Abstract
Goals
Test whether ursodeoxycholic acid is inhibitory to Clostridium difficile and can be used in the treatment of C. difficile associated ileal pouchitis.
Background
Restoration of secondary bile metabolism may be the key mechanism for fecal microbiota transplantation (FMT) in treating recurrent C. difficile infections (RCDI). Therefore, it is possible that exogenous administration of inhibitory bile acids may be used directly as non-antibiotic therapeutics for this indication. The need for such a treatment alternative is especially great in patients with refractory C. difficile associated pouchitis, where the efficacy of FMT may be limited.
Study
We measured the ability of ursodeoxycholic acid (UDCA) to suppress germination and vegetative growth of 11 clinical isolate strains of C. difficile from patients treated with FMT for RCDI. In addition, we used oral UDCA to treat a patient with RCDI pouchitis that proved refractory to multiple antibiotic treatments and FMT.
Results
UDCA was found inhibitory to germination and vegetative growth of all C. difficile strains tested. Fecal concentrations of UDCA from the patient with RCDI pouchitis exceeded levels necessary to inhibit germination and growth of C. difficile in vitro. The patient has remained infection-free for over ten months following initiation of UDCA.
Conclusions
UDCA can be considered as a therapeutic option in patients with C. difficile associated pouchitis. Further studies need to be done to define the optimal dose and duration of such treatment. In addition, bile acid derivatives inhibitory to C. difficile that are able to achieve high intracolonic concentrations may be developed as therapeutics for RCDI colitis.
Keywords: Clostridium difficile, pouchitis, bile acids
Introduction
Clostridium difficile infection (CDI) is an antibiotic-associated diarrheal disease that is currently estimated to be the most common nosocomial infection in the US [1-4]. Antibiotics constitute the standard therapy for CDI, but a significant fraction of patients fail to clear the infection, resulting in spontaneous relapse [5, 6]. Chances of cure diminish with every relapse, and eventually patients develop recurrent CDI (RCDI) syndrome, which can last indefinitely [6]. Recently, fecal microbiota transplantation (FMT) has emerged as a highly effective treatment of RCDI [7, 8]. Unlike antibiotics, which inhibit and disrupt gut microbiota, this treatment normalizes the microbial community structure in the colon and restores microbiota-associated protective mechanisms [9-11].
Although CDI is primarily a colonic disease, it is also associated with diarrheal symptoms in 10% of patients with a surgically created ileal pouch reservoir after colectomy [12]. Similar to colonic CDI, ileal pouch CDI can become recurrent and difficult to eradicate with antibiotics. Thus, there is a need for alternative approaches. FMT has been suggested for R-CDI pouchitis [12], but its efficacy in this situation is currently unknown. It is possible that altered anatomy does not allow for optimal engraftment of donor microbiota or its normal protective activity. In our experience, large segmental partial colectomy has been associated with initial FMT failure [13], and other investigators have noted difficulty curing RCDI with FMT following subtotal colectomy [14].
Although FMT may combat C. difficile by multiple mechanisms, including competitive niche exclusion, elaboration of targeted bacteriocins, and upregulation of host immunity, more recent investigations have built a compelling case for a central role of secondary bile acid metabolism in curing CDI [15]. Thus, taurocholic acid, which is one of the major primary bile acids, is used routinely in growth media of C. difficile [16, 17], and both taurocholate and cholate are germinants of C. difficile spores [18]. In contrast, lithocholic acid, one of the major secondary bile acids, is a potent inhibitor of C. difficile spore germination [19, 20]. We had previously demonstrated that patients with RCDI completely lack secondary bile acids in their feces, but have increased levels of taurocholic acid [10]. Within days following FMT, however, the fecal levels of secondary bile acids increase and primary bile acids drop to levels found in the donors [10].
