Abstract
Background
Exercise intolerance is a hallmark symptom of heart failure patients with preserved ejection fraction (HFpEF), which may be related to an impaired ability to appropriately increase blood flow to the exercising muscle.
Methods
We evaluated leg blood flow (LBF, ultrasound Doppler), heart rate (HR), stroke volume (SV), cardiac output (CO), and mean arterial blood pressure (MAP, photoplethysmography) during dynamic, single leg knee-extensor (KE) exercise in HFpEF patients (n = 21; 68 ± 2 yrs) and healthy controls (n = 20; 71 ± 2 yrs).
Results
HFpEF patients exhibited a marked attrition during KE exercise, with only 60% able to complete the exercise protocol. In participants who completed all exercise intensities (0-5-10-15W; HFpEF, n = 13; Controls, n = 16), LBF was not different at 0W and 5W, but was 15-25% lower in HFpEF compared to controls at 10W and 15W (P < 0.001). Likewise, leg vascular conductance (LVC), an index of vasodilation, was not different at 0W and 5W, but was 15-20% lower in HFpEF compared to controls at 10W and 15W (P < 0.05). In contrast to these peripheral deficits, exercise-induced changes in central variables (HR, SV, CO), as well as MAP, were similar between groups.
Conclusions
These data reveal a marked reduction in LBF and LVC in HFpEF patients during exercise that cannot be attributed to a disease-related alteration in central hemodynamics, suggesting that impaired vasodilation in the exercising skeletal muscle vasculature may play a key role in the exercise intolerance associated with this patient population.
Keywords: Heart Failure, Blood Flow, Exercise, Vasodilation, HFpEF
INTRODUCTION
In the U.S. alone, heart failure (HF) afflicts over 5 million people (1) and places a considerable burden on the health care system, at a cost exceeding $30 billion annually (2). Although HF has traditionally been associated with reduced ejection fraction (HFrEF), greater than one-half of HF patients actually exhibit normal or “preserved” ejection fraction (HFpEF) (3,4). Interestingly, the prognosis for HFpEF patients is similar to that of HFrEF, (3,5), but in contrast to HFrEF, there is no optimal treatment strategy for HFpEF patients (6,7), and there have been no improvement in clinical outcomes in this cohort over the past two decades (5). Given that the prevalence of HFpEF continues to rise at a rate of 1% per year relative to HFrEF (5), this represents an ever-increasing public health issue.
Severe exercise intolerance is a hallmark symptom of HF, and previous studies have documented similar magnitudes of exercise intolerance in HFpEF and HFrEF (8); however, unlike HFrEF, the mechanisms underlying exercise intolerance in HFpEF have not been thoroughly investigated (9). Clearly, cardiac abnormalities including increased left ventricular stiffness and the associated elevation in chamber filling pressures (10,11) may contribute to the symptoms of exercise intolerance in HFpEF (12), particularly during whole body dynamic exercise (13). However, a growing number of studies have reported minimal impairments in central hemodynamics during exercise in HFpEF, implicating peripheral, non-cardiac mechanisms as important contributors to exercise intolerance in this cohort (14-16). Indeed, there is emerging evidence supportive of disease-related changes in skeletal muscle fiber type composition and resultant alterations in muscle function in HFpEF patients (14,15), in line with the proposed systemic nature of HFpEF pathophysiology. In addition, exercise training studies have reported improvements in peak O2 consumption in the absence of changes in central hemodynamics in HFpEF (17,18), further illustrating the extent to which non-cardiac mechanisms may contribute to exercise intolerance in this cohort.
Considering this evidence of peripheral dysfunction in HFpEF, it is somewhat surprising how few studies to date have examined disease-related changes in the regulation of skeletal muscle blood flow during exercise. Puntawangkoon et al. (19) observed a reduction in superficial femoral artery blood flow upon cessation of supine cycling exercise in HFpEF patients compared to controls despite similar flow in the ascending and descending aorta, suggesting impaired distribution of cardiac output (CO) in the HFpEF group. More recently, Borlaug et al. (20) reported an impaired reduction in systemic vascular resistance during submaximal cycling exercise in HFpEF patients compared to hypertensive control patients, suggesting a disease-related change in “vasodilatory reserve”. However, to our knowledge, no studies to date have attempted direct measurements of blood flow in the exercising muscle of HFpEF patients.
Also noteworthy is the fact that each of these previous studies (19,20) utilized cycle ergometry exercise, a modality that induces significant cardiopulmonary stress and therefore makes difficult the task of isolating central and peripheral contributions to perfusion of the exercising limbs. This limitation may be overcome through use of knee-extensor (KE) exercise, a small muscle mass model that does not provoke significant cardiopulmonary stress (21). While members of our group (22-24) and others (25,26) have utilized KE exercise to examine the regional regulation of exercising leg blood flow in HFrEF, this exercise model has not been employed to examine peripheral hemodynamics in the HFpEF patient population. In view of the well-defined relationship between blood flow, O2 uptake, and exercise capacity (27,28), disease-related changes in the regulation of skeletal muscle blood flow may be an important contributor to exercise intolerance in this patient group.
Therefore, using the small muscle mass KE exercise paradigm, we sought to evaluate exercise-induced changes in central and peripheral hemodynamics in HFpEF patients compared to healthy controls. We hypothesized that exercise-induced increases in cardiac output would be similar between groups, but that vasodilation in the active skeletal muscle would be attenuated in HFpEF patients compared to controls. If proven correct, such findings could have significant implications for our understanding of exercise intolerance in this growing patient population.
