28-2.28) P=0.0003 | 1.25 (1.03-1.52) P=0.03 |
| SBP (per 10 mmHg) | – | – | – | 1.20 (1.00-1.44) P=0.049 |
| LDL-C (per 10 mg/dL) | – | 0.88 (0.79-0.98) P=0.02 | – | – |
| eIS (per SD [1.58 mg/kg-1 min-1]) | 0.54 (0.35-0.83) P=0.005 | 0.69 (0.50-0.95) P=0.02 | 0.65 (0.42-0.99) P=0.049 | 0.59 (0.46-0.77) P<0.0001 |
Data is presented as OR and 95% CI. OR represent the increase in odds of developing albuminuria, DR, PDR and CACp for every unit increase in the independent variable. These are stepwise models so only variables which entered the models are presented.
To further test the independence of the associations between eIS and the vascular complications, we also adjusted for antihypertensive medication, and the associations remained significant between eIS and albuminuria (OR: 0.56, 95% CI 0.37-0.87, p=0.009), DR (OR: 0.71, 95% 0.52-0.98, p=0.03) and CACp (OR: 0.53, 95% CI 0.41-0.68, p<0.0001), but was attenuated in PDR (OR: 0.69, 95% 0.45-1.08, p=0.10).
Discussion
Greater eIS at baseline independently predicted lower odds of developing albuminuria, DR, PDR and CACp in a contemporary cohort of adults with T1D. A major challenge in preventing vascular complications of T1D is the difficulty in accurately identifying high risk patients and the need for additional targets to supplement the conventional therapies. There are clinical trials underway exploring the role of metformin in preventing cardiorenal complications of T1D (REMOVAL [NCT01483560] and EMERALD [NCT01808690]), but insulin sensitivity may prove to be an equally important target in the prevention of DR and PDR.
The American Diabetes Association recommends that most adults with T1D should achieve a HbA1C <7.0% (<53 mmol/mol), BP <130/80 mm Hg, and LDL-C <100 mg/dL (25). We have previously reported that only 6% of adults with T1D in CACTI achieved all three ABC goals (26), and not a single subject met the American Heart Association's 7 metrics for ideal cardiovascular health (ICH) (27). The suboptimal ABC and ICH control may be reflective of unattainable goals for subjects with T1D or the lack of sufficiently effective strategies to achieve these goals. While there is strong evidence showing the benefits of ABC and ICH control in reducing vascular complications in T1D (1, 27), optimal control does not abolish the risk for complications. For these reasons there is a call for novel therapeutic targets to supplement conventional therapies.
Orchard et al. demonstrated that estimated glucose disposal rate (eGDR) predicted overt nephropathy in adults with T1D in the EDC cohort (28) over a decade ago. We have also previously demonstrated a cross-sectional association between measured insulin sensitivity and CAC in a small subset of subjects with T1D in CACTI who underwent hyperinsulinemic-euglycemic clamp studies (10). In support of this finding, we here report a strong independent association between eIS at baseline and CACp over 6 years in the full T1D cohort. Furthermore, we report for the first time that eIS at baseline also predicts lower odds of developing DR and PDR independent of other established risk factors. Diabetic retinopathy (DR) remains the most common cause of new onset blindness in adults (7). The prevalence of PDR and DR in CACTI at baseline were 27% and 33% of men and 19% and 26% of women respectively which is consistent with the prevalence reported by several population-based studies (29).