Ursodeoxycholic acid (UDCA) is a minor secondary bile acid in humans that is structurally related to lithocholic acid and approved for clinical use [21]. Given this relationship, we thought it might have therapeutic potential in RCDI as an inhibitor of C. difficile spore germination. A major limitation of UDCA for this indication is its rapid uptake into the enterohepatic circulation in the small intestine, resulting in low achievable intracolonic concentrations. However, patients with CDI-induced pouchitis may not have the same limitations and may be more appropriate for treatment with UDCA.
Here we present successful use of this drug to control C. difficile in a patient suffering from refractory RCDI pouchitis and explore in vitro activity of UDCA against a library of ten clinical isolate strains of C. difficile from patients suffering RCDI colitis.
Materials and Methods
Isolation of C. difficile from patient feces
C. difficile from patient feces was isolated by serial dilution onto taurocholatecycloserine-cefoxitin-fructose agar (TCCFA) followed by 48 hr anaerobic growth at 37°C [22]. Colonies were identified by their production of L-proline aminopeptidase, which differentiates C. difficile from other Clostridia, using the PRO kit test (Remel, USA) and confirmed by sequencing the 16S rRNA gene using the primer set 515F-806R [23]. Confirmed isolates were grown in CCFB anaerobically for 48 hr and stored in 25% glycerol at −80°C.
C. difficile spore preparation
C. difficile cells from frozen stocks were inoculated into CCFB medium and grown anaerobically at 37°C for 48 hr. Cultures were plated onto brain heart infusion with 5 g/L yeast extract and 0.1% L-cysteine (BHIS) and grown for 7 d at 37°C under anaerobic conditions., Colonies from each plate were scraped into 1 mL of ice-cold water and incubated at 4°C overnight to release spores [20]. A 3 mL suspension was loaded onto 10 mL of 50% (w/v) sucrose in a 15 mL conical tube and centrifuged in a swinging bucket rotor at 3200 × g for 20 min at 4°C. Sucrose and vegetative cells above the spore pellet were removed, and the pellet was washed 5 times in ice-cold water to remove remaining sucrose. Spores were examined under phase-contrast microscopy to determine purity; spore samples with >99% purity (<1% vegetative cells) were stored at 4°C.
C. difficile spore germination
Germination assays were done as previously described by Sorg and Sonenshein, 2010. Spores were heated to 65°C for 30 min and inoculated into BHIS, with or without UDCA and/or taurocholic acid, within an anaerobic bag flushed and filled with N2 gas. Optical density at 600 nm (OD600) was measured initially (OD600(t0)) and every minute for 20 min (OD600(t)) using an EL808 Microplate Reader (Biotek Instruments, Inc., Winooski, VT). Relative OD600 for each time point was calculated as OD600(t)/OD600(t0). Experiments were performed in triplicate.
Growth of C. difficile vegetative cells
Cells from frozen stocks were inoculated into BHIS broth with 0.1% (w/v) taurocholate and incubated overnight at 37°C under anaerobic conditions. Vegetative cells were inoculated into tubes containing BHIS, with or without UDCA, normalized to an OD600 of 0.005, and grown anaerobically at 37°C. Measurements of OD600 were collected each hour for 12 hr following inoculation, and a final OD600 was measured after 24 hr growth. Experiments were performed in triplicate.
Measurement of UDCA and taurocholic acid in feces
Fecal samples were spiked with oleanolic acid (internal standard), suspended in 10 volumes of 50% aqueous acetonitrile, and extracted by vortexing and sonication for 10 min. The suspension was centrifuged twice at 18,000 × g for 10 min. The supernatant was passed through a 2 μm filter and subjected to LC-MS analysis. A 5 μl aliquot was injected into an Acquity™ UPLC system (Waters, Milford, MA) and separated by a mobile phase gradient ranging from water to 95% aqueous acetonitrile containing 0.1% formic acid over a 10-min run. LC eluant was introduced into a Xevo-G2-S QTOF mass spectrometer (Waters) for metabolite identification and quantification. Capillary and cone voltage for electrospray ionization (ESI) were maintained at −1.5 kV and −35 V for negative-mode detection, respectively. Source temperature and desolvation temperature were 120 °C and 350 °C, respectively. Nitrogen was used as a cone (50 l/h) and desolvation gas (800 l/h), and argon was included as the collision gas. The mass spectrometer was calibrated with sodium formate solution (range m/z 50-1000) and monitored by the intermittent injection of the lock mass leucine enkephalin ([M-H]− = 554.2615 m/z) in real-time. Mass chromatograms and mass spectral data were acquired and processed by MassLynx™ software (Waters) in centroided format. UDCA and taurocholic acid were identified by comparing their accurate masses, tandem MS (MS/MS) fragments, and retention times with authentic standards. Concentrations in fecal samples were determined using corresponding standard curves and QuanLynx™ software (Waters).