METHODS
Participants
HFpEF patients were recruited from the HF clinics at the University of Utah and the Salt Lake City Veterans Affairs Medical Center (VAMC), and healthy controls were recruited from the greater Salt Lake City community. Patient inclusion criteria were consistent with the TOPCAT trial (29), which is as follows; (1) HF defined by the presence of ≥1 symptom at the time of screening (paroxysmal nocturnal dyspnea, orthopnea, dyspnea on exertion) and 1 sign (edema, elevation in jugular venous distention) in the previous 12 months; (2) LVEF ≥45%; (3) controlled systolic blood pressure; and (4) either ≥1 hospitalization in the previous 12 months for which HF was a major component of hospitalization, or B-type natriuretic peptide (BNP) in the previous 60 days ≥100 pg/mL. Exclusion criteria for the HFpEF group included significant valvular heart disease, acute atrial fibrillation, body mass index (BMI) >45, and any orthopedic limitations that would prevent performance of KE exercise. For the control group, participants were not taking any prescription medications and were free of overt cardiovascular disease, as indicated by a health history. All participants were non-smokers. All procedures were approved by The University of Utah and the Salt Lake City VAMC Institutional Review Boards, and studies were performed at the VA Salt Lake City Geriatric Research, Education, and Clinical Center in the Utah Vascular Research Laboratory.
Protocols
On the experimental day, participants reported to the laboratory approximately 8 hours postprandial, and a fasting glucose and lipid panel was performed on blood drawn from an antecubital vein in all participants using standard methods. Data collection took place in a thermoneutral environment with participants in the semi-recumbent position (~60° reclined). A subset of patients (n = 10) returned to the laboratory within two weeks of the experimental day for 6-min walk (6MW) test (30) and gait speed (31) assessments to characterize functional capacity. The 6MW has been shown to predict survival and peak O2 consumption in heart failure patients (30), and gait speed is associated with survival in aged humans (31).
Knee-Extensor Exercise
The KE exercise paradigm utilized in this investigation has been described previously in detail (21,32,33). Briefly, participants sat in a semi-recumbent position on an adjustable chair in front of a modified cycle ergometer (model 828E; Monark Exercise AB, Vansbro, Sweden). A custom made metal boot, connected by a metal bar to the crank arm of the ergometer, held the subject’s foot and lower leg, enabling participants to turn the crank of the ergometer by extending their leg. Resistance was applied directly to the flywheel to elicit incremental work rates [0 (i.e. unweighted), 5, 10, and 15 W, 1 Hz]. Participants exercised for 3 min at each level, and each exercise bout was separated by a 3 min recovery period.
Measurements
Ultrasound Doppler
Blood velocity and vessel diameter of the common femoral artery were determined in the exercising leg using a Logiq 7 ultrasound Doppler system (GE Medical Systems, Milwaukee, WI). The artery was insonated 2 to 3 cm proximal to the bifurcation of the superficial and deep branches. Blood velocity was collected at a Doppler frequency of 5 MHz in high-pulsed repetition frequency mode (2 to 25kHz). Sample volume was optimized in relation to vessel diameter and centered within the vessel. Vessel diameter was obtained during end diastole (corresponding to an R wave documented by the simultaneous ECG signal; Logic 7) using the same transducer at an imaging frequency ranging from 9 to 14 MHz. An angle of insonation of ≤ 60 degrees (34) was achieved for all measurements. Commercially available software (Logic 7) was used to calculate arterial diameter as well as angle-corrected, time-averaged, and intensity-weighted mean blood velocity (Vmean). LBF was calculated with the formula: LBF (ml/min) = (Vmean × π (vessel diameter/2)2 × 60) and leg vascular conductance (LVC) was calculated as: LVC (ml/min/mmHg) = LBF / mean arterial pressure (MAP).
Central Cardiovascular Variables
Heart Rate (HR) was monitored continuously from a standard three-lead ECG recorded in duplicate on the data acquisition device (Biopac, Goleta, CA, U.S.A.) and the Logiq 7. HR and beat-to-beat arterial blood pressure was determined non-invasively using photoplethysmography (Finapres Medical Systems BV, Amsterdam, The Netherlands). Using the arterial waveform, SV was calculated using the Modelflow method (Beatscope version 1.1; Finapres Medical Systems BV, Amsterdam, The Netherlands) (35), which has been documented to accurately track SV during a variety of experimental protocols, including exercise (36-39). In heart disease patients, this methodology has been shown to accurately track changes in cardiac output in <95% of cases when compared to the conventional thermodilution technique (40). MAP was calculated as: MAP (mmHg) = diastolic arterial pressure + (pulse pressure · 0.33). CO was calculated as: CO (L/min) = SV × HR. Using the Du Bois formula, body surface area (BSA) was calculated as: BSA = 0.007184 × Weight (W)0.425 × Height (H)0.725. Using BSA, stroke index (SI) and cardiac index (CI) were calculated as: SI (ml/min/m2) = SV / BSA and CI (L/min/m2) = CO / BSA.
Physical Activity
Daily physical activity was assessed in a subset of patients (n = 10) and healthy controls (n = 11) with an accelerometer (GT1M; Actigraph, Pensacola, FL) that participants wore continuously for 7 days. Previous work has demonstrated that this accelerometer is accurate and provides a reliable estimation of daily physical activity (41,42).
Data Analysis and Statistical Approach
All statistical analysis was performed using commercially available software (SigmaStat 3.10, Systat Software, Point Richmond, CA). A Student’s unpaired t-test was used to determine mean differences for subject characteristics and resting cardiovascular and hemodynamic variables, and a two-way ANOVA was used to compare differences between groups across exercise intensities. When a significant main effect was observed, a Holm-Sidak post hoc analysis was performed. Group data are presented as mean ± SEM, and exact P-values are reported unless otherwise noted.
RESULTS
Subject Characteristics
Anthropometric data and general characteristics for both patients and controls are shown in Table 1. Patients and healthy controls were well-matched for age and sex. HFpEF patients were similar in stature, but had both a greater body mass and a body mass index compared to healthy controls. Disease-specific characteristics as well as pharmacological therapeutic information for the HFpEF patients are shown in Table 2. The patients displayed characteristics common to the HFpEF phenotype including advanced age, obesity, diabetes, and exercise intolerance [determined by the 6MW distance and New York Heart Association (NYHA) classification], and were on standard pharmaceutical therapies. Resting diastolic function and cardiac dimensions assessed by Doppler and echocardiographic techniques (43) were also consistent with the HFpEF phenotype.