Reduced insulin sensitivity as a unified risk factor for the development of both micro-and macrovascular complications does not necessarily imply causation, but there is increasing evidence implicating reduced insulin sensitivity in the pathogenesis of vascular complications in T1D (30). The exact mechanism of reduced insulin sensitivity in T1D remains unclear. Several factors have been implicated including prolonged exposure to supraphysiologic levels of exogenous insulin, weight gain caused by intensive insulin therapy and perhaps most importantly similar genetic and environmental factors that lead to type 2 diabetes (13, 31). Another possible mechanistic pathway linking reduced insulin sensitivity to vascular complications in T1D is via insulin's effects on overall non-essential fatty acid exposure and lipotoxicity in development of macro- and microangiopathy. There are robust data demonstrating that subjects with T1D with insulin resistance and/or family history of type 2 diabetes are at greater risk of micro- and macrovascular complications (32). Furthermore, high prevalence of metabolic syndrome (38% in men and 40% in women) has been reported in subjects with T1D (33). Insulin sensitivity holds promise as an independent therapeutic target to reduce vascular complications in T1D, with both lifestyle changes (diet and exercise) and drugs such as metformin (34). The REducing With MetfOrmin Vascular Adverse Lesions in Type 1 Diabetes (REMOVAL, NCT01483560) (35) and Effects of Metformin on Cardiovascular Function in Adolescents With Type 1 Diabetes (EMERALD, NCT01808690) are ongoing double-blind randomized clinical trials with metformin to improve insulin sensitivity in subjects with T1D in an attempt to prevent vascular complications.
There are limitations of this study worth mentioning, including the limited number of observations for incident albuminuria and incident PDR. DR and PDR were also self- reported and could have been affected by poor recall, but self-reporting of DR has recently been validated for subjects with T1D with sensitivity and specificity greater than 90% (23, 24). Moreover, we did not have data on diabetic neuropathy, another important microvascular complication in T1D. Our study utilizes the eIS equation derived from the largest euglycemic-hyperinsulinemic clamp study in adults both with and without T1D (20) and improved on the performance of previous eIS equations in individuals with and without T1D (18, 28) . Additionally, a direct measure of insulin sensitivity would have been too cumbersome for use in a large-scale clinical study like CACTI. More importantly our main goal was to explore the utility of eIS in predicting vascular complications in adults with T1D. We adjusted for a variety of important confounding variables, but cannot rule out the presence of unknown risk factors that may have biased or confounded the present analyses. Results from this study may not be generalizable to significantly younger or older subjects with T1D. We also acknowledge that microalbuminuria as a proxy for diabetic nephropathy is not without controversy (36). We have previously reported an association between eIS, albuminuria and rapid GFR decline (34), but this paper is novel in that it explores the associations between eIS and CACp, DR and PDR.
In summary, greater eIS at baseline appears to be protective against the development and progression of both micro- and macrovascular complications of T1D. For that reason, estimated insulin sensitivity may supplement conventional risk factors in identifying people at risk of vascular complications in clinical care. Despite the BARI-2D study (37) showing no benefit of insulin sensitizing strategy on nephropathy in older adults with established coronary artery disease with type 2 diabetes, modification of insulin sensitivity holds promise as a novel target to reduce vascular complications in T1D. Translation of insulin sensitivity into clinical practice as a therapeutic target requires investment in adequately powered clinical trials to capture important long-term vascular outcomes.
Acknowledgments
Support for this study was provided by NHLBI grant R01 HL61753, HL79611, and HL113029, JDRF grant 17-2013-313, and DERC Clinical Investigation Core P30 DK57516. The study was performed at the Adult CTRC at UCD support by NIH-M01-RR00051, at the Barbara Davis Center for Childhood Diabetes and at Colorado Heart Imaging Center in Denver, CO. Dr. Snell-Bergeon by an American Diabetes Association Junior Faculty Award (1-10-JF-50).
Drs. Petter Bjornstad, Laura Pyle and Janet K. Snell-Bergeon are guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis
Footnotes
Author Contributions: PB researched, wrote, contributed to discussion, and reviewed/edited the manuscript; DMM researched, contributed to discussion, and reviewed/edited the manuscript; LMD researched, wrote, contributed to discussion, and reviewed/edited the manuscript; LP contributed to discussion, performed statistical analyses and reviewed/edited the manuscript; MR designed the CACTI Study, researched, contributed to the discussion and reviewed/edited the manuscript; RJJ contributed to the discussion and reviewed/edited the manuscript; JKSB researched, contributed to the discussion, reviewed/edited the manuscript.