DNA extraction
DNA from each sample (0.25 to 0.50 g) was extracted using the PowerSoil® DNA Isolation Kit (MO BIO, Carlsbad, CA) according to the manufacturer's instructions. Samples with high water content (Bristol stool scale types 5 to 7) were centrifuged at 12,000 RPM for 3 min to pellet solids, which were then used for DNA extraction. Each sample was extracted in triplicate, and each replicate was eluted in 50 μl of 10mM Tris-HCl buffer (pH 8.0) and pooled. DNA concentrations of extracted samples were measured with a QuBit® DNA quantification system (Invitrogen, Carlsbad, CA) using QuBit high sensitivity assay reagents. All extracted DNA samples were stored at −20°C until amplification.
PCR amplification and sequencing
Fecal DNA samples were used as template for PCR amplification of the V5-V6 region of the 16S rRNA gene using a V5F/V6R Nextera primer pair (Table 6.1). Following amplification with these primers, PCR products were diluted 1:100 and amplified using Illumina indexing primers, which include flowcell adapters (Table 6.1). Pooled, size-selected PCR products from the second reaction were denatured with NaOH, diluted to 8 pM in the Illumina HT1 buffer (Illumina, San Diego, CA), spiked with 15% PhiX, and heat denatured at 96°C for 2 min immediately prior to loading onto the sequencer. A MiSeq 600 cycle v3 kit was used to sequence the samples.
Sequence processing and analysis
Reads in each pair for each sequencing run overlapped and paired ends were merged. The hamming distance (number of substitutions) was calculated for sliding overlaps of the two reads in a pair to find the best overlap (lowest hamming distance with a minimal overlap of 25 nucleotides and 98% identity). Merged sequences were binned according to barcode sequence and both barcode and amplicon primer sequences were trimmed using custom Perl scripts. Sequence data was processed and analyzed using the mothur program [24]. To ensure high quality data for analysis, sequence reads containing: ambiguous bases; homopolymers >8 bp; more than one mismatch in the primer sequence; or an average per base quality score below 35 within each 50 bp window were removed. Sequences that only appeared once in the total set were assumed to be a result of sequencing error and removed from the analysis. Chimeric sequences were removed from the data set using the UCHIME algorithm within the mothur program [25]. A random subset of 25,189 sequences per sample were used to balance read numbers and clustered into operational taxonomic units (OTUs). Taxonomy was assigned at a cutoff value of ≥97%, using a 16S rRNA database prepared from the Ribosomal Database Project (RDP) 9, using the Bayesian method with a bootstrap algorithm (100 iterations) and a probability cutoff of 0.60 [26]. Principal coordinate analysis was performed using a tree generated via Bray-Curtis method, and Shannon diversity indices were calculated using mothur.
Study approval
The study was approved by the University of Minnesota Institutional Review Board (IRB 0901M56962). The patient provided informed consent for participation in the study.