Table 1. Subject characteristics.
| Controls | HFpEF | |
|---|---|---|
| Age (yrs) | 71 ± 2 | 68 ± 2 |
| Participants, n (male:female) | 20 (10:10) | 21 (11:10) |
| Body mass (kg) | 68 ± 4 | 102 ± 6* |
| Stature (m) | 1.70 ± 0.02 | 1.71 ± 0.03 |
| Body mass index (kg/m2) | 23 ± 1 | 35 ± 2* |
| Glucose (mg/dL) | 88 ± 2 | 109 ± 21 |
| Triglycerides (mg/dL) | 117 ± 24 | 139 ± 12 |
| HDL (mg/dL) | 59 ± 7 | 45 ± 6 |
| LDL (mg/dL) | 120 ± 9 | 89 ± 18 |
| Physical Activity | ||
| Steps (counts/day) | 7,070 ± 1,318 | 2,150 ± 577* |
| Total physical activity (counts/min) | 193 ± 37 | 89 ± 16* |
| Sedentary (min) | 1,212 ± 31 | 1,266 ± 14 |
| Low (min) | 178 ± 21 | 108 ± 17* |
| Moderate (min) | 36 ± 12 | 6 ± 2* |
| High (min) | 1 ± 1 | 0 ± 0 |
| Very high (min) | 0 ± 0 | 0 ± 0 |
| Daily sedentary time (% total) | 85 ± 2 | 92 ± 1* |
Heart failure with preserved ejection fraction, HFpEF; high-density lipoprotein, HDL; low-density lipoprotein, LDL.
Significant difference between groups (P < 0.05).
Table 2. Heart failure with preserved ejection fraction (HFpEF) specific characteristics and pharmacotherapy.
| Disease-specific characteristics | |
| NYHA Class II | 11 (52%) |
| NYHA Class III | 8 (38%) |
| NYHA Class IV | 2 (10%) |
| Diabetic | 10 (48%) |
| Hypertensive | 17 (81%) |
| Echocardiographic and Doppler analysis | |
| Left ventricular ejection fraction (%) | 61 ± 1 |
| E-wave velocity (cm/s) | 89 ± 8 |
| A-wave velocity (cm/s) | 100 ± 11 |
| E/A ratio | 1.14 ± 0.19 |
| E/E′ (medial) | 19 ± 3 |
| E/E′ ratio (lateral) | 13 ± 2 |
| Mitral E-wave deceleration time (ms) | 216 ± 19 |
| Functional capacity | |
| Six min walk (m) | 414 ± 68 |
| Gait speed (m/s) | 0.94 ± 0.07 |
| Medications | |
| β blocker | 9 (43 %) |
| ACEi or ARB | 8 (38%) |
| Loop diuretic | 20 (95%) |
| Aldosterone antagonist | 14 (67%) |
| Statin | 16 (76%) |
| Nitrates | 5 (24%) |
New York Heart Association, NYHA.
Despite the utilization of a small muscle mass and the very low absolute exercise intensity utilized in the current study, not all participants were able to complete every stage of exercise (0W: HFpEF n = 21, Controls, n = 20; 5W: HFpEF n = 16, Controls n = 20; 10W: HFpEF n = 15, Controls n = 17; 15W: HFpEF, n = 13; Controls, n = 16). Those participants who were unable to complete the entire exercise protocol were excluded from analysis.
Central and Peripheral responses during KE Exercise
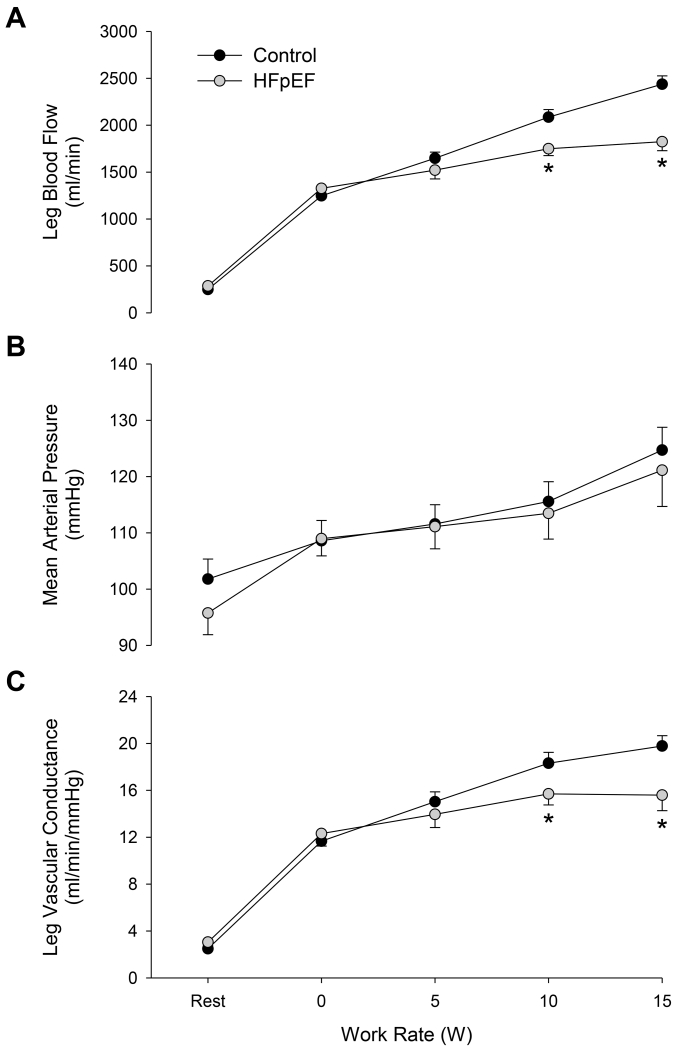
At rest, no differences in LBF, MAP, or LVC were observed between groups. During KE exercise, LBF was lower in HFpEF patients at the two highest workloads (10 and 15 W; P ≤ 0.001; Figure 1A), while MAP was not different at any work rate in patients compared to controls (Figure 1B). Similar to the LBF responses, LVC was not different during the initial stages of exercise, but was significantly reduced at the 10 and 15 W KE exercise intensities in HFpEF patients compared to controls (Figure 1C).
