Abstract
Introduction
Despite significant advances in intravascular stent technology, safe prevention of stent thrombosis over prolonged periods after initial deployment persists as a medical need to reduce device failure. The objective of this project was to assess the potential of perfluorocarbon nanoparticles conjugated with the direct thrombin inhibitor D-phenylalanyl-L-prolyl-L-arginyl chloromethylketone (PPACK-NP) to inhibit stent thrombosis.
Methods
In a static model of stent thrombosis, 3 mm × 3 mm pieces of stainless steel coronary stents were cut and adsorbed with thrombin to create a procoagulant surface that would facilitate thrombus development. Following treatment with PPACK-NP or control NP, stents were exposed to platelet poor plasma (PPP) or platelet rich plasma (PRP) for set time points up to 60 minutes. Measurements of final clot weight in grams were utilized for assessing the effect of nanoparticle treatment on limiting thrombosis. Additionally, groups of stents were exposed to flowing plasma containing various treatments (saline, free PPACK, control NP and PPACK-NP) and generated thrombi were stained and imaged to investigate the treatment effects of PPACK-NP under flow conditions.
Results
The static model of stent thrombosis utilized in this study indicated a significant reduction in thrombus deposition with PPACK-NP treatment (0.00067 ± 0.00026g, N=3) compared to control NP (0.0098 ± 0.0015g, N=3, p = 0.026) in PPP. Exposure to PRP demonstrated similar effects with PPACK-NP treatment (0.00033 ± 0.00012g, N=3) versus control NP treatment (0.0045 ± 0.00012g, N=3, p = 0.000017). In additional studies, stents were exposed to both platelet rich plasma pretreated with vorapaxar and PPACK-NP, which illustrated adjunctive benefit to oral platelet inhibitors for prevention of stent thrombosis. Additionally, an in vitro model of stent thrombosis under flow conditions established that PPACK-NP treatment significantly inhibited thrombus deposition on stents.
Conclusion
This study demonstrates that anti-thrombin perfluorocarbon nanoparticles exert marked focal anti-thrombin activity to prevent intravascular stent thrombosis and occlusion.
Introduction
Stroke, myocardial infarction, and limb loss from peripheral vascular disease are significant causes of cardiovascular morbidity and mortality in the U.S. that often are treated with interventions requiring one or more stents.1 Over the past few decades, significant technological advances have been made in these interventional treatments, yet all such therapies continue to fail a significant portion of patients due to thrombosis as the final common endpoint. In fact, recent reports from the Dual Antiplatelet Therapy (DAPT) study have demonstrated that the current standard of dual antiplatelet therapy (P2Y12-receptor inhibitor plus aspirin) for prevention of thrombotic complications following placement of drug-eluting stents may require extended use beyond 1 year following stenting to prevent thrombosis, instead of the commonly prescribed 6–12 months.2, Despite this demonstration of a reduction of risk of stent thrombosis and major adverse cardiovascular and cerebrovascular events with therapy for >1 year, the prolonged treatment regimen harbors an increased risk of bleeding, suggesting a clear medical need for new antithrombotic and antiplatelet agents that provide increased efficacy in preventing thrombotic complications, while minimizing bleeding risk. These studies also highlight the clinically significant rate of stent thrombosis for intravascular stents in the carotid, coronary and peripheral circulation. Intravascular stents continue to experience thrombosis at rates in coronary circulation of 0.9% at 30 days post-implantation with second-generation drug eluting stents3, but highly variable rates in the peripheral circulation with failure rates up to 25%.4,5 Significantly, the immediate incidence of death or myocardial infarction is 64.4% for coronary stent thrombosis3 and in the peripheral circulation stent thrombosis can result in limb loss, stroke, renal failure or bowel ischemia. Aggressive and prolonged pharmacological therapy with platelet inhibitors is required to mitigate the prothrombotic tendency of stents until they become endothelialized and more resistant to thrombosis, but at the cost of significant and sustained bleeding risk. Indeed the published data indicate a severe bleeding risk of 1.7% and moderate bleeding risk of 2.1% over 28 months with the highest risk during the first year and significant additional mortality with moderate bleeding events.6 We propose an alternative solution to maintaining stent patency while minimizing bleeding risk by exerting a localized anticlotting effect devoid of any sustained systemic anticoagulant effect. To address this clinical need, this study assessed the ability of anti-thrombin PFC NPs to prevent thrombosis on thrombin treated stents in both static and flow in-vitro models.