Results
Case presentation
The patient was first seen in our clinic as a 53-year old woman, 6 years status post total colectomy performed for refractory ulcerative colitis. Following her surgery, she suffered from chronic, incessant, diarrhea averaging approximately 10-15 liquid bowel movements over 24 hours despite use of antidiarrheal agents, resulting in extreme fatigue and inability to get sufficient sleep. Laboratory evaluation demonstrated iron deficiency anemia (hemoglobin 10.2 g/dL, serum iron < 10 μg/dL, iron binding capacity 320 μg/dL, ferritin 4 ng/mL) and protein-losing enteropathy, with serum albumin of 2.3 g/dL and moderately decreased levels of immunoglobulins G, A, and M. Endoscopic evaluation demonstrated ulceration with exudate, edema, and friability with active chronic inflammatory infiltration on histopathology in the ileal-anal pouch. Ulcers were also noted in the proximal small bowel and a stricture in the distal small bowel resulted in a retained video capsule that was retrieved endoscopically. These findings were most consistent with Crohn's disease, or possibly post-colectomy enteritis, and the patient was started on anti-tumor necrosis factor therapy. Although there was modest overall clinical improvement attributed primarily to intravenous iron replacement, her diarrhea remained unchanged. Her stool tested positive for C. difficile toxin B, despite absence of any recent antibiotic exposure. The patient was started on oral vancomycin and she reported marked improvement in her diarrhea, including the firmest stool consistency since her colectomy.
Unfortunately, symptoms recurred within a week of stopping vancomycin, after a two week treatment course. Additional attempts to clear the infection included two more courses of vancomycin, including a 2-month tapered regimen, and a course of fidaxomicin. Antibiotics were then discontinued and FMT was performed by endoscopic infusion of standardized fecal microbiota directly into the pouch and the proximal small bowel. Subsequently the patient tested negative for C. difficile at 2 and 3 weeks post-FMT, but did not experience improvement in diarrheal symptoms. Relapse of the infection was documented at one month post FMT. Vancomycin was restarted at 125 mg four times daily, tapered down to 125 mg once daily, and maintained at that dosage for 6 months. Despite clinical improvement in the initial months, her diarrhea progressively became worse and ultimately returned to the original state, despite control of C. difficile infection by vancomycin. Vancomycin was at this time discontinued, and the patient's stool again tested positive for C. difficile toxin B. Vancomycin was restarted.
Germination and growth of C. difficile isolated from CDI pouchitis patient is inhibited by UDCA
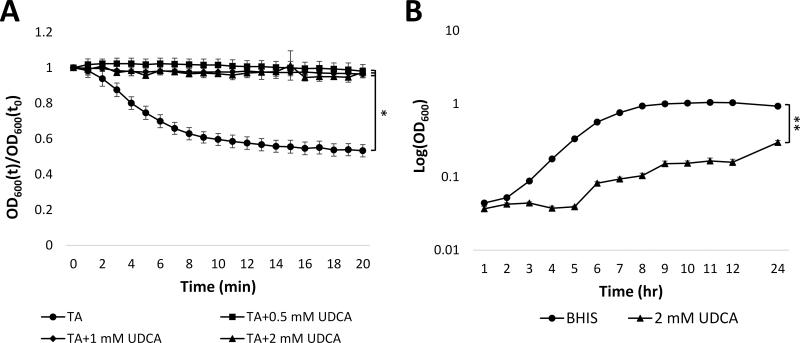
We considered using UDCA as a possible non-antibiotic treatment of RCDI pouchitis in this patient. As a pre-clinical test of this approach, we isolated C. difficile from the patient's feces collected during active infection and examined whether UDCA could inhibit germination of spores generated from this isolate. We used a well-established spectrophotometric assay that relies on the refractive nature of bacterial spore coats [18-20, 27] to examine germination of this isolate when spores were exposed to taurocholic acid, a known germinant, alone and in the presence of a range of concentrations of UDCA. Germination of spores is associated with a drop in OD600 due to degradation of the spore coats. As expected, that is what occurred when spores were exposed to taurocholic acid (Fig. 1A). However, the taurocholate-induced drop in OD600 was abrogated by addition of UDCA at all concentrations tested (0.5, 1, or 2 mM), suggesting that concentrations of UDCA as low as 0.5 mM could prevent germination of C. difficile spores from this patient's isolate. .