FIGURE 1.
Leg blood flow (panel A), mean arterial blood pressure (panel B), and leg vascular conductance (panel C) in heart failure patients with preserved ejection fraction (HFpEF) and healthy controls at rest and during single leg knee extensor exercise at 0, 5, 10, and 15 W. *Significant difference between groups, P < 0.05.
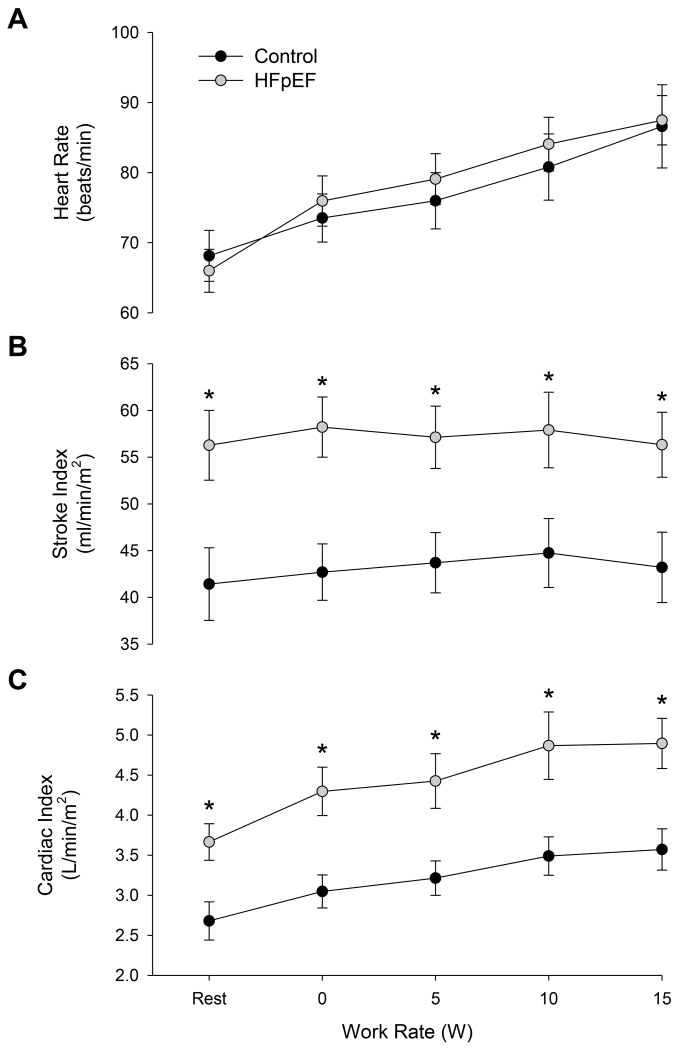
Central hemodynamic responses during exercise are illustrated in Figure 2. Resting HR was not different between patients and controls (P = 0.72), but resting SV (HFpEF: 131 ± 10 ml/min; Controls: 76 ± 7 ml/min, P < 0.001) and CO (HFpEF: 8.6 ± 0.7 L/min; Controls: 4.8 ± 0.4 L/min; P < 0.001) were higher in patients. HR increased with each level of exercise (P < 0.001) and did not differ between patients and controls (P = 0.792). CO also increased with work rate in both groups (P < 0.001), while SV remained unchanged across exercise intensities (P = 0.223). SI was higher in HFpEF patients compared with controls at rest and during exercise, but did not change significantly during exercise (P = 0.255) (Figure 2B). Likewise, CI was higher in HFpEF patients compared with controls at rest and during exercise, and increased as a consequence of exercise (Figure 2C).
FIGURE 2.
Heart rate (panel A), stroke index (panel B), and cardiac index (panel C) in heart failure patients with preserved ejection fraction (HFpEF) and healthy controls at rest and during single leg knee extensor exercise at 0, 5, 10, and 15 W. *Significant difference between groups, P < 0.05.
Physical Activity Level
Physical activity assessment demonstrated that HFpEF patients take fewer steps (P < 0.01) and are less physically active than controls, spending over 90% of their day in a sedentary state (Table 1). 6MW and gait speed were within the expected range for the patient group (Table 2), confirming reduced functional capacity in this cohort.
DISCUSSION
Through assessment of both central and peripheral hemodynamics during isolated, small muscle mass exercise, this study has provided new insight into the cardiovascular response to exercise in HFpEF patients compared to healthy, age-matched controls. An important initial, qualitative observation was that despite the use of a small muscle mass paradigm, HFpEF patients exhibited a marked exercise intolerance compared to controls, with almost 25% of patients unable to continue beyond unweighted KE exercise. In those patients able to complete the entire exercise protocol, LBF and LVC were similar to controls during low intensity KE exercise, but were reduced ~15-25% at the two highest levels of exercise. To our knowledge, this is the first study to evaluate skeletal muscle blood flow during dynamic exercise in HFpEF patients, and to identify attenuated LBF and LVC in this patient group. In contrast to this decrement in peripheral hemodynamic responses, exercise-induced changes in central hemodynamic variables (HR, SV, CO) and MAP were similar between patients and controls. Together, these findings provide new evidence of a disease-related dysregulation in the exercising skeletal muscle vasculature of HFpEF patients that cannot be attributed to cardiac dysfunction, potentially providing further insight into the peripheral factors that contribute to exercise intolerance in this patient population.