The agents utilized in this study consist of perfluorocarbon nanoparticles (PFC-NP) with a diameter of 160.5 ± 2.6 nm that were conjugated to the direct thrombin inhibitor PPACK (D-phenylalanyl-L-prolyl-L-arginyl chloromethylketone) to form antithrombotic nanoparticles, PPACK-NP (Fig. 1A).7 These nanoparticles were formulated to carry ~13650 PPACK moieties per particle, and already have demonstrated favorable safety profiles in vivo with respect to bleeding risk, with APTT and mouse tail vein bleeding times normalized within 60 minutes following intravenous administration. Furthermore, PPACK-NP are effective antithrombotic agents in mouse models of arterial thrombosis, where prior work has demonstrated that PPACK-NP treatment more than doubles the time to total thrombotic occlusion of the carotid artery after dye-laser injury by directly inhibiting thrombin activity at the site of injury. Additionally, as thrombin accumulated, NP-PPACK continually bound and inactivated all exposed thrombin molecules ultimately forming an “anti-clotting” surface (Fig. 1B).8 The anti-thrombin activity of the nanoparticle PPACK system in vivo is greatly improved over that of free PPACK due to an increased circulating half-life (t1/2 ~ 3 min for free inhibitor9 vs. t1/2 ~ 105 min for PPACK-NP as a direct consequence of conjugation of the active PPACK moiety to the stable nanoparticle.10 Even though PPACK is a potent direct and irreversible inhibitor of thrombin by covalently coupling to the active site of the protease, its lack of acceptable pharmacokinetics render it essentially useless as a clinical anticoagulant unless coupled to a nanosystem to extend its clearance time.
Figure 1.
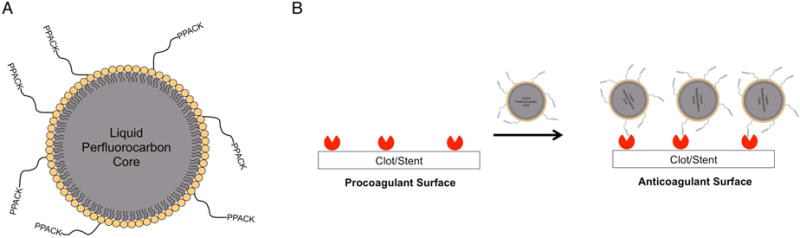
(A) Schematic of perfluorocarbon nanoparticle with surface conjugated PPACK (PPACK-NP). (B) Following implantation, activated thrombin adsorbs to vascular stents forming a pro-thrombotic coating. NP-PPACK binds to and inactivates this adsorbed thrombin transforming the stent surface with a functionally anti-thrombotic coating.
Herein, we sought to define the utility of PPACK-NP for inhibiting stent thrombosis in both static and dynamic models of thrombosis in vitro. PPACK-NP were assayed under conditions of stent exposure to platelet poor (PPP) and platelet rich plasma (PRP), and also tested in conjunction with Vorapaxar treatment to delineate an additive antithrombotic effect of PPACK-NP, which we hypothesize will occur through decreased thrombin mediated protease activated receptor-1 (PAR-1) platelet activation with PPACK-NP and through Voraxapar as it directly blocks PAR-1 along with diminished activity of thrombin in cleaving fibrinogen as a consequence of PPACK-NP therapy, in the prevention of stent thrombosis. Dynamic models of stent thrombosis were utilized to generate thrombi on stents under flow conditions, and PPACK-NP were tested for their ability to prevent the formation of an occlusive thrombus following infusion of thrombin within a flow loop. The goal was to elucidate the beneficial effects of PPACK-NP in the prevention of stent thrombosis that might support further development of both novel nanoparticle coatings for stents and subsequent intravenous treatment regimens to prevent or ameliorate thrombosis of stents or other intravascular prosthetics.
Methods
Nanoparticle Formulation
Nanoparticles were synthesized according to previously established emulsification techniques.7,8 Briefly, the precursor PFC-NP were composed of a 20% (vol/vol) perfluoro-15-crown-5-ether (CE) core, 2% (wt/vol) surfactant, 1.7% (wt/vol) glycerin, and water. The surfactant used in the formulation consisted of 99% egg phosphatidylcholine, EPC (Avanti Polar Lipids, Alabaster, AL) and 1 mol% 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[carboxy(polyethylene glycol)-2000], DSPE-PEG2000-COOH (Avanti Polar Lipids, Alabaster, AL). Following emulsification of the surfactant, CE, glycerin and water, the resulting precursor PFC NP was activated for coupling to the free carboxyl groups using 2 mg/mL 1-ethyl-3-(3-dimethylaminopropyl carbodiimide HCl, EDC (Pierce, Rockford, IL). Amine coupling of PPACK to the nanoparticle surface was accomplished with the subsequent addition of 12.5 mg/ml PPACK (American Peptide Company, Sunnyvale, CA) to the activated precursor nanoparticle and the conjugation reaction was allowed to proceed overnight. Following conjugation, the excess EDC and uncoupled PPACK was removed from the nanoparticle suspension using dialysis tubing of MWCO 3000–5000 (Spectrum Laboratories, Rancho Dominguez, CA) and allowed to filter for 4 hours.
Static Model of Stent Thrombosis
For the static in vitro model of stent thrombosis, expired Guidant® bare metal stents constructed of 316L stainless steel, were acquired from the Washington University in Saint Louis’ Department of Medicine. Stents were expanded and cut into 3 × 3 mm sections and placed into wells of a 12-well plate. To model potential biofouling and accumulation of prothrombotic material on stents following deployment, the stent sections were incubated with 10 μl of 1 U/μl bovine thrombin (Sigma-Aldrich, St. Louis, MO) for 1 hour. Following incubation, the stents were rinsed with saline to remove any unadsorbed thrombin. To confirm the presence of thrombin on the stent surface, the activity of adsorbed thrombin on the stent surface was tested against a chromogenic substrate for thrombin, S-2238 (Diapharma, West Chester, OH). Stents with or without adsorbed thrombin were incubated with 100 μl of 333 μM S-2238 for 30 minutes, after which the reacted S-2238 solution was transferred to wells of a 96-well plate and absorbance was measured at 405 nm.
