Abstract
IDH1 and IDH2 are homodimeric enzymes that catalyze the conversion of isocitrate to α-ketoglutarate (α-KG) and concomitantly produce reduced nicotinamide adenine dinucleotide phosphate (NADPH) from NADP+. Mutations in the genes encoding IDH1 and IDH2 have recently been found in a variety of human cancers, most commonly glioma, acute myeloid leukemia (AML), chondrosarcoma, and intrahepatic cholangiocarcinoma. The mutant protein loses its normal enzymatic activity and gains a new ability to produce the ‘oncometabolite’ R(−)-2-hydroxyglutarate (2HG). 2-HG competitively inhibits α-KG-dependent enzymes which play crucial roles in gene regulation and tissue homeostasis. Expression of mutant IDH impairs cellular differentiation in various cell lineages and promotes tumor development in cooperation with other cancer genes. First-generation inhibitors of mutant IDH have entered clinical trials and have shown encouraging results in patients with IDH2 mutant AML. This article summarizes recent progress in our understanding of the role of mutant IDH in tumorigenesis.
BACKGROUND
The first identification of a cancer-associated isocitrate dehydrogenase (IDH) mutation was in a patient with colorectal cancer, and emerged from one of the earliest comprehensive analyses of mutations in protein coding genes (1). Two years later, the same group reported a substantially higher frequency of IDH mutations (12%) when they applied whole genome sequencing to a small number of glioblastomas (GBMs), the most common malignant brain tumor in adults (2). Interestingly, the majority of IDH mutant GBMs (5/6) were from patients whose tumors had developed overtime from lower grade (WHO grade II and WHO grade III) tumors. This seminal finding was confirmed in a follow up study with a much larger number of tumors, which reported IDH mutations found in the vast majority (> 70%) of WHO grade II and WHO grade III gliomas (3).
Since these initial studies, many human cancers were examined for the presence of mutations in IDH1 and IDH2. IDH mutations were observed in a number of hematopoietic neoplasms, most commonly acute myeloid leukemia (AML) (~10–15%)(4–6) and angio-immunoblastic T-cell lymphoma (~20%)(7). IDH mutations were also found in chondrosarcoma (~50%)(8), intrahepatic cholangiocarcinoma (~15–20%)(9), and - at lower frequency (< 5%) – in other solid tumors (e.g., GBM, colorectal cancer, esophageal cancer, bladder cancer, melanoma, prostate carcinoma, breast adenocarcinoma)(10). Somatic heterozygous mutations in IDH1 or IDH2 were also found in the majority of enchondromas and spindle cell hemangiomas in patients with the Ollier disease and Maffuci syndrome, non-hereditary skeletal disorders (11).
More recent DNA resequencing projects have provided additional information regarding the timing of IDH mutations during tumor development. Analyzing over 300 gliomas, Watanabe et al. found that in 51 cases with multiple biopsies, neither acquisition of a mutation in TP53 nor loss of 1p/19q occurred prior to a mutation in IDH1 (12). Further analysis of matched biopsy pairs, collected from glioma patients at the initial diagnosis and the time of tumor recurrence showed that IDH1R132H was the only mutation that was consistently present in both the initial and recurrent biopsy specimen (13). In leukemia patients, IDH2 mutations were observed in the absence of NPM1c mutations in both mature and progenitor cell populations, suggesting that IDH2 mutation might be an early and perhaps pre-leukemic event (14, 15).
The vast majority of cancer-associated mutations in IDH1 and IDH2 map to an arginine within the catalytic pocket of the enzyme. Mutations in IDH1 mostly occur at arginine 132, with substitutions including R132H, R132C, R132L, R132S and R132G. Mutations in IDH2 typically occur at arginine 172 or arginine 140 (which is analogous to R132 in IDH1). The clustering of cancer-associated IDH mutations in the functional domain of the enzyme suggested that these mutations might endow the mutant protein with a novel and presumably oncogenic enzymatic activity. This question has been explored through untargeted metabolomic profiling of cells engineered to express the mutant enzyme. Compared to parental cells, cells expressing the IDH1R132H mutant enzyme were found to produce the R(−) enantiomer of the metabolite 2-hydroyglutarate (R-2-HG), which accumulates in IDH mutant human gliomas (16) and leukemias (5, 17). Production of R-2-HG involves direct conversion from α-KG and relies on the presence of a wild type allele (18), likely explaining the rareness of loss of heterozygosity.
The identification of an ‘oncometabolite” in IDH mutant tumors strengthened the hypothesis that IDH mutations are oncogenic, and led many investigators to examine the ability of mutant IDH to transform non-malignant cells. Expression of mutant Idh in mouse myeloid progenitor 32D cells and primary mouse bone marrow cells impaired hematopoietic differentiation and increased stem/progenitor cell marker expression, suggesting a pro-leukemogenic effect (19). A more recent study reported that retrovirally mediated expression of mutant Idh2R140Q in murine primary hematopoeitic bone marrow stem and progenitor cell populations induced myeloproliferative-like neoplasms, T-cell lymphoma or B-cell lymphoma when transplanted into irradiated mice (20). However, these hematological malignancies occurred at low penetrance and with long latency, suggesting that they did not arise solely due to mutant Idh2 expression. Expression of mutant Idh2 in a nontransformed mesenchymal multipotent mouse cell line (C3H, 10T) impaired their differentiation into adipocytic and chondrocytic lineages and resulted in loss of contact inhibition and tumor formation in vivo (21). In immortalized human astrocytes, expression of mutant IDH, but not wildtype IDH or a catalytically-inactive IDH mutant promoted their anchorage-independent growth (22).