Duality of interest: Drs. Bjornstad, Snell-Bergeon, Johnson, Rewers, Pyle and Maahs, and Ms. Duca have no conflict of interest to disclose.
References
- 1.Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988-2010. Diabetes care. 2013;36(8):2271–9. doi: 10.2337/dc12-2258. Epub 2013/02/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby P, Nathan DM, Abraham K, Brunzell JD, Fradkin JE, Haffner SM, et al. Report of the National Heart, Lung, and Blood Institute-National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Cardiovascular Complications of Type 1 Diabetes Mellitus. Circulation. 2005;111(25):3489–93. doi: 10.1161/CIRCULATIONAHA.104.529651. Epub 2005/06/29. [DOI] [PubMed] [Google Scholar]
- 3.de Ferranti SD, de Boer IH, Fonseca V, Fox CS, Golden SH, Lavie CJ, et al. Type 1 Diabetes Mellitus and Cardiovascular Disease: A Scientific Statement From the American Heart Association and American Diabetes Association. Circulation. 2014 doi: 10.1161/CIR.0000000000000034. Epub 2014/08/13. [DOI] [PubMed] [Google Scholar]
- 4.Krolewski AS, Kosinski EJ, Warram JH, Leland OS, Busick EJ, Asmal AC, et al. Magnitude and determinants of coronary artery disease in juvenile-onset, insulin-dependent diabetes mellitus. Am J Cardiol. 1987;59(8):750–5. doi: 10.1016/0002-9149(87)91086-1. Epub 1987/04/01. [DOI] [PubMed] [Google Scholar]
- 5.Olson JC, Edmundowicz D, Becker DJ, Kuller LH, Orchard TJ. Coronary calcium in adults with type 1 diabetes: a stronger correlate of clinical coronary artery disease in men than in women. Diabetes. 2000;49(9):1571–8. doi: 10.2337/diabetes.49.9.1571. Epub 2000/09/02. [DOI] [PubMed] [Google Scholar]
- 6.Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Ishani A, et al. US Renal Data System 2010 Annual Data Report. Am J Kidney Dis. 2011;57(1 Suppl 1):A8, e1–526. doi: 10.1053/j.ajkd.2010.10.007. Epub 2010/12/28. [DOI] [PubMed] [Google Scholar]
- 7.Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, et al. Diabetic retinopathy. Diabetes care. 2003;26(1):226–9. doi: 10.2337/diacare.26.1.226. Epub 2002/12/28. [DOI] [PubMed] [Google Scholar]
- 8.Orchard TJ, Secrest AM, Miller RG, Costacou T. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia. 2010;53(11):2312–9. doi: 10.1007/s00125-010-1860-3. Epub 2010/07/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snell-Bergeon JK, Hokanson JE, Jensen L, MacKenzie T, Kinney G, Dabelea D, et al. Progression of coronary artery calcification in type 1 diabetes: the importance of glycemic control. Diabetes care. 2003;26(10):2923–8. doi: 10.2337/diacare.26.10.2923. Epub 2003/09/30. [DOI] [PubMed] [Google Scholar]
- 10.Schauer IE, Snell-Bergeon JK, Bergman BC, Maahs DM, Kretowski A, Eckel RH, et al. Insulin Resistance, Defective Insulin-Mediated Fatty Acid Suppression, and Coronary Artery Calcification in Subjects With and Without Type 1 Diabetes. Diabetes. 2011;60(1):306–14. doi: 10.2337/db10-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nadeau KJ, Regensteiner JG, Bauer TA, Brown MS, Dorosz JL, Hull A, et al. Insulin Resistance in Adolescents with Type 1 Diabetes and Its Relationship to Cardiovascular Function. Journal of Clinical Endocrinology & Metabolism. 2010;95(2):513–21. doi: 10.1210/jc.2009-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westreich KD, Mottl AK. Is insulin resistance a useful predictor of outcomes in diabetic kidney disease? Journal of diabetes and its complications. 2015;29(8):971–3. doi: 10.1016/j.jdiacomp.2015.07.024. Epub 2015/09/06. [DOI] [PubMed] [Google Scholar]