To test the efficacy of PPACK-NP treatment in this static model, thrombin-adsorbed stent sections were treated with 10 μl of either saline, free PPACK (12.5 mg/ml), control NP, or PPACK-NP and allowed to incubate for 1 hour. In this and subsequent experiments, the control NP used were the precursor PFC NP prior to amine coupling of PPACK. Following treatment, the stents were then washed with saline to remove any non-stent associated nanoparticles. The treated stents were then exposed to, platelet poor plasma (PPP), generated through the addition of 500 mM CaCl2 to aliquots of expired human plasma. In the investigation of the effect of dual treatment with platelet inhibitors, platelet rich plasma (PRP) was made through the dilution of expired human platelet concentrate (ZenBio, Research Triangle Park, NC) in PPP, resulting in a total platelet concentration of 300,000 platelets/ml. For test groups including platelet inhibitors, PRP was pretreated with Vorapaxar (Axon Medchem LLC, Reston, VA) at a final concentration of 50 nM. The treated stents were incubated with 10 μl of PPP, PRP or PRP/Vorapaxar for designated time points of 5, 15, 30, and 60 minutes at 37°C, N=3 for each group at each time point. At each of these time points, stents were weighed to determine clot weight following plasma incubation.
Ultrasound Imaging of Thrombosed Stent Sections
Samples were placed in room temperature saline at the focal zone of a high-frequency ultrasound scanner (Vevo 660 with RMV-704 40-MHz probe, VisualSonics, Toronto, ON, Canada). The probe was mounted to a computer-controlled gantry to permit scanning in multiple image planes, where each plane corresponded spatially to a cross-sectional view that was 8.0 mm wide and 1.9 mm deep. Raw radio-frequency (RF) data were acquired and stored as the probe was scanned over the sample surface. The backscattered energy at each point in the digitized waveforms making up an image frame was computed from the log of the sum of the squared RF amplitudes in a center-weighted moving window. These values were mapped to grayscale and scaled to the appropriate physical dimensions to form cross-sectional images of the sample, with acoustically ‘bright’ areas represented in white and non-echogenic regions in black.
Scanning Electron Microscopy
Stent sections harboring thrombi were prepared for scanning electron microscopy to visualize the presence of deposited fibrin and platelets on the stent surface. Following clot growth, stent sections were each placed into wells of a 12-well plate containing 1 ml of freshly prepared 2% gluteraldehyde (Sigma-Aldrich, St. Louis, MO) overnight at 4°C for fixation. After fixation, the stent sections were serially dehydrated in 1 hour incubations with 10%, 30%, 50%, 70%, 90% and 100% (3×) ethanol. Following dehydration, the stent sections were placed in a vacuum dessicator overnight to remove any residual moisture. The stent sections were then sputter coated with gold for 90 seconds followed by imaging with a Hitachi S-2600H and FEI Nova Nano 2300. ImageJ v1.47 was utilized to determine the diameter of nanoparticles visualized on stents.
In Vitro Flow Model of Stent Thrombosis
To test the ability of PPACK-NP to prevent stent thrombosis under flow conditions, a flow loop was utilized to simulate thrombus formation under flow conditions. The stent was deployed in Tygon tubing and 15 ml of PRP (150,000 platelets/ml) containing 50 μl of either saline, free PPACK (12.5 mg/ml), control NP, or PPACK-NP was allowed to flow in a loop using a peristaltic pump at 50 ml/min. After continuous perfusion of the flow loop with plasma, 10 μl of 1 U/μl bovine thrombin was infused into the flow loop and the plasma was allowed to flow for 10 mins. After 10 minutes, stents were removed from the flow loop, fixed overnight in 10% formalin and stained with picrosirius red for 30 minutes to obtain images of thrombus deposition on the stent.
Effect of PPACK-NP on aortic endothelial cell proliferation
Human aortic endothelial cells (HAECs) were acquired from Lifeline Cell Technology (Frederick MD). HAECs were cultured in VascuLife EnGS Complete Medium (Lifeline Cell Technology) prior to detachment with 0.05% trypsin-EDTA and plating in a 96 well plate at 10,000 cells/well. Following overnight cell attachment, the growth medium was replaced with EnGS complete medium with 1% penicillin-streptomycin-amphotericin (PSA, LS-1085, Lifeline Cell Technology) containing 1 U/ml human thrombin (Haematologic Technologies, Essex Junction, VT) plus equimolar amounts of free PPACK, precursor NP and PPACK-NP. The cells were allowed to incubate for 4 days with gentle shaking to prevent precipitation of nanoparticles. Following 4 days of incubation, proliferation of HAECs was determined using an XTT assay (Biotium Inc., Hayward, CA) as per the manufacturers instructions.
Statistics
All statistical tests were performed on R, version 3.0.1. For the static model of stent thrombosis, the Student’s t-test was utilized, where p < 0.05 denotes statistical significance. All error bars are depicted as standard error of the mean.