Peripheral Hemodynamic Responses during Exercise in HFpEF
While exercise intolerance is one of the defining symptoms in the clinical presentation of HFpEF, the factors responsible for this aspect of the disease remain poorly understood, and may span a host of disease-related changes in cardiac, pulmonary, autonomic, and vascular function. Indeed, the concept of an impaired “ventricular-arterial coupling” in HFpEF has emerged in an effort to describe the combined effect of impaired cardiac chronotropy, contractility, and peripheral vasodilation on exercise intolerance (13), emphasizing the multi-faceted nature of hemodynamic dysfunction experienced by this patient group. Thus, with recognition that the cardiovascular response to exercise includes both central and peripheral components, in the present study we employed a small muscle mass exercise modality that evokes minimal cardiopulmonary stress to examine exercising limb hemodynamic responses in relative isolation from the potentially confounding effects of impaired cardiac function. Using this reductionist approach, we have identified a significant attenuation in LBF (Figure 1A) and LVC (Figure 1C) across multiple exercise intensities, with the greatest reduction at the highest (15W) absolute work rate in the HFpEF group compared to control group.
To our knowledge, this is the first study to assess exercising limb blood flow in HFpEF, and to report an attenuated hyperemia in the exercising skeletal muscle vasculature in this cohort. Of the few studies to date examining maladaptations in the peripheral circulation in HFpEF, impaired “vasodilator reserve” has been implicated as a key factor contributing to the lowered exercise capacity in HFpEF. Specifically, Borlaug et al. (20) compared HFpEF patients with control participants who exhibited similar characteristics and comorbidities in an attempt to elucidate mechanisms underlying the exercise limitation in HFpEF patients, and observed an impaired capacity to reduce systemic vascular resistance during submaximal (25W) cycling exercise in patients compared to controls, a finding replicated in other work from this group (16,44). However, a key limitation of each of these investigations was the use of systemic vascular resistance index (SVRI; calculated from cardiac output and arterial blood pressure) (16,20,44) to quantify skeletal muscle vasodilation. While this parameter may serve to describe one aspect of the systemic cardiovascular response to exercise, it provides little information about local blood flow regulation or the magnitude of skeletal muscle vasodilation during exercise. Thus, the current finding that exercise-induced increases in LBF and LVC are attenuated in HFpEF patients compared to healthy controls extends this previous work (16,20,44), providing new and directly measured evidence of impaired vasodilation in the exercising limb of this patient group.
The relationship between exercising skeletal muscle blood flow and exercise intolerance in HF should not be underestimated. Indeed, in HFrEF patients, it has been reported that modest increases in muscle perfusion induced by respiratory muscle unloading lead to a significant improvement in exercise tolerance (45,46). A recent study in HFrEF patients from our own group lend further support to this relationship, identifying a marked improvement in O2 delivery and end-exercise skeletal muscle fatigue when exercising limb blood flow was increased by the inhibition of group III/IV muscle afferent fibers (47). In this light, it is likely that the reduction in LBF in the present study is of functional significance, and may contribute to exercise intolerance in HFpEF patients. However, it is important to note that LBF was not different at 0 and 5W in HFpEF patients and healthy controls, yet ~25% of HFpEF patients reached volitional exhaustion after these two exercise stages. This observation not only highlights the heterogeneity of exercise capacity in HFpEF, but also suggests that mechanisms in addition to impaired exercising muscle blood flow likely contribute to the severe exercise intolerance in this patient group.
Central Hemodynamic Responses during Exercise in HFpEF
By definition, ejection fraction is normal in HFpEF. However, this does not preclude the potential for disease-related changes in diastolic and systolic cardiac function to contribute to exercise intolerance in this cohort. Indeed, in an early investigation into central hemodynamic responses to exercise in HFpEF patients, Kitzman et al. (12) reported a blunted peak O2 consumption during maximal upright cycling exercise in patients which was related to smaller stroke volume and end diastolic volume (EDV) compared to controls. However, more recent work indicates EDV volume during exercise is similar in HFpEF patients and hypertensive controls, suggesting instead that reduced CO reserve due to chronotropic incompetence is a key factor contributing to exercise intolerance in this cohort. It should be noted that these studies have in common the use of an upright cycling paradigm, a large muscle mass exercise modality that imposes significant central hemodynamic stress that may amplify cardiac limitations, masking potential dysfunction of the peripheral vasculature. KE exercise was employed in the current study to address this potentially confounding issue, with the expectation that this exercise modality would provoke a similar cardiac response in HFpEF patients and controls. In support of this hypothesis, we observed similar exercise-induced changes in HR (Figure 2A), SV (Figure 2B), and CO (Figure 2C) across all intensities of KE exercise in HFpEF patients and controls. These comparable cardiac responses between groups indicate that the observed impairment in LBF (Figure 1A) and LVC (Figure 1C) in the HFpEF patient group cannot be attributed to differences in central hemodynamics, implicating impaired skeletal muscle vasodilatory ability as the primary factor responsible for the observed decrement in peripheral hemodynamics in this cohort.
The observed reduction in LBF and LVC in the face of comparable central hemodynamic responses during exercise in HFpEF raises the question of whether CO is properly distributed in this patient group. Initial evidence of altered CO distribution is provided by a recent study using phase contrast magnetic resonance imaging to assess aortic and superficial femoral blood flow following submaximal cycling exercise in HFpEF and HFrEF compared with healthy controls (19). In this study, lower femoral artery blood flow was reported upon cessation of exercise in HFpEF patients despite similar ascending and descending aortic blood flow, a difference that persisted even after adjusting for differences in ejection fraction between groups (19). These findings suggest altered blood flow distribution, not impaired CO, is key to reduced blood flow in these patients during submaximal cycling (19). With the inclusion of multiple exercise intensities, a small muscle mass exercise modality, and continuous measurements of central and peripheral hemodynamics during exercise, the current data build upon these previous findings to support the concept of a possible maldistribution of CO during exercise in HFpEF, though additional measurements in non-exercising vascular beds are required to further explore this possibility.