Figure 1. UDCA inhibits germination and growth of C. difficile isolated from patient stool.
A) Relative OD600 of spores isolated from patient exposed to 0.5 mM (■), 1 mM (♦), or 2 mM (▲) UDCA in the presence of 2 mM TA vs. 2 mM TA alone (●). OD600(t)/OD600(t0) = OD600 normalized to initial OD600 (relative OD600). B) Growth of vegetative cells from C. difficile isolate in BHIS alone (●) or BHIS with 2 mM UDCA (▲). * = p<0.01, ** = p<0.0001. TA: taurocholate; UDCA: ursodeoxycholic acid. Data represent mean ± SEM.
We also evaluated whether UDCA could inhibit growth of vegetative C. difficile. To assess growth in the presence of UDCA, overnight cultures were grown with TA to induce germination, and resulting vegetative cells were inoculated (to OD600 = 0.005) into BHIS medium or BHIS containing 2 mM UDCA. After 24 hours, the growth of cells exposed to UDCA was significantly (p < 0.0001) lower than cell grown in BHIS alone (Fig. 1B), indicating that UDCA inhibited vegetative growth of this isolate of C. difficile. Overall, these findings implied that UDCA could be an effective therapy to prevent CDI recurrence in this patient.
Successful treatment of recurrent CDI pouchitis with oral UDCA
Based on these encouraging in vitro results, the patient was prescribed oral UDCA (300 mg twice daily), which was well tolerated. After two weeks, the UDCA dose was increased to 300 mg four times daily (20 mg/kg/day) and oral vancomycin (125 mg/day) was discontinued. The frequency of diarrhea decreased and firm bowel movements were reported. Furthermore, the stool remained negative for C. difficile by PCR tests for toxin B over 12 months following termination of vancomycin. Endoscopic examination of the pouch at six months and one year showed no signs of inflammation. UDCA dose was decreased to 300 mg twice daily at seven months without relapse of CDI. The patient refused to discontinue UDCA altogether in fear of CDI relapse.
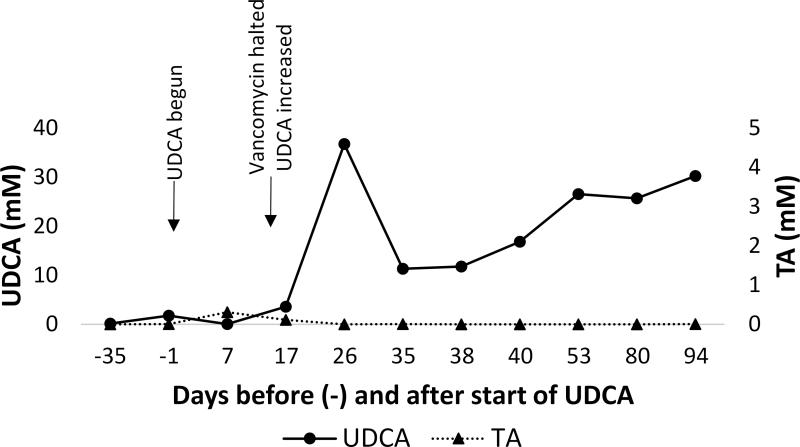
The concentrations of UDCA and taurocholic acid were measured in the patient's feces before and up to 94 d after initiation of therapy. There was a marked increase in UDCA and decrease in taurocholic acid following UDCA dose increase (Fig. 2). The fecal concentration of UDCA exceeded the therapeutic dose suggested by our in vitro studies in suppression of taurocholate-induced C. difficile spore germination.
Figure 2. UDCA is detectable in patient feces.
Concentration of UDCA (●) and taurocholate (▲) in patient feces before and after initiation of UDCA therapy. Timing of significant clinical events is noted. TA: taurocholate; UDCA: ursodeoxycholic acid.