- 13.Cleland SJ, Fisher BM, Colhoun HM, Sattar N, Petrie JR. Insulin resistance in type 1 diabetes: what is ‘double diabetes’ and what are the risks? Diabetologia. 2013;56(7):1462–70. doi: 10.1007/s00125-013-2904-2. Epub 2013/04/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bjornstad P, Cherney D, Maahs DM. Early diabetic nephropathy in type 1 diabetes: new insights. Curr Opin Endocrinol Diabetes Obes. 2014;21(4):279–86. doi: 10.1097/MED.0000000000000074. Epub 2014/07/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bjornstad P, Maahs DM, Rivard CJ, Pyle L, Rewers M, Johnson RJ, et al. Serum uric acid predicts vascular complications in adults with type 1 diabetes: the coronary artery calcification in type 1 diabetes study. Acta diabetologica. 2014 doi: 10.1007/s00592-014-0611-1. Epub 2014/06/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjornstad P, Snell-Bergeon JK, McFann K, Wadwa RP, Rewers M, Rivard CJ, et al. Serum uric acid and insulin sensitivity in adolescents and adults with and without type 1 diabetes. Journal of diabetes and its complications. 2014;28(3):298–304. doi: 10.1016/j.jdiacomp.2013.12.007. Epub 2014/01/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bjornstad P, Maahs DM, Cherney DZ, Cree-Green M, West A, Pyle L, et al. Insulin Sensitivity Is an Important Determinant of Renal Health in Adolescents With Type 2 Diabetes. Diabetes care. 2014 doi: 10.2337/dc14-1331. Epub 2014/07/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duca LM, Maahs DM, Schauer IE, Bergman BC, Nadeau KJ, Bjornstad P, et al. Development and validation of a method to estimate insulin sensitivity in patients with and without type 1 diabetes. The Journal of clinical endocrinology and metabolism. 2015:jc20153272. doi: 10.1210/jc.2015-3272. Epub 2015/12/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maahs DM, Kinney GL, Wadwa P, Snell-Bergeon JK, Dabelea D, Hokanson J, et al. Hypertension prevalence, awareness, treatment, and control in an adult type 1 diabetes population and a comparable general population. Diabetes care. 2005;28(2):301–6. doi: 10.2337/diacare.28.2.301. Epub 2005/01/29. [DOI] [PubMed] [Google Scholar]
- 20.Duca LM, Maahs DM, Schauer I, Bergman B, Nadeau K, Bjornstad P, et al. Development and validation of a method to estimate insulin sensitivity in patients with and without type 1 diabetes Journal of Clinical Endocrinology & Metabolism 2015;In Press. doi: 10.1210/jc.2015-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. The New England journal of medicine. 2012;367(1):20–9. doi: 10.1056/NEJMoa1114248. Epub 2012/07/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maahs DM, Jalal D, McFann K, Rewers M, Snell-Bergeon JK. Systematic shifts in cystatin C between 2006 and 2010. Clin J Am Soc Nephrol. 2011;6(8):1952–5. doi: 10.2215/CJN.11271210. Epub 2011/07/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grassi MA, Sun W, Gangaputra S, Cleary PA, Hubbard L, Lachin JM, et al. Validity of Self-Report in Type 1 Diabetic Subjects for Laser Treatment of Retinopathy. Ophthalmology. 2013 doi: 10.1016/j.ophtha.2013.06.002. Epub 2013/07/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grassi MA, Mazzulla DA, Knudtson MD, Huang WW, Lee KE, Klein BE, et al. Patient self-report of prior laser treatment reliably indicates presence of severe diabetic retinopathy. Am J Ophthalmol. 2009;147(3):501–4. doi: 10.1016/j.ajo.2008.09.016. Epub 2008/12/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Professional Practice Committee for the Standards of Medical Care in Diabetes-2016. Diabetes Care. 2016;39(Suppl 1):S107–8. doi: 10.2337/dc16-S018. Epub 2015/12/24. [DOI] [PubMed] [Google Scholar]