Results
Evaluation of PPACK-NP in a static model of stent thrombosis
We initially seeded sections of stents with thrombin to facilitate the further accumulation of procoagulant material on the stent surface. Thrombin adsorption to the stent surface was quantified through incubation of thrombin treated stents with S-2238, a chromogenic substrate that liberates a p-nitroanilide group following specific cleavage by thrombin, thus producing an absorbance peak at 405 nm. Thrombin-treated stents significantly increased the amount of S-2238 cleavage when compared to saline controls (p = 0.00000052, n = 3 per group), confirming the presence of thrombin on stent surfaces (Fig. 2A).
Figure 2.
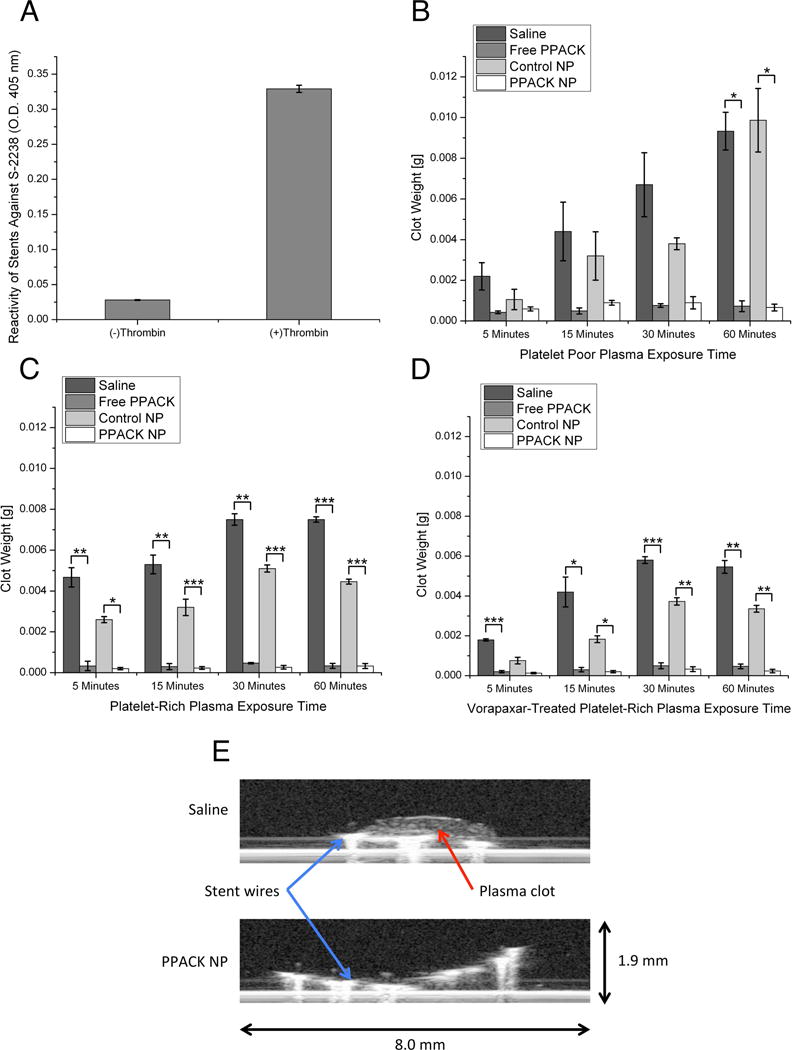
(A) S-2238 incubation confirms thrombin adsorption to stent surfaces. (B) Incubation of treated stents with platelet poor plasma results in significant clot deposition following 60 minutes of exposure with non-inhibitor treated stents compared to lack of clot growth on PPACK-NP treated stents. (C) Rapid thrombus deposition following 5 minutes of platelet-rich plasma exposure, compared to no growth on PPACK-NP treated stents for the duration of the experiment. (D) Pretreatment with vorapaxar demonstrates little benefit in preventing stent thrombosis alone, with significantly less clot deposition when used in combination with PPACK-NP. (E) Ultrasound imaging of platelet poor plasma exposed stents confirms lack of clot deposition on PPACK-NP treated stents compared to saline controls. In all panels, *p<0.05, **p<0.005, ***p<0.0005.
The efficacy of PPACK-NP was assayed in comparison to saline, free PPACK and plain NP controls. Initial work was performed with platelet poor plasma (PPP) to isolate the effect of PPACK-NP for inhibiting the formation of primarily fibrin clots (Fig. 2B). In the absence of thrombin inhibiting compounds (PPACK or PPACK-NP), we observed that treatment with saline and control NP resulted in a gradual time-dependent increase in clot weight. Treatment of thrombin-adsorbed stents with PPACK or PPACK-NP results in minimal fibrin clot formation, which was indicative of passivation of the procoagulant stent surface.
As thrombin exerts pleiotropic thrombotic and signaling effects, we aimed to explore the role of PPACK-NP for inhibiting platelet activation on stents. Platelet-rich plasma (PRP) was generated through supplementation of platelet concentrate into samples of PPP, with a final concentration of 300,000 platelets/ml used in each experiment. Additionally, separate groups of stents were exposed to Vorapaxar treated plasma to delineate the benefit of PPACK-NP in conjunction with an established platelet inhibitor. PRP exposure to stents (Fig. 2C) resulted in rapid clot formation within 5 minutes of exposure and plateaued for the duration of the experiment in saline and control NP treated groups. PPACK and PPACK-NP treatments resulted in little to no clot deposition on stents following 60 minutes of PRP exposure. This lack of clot deposition on stents was also observed in stents treated with PPACK and PPACK-NP after exposure to platelet-inhibitor treated plasma. In the case of Vorapaxar-treated plasma, PPACK and PPACK-NP inhibited stent thrombosis with greater efficacy than did Vorapaxar alone (Fig. 2D).