Bacterial composition in the patient's feces, as measured by 16S rRNA gene profiling, before and after initiation of UDCA (for a period of three months of observation) retained signs of antibiotic-induced disruption, including major expansion of the Enterobacteraceae family and complete absence of members of Bacteroidetes phylum (Fig. S1). This is similar to the general microbiome signature seen in patients with RCDI prior to FMT [10, 28]. Microbial alpha diversity was low compared to fecal samples from healthy individuals (Fig. S2); however, beta microbial diversity during the follow-up period was high, suggestive of erratic compositional swings. Interestingly, a large abundance of Fusobacteraceae was seen during the first month during the UDCA treatment, a microbial family previously reported to be associated with inflammatory pouchitis [29]. Later time points were notable for decreased abundance of Fusobacteraceae and an increase in Lachnospiraceae family members, a pattern associated with less pouch inflammation [29].
UDCA inhibits germination and vegetative growth of multiple C. difficile strains
In order to more systematically explore UDCA as a therapeutic agent for RCDI, we tested whether it could inhibit germination of C difficile spores from 10 clinical isolates previously obtained from RCDI patients within our program (Supplemental Table 1) which included a North American pulsed-field gel electrophoresis type 1 (NAP1) isolate, one of the known hypervirulent strains. We found that UDCA inhibited germination of all other 11 clinical isolates (Fig. 3A) in our library at all concentrations tested (0.5, 1.0. 2.0 mM).
Figure 3. UDCA inhibits C. difficile spore germination.
A) Mean relative OD600 of spores from 10 isolates after 20 min exposure to 0.5, 1, or 2 mM UDCA in the presence of 2 mM TA vs. 2 mM TA alone. OD600(t)/OD600(t0) = OD600 normalized to initial OD600 (relative OD600); * = p<0.01. TA: taurocholate; UDCA: ursodeoxycholic acid. Data represent mean ± SEM. B) Mean OD600 at 24 hr across 10 isolates. UDCA: ursodeoxycholic acid. * = p<0.0001. Data represent mean ± SEM.
Next we examined whether the drug could also affect vegetative growth of the individual C. difficile isolates. As above, overnight cultures of vegetative cells from each isolate were inoculated into tubes containing BHIS or BHIS with 2 mM UDCA. Isolates grown with UDCA showed substantially delayed growth compared to control cultures, and after 24 hours the OD600 of cultures grown with UDCA was significantly lower than controls for all C. difficile isolates in the collection (Fig. 3B and Fig. S3). These findings demonstrate that inhibition of both C. difficile vegetative growth and spore germination is a general property of UDCA.
Discussion
Recent mechanistic investigations have built a compelling case for the importance of secondary bile acid metabolism in control of CDI. Taurocholate, one of the major primary bile acids, is routinely used in C. difficile culture media [16, 17], and both taurocholate and cholate are known to induce germination of the bacterium [18]. In contrast, lithocholic acid, one of the dominant secondary bile acids is a known inhibitor of C. difficile germination [19, 20]. Recently, the germination-specific protease, CspC, was identified as a bile acid germinant receptor and its deletion in C. difficile resulted in decreased pathogenicity in a hamster model of CDI [30]. CDI susceptibility in mice induced by antibiotics is correlated with the extent of decrease in fecal concentrations of secondary bile acids [31-34]. We have previously shown that secondary bile acids are entirely absent in the feces of patients with refractory RCDI, primary bile acids are abundant, and FMT promptly normalizes the fecal bile acid composition to approximate that of the donor, at least in patients with intact colons [10].