Further insights into the role of mutant IDH in tumor initiation have emerged from experiments with genetically engineered mice. Tamoxifen-induced global expression of Idh2R140Q or R172K, driven from the chicken β-actin promoter, resulted in cardiomyopathy, white matter abnormalities throughout the central nervous system, and muscular dystrophy; mice engineered to express mutant Idh2 in specific tissues reportedly developed carcinomas with very long latencies (23). In another model, mice who expressed a doxycycline-inducible Idh2R140Q allele from the Collagen A1 locus did not develop leukemia, even after one year of continuous doxycycline treatment (24). The most common cancer-associated IDH mutation, IDH1R132H, has also been inserted into the endogenous murine Idh1 locus and expression of the mutant enzyme subsequently targeted to specific cell populations. Expression of Idh1R132H in hematopoietic cells (using the LysM and Vav promoters to express Cre-recombinase) resulted in increased numbers of hematopoietic progenitors, but no overt leukemia (25). Expression of Idh1R132H in nestin (Nes)-expressing neural stem cells resulted in perinatal lethality due to cerebral hemorrhage (26). Expression of Idh1R132H in GFAP-expressing astrocytes resulted in impaired collagen maturation and basement membrane function, but again no tumors. Tamoxifen-inducible expression of mutant Idh1 in chondrocytes (using the Col2A1 promoter to express Cre recombinase) resulted in enchondromas, benign cartilage tumors and precursors to malignant chondrosarcomas (27). Doxycycline-induced expression of mutant Idh2 (Idh2R140Q or Idh2R172K) increased hepatocyte proliferation in a liver injury-model, but was not sufficient to induce tumors (28).
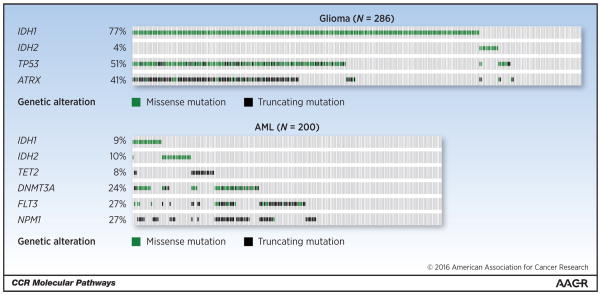
More recent studies have examined whether IDH mutations might collaborate with other genetic events to induce cancer in mice. Using a mouse transplantation assay, Chaturvedi et al. found that mutant IDH1 was not sufficient to transform hematopoietic cells but accelerated the onset of leukemia in cooperation with HoxA9 (29). Chen et al. applied a mosaic mouse modeling approach in which hematopoietic stem and progenitor cells (HPSC) from Flt3-ITD or NrasG12D mice were transduced with a retroviral vector expressing mutant Idh2 and then assessed for tumorigenic potential following transplantation into syngeneic recipient mice. Idh2 mutants were found to cooperate with Flt3 or Nras alleles to drive leukemia formation (30). Cooperativity between mutant Idh2R140Q and other leukemia-relevant pathway alterations (e.g. Flt3-ITD or homeobox proteins HoxA9 and Meis1a) was confirmed in the above-mentioned mouse model (24) and, more recently, another mosaic mouse modeling approach (31). Saha et al. showed that Idh2R172K cooperated with mutant KRAS (KrasG12D) to induce intrahepatic cholangio-carcinomas (28). In aggregate, these studies demonstrate that mutant IDH cooperates with other oncogenic events to initiate cancer, consistent with the finding that IDH mutant human cancers typically harbor alterations in multiple other cancer genes. In glioma, for example, IDH mutations are associated with missense mutations in ATRX, TP53, and TERT (diffuse astrocytomas) or co-deletion of chromosome arms 1p and 19q (oligodendrogliomas) (32, 33). In AML patients with a normal cytogenetic profile, IDH mutations are associated with mutations in nucleophosmin (NPM1), FLT3-ITD, and DNMT3A (34, 35) (Figure 1).
Figure 1. Co-occuring genetic lesions in IDH-mutant glioma and AML.
Oncoprint figure (generated using Memorial Sloan Kettering cBio Portal)(10) showing frequency of commonly co-occuring genetic lesions in glioma (top) and AML (bottom). Note that TET2 is shown to illustrate near complete mutual exclusivity with mutations in IDH1/2. Results are based upon data generated by the TCGA Research Network: Glioma data set (32); AML data set (73). For simplicity changes in copy number were not included.