Physical Activity
Average daily step count for HFpEF patients was ~30% of the healthy control participants (Table 1), highlighting an extremely low level of ambulation in these patients. While the absence of physical activity in these patients is likely a consequence of their condition, this level of inactivity may also be a key contributor to the pathophysiology of their disease through vascular deconditioning related to disuse in skeletal muscle (48). Furthermore, the lack of physical activity may exacerbate many of their symptoms over time through further deconditioning and associated reductions in muscle strength and function, leading to a downward spiral of morbidity. Thus, it is possible that the observed reduction in LBF (Figure 1A) and LVC (Figure 1C) during exercise is a significant influence to this transition towards a sedentary lifestyle in HF patients. In support of a role of reduced LBF in exercise intolerance, our group has reported significant improvements in exercise tolerance, as quantified by assessment of exercise-induced skeletal muscle fatigue and perceived exertion, when exercising LBF is increased acutely in HFrEF patients (47). Together, these previous and present findings suggest that impairments in skeletal muscle vasodilatory ability may represent a significant deterrent to physical activity in HF, and may thus play an important role in the progression towards adoption of a sedentary lifestyle that is so detrimental in this cohort.
Perspectives
The current finding of reduced LBF and LVC in the face of preserved central hemodynamic responses raises the question of what peripheral mechanisms may be responsible for the impaired exercising muscle vasodilation in HFpEF patients. While the present study was not designed to elucidate the mechanisms underlying this response, one putative pathway of particular interest, based in part on observations in HFrEF, is sympathetic vasoconstriction. Indeed, excessive sympathoexcitation is well described in HFrEF (49,50), and is now recognized as significant factor in disease progression and survival (51). With respect to the regulation of LBF, elevated resting and reflex-mediated increases in muscle sympathetic nerve activity (MSNA) have been shown to be important mediators of the attenuated limb blood flow during exercise in HFrEF patients (52). In contrast, and somewhat surprisingly, very little is currently known with respect to disease-related changes in sympathoexcitation in HFpEF patients, particularly during exercise. Borlaug et al. (20) reported that the rise in plasma norepinephrine (NE) during submaximal cycling exercise was the same in HFpEF patients relative to control participants, though the inability of this quantitative approach to evaluate NE uptake, release, and spillover suggests that caution is warranted when interpreting these findings. Considering that increased age, obesity, and hypertension are common characteristics of the HFpEF phenotype (20), and that each of these conditions is associated with elevated resting MSNA (53-55), it is tempting to speculate that sustained sympathetic vasoconstriction may contribute to the observed reduction in exercising LBF and LVC in HFpEF. Further studies are warranted to explore this intriguing possibility.
Experimental Considerations
In the present study, we enrolled HFpEF patients without regard to existing comorbidities such as hypertension, diabetes, and coronary artery disease. While this approach may introduce some heterogeneity to the outcome measures, it provided an opportunity to study the pathophysiology of HFpEF in a manner that fairly represents the diverse nature of this patient population. It is also acknowledged that the sample size of the present study was relatively small, though a sufficient number of subjects were enrolled to achieve adequate statistical power in the major variables. We also recognize that body mass of the HFpEF patients enrolled in this study was significantly higher than the control group, raising the question of whether obesity, per se, represents a confounding factor for data interpretation. However, this concern is somewhat mitigated by a recent study by Limberg et al. (56) that failed to identify a difference in limb blood flow during both handgrip and knee-extensor exercise in lean versus obese individuals. This observation, coupled with the known lack of correlation between leg blood flow and quadriceps muscle mass across work rates during knee extensor exercise (57), suggests that the observed decrement in skeletal muscle vasodilation during exercise in HFpEF in likely not explained by higher body mass in this cohort. Finally, we acknowledge that use of the Modelflow method for estimation of stroke volume may not provide the same level of precision as that provided by more invasive techniques, though it is noteworthy that good agreement in tracking CO changes has recently been documented with these two methodologies in heart disease patients (40).
Conclusions
HFpEF patients exhibit an impaired exercise-induced hyperemic response during exercise, and importantly, this response cannot be attributed to a disease-related impairment in central hemodynamics. These findings indicate that impaired vasodilation in the exercising skeletal muscle vasculature plays a key role in exercise intolerance in this patient population.
Clinical Perspectives
Severe exercise intolerance is a hallmark symptom of HFpEF (8). While regular physical activity has been documented to improve functional capacity, overall quality of life, fatigue, and dyspnea in HFpEF patients (58), the development of symptoms upon exertion remains a significant barrier to engaging in regular physical activity. In this light, the present findings of impaired vasodilatory ability within the exercising skeletal muscle provide new insight into the mechanisms of exercise intolerance in HFpEF, and identifies a potential therapeutic target for improving exercise intolerance in this patient group.
Acknowledgments
Funding Sources. Funded in part by grants from the National Institutes of Health (HL091830 and HL118313) and the Department of Veterans Affairs Rehabilitation Research and Development Service (RX001433, RX001418, RX001697, and RX000182).