Ultrasound imaging of clots revealed a dense clot deposited on the surface of stents treated with saline, compared to imperceptible clot deposition in stents treated with PPACK-NP (Fig. 2E). The morphology of platelet-rich clots was observed by scanning electron microscopy of stents following 60 minutes of PRP exposure for each stent treatment group. SEM evaluation revealed a dense fibrin network formed on stent surfaces treated with saline (Fig. 3A) and control nanoparticles (Fig. 3B), with platelets embedded within fibrin clots in the saline treated stents. PPACK treated stents (Fig. 3C) demonstrated only a very thin layer of fibrous material deposited on the stent surface, consistent with clot weight measurements confirming very little accumulation of clot on stent surfaces in the static model of stent thrombosis. Interestingly, in PPACK-NP treated stents (Fig. 3D), the surface of the treated stents showed the deposition of circular nanostructures and a lack of fibrin or platelets. We hypothesized that the circular particles on the surface of the stent were particles that had adhered to the stent surface, with their flat appearance on SEM due to the vacuum drying step in SEM preparation that results in evaporation of the perfluorocarbon core and subsequent collapse of the particle, leaving a flattened deposit of the collapsed PFC-NP lipid monolayer. The diameter of these deposited particles was observed to be 185.46 ± 2.92 nm, as measured by ImageJ.
Figure 3.
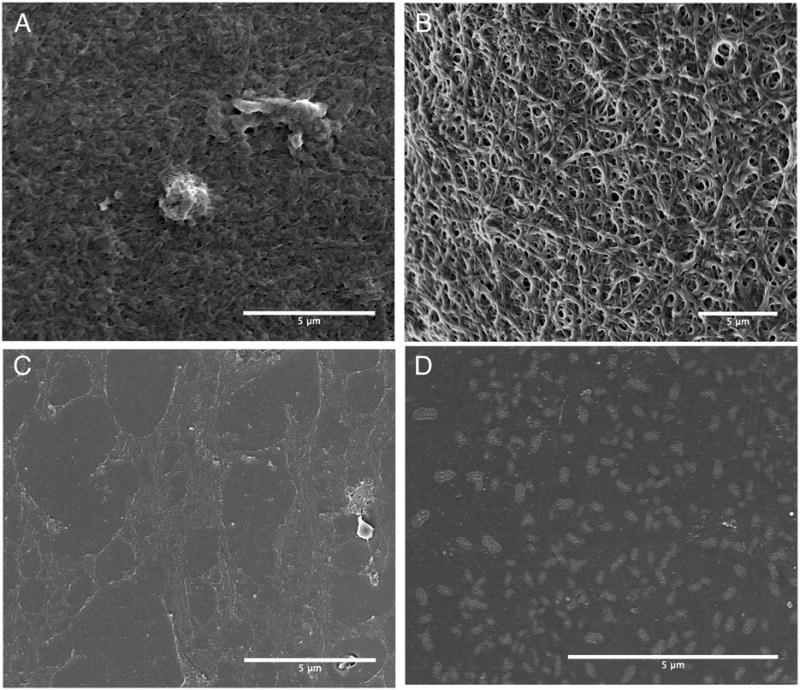
Scanning electron microscopy of platelet rich plasma exposed stents treated with (A) Saline, (B) Control NP, (C) free PPACK, and (D) PPACK-NP. Stents treated with saline or control NP show deposition of a dense fibrin mesh compared to PPACK or PPACK-NP treated stents. SEM confirms the deposition of PPACK-NP on the stent surface with a measured diameter of 185.46 ± 2.92 nm.
Following the evaluation of PPACK-NP in the static stent thrombosis model, we investigated the ability of PPACK-NP to prevent clot formation in flowing, platelet rich plasma (Fig. 4). In this model, a flow loop circuit was perfused with platelet-rich plasma at 150,000 platelets/ml together with selected nanoparticle or control solutions. Following complete perfusion of the flow loop, 10 units of thrombin was infused and allowed to flow for 10 minutes, after which the stents were removed and stained with picrosirius red to observe clot deposition. The results of this experiment demonstrate the ability of PPACK-NP to prevent clot deposition on prothrombotic materials such as a bare metal stent.
Figure 4.

Picrosirus red staining of thrombosed stents following 10 minutes of stent exposure to flowing plasma containing each treatment group and 10 units of thrombin. In saline and control NP treated plasma, occlusive thrombi were deposited on stents with minimal deposition on stents in PPACK and PPACK-NP treated plasma.
Because reendothelialization of denuded vascular regions and stent surfaces is crucial to the wound healing response following stent implantation, we sought to define the effect of PPACK-NP on endothelial proliferation. Proliferation was measured using an XTT assay (Fig. 5) that revealed a modest but significant decrease in proliferation after 4 days of continuous exposure to thrombin (12.45% decrease, p = 0.011) or thrombin and control NP (11.24%, p=0.049). PPACK and PPACK-NP treatment restored baseline endothelial proliferation levels under these conditions suggesting an additional benefit of anti-thrombin nanoparticle in accelerating local endothelial proliferation.