TET2 - Tet Methylcytosine Dioxygenase 2; DNMT3A - DNA (Cytosine-5-)-Methyltransferase 3 Alpha; FLT3 - Fms-Related Tyrosine Kinase 3; NPM1 - Nucleophosmin (Nucleolar Phosphoprotein B23, Numatrin); TP53 - Tumor Protein P53; ATRX - Alpha Thalassemia/Mental Retardation Syndrome X-Linked.
Recent studies have begun to address the question whether the activity of the mutant IDH enzyme remains important for the growth of IDH mutant cancers once they are fully established. Studies in experimental cancer models suggest that this is indeed the case, with the strongest evidence coming from leukemia models. In TF-1 human erythroleukemia cells, ectopic expression of mutant IDH promotes growth factor independence and this phenotype can be reversed with a small molecule inhibitor of mutant IDH (36, 37). Ex-vivo treatment of freshly isolated IDH mutant leukemic blasts with a mutant selective IDH inhibitor induces a cellular differentiation program (36). Pharmacological inhibition of the mutant IDH enzyme blocks colony formation of human AML cells but not of normal CD34(+) bone marrow cells (29). In a genetically engineered leukemia model, pharmacologic or genetic inhibition of mutant Idh2 triggered the differentiation and death of AML cells (30), and doxycycline-induced silencing of mutant Idh2 similarly eliminated Idh2R140Q/Hoxa9 or Idh2R140Q/Meis1a-driven leukemia cells (24).
CLINICAL-TRANSLATIONAL ADVANCES
The above mentioned results in experimental leukemia models are supported by the preliminary findings of an ongoing Phase 1 clinical trial, which showed that the mutant IDH2 inhibitor AG-221 produces clinical responses, including complete and durable responses, in about 40% of patients with AML and MDS (38).
The contribution of the mutant enzyme for the maintenance of IDH mutant solid tumors remains currently unknown and further insights are likely to emerge from an ongoing single-arm dose escalation study (ClinicalTrials.gov NCT02073994) with the mutant-selective IDH1 inhibitor AG-120 (39). Data from experimental models suggest that IDH1-mutant solid tumors remain, at least in part, dependent on the activity of the mutant enzyme, In HT1080 human fibrosarcoma cells, RNAi-mediated suppression of endogenous mutant IDH1 significantly inhibited anchorage-independent growth (40). Knocking out the endogenous mutant IDH1 gene using TALEN technology similarly impaired anchorage-independent growth and in-vivo growth of IDH-mutant human sarcoma cells (41). In IDH1 mutant glioma cells, pharmacological blockade of the mutant enzyme retarded their growth in soft-agar and in mice (42).
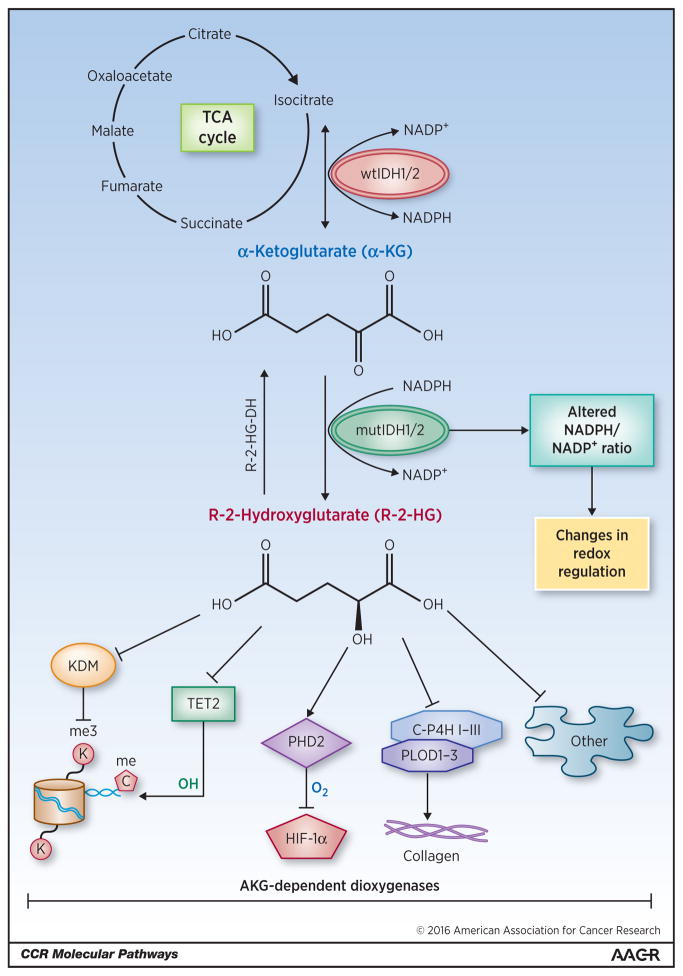
Targeted therapy of IDH mutant cancers is likely to grow beyond inhibition of the mutant enzyme itself and may include strategies directed against the epigenetic and metabolic changes that are associated with IDH mutations. Current data suggests that the R-2-HG ‘oncometabolite’ is responsible for many, if not all, biological effects of cancer-associated IDH mutations. Cell-permeable esters of R-2-HG phenocopy the effects of mutant IDH in a range of experimental models (43) and ectopic expression of the dehydrogenase (44) that converts R-2-HG into α-KG and thus counteracts the activity of the mutant IDH enzyme is sufficient to reverse the cellular effects of cancer-associated IDH mutants (45). R-2-HG competitively inhibits a large family of α-KG-dependent enzymes, a protein family with over 60 members. These include the ten-eleven translocation (TET) family of 5-methyl cytosine hydroxylases, the jumonji domain containing (JmjC) family of histone lysine demethylases (46, 47), enzymes involved in nucleic acid metabolism (AlkB, FTO), and many enzymes with still unknown functions (48) (Figure 2).