Footnotes
Disclosures. None
REFERENCES
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circ. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–19. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatia R, Tu J, Lee D, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–9. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 4.Bursi F, Weston S, Redfield M, et al. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–16. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 5.Owan T, Hodge D, Herges R, Jacobsen S, Roger V, Redfield M. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 6.Paulus WJ, van Ballegoij JJ. Treatment of heart failure with normal ejection fraction: an inconvenient truth! J Am Coll Cardiol. 2010;55:526–37. doi: 10.1016/j.jacc.2009.06.067. [DOI] [PubMed] [Google Scholar]
- 7.Maeder MT, Kaye DM. Heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol. 2009;53:905–18. doi: 10.1016/j.jacc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Kitzman DW, Little WC, Brubaker PH, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA: the journal of the American Medical Association. 2002;288:2144–50. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 9.Haykowsky MJ, Tomczak CR, Scott JM, Paterson DI, Kitzman DW. Determinants of exercise intolerance in patients with heart failure and reduced or preserved ejection fraction. J Appl Physiol (1985) 2015;119:739–44. doi: 10.1152/japplphysiol.00049.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westermann D, Kasner M, Steendijk P, et al. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circ. 2008;117:2051–60. doi: 10.1161/CIRCULATIONAHA.107.716886. [DOI] [PubMed] [Google Scholar]
- 11.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–9. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 12.Kitzman D, Higginbotham M, Cobb F, Sheikh K, Sullivan M. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol. 1991;17:1065–72. doi: 10.1016/0735-1097(91)90832-t. [DOI] [PubMed] [Google Scholar]
- 13.Borlaug BA, Jaber WA, Ommen SR, Lam CS, Redfield MM, Nishimura RA. Diastolic relaxation and compliance reserve during dynamic exercise in heart failure with preserved ejection fraction. Heart. 2011;97:964–9. doi: 10.1136/hrt.2010.212787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitzman DW, Nicklas BJ, Kraus WE, et al. Skeletal Muscle Abnormalities and Exercise Intolerance in Older Patients with Heart Failure and Preserved Ejection Fraction. Am J Physiol Heart Circ Physiol. 2014 doi: 10.1152/ajpheart.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haykowsky MJ, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J, Kitzman DW. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol. 2014;113:1211–6. doi: 10.1016/j.amjcard.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borlaug BA, Olson TP, Lam CS, et al. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–54. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujimoto N, Prasad A, Hastings JL, et al. Cardiovascular effects of 1 year of progressive endurance exercise training in patients with heart failure with preserved ejection fraction. Am Heart J. 2012;164:869–77. doi: 10.1016/j.ahj.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60:120–8. doi: 10.1016/j.jacc.2012.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puntawangkoon C, Kitzman DW, Kritchevsky SB, et al. Reduced peripheral arterial blood flow with preserved cardiac output during submaximal bicycle exercise in elderly heart failure. Journal of cardiovascular magnetic resonance: official journal of the Society for Cardiovascular Magnetic Resonance. 2009;11:48. doi: 10.1186/1532-429X-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borlaug BA, Melenovsky V, Russell SD, et al. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circ. 2006;114:2138–47. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 21.Andersen P, Adams RP, Sjogaard G, Thorboe A, Saltin B. Dynamic knee extension as model for study of isolated exercising muscle in humans. J Appl Physiol. 1985;59:1647–1653. doi: 10.1152/jappl.1985.59.5.1647. [DOI] [PubMed] [Google Scholar]
- 22.Esposito F, Reese V, Shabetai R, Wagner PD, Richardson RS. Isolated quadriceps training increases maximal exercise capacity in chronic heart failure: the role of skeletal muscle convective and diffusive oxygen transport. J Am Coll Cardiol. 2011;58:1353–62. doi: 10.1016/j.jacc.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esposito F, Wagner PD, Richardson RS. Incremental large and small muscle mass exercise in patients with heart failure: evidence of preserved peripheral haemodynamics and metabolism. Acta Physiol (Oxf) 2015;213:688–9. doi: 10.1111/apha.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrett-O’Keefe Z, Lee JF, Berbert A, et al. Hemodynamic responses to small muscle mass exercise in heart failure patients with reduced ejection fraction. Am J Physiol Heart Circ Physiol. 2014;307:H1512–20. doi: 10.1152/ajpheart.00527.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LeJemtel TH, Maskin CS, Lucido D, Chadwick BJ. Failure to augment maximal limb blood flow in response to one-leg versus two-leg exercise in patients with severe heart failure. Circ. 1986;74:245–51. doi: 10.1161/01.cir.74.2.245. [DOI] [PubMed] [Google Scholar]
- 26.Magnusson G, Gordon A, Kaijser L, et al. High intensity knee extensor training, in patients with chronic heart failure: Major skeletal muscle improvement. Eur Heart J. 1996;17:1048–1055. doi: 10.1093/oxfordjournals.eurheartj.a015001. [DOI] [PubMed] [Google Scholar]
- 27.Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. The Journal of physiology. 1985;366:233–49. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poole DC, Richardson RS. Determinants of oxygen uptake. Implications for exercise testing. Sports Med. 1997;24:308–20. doi: 10.2165/00007256-199724050-00003. [DOI] [PubMed] [Google Scholar]
- 29.Desai AS, Lewis EF, Li R, et al. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J. 2011;162:966–972. e10. doi: 10.1016/j.ahj.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Cahalin LP, Mathier MA, Semigran MJ, Dec GW, DiSalvo TG. The six-minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest. 1996;110:325–32. doi: 10.1378/chest.110.2.325. [DOI] [PubMed] [Google Scholar]