Figure 5.
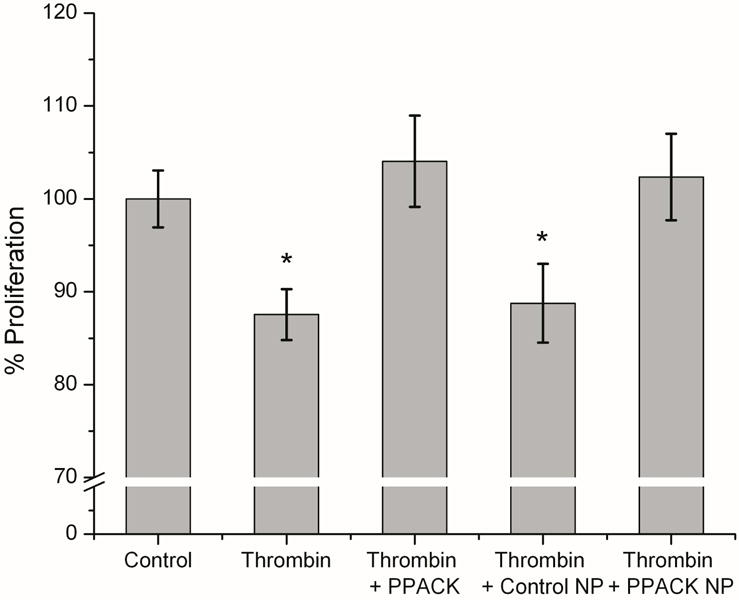
Thrombin inhibition allows for maintenance of normal cell viability and proliferation in human aortic endothelial cells compared to non-inhibitor controls. In saline and control NP treated groups, proliferation of endothelial cells was significantly decreased compared to baseline levels of proliferation following 4 days of thrombin exposure.
Discussion
Previous experiments have attributed a potential mechanistic explanation for the efficacy of antithrombin nanoparticles, where the presentation of multiple copies of thrombin inhibiting peptides allows for the binding and retention of nanoparticles to clot-bound thrombin. This retention of particles allows for the generation of an “anti-clotting” surface on top of a fresh thrombus that delays or prevents future clot growth. This phenomenon represents an additional benefit of antithrombin nanoparticle treatment, as treatment with free inhibitor only allows for 1:1 binding and would not establish such an antithrombotic surface.8,11 Furthermore, as each particle can inactivate thousands of thrombin molecules, the continued surveillance and inactivation of local thrombin results in a sustained thrombin “sponge” effect.
In static conditions, PPACK-NP treated stents exhibited markedly reduced clot burdens than did saline or control nanoparticle both in platelet rich and platelet poor plasma. Ultrasound imaging of stents along with scanning electron microscopy clearly revealed that PPACK and PPACK-NP treated stents presented with essentially no clot deposition as compared to the dense fibrin thrombi formed in control nanoparticle and saline control groups (Fig. 2B&C). Furthermore, as antiplatelet therapy is a commonly prescribed as an adjunct to increase stent patency, we investigated the effects of PPACK-NP on treated stents following exposure to platelet inhibitor-treated PRP. As thrombin served as the agonist in these experiments, we tested Vorapaxar (Zontivity, Merck), a recently FDA-approved direct protease-activated receptor-1 antagonist12 to block the activation of platelets by thrombin.13 Only a minor effect of Vorapaxar was observed in preventing thrombus formation as compared to thrombus inhibition with PPACK or PPACK-NP (Fig. 2D). This superiority of PPACK-NP treated stents over and above Vorapaxar alone suggests that even in the event of diminished platelet activation due to vorapaxar treatment, the uninhibited effects of thrombin on fibrinogen cleavage still prevails in formation of thrombi at injury sites. These results illustrate a strong potential adjunctive benefit for the use of PPACK-NP either alone or in conjunction with other standard therapies.
We further explored this activity in an in vitro flow loop model to simulate a clinically relevant scenario. Picrosirus red staining of stents following exposure to circulating PRP demonstrated total occlusion of stents after saline and plain NP treatment, as compared to no thrombosis in PPACK or PPACK-NP treated stents (Fig. 4). The present results suggest a potential clinical utility for PPACK-NP as either stent coatings or through periodic intravenous administration that would allow for effective antithrombotic activity with minimal bleeding side effects, as previously demonstrated.7
Endothelialization of stents following implantation is a crucial step in healing of injury sites14, and as such, we investigated the effect of PPACK-NP on endothelial proliferation. Thrombin has previously been shown to promote wound healing through generation of VEGF, and with inhibition of thrombin being potentially detrimental to endothelialization. However, several groups have shown a concentration dependent and cell-type dependent effects of thrombin on endothelial cell proliferation, where high concentrations of thrombin (>1U/ml) have a detrimental effect on proliferation of aortic endothelial cells, as opposed to umbilical vein endothelial cells.15–17 Accordingly, we utilized human aortic endothelial cells for measurements of proliferation in response to thrombin stimulation at 1U/ml for 4 days. A modest thrombin-dependent decrease in proliferation was observed for the control group, but basal cell proliferation/viability was restored in the PPACK and PPACK-NP treated groups. These results indicate that thrombin inhibition with PPACK-NP in this in vitro experiment preserved endothelial proliferative potential on the face of high local concentrations of thrombin such as those found in atherosclerotic plaques and vessel segments undergoing stenting.18 Additionally, it is possible that treatment with PPACK-NP may have other related effects on the endothelium, namely effects on cell migration and survival. Thrombin’s role in vascular pathophysiology, for example, includes the upregulation of NADPH oxidase secretion by vascular smooth muscles cells19 that may contribute to reactive oxygen species (ROS)-mediated endothelial damage20 and inhibition of endothelial cell migration.21 Thus along with the present data, and the effects of thrombin on endothelial proliferation, migration and survival published in the literature, there may be a benefit to thrombin inhibition with PPACK-NP, however this effect remains to be delineated and is the subject of future studies with this therapeutic.