Figure 2. Molecular mechanisms of IDH-associated tumorigenesis.
Wild-type IDH1/2 (wtIDH1/2) converts isocitrate (generated through the citric acid (TCA) cycle) into α-KG, producing NADPH in the process. Mutant IDH1/2 (mutIDH1/2) converts α-KG to the oncometabolite R-2-HG, consuming NADPH. R-2-HG inhibits members of the protein family of α-KG-dependent dioxygenases. Epigenetic modifications result from inhibition of histone lysine demethylases (KDMs) and the 5-methyl cytosine hydroxylase TET2. Inhibition of prolyl-hydroxylation impairs collagen maturation. R-2-HG has been reported to activate the enzyme prolyl-hydroxylase 2 (PHD2) that inhibits hypoxia-inducible factor 1-alpha (HIF-1α) (22), although other studies suggest that it may be inhibited (46, 47). Mutant IDH may also change the cellular redox environment by altering the ratio of NADPH to NADP+. IDH3 has been omitted from this figure for simplicity.
It is currently unknown which α-KG-dependent enzymes function as context-dependent tumor suppressors and are inhibited at the relevant R-2-HG concentrations in human tumors. Inhibition of TET2 is likely to mediate the effects of mutant IDH in AML, given the well documented role of TET2 in hematopoietic differentiation, the almost completely mutual exclusivity of TET2 and IDH mutations in AML (Figure 1), the decreased 5-hydroxymethylcytosine levels in IDH mutant AMLs, and the identification of TET2 as candidate effector of mutant IDH in a short hairpin RNA library screen of α-KG-dependent dioxygenases (19, 37, 49–51). Inhibition of the histone demethylases JMJD2A and JMJD2C likely contributes to the effects of mutant IDH on cellular differentiation in many cell lineages. These enzymes are particularly sensitive to inhibition by R-2-HG (46, 47), and knockdown of Jmjd2C (also known as Kdm4c) was sufficient to phenocopy the effects of mutant Idh on adipocyte differentiation in 3T3-L1 cells (43). In fact, it seems plausible than many hallmarks of mutant IDH-associated human malignancies, such as restricted cellular differentiation, DNA hypermethylation result from coordinate effects of R-2-HG on DNA and histone methylation (43, 52). These findings raise the intriguing question of whether IDH mutant cancers cells might show an increased sensitivity to the DNA methyltransferase inhibitors (DNMTIs) decitabine or 5-azacytidine (53–55) or drugs targeting histone modifications.
It is less clear whether epigenetic alterations are also responsible for the oncogenic effects of mutant IDH in solid tumors. We observed that growth inhibition of IDH1 mutant glioma xenografts by a mutant selective IDH1 inhibitor was not clearly linked to epigenetic changes in tumor tissue (42). Similarly, ectopic expression of the R-2-HG-dehydrogenase in HT1080 sarcoma cells effectively inhibited their growth in vivo without clear changes in histone methylation marks or TET2-induced 5-hmC production (41). It is plausible that the oncogenic effects of mutant IDH in solid tumors are linked to metabolic alterations associated with IDH mutations including reductions in α-KG levels, changes in the NADP+/NADPH ratio and mitochondrial bioenergetics, and an impaired ability of the IDH enzyme to catalyze the reverse carboxylation of α-KG to form isocitrate. These metabolic changes can have a profound effect on the ability of cells to proliferate, differentiate, and escape cell death, and may introduce therapeutic vulnerabilities (56–64). Recent studies reported an enhanced susceptibility of IDH1 mutant cancers to inhibitors of BCL-2 (63), due to the effect of mutant IDH on mitochondrial bioenergetics, or to depletion of the coenzyme NAD+ (65).