- 31.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. Jama. 2011;305:50–8. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barrett-O’Keefe Z, Ives SJ, Trinity JD, et al. Taming the “sleeping giant”: the role of endothelin-1 in the regulation of skeletal muscle blood flow and arterial blood pressure during exercise. Am J Physiol Heart Circ Physiol. 2013;304:H162–169. doi: 10.1152/ajpheart.00603.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wray DW, Uberoi A, Lawrenson L, Richardson RS. Heterogeneous limb vascular responsiveness to shear stimuli during dynamic exercise in humans. J Appl Physiol. 2005;99:81–86. doi: 10.1152/japplphysiol.01285.2004. [DOI] [PubMed] [Google Scholar]
- 34.Logason K, Barlin T, Jonsson ML, Bostrom A, Hardemark HG, Karacagil S. The importance of Doppler angle of insonation on differentiation between 50-69% and 70-99% carotid artery stenosis. European journal of vascular and endovascular surgery: the official journal of the European Society for Vascular Surgery. 2001;21:311–3. doi: 10.1053/ejvs.2001.1331. [DOI] [PubMed] [Google Scholar]
- 35.Bogert LW, van Lieshout JJ. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Exp Physiol. 2005;90:437–46. doi: 10.1113/expphysiol.2005.030262. [DOI] [PubMed] [Google Scholar]
- 36.de Vaal JB, de Wilde RB, van den Berg PC, Schreuder JJ, Jansen JR. Less invasive determination of cardiac output from the arterial pressure by aortic diameter-calibrated pulse contour. Br J Anaesth. 2005;95:326–31. doi: 10.1093/bja/aei189. [DOI] [PubMed] [Google Scholar]
- 37.de Wilde RB, Geerts BF, Cui J, van den Berg PC, Jansen JR. Performance of three minimally invasive cardiac output monitoring systems. Anaesthesia. 2009;64:762–9. doi: 10.1111/j.1365-2044.2009.05934.x. [DOI] [PubMed] [Google Scholar]
- 38.Sugawara J, Tanabe T, Miyachi M, et al. Non-invasive assessment of cardiac output during exercise in healthy young humans: comparison between Modelflow method and Doppler echocardiography method. Acta Physiol Scand. 2003;179:361–6. doi: 10.1046/j.0001-6772.2003.01211.x. [DOI] [PubMed] [Google Scholar]
- 39.van Lieshout JJ, Toska K, van Lieshout EJ, Eriksen M, Walloe L, Wesseling KH. Beat-to-beat noninvasive stroke volume from arterial pressure and Doppler ultrasound. Eur J Appl Physiol. 2003;90:131–7. doi: 10.1007/s00421-003-0901-8. [DOI] [PubMed] [Google Scholar]
- 40.de Wilde RB, Schreuder JJ, van den Berg PC, Jansen JR. An evaluation of cardiac output by five arterial pulse contour techniques during cardiac surgery. Anaesthesia. 2007;62:760–8. doi: 10.1111/j.1365-2044.2007.05135.x. [DOI] [PubMed] [Google Scholar]
- 41.Welk GJ, Blair SN, Wood K, Jones S, Thompson RW. A comparative evaluation of three accelerometry-based physical activity monitors. Med Sci Sports Exerc. 2000;32:S489–97. doi: 10.1097/00005768-200009001-00008. [DOI] [PubMed] [Google Scholar]
- 42.Abel MG, Hannon JC, Sell K, Lillie T, Conlin G, Anderson D. Validation of the Kenz Lifecorder EX and ActiGraph GT1M accelerometers for walking and running in adults. Appl Physiol Nutr Metab. 2008;33:1155–64. doi: 10.1139/h08-103. [DOI] [PubMed] [Google Scholar]
- 43.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circulation Heart failure. 2010;3:588–95. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borghi-Silva A, Carrascosa C, Oliveira CC, et al. Effects of respiratory muscle unloading on leg muscle oxygenation and blood volume during high-intensity exercise in chronic heart failure. Am J Physiol Heart Circ Physiol. 2008;294:H2465–72. doi: 10.1152/ajpheart.91520.2007. [DOI] [PubMed] [Google Scholar]
- 46.Mancini D, Donchez L, Levine S. Acute unloading of the work of breathing extends exercise duration in patients with heart failure. J Am Coll Cardiol. 1997;29:590–6. doi: 10.1016/s0735-1097(96)00556-6. [DOI] [PubMed] [Google Scholar]
- 47.Amann M, Venturelli M, Ives SJ, et al. Group III/IV muscle afferents impair limb blood in patients with chronic heart failure. Int J Cardiol. 2014;174:368–75. doi: 10.1016/j.ijcard.2014.04.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silber DH, Sinoway LI. Reversible impairment of forearm vasodilation after forearm casting. J Appl Physiol. 1990;68:1945–1949. doi: 10.1152/jappl.1990.68.5.1945. [DOI] [PubMed] [Google Scholar]
- 49.Leimbach WN, Jr., Wallin BG, Victor RG, Aylward PE, Sundlof G, Mark AL. Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circ. 1986;73:913–9. doi: 10.1161/01.cir.73.5.913. [DOI] [PubMed] [Google Scholar]
- 50.Notarius CF, Millar PJ, Murai H, et al. Divergent muscle sympathetic responses to dynamic leg exercise in heart failure and age-matched healthy subjects. J Physiol. 2015;593:715–22. doi: 10.1113/jphysiol.2014.281873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaye DM, Lefkovits J, Jennings GL, Bergin P, Broughton A, Esler MD. Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol. 1995;26:1257–63. doi: 10.1016/0735-1097(95)00332-0. [DOI] [PubMed] [Google Scholar]
- 52.Alves MJ, Rondon MU, Santos AC, et al. Sympathetic nerve activity restrains reflex vasodilatation in heart failure. Clinical autonomic research: official journal of the Clinical Autonomic Research Society. 2007;17:364–9. doi: 10.1007/s10286-007-0448-6. [DOI] [PubMed] [Google Scholar]
- 53.Grassi G, Seravalle G, Quarti-Trevano F, Dell’Oro R, Bolla G, Mancia G. Effects of hypertension and obesity on the sympathetic activation of heart failure patients. Hyper. 2003;42:873–7. doi: 10.1161/01.HYP.0000098660.26184.63. [DOI] [PubMed] [Google Scholar]
- 54.Grassi G, Dell’Oro R, Facchini A, Quarti Trevano F, Bolla GB, Mancia G. Effect of central and peripheral body fat distribution on sympathetic and baroreflex function in obese normotensives. J Hypertens. 2004;22:2363–9. doi: 10.1097/00004872-200412000-00019. [DOI] [PubMed] [Google Scholar]
- 55.Ng AV, Callister R, Johnson DG, Seals DR. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hyper. 1993;21:498–503. doi: 10.1161/01.hyp.21.4.498. [DOI] [PubMed] [Google Scholar]
- 56.Limberg JK, De Vita MD, Blain GM, Schrage WG. Muscle blood flow responses to dynamic exercise in young obese humans. J Appl Physiol (1985) 2010;108:349–55. doi: 10.1152/japplphysiol.00551.2009. [DOI] [PubMed] [Google Scholar]
- 57.Garten RS, Groot HJ, Rossman MJ, Gifford JR, Richardson RS. The role of muscle mass in exercise-induced hyperemia. J Appl Physiol (1985) 2014;116:1204–9. doi: 10.1152/japplphysiol.00103.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Asrar Ul Haq M, Goh CY, Levinger I, Wong C, Hare DL. Clinical utility of exercise training in heart failure with reduced and preserved ejection fraction. Clin Med Insights Cardiol. 2015;9:1–9. doi: 10.4137/CMC.S21372. [DOI] [PMC free article] [PubMed] [Google Scholar]