Current recommended systemic strategies for post-stent therapy include anticoagulation and antiplatelet therapy. There has been considerable interest in modification of the implant itself with strategies including coatings, surface modifications, and techniques to more rapidly endothelialize the surface. Specific anti-thrombotic surface modifications with the addition of heparin and bivalirudin have shown promise in prior studies.14,22 Of note, Zilver PTX was recently approved and is the first drug-eluting stent approved for treatment of peripheral arterial disease.23 Unfortunately, drug-eluting devices are plagued by early thrombosis and require prolonged dual antiplatelet therapy with its attendant bleeding risks and costs. Our approach offers a flexible method to employ a potent biocompatible nanoparticle that is functionalized with > 10,000 copies of a relevant anticlotting agent per particle, which could be PPACK in this case, or any other moiety as we have shown already for bivalirudin.8
The study has some limitations that merit discussion. As this is an in-vitro experiment with controlled variables, these results cannot be immediately extrapolated to similar efficacy in in-vivo models or in patients, where flow conditions may differ. Additionally, in the static experiment the clots were followed out to 60 minutes, but to more accurately depict clinical situations more prolonged observations would be needed. Furthermore, bare metal stents are less commonly employed clinically and the effect of these NPs on drug eluting stents will require examination. Finally, persistence of the agent at the site of stenting or vessel injury will need to be assessed.
Conclusion
This pilot study has shown that anti-thrombin perfluorocarbon nanoparticles can inhibit stent thrombosis in static and flow loop in-vitro models. Future work will explore efficacy in vivo using rabbit models of thrombosis following vascular device implantation to validate the promise of a potentially safer adjunctive solution to the clinical problem of intravascular stent thrombosis.
Clinical Relevance.
Despite significant improvements in procedural technique and stent technology, intravascular stent thrombosis occurs with devastating end organ consequences such as limb loss. The findings of this study demonstrate that a targeted, nanomedicine-based, anti-thrombin approach results in decreased clotting of ex-vivo stents, decreased clotting of intravascular stents in flow loops and improved endothelial cell proliferation. Ultimately, this strategy may provide physicians with a treatment to decrease intravascular stent thrombosis and maintain endothelial cell viability without the systemic bleeding risks associated dual antiplatelet therapy and systemic anticoagulation.
Acknowledgments
The authors would like to acknowledge the contributions of Remya Nair of the Washington University Nano Research Facility for assistance with sample preparation and SEM image acquisition. This study was supported in part by the NIH grants HL073646, HL112303 and the James R. Hornsby Family Dream Garden Investment Partnership to S.A.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. AHA statistical update. Circulation. Am Heart Assoc. 2013;129:e28–92. [Google Scholar]
- 2.Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014 Dec 4;371(23):2155–66. doi: 10.1056/NEJMoa1409312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cutlip DE, Baim DS, Ho KK, Popma JJ, Lansky AJ, Cohen DJ, et al. Stent thrombosis in the modern era: a pooled analysis of multicenter coronary stent clinical trials. Circulation. 2001 Apr 17;103(15):1967–71. doi: 10.1161/01.cir.103.15.1967. [DOI] [PubMed] [Google Scholar]
- 4.Ong DS, Jang I-K. Causes, assessment, and treatment of stent thrombosis-intravascular imaging insights. Nat Rev Cardiol. 2015 Jun;12(6):325–36. doi: 10.1038/nrcardio.2015.32. [DOI] [PubMed] [Google Scholar]
- 5.Thukkani AK, Kinlay S. Endovascular intervention for peripheral artery disease. Circ Res. 2015 Apr 24;116(9):1599–613. doi: 10.1161/CIRCRESAHA.116.303503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger PB, Bhatt DL, Fuster V, Steg PG, Fox KAA, Shao M, et al. Bleeding complications with dual antiplatelet therapy among patients with stable vascular disease or risk factors for vascular disease: results from the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial. Circulation. 2010 Jun 15;121(23):2575–83. doi: 10.1161/CIRCULATIONAHA.109.895342. [DOI] [PubMed] [Google Scholar]