The success of therapeutic strategies to curb the growth of IDH mutant cancers will hinge on a deeper understanding of the molecular mechanisms of IDH-associated tumorigenesis and R-2-HG effector pathways in each cancer type. In addition to the epigenetic and metabolic effects of mutant IDH outlined above, the (R)-enantiomer of 2-hydroxyglutarate has been reported to stimulate the activity of the EGLN prolyl 4-hydroxylases, leading to diminished levels of hypoxia-inducible transcription factor (HIF) and enhanced soft agar growth of human astrocytes (66). Consistent with this result, the expression of HIF1α-responsive genes, including many essential for glycolysis appears to be lower in IDH mutant gliomas (67), perhaps explaining the often poor retention of the radiotracer 2-[(18)F] Fluoro-2-deoxy-D-glucose (FDG) by these tumors. The group of α-KG-dependent dioxygenases also includes enzymes that hydroxylate proline and lysine residues in collagen and promote its stability. It is intriguing to speculate that the observed brain hemorrhages in Nes-Cre Idh1R132H mice might have resulted from impaired collagen maturation and basement membrane architecture (68).
A remarkable body of knowledge has been generated in the few years since the first description of cancer associated IDH mutants and the future seems bright for the development of rationale therapeutic approaches of IDH mutant cancers. The ability to monitor the activity of the mutant IDH enzyme through quantification of 2-HG levels in blood (69) or Magnetic Resonance Spectroscopy in tumors (70–72) will be instrumental to develop such strategies.
Acknowledgments
GRANT SUPPORT
Research reported in this publication was supported by the NIH under award numbers R01NS080944 (to I.K. Mellinghoff), T32CA160001 (to O. Clark), and P30CA008748 (to O. Clark and I.K. Mellinghoff, through their institution). I.K. Mellinghoff was supported by the Ben and Catherine Ivy Foundation and the Defeat GBM Research Collaborative of the National Brain Tumor Society.
The authors wish to thank Dr. Sara Kubek for her critical input to graphic illustration, as well Drs. Donna Nichol and Paolo Codega for help and advice with the manuscript.
Footnotes
Disclosure of Potential Conflicts of Interest
K. Yen has ownership interest (including patents) in Agios Pharmaceuticals. No potential conflicts of interest were disclosed by the other authors.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–74. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 2.Parsons DW, Jones S, Zhang X, Lin JCH, Leary RJ, Angenendt P, et al. An Integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–12. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. New England Journal of Medicine. 2009;360:765–73. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mardis ER, Ding L, Dooling DJ, Larson DE, McLellan MD, Chen K, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. The New England journal of medicine. 2009;361:1058–66. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gross S, Cairns RA, Minden MD, Driggers EM, Bittinger MA, Jang HG, et al. Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. The Journal of experimental medicine. 2010;207:339–44. doi: 10.1084/jem.20092506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcucci G, Maharry K, Wu YZ, Radmacher MD, Mrozek K, Margeson D, et al. IDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. Journal of clinical oncology. 2010;28:2348–55. doi: 10.1200/JCO.2009.27.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cairns RA, Iqbal J, Lemonnier F, Kucuk C, de Leval L, Jais J-P, et al. IDH2 mutations are frequent in angioimmunoblastic T-cell lymphoma. Blood. 2012;119:1901–3. doi: 10.1182/blood-2011-11-391748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amary MF, Bacsi K, Maggiani F, Damato S, Halai D, Berisha F, et al. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. The Journal of pathology. 2011;224:334–43. doi: 10.1002/path.2913. [DOI] [PubMed] [Google Scholar]
- 9.Borger DR, Tanabe KK, Fan KC, Lopez HU, Fantin VR, Straley KS, et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. The Oncologist. 2012;17:72–9. doi: 10.1634/theoncologist.2011-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer discovery. 2012;2:401–4. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pansuriya TC, van Eijk R, d’Adamo P, van Ruler MA, Kuijjer ML, Oosting J, et al. Somatic mosaic IDH1 and IDH2 mutations are associated with enchondroma and spindle cell hemangioma in Ollier disease and Maffucci syndrome. Nature genetics. 2011;43:1256–61. doi: 10.1038/ng.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe T, Nobusawa S, Kleihues P, Ohgaki H. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. The American Journal of Pathology. 2010;174:1149–53. doi: 10.2353/ajpath.2009.080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson BE, Mazor T, Hong C, Barnes M, Aihara K, McLean CY, et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343:189–93. doi: 10.1126/science.1239947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corces-Zimmerman MR, Hong WJ, Weissman IL, Medeiros BC, Majeti R. Preleukemic mutations in human acute myeloid leukemia affect epigenetic regulators and persist in remission. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:2548–53. doi: 10.1073/pnas.1324297111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shlush LI, Zandi S, Mitchell A, Chen WC, Brandwein JM, Gupta V, et al. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature. 2014:1–13. doi: 10.1038/nature13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–44. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward PS, Patel J, Wise DR, Abdel-Wahab O, Bennett BD, Coller HA, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer cell. 2010;17:225–34. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin G, Reitman ZJ, Duncan CG, Spasojevic I, Gooden DM, Rasheed BA, et al. Disruption of wild-type IDH1 suppresses D-2-hydroxyglutarate production in IDH1-mutated gliomas. Cancer Research. 2013;73:496–501. doi: 10.1158/0008-5472.CAN-12-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Figueroa ME, Abdel-Wahab O, Lu C, Ward PS, Patel J, Shih A, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer cell. 2010;18:553–67. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mylonas E, Janin M, Bawa O, Opolon P, David M, Quivoron C, et al. Isocitrate dehydrogenase (IDH)2 R140Q mutation induces myeloid and lymphoid neoplasms in mice. 2014:1–3. doi: 10.1038/leu.2014.18. [DOI] [PubMed] [Google Scholar]
- 21.Lu C, Lu C, Venneti S, Venneti S, Akalin A, Akalin A, et al. Induction of sarcomas by mutant IDH2. Genes Development. 2013;27:1986–98. doi: 10.1101/gad.226753.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koivunen P, Koivunen P, Lee S, Lee S, Duncan CG, Duncan CG, et al. Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature. 2012:1–7. doi: 10.1038/nature10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akbay EA, Moslehi J, Christensen CL, Saha S, Tchaicha JH, Ramkissoon SH, et al. D-2-hydroxyglutarate produced by mutant IDH2 causes cardiomyopathy and neurodegeneration in mice. Genes Development. 2014;28:479–90. doi: 10.1101/gad.231233.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kats LM, Reschke M, Taulli R, Pozdnyakova O, Burgess K, Bhargava P, et al. Proto-oncogenic role of mutant IDH2 in leukemia initiation and maintenance. Stem Cell. 2014:1–13. doi: 10.1016/j.stem.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sasaki M, Knobbe CB, Munger JC, Lind EF, Brenner D, Brustle A, et al. IDH1(R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature. 2012:1–7. doi: 10.1038/nature11323. [DOI] [PMC free article] [PubMed]
- 26.Sasaki M, Sasaki M, Knobbe CB, Knobbe CB, Itsumi M, Itsumi M, et al. D-2-hydroxyglutarate produced by mutant IDH1 perturbs collagen maturation and basement membrane function. Genes Development. 2012;26:2038–49. doi: 10.1101/gad.198200.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirata M, Sasaki M, Cairns RA, Inoue S, Puviindran V, Li WY, et al. Mutant IDH is sufficient to initiate enchondromatosis in mice. Proceedings of the National Academy of Sciences. 2015;112:2829–34. doi: 10.1073/pnas.1424400112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saha SK, Parachoniak CA, Ghanta KS, Fitamant J, Ross KN, Najem MS, et al. Mutant IDH inhibits HNF-4α to block hepatocyte differentiation and promote biliary cancer. Nature. 2014:1–18. doi: 10.1038/nature13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaturvedi A, Araujo Cruz MM, Jyotsana N, Sharma A, Yun H, Gorlich K, et al. Mutant IDH1 promotes leukemogenesis in vivo and can be specifically targeted in human AML. Blood. 2013;122:2877–87. doi: 10.1182/blood-2013-03-491571. [DOI] [PubMed] [Google Scholar]
- 30.Chen C, Chen C, Liu Y, Liu Y, Lu C, Lu C, et al. Cancer-associated IDH2 mutants drive an acute myeloid leukemia that is susceptible to Brd4 inhibition. Genes Development. 2013;27:1974–85. doi: 10.1101/gad.226613.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogawara Y, Katsumoto T, Aikawa Y, Shima Y, Kagiyama Y, Soga T, et al. IDH2 and NPM1 mutations cooperate to activate Hoxa9/Meis1 and hypoxia pathways in acute myeloid leukemia. Cancer research. 2015;75:2005–16. doi: 10.1158/0008-5472.CAN-14-2200. [DOI] [PubMed] [Google Scholar]
- 32.Cancer Genome Atlas Research N. Brat DJ, Verhaak RG, Aldape KD, Yung WK, Salama SR, et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. The New England journal of medicine. 2015;372:2481–98. doi: 10.1056/NEJMoa1402121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eckel-Passow JE, Lachance DH, Molinaro AM, Walsh KM, Decker PA, Sicotte H, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. The New England journal of medicine. 2015;372:2499–508. doi: 10.1056/NEJMoa1407279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paschka P, Schlenk RF, Gaidzik VI, Habdank M, Kronke J, Bullinger L, et al. IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with NPM1 mutation without FLT3 internal tandem duplication. Journal of clinical oncology. 2010;28:3636–43. doi: 10.1200/JCO.2010.28.3762. [DOI] [PubMed] [Google Scholar]