- 26.Bjornstad P, Maahs DM, Rewers M, Johnson RJ, Snell-Bergeon JK. ABC goal achievement predicts microvascular but not macrovascular complications over 6-years in adults with type 1 diabetes: The Coronary Artery Calcification in Type 1 Diabetes Study. Journal of diabetes and its complications. 2014 doi: 10.1016/j.jdiacomp.2014.06.017. Epub 2014/10/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alman AC, Maahs DM, Rewers MJ, Snell-Bergeon JK. Ideal cardiovascular health and the prevalence and progression of coronary artery calcification in adults with and without type 1 diabetes. Diabetes care. 2013 doi: 10.2337/dc13-0997. Epub 2013/10/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orchard TJ, Chang YF, Ferrell RE, Petro N, Ellis DE. Nephropathy in type 1 diabetes: a manifestation of insulin resistance and multiple genetic susceptibilities? Further evidence from the Pittsburgh Epidemiology of Diabetes Complication Study. Kidney Int. 2002;62(3):963–70. doi: 10.1046/j.1523-1755.2002.00507.x. Epub 2002/08/08. [DOI] [PubMed] [Google Scholar]
- 29.Gunnlaugsdottir E, Halldorsdottir S, Klein R, Eiriksdottir G, Klein BE, Benediktsson R, et al. Retinopathy in old persons with and without diabetes mellitus: the Age, Gene/Environment Susceptibility--Reykjavik Study (AGES-R) Diabetologia. 2012;55(3):671–80. doi: 10.1007/s00125-011-2395-y. Epub 2011/12/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bjornstad P, Snell-Bergeon JK, Nadeau KJ, Maahs DM. Insulin sensitivity and complications in type 1 diabetes: New insights. World J Diabetes. 2015;6(1):8–16. doi: 10.4239/wjd.v6.i1.8. Epub 2015/02/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440(7086):944–8. doi: 10.1038/nature04634. Epub 2006/04/14. [DOI] [PubMed] [Google Scholar]
- 32.Bjornstad P, Snell-Bergeon J, Nadeau K, Maahs D. Insulin sensitivity and complications in type 1 diabetes: new insights. World J Diabetes. 2014 doi: 10.4239/wjd.v6.i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thorn LM, Forsblom C, Fagerudd J, Thomas MC, Pettersson-Fernholm K, Saraheimo M, et al. Metabolic syndrome in type 1 diabetes: association with diabetic nephropathy and glycemic control (the FinnDiane study) Diabetes care. 2005;28(8):2019–24. doi: 10.2337/diacare.28.8.2019. Epub 2005/07/27. [DOI] [PubMed] [Google Scholar]
- 34.Bjornstad P, Snell-Bergeon JK, Rewers M, Jalal D, Chonchol MB, Johnson RJ, et al. Early Diabetic Nephropathy: A complication of reduced insulin sensitivity in type 1 diabetes. Diabetes care. 2013 doi: 10.2337/dc13-0631. Epub 2013/09/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petrie JR. REducing With MetfOrmin Vascular Adverse Lesions in Type 1 Diabetes (REMOVAL) 2013 [Google Scholar]
- 36.Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. The New England journal of medicine. 2003;348(23):2285–93. doi: 10.1056/NEJMoa021835. Epub 2003/06/06. [DOI] [PubMed] [Google Scholar]
- 37.Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. The New England journal of medicine. 2009;360(24):2503–15. doi: 10.1056/NEJMoa0805796. Epub 2009/06/09. [DOI] [PMC free article] [PubMed] [Google Scholar]