- 7.Myerson J, He L, Lanza G, Tollefsen D, Wickline S. Thrombin-inhibiting perfluorocarbon nanoparticles provide a novel strategy for the treatment and magnetic resonance imaging of acute thrombosis. J Thromb Haemost. 2011 Jul 1;9(7):1292–300. doi: 10.1111/j.1538-7836.2011.04339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myerson JW, He L, Allen JS, Williams T, Lanza G, Tollefsen D, et al. Thrombin-inhibiting nanoparticles rapidly constitute versatile and detectable anticlotting surfaces. Nanotechnology. 2014 Oct 3;25(39):395101–1. doi: 10.1088/0957-4484/25/39/395101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaiser B. Pharmacology of synthetic thrombin inhibitors of the tripeptide type. Cardiovascular Drug Reviews. 1992 [Google Scholar]
- 10.Myerson JW. Thrombin-Inhibiting Perfluorocarbon Nanoparticles: A New Class of Therapeutic for Acute Thrombosis Treatment and Diagnosis. 2014 [Google Scholar]
- 11.Palekar RU, Myerson JW, Schlesinger PH, Sadler JE, Pan H, Wickline SA. Thrombin-targeted liposomes establish a sustained localized anticlotting barrier against acute thrombosis. Mol Pharmaceutics. 2013 Nov 4;10(11):4168–75. doi: 10.1021/mp400210q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magnani G, Bonaca MP, Braunwald E, Dalby AJ, Fox KAA, Murphy SA, et al. Efficacy and safety of vorapaxar as approved for clinical use in the United States. J Am Heart Assoc. 2015 Mar;4(3):e001505. doi: 10.1161/JAHA.114.001505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatterjee S, Sharma A, Mukherjee D. PAR-1 antagonists: current state of evidence. J Thromb Thrombolysis. 2012 May 29;35(1):1–9. doi: 10.1007/s11239-012-0752-4. [DOI] [PubMed] [Google Scholar]
- 14.Zhang K, Liu T, Li J-A, Chen J-Y, Wang J, Huang N. Surface modification of implanted cardiovascular metal stents: from antithrombosis and antirestenosis to endothelialization. J Biomed Mater Res A. 2014 Feb 1;102(2):588–609. doi: 10.1002/jbm.a.34714. [DOI] [PubMed] [Google Scholar]
- 15.Borrelli V, Sterpetti AV, Coluccia P, Randone B, Cavallaro A, Santoro D’Angelo L, et al. Bimodal Concentration-Dependent Effect of Thrombin on Endothelial Cell Proliferation and Growth Factor Release in Culture. Journal of Surgical Research. 2001 Oct;100(2):154–60. doi: 10.1006/jsre.2001.6231. [DOI] [PubMed] [Google Scholar]
- 16.Wang H-S, Li F, Runge MS, Chaikof EL. Endothelial Cells Exhibit Differential Chemokinetic and Mitogenic Responsiveness to α-Thrombin. Journal of Surgical Research. 1997 Mar;68(2):139–44. doi: 10.1006/jsre.1997.5044. [DOI] [PubMed] [Google Scholar]
- 17.Wang B, Pearson T, Manning G, Donnelly R. In vitro study of thrombin on tubule formation and regulators of angiogenesis. Clin Appl Thromb Hemost. 2010 Dec 1;16(6):674–8. doi: 10.1177/1076029609354332. [DOI] [PubMed] [Google Scholar]
- 18.Borissoff JI, Heeneman S, Kilinç E, Kassák P, van Oerle R, Winckers K, et al. Early atherosclerosis exhibits an enhanced procoagulant state. Circulation. 2010 Aug 24;122(8):821–30. doi: 10.1161/CIRCULATIONAHA.109.907121. [DOI] [PubMed] [Google Scholar]
- 19.Jagadeesha DK, Takapoo M, Banfi B, Bhalla RC, Miller FJ. Nox1 transactivation of epidermal growth factor receptor promotes N-cadherin shedding and smooth muscle cell migration. Cardiovascular Research. 2012 Mar 1;93(3):406–13. doi: 10.1093/cvr/cvr308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Victor VM, Rocha M, Solá E, Bañuls C, Garcia-Malpartida K, Hernández-Mijares A. Oxidative stress, endothelial dysfunction and atherosclerosis. curr pharm des. 2009;15(26):2988–3002. doi: 10.2174/138161209789058093. [DOI] [PubMed] [Google Scholar]
- 21.van Aalst JA, Zhang D-M, Miyazaki K, Colles SM, Fox PL, Graham LM. Role of reactive oxygen species in inhibition of endothelial cell migration by oxidized low-density lipoprotein. Journal of Vascular Surgery. 2004 Dec;40(6):1208–15. doi: 10.1016/j.jvs.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Wöhrle J, Brodie B, Witzenbichler B, Dudek D, Kornowski R, Metzger C, et al. Impact of Bivalirudin and Paclitaxel-Eluting Stents on Outcomes in Patients Undergoing Primary Percutaneous Coronary Intervention of the Left Anterior Descending Artery. Am J Cardiol Elsevier. 2013 Sep;112(6):753–60. doi: 10.1016/j.amjcard.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Chan YC, Cheng SW. Drug-eluting stents and balloons in peripheral arterial disease: evidence so far. Int J Clin Pract. 2011 Jun 1;65(6):664–8. doi: 10.1111/j.1742-1241.2011.02639.x. [DOI] [PubMed] [Google Scholar]