- 35.DiNardo CD, Ravandi F, Agresta S, Konopleva M, Takahashi K, Kadia T, et al. Characteristics, clinical outcome, and prognostic significance of IDH mutations in AML. American journal of hematology. 2015;90:732–6. doi: 10.1002/ajh.24072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang F, Travins J, DeLaBarre B, Penard-Lacronique V, Schalm S, Hansen E, et al. Targeted inhibition of mutant IDH2 in leukemia cells induces cellular differentiation. Science. 2013;340:622–6. doi: 10.1126/science.1234769. [DOI] [PubMed] [Google Scholar]
- 37.Losman JA, Looper RE, Koivunen P, Lee S, Schneider RK, McMahon C, et al. (R)-2-hydroxyglutarate is sufficient to promote leukemogenesis and its effects are reversible. Science. 2013;339:1621–5. doi: 10.1126/science.1231677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DiNardo C, Stein EM, Altman JK, Collins R, DeAngelo DJ, Fathi AT, et al. AG-221, an oral, selective, first-in-class, potent inhibitor of the IDH2 mutant enzyme, induced durable responses in a phase 1 study of IDH2 mutation-positive advanced hematologic malignancies [abstract]. Proceedings of the 20th Congress of the European Hematology Association; 2015 June 11–14; Vienna, Austria. The Hague (The Netherlands): EHA; 2015. Abstract nr P569. [Google Scholar]
- 39.Burris H, Mellinghoff IK, Maher E, Wen PY, Beeram M, Touat M, et al. The first reported results of AG-120, a first-in-class, potent inhibitor of the IDH1 mutant protein, in a phase 1 study of patients with advanced IDH1-mutant solid tumors, including gliomas [abstract]. Mol Cancer Ther; Proceedings of the AACR-NCI-EORTC International Conference: Molecular Targets and Cancer Therapeutics; 2015 Nov 5–9; Boston, MA. Philadelphia (PA): AACR; 2015. p. Abstract nr PL04–05. [Google Scholar]
- 40.Jin G, Pirozzi CJ, Chen LH, Lopez GY, Duncan CG, Feng J, et al. Mutant IDH1 is required for IDH1 mutated tumor cell growth. Oncotarget. 2012;3:774–82. doi: 10.18632/oncotarget.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma S, Jiang B, Deng W, Gu ZK, Wu FZ, Li T, et al. D-2-hydroxyglutarate is essential for maintaining oncogenic property of mutant IDH-containing cancer cells but dispensable for cell growth. Oncotarget. 2015;6:8606–20. doi: 10.18632/oncotarget.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rohle D, Popovici-Muller J, Palaskas N, Turcan S, Grommes C, Campos C, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science. 2013;340:626–30. doi: 10.1126/science.1236062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu C, Ward PS, Kapoor GS, Rohle D, Turcan S, Abdel-Wahab O, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483:474–8. doi: 10.1038/nature10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Achouri Y, Noel G, Vertommen D, Rider MH, Veiga-Da-Cunha M, Van Schaftingen E. Identification of a dehydrogenase acting on D-2-hydroxyglutarate. The Biochemical journal. 2004;381:35–42. doi: 10.1042/BJ20031933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reitman ZJ, Sinenko SA, Spana EP, Yan H. Genetic dissection of leukemia-associated IDH1 and IDH2 mutants and D-2-hydroxyglutarate in Drosophila. Blood. 2015;125:336–45. doi: 10.1182/blood-2014-05-577940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chowdhury R, Yeoh KK, Tian YM, Hillringhaus L, Bagg EA, Rose NR, et al. The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO reports. 2011;12:463–9. doi: 10.1038/embor.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim SH, et al. Oncometabolite 2-Hydroxyglutarate Is a Competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer cell. 2011;19:17–30. doi: 10.1016/j.ccr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loenarz C, Schofield CJ. Physiological and biochemical aspects of hydroxylations and demethylations catalyzed by human 2-oxoglutarate oxygenases. Trends in biochemical sciences. 2011;36:7–18. doi: 10.1016/j.tibs.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 49.Delhommeau F, Dupont S, Della Valle V, James C, Trannoy S, Masse A, et al. Mutation in TET2 in myeloid cancers. The New England journal of medicine. 2009;360:2289–301. doi: 10.1056/NEJMoa0810069. [DOI] [PubMed] [Google Scholar]
- 50.Moran-Crusio K, Reavie L, Shih A, Abdel-Wahab O, Ndiaye-Lobry D, Lobry C, et al. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer cell. 2011;20:11–24. doi: 10.1016/j.ccr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kroeze LI, Aslanyan MG, van Rooij A, Koorenhof-Scheele TN, Massop M, Carell T, et al. Characterization of acute myeloid leukemia based on levels of global hydroxymethylation. Blood. 2014;124:1110–8. doi: 10.1182/blood-2013-08-518514. [DOI] [PubMed] [Google Scholar]
- 52.Noushmehr H, Weisenberger DJ, Diefes K, Phillips HS, Pujara K, Berman BP, et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer cell. 2010;17:510–22. doi: 10.1016/j.ccr.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turcan S, Fabius AW, Borodovsky A, Pedraza A, Brennan C, Huse J, et al. Efficient induction of differentiation and growth inhibition in IDH1 mutant glioma cells by the DNMT inhibitor decitabine. Oncotarget. 2013;4:1729–36. doi: 10.18632/oncotarget.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borodovsky A, Salmasi V, Turcan S, Fabius AW, Baia GS, Eberhart CG, et al. 5-azacytidine reduces methylation, promotes differentiation and induces tumor regression in a patient-derived IDH1 mutant glioma xenograft. Oncotarget. 2013;4:1737–47. doi: 10.18632/oncotarget.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Flavahan WA, Drier Y, Liau BB, Gillespie SM, Venteicher AS, Stemmer-Rachamimov AO, et al. Insulator dysfunction and oncogene activation in IDH mutant gliomas. Nature. 2016;529:110–4. doi: 10.1038/nature16490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cairns RA, Mak TW. Oncogenic isocitrate dehydrogenase mutations: mechanisms, models, and clinical opportunities. Cancer discovery. 2013;3:730–41. doi: 10.1158/2159-8290.CD-13-0083. [DOI] [PubMed] [Google Scholar]
- 57.Parker SJ, Metallo CM. Metabolic consequences of oncogenic IDH mutations. Pharmacology & therapeutics. 2015;152:54–62. doi: 10.1016/j.pharmthera.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reitman ZJ, Jin G, Karoly ED, Spasojevic I, Yang J, Kinzler KW, et al. Profiling the effects of isocitrate dehydrogenase 1 and 2 mutations on the cellular metabolome. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3270–5. doi: 10.1073/pnas.1019393108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oizel K, Gratas C, Nadaradjane A, Oliver L, Vallette FM, Pecqueur C. D-2-Hydroxyglutarate does not mimic all the IDH mutation effects, in particular the reduced etoposide-triggered apoptosis mediated by an alteration in mitochondrial NADH. Cell death & disease. 2015;6:e1704. doi: 10.1038/cddis.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shi J, Sun B, Shi W, Zuo H, Cui D, Ni L, et al. Decreasing GSH and increasing ROS in chemosensitivity gliomas with IDH1 mutation. Tumour biology. 2015;36:655–62. doi: 10.1007/s13277-014-2644-z. [DOI] [PubMed] [Google Scholar]
- 61.Carey BW, Finley LW, Cross JR, Allis CD, Thompson CB. Intracellular alpha-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature. 2015;518:413–6. doi: 10.1038/nature13981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Izquierdo-Garcia JL, Viswanath P, Eriksson P, Cai L, Radoul M, Chaumeil MM, et al. IDH1 mutation induces reprogramming of pyruvate metabolism. Cancer research. 2015;75:2999–3009. doi: 10.1158/0008-5472.CAN-15-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chan SM, Thomas D, Corces-Zimmerman MR, Xavy S, Rastogi S, Hong WJ, et al. Isocitrate dehydrogenase 1 and 2 mutations induce BCL-2 dependence in acute myeloid leukemia. Nature medicine. 2015;21:178–84. doi: 10.1038/nm.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leonardi R, Subramanian C, Jackowski S, Rock CO. Cancer-associated isocitrate dehydrogenase mutations inactivate NADPH-dependent reductive carboxylation. The Journal of biological chemistry. 2012;287:14615–20. doi: 10.1074/jbc.C112.353946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tateishi K, Wakimoto H, Iafrate AJ, Tanaka S, Loebel F, Lelic N, et al. Extreme vulnerability of IDH1 mutant cancers to NAD+ depletion. Cancer cell. 2015;28:773–84. doi: 10.1016/j.ccell.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koivunen P, Lee S, Duncan CG, Lopez G, Lu G, Ramkissoon S, et al. Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature. 2012;483:484–8. doi: 10.1038/nature10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chesnelong C, Chaumeil MM, Blough MD, Al-Najjar M, Stechishin OD, Chan JA, et al. Lactate dehydrogenase A silencing in IDH mutant gliomas. Neuro-oncology. 2014;16:686–95. doi: 10.1093/neuonc/not243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sasaki M, Knobbe CB, Itsumi M, Elia AJ, Harris IS, Chio II, et al. D-2-hydroxyglutarate produced by mutant IDH1 perturbs collagen maturation and basement membrane function. Genes & development. 2012;26:2038–49. doi: 10.1101/gad.198200.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fathi AT, Sadrzadeh H, Borger DR, Ballen KK, Amrein PC, Attar EC, et al. Prospective serial evaluation of 2-hydroxyglutarate, during treatment of newly diagnosed acute myeloid leukemia, to assess disease activity and therapeutic response. Blood. 2012;120:4649–52. doi: 10.1182/blood-2012-06-438267. [DOI] [PubMed] [Google Scholar]
- 70.Choi C, Ganji SK, DeBerardinis RJ, Hatanpaa KJ, Rakheja D, Kovacs Z, et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nature medicine. 2012;18:624–9. doi: 10.1038/nm.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Andronesi OC, Loebel F, Bogner W, Marjanska M, Vander Heiden MG, Iafrate AJ, et al. Treatment response assessment in IDH-mutant glioma patients by non-invasive 3D functional spectroscopic mapping of 2-hydroxyglutarate. Clinical cancer research. 2015 Nov 3; doi: 10.1158/1078-0432.CCR-15-0656. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de la Fuente MI, Young RJ, Rubel J, Rosenblum M, Tisnado J, Briggs S, et al. Integration of 2-hydroxyglutarate-proton magnetic resonance spectroscopy into clinical practice for disease monitoring in isocitrate dehydrogenase-mutant glioma. Neuro-oncology. 2015 Dec 20; doi: 10.1093/neuonc/nov307. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cancer Genome Atlas Research Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. The New England journal of medicine. 2013;368:2059–74. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]