RCDI pouchitis may present an especially difficult challenge for FMT because the pouch, a limited reservoir draining the small bowel, may be exposed to relatively high concentrations of primary bile acids, taurocholate and cholate, and may not provide sufficient space for microbiota engraftment and restoration of secondary bile acid metabolism. Experience in using FMT in treating RCDI pouchitis has been very limited. Patel and colleagues reported use of FMT in one case [35], but the patient did not respond symptomatically and was continued on anti-CDI antibiotics for a month after the procedure, with ultimate resolution of CDI. Our own previous anecdotal experience in a patient with large segment partial colectomy has been associated with initial FMT failure [13], and other investigators have noted difficulty in tcuring RCDI with FMT following subtotal colectomy [14]. The patient described here also failed to clear RCDI following FMT; unfortunately, we were not able to collect immediate pre- and post-FMT fecal samples in this patient to evaluate microbiota engraftment or bile acid composition. In the future it will be interesting to examine capacity for bacterial 7α-dehydoxylation of bile acids, which is the key step in secondary bile acid metabolism that is critical for control of CDI [33], in pouch patients following a total or subtotal colectomy.
UDCA is a minor human secondary bile acid related to lithocholic acid as a derivative of chenodeoxycholic acid. It is absorbed into the enterohepatic circulation leading to beneficial effects on hepatocytes and cholangiocytes in cholestatic liver disease, which is the primary indication for its clinical use currently [36]. Its absorption in the distal small bowel also limits the achievement of the intracolonic concentrations necessary to inhibit C. difficile. However, we thought this limitation would not be prohibitive in a patient with an ileal pouch reservoir following a colectomy. Indeed, we were able to achieve fecal concentrations of UDCA at least 10-fold higher than were found sufficient to inhibit taurocholate-induced germination of C. difficile spores in vitro, despite the day-to-day variability that is characteristic of fecal spot measurements. Fecal microbial composition retained the general imprint of antibiotic treatments, such as expansion of Enterobacteraceae, after initiation of UDCA [29], making it highly unlikely that some spontaneous microbiota recovery contributed to C. difficile suppression. UDCA treatment was associated with a decrease in abundance of Fusobacteraceae and an increase in Lachnospiraceae families over time, a pattern seen with less pouch inflammation [29]. In fact, endoscopic examination did confirm resolution of pouchitis.
Although our clinical experience in treating refractory RCDI pouchitis with UDCA is thus far limited to a single patient case, we tested the potential of UDCA to inhibit spore germination and vegetative growth of multiple C. difficile strains, all of which were clinical isolates from patients with refractory RCDI who were treated with FMT within our program. This is especially notable since considerable diversity among C. difficile isolates has been reported previously with respect to bile acid-induced stimulation and inhibition of spore germination and outgrowth in vitro [27]. We found that the UDCA was inhibitory to both spore germination and vegetative growth of all C. difficile strains found in our collection, although minor variability in dose response was also observed among individual strains. Our results suggest that UDCA may be used as a therapeutic agent for treating RCDI pouchitis. Additional studies are needed to determine the optimal dosage and duration of treatment. These results also support the idea that bile acid derivatives inhibitory to C. difficile that are not absorbed into the enterohepatic circulation could be developed to treat RCDI colitis. Such agents might be especially useful for the many patients who derive minimal benefit from FMT because of their frequent requirement for antibiotic treatments for non-CDI indications.
Supplementary Material
Acknowledgements
This work was supported NIH grant 1R21-AI114722-01 (AK, MJS, CC) and NIH Training Grant UL1TR00114 to University of Minnesota CTSI (ARW).
Footnotes
None of the authors have a conflict of interest to declare relevant to this work. Ursodeoxycholic acid is not labeled for treatment of Clostridium difficile infection.
References
- 1.Zilberberg MD, Shorr AF, Kollef MH. Growth and geographic variation in hospitalizations with resistant infections, United States, 2000-2005. Emerg Infect Dis. 2008;14:1756–1758. doi: 10.3201/eid1411.080337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller BA, Chen LF, Sexton DJ, et al. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile Infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol. 2011;32:387–390. doi: 10.1086/659156. [DOI] [PubMed] [Google Scholar]
- 3.Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(Suppl 2):S88–92. doi: 10.1093/cid/cis335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly CP, LaMont JT. Clostridium difficile--more difficult than ever. N Engl J Med. 2008;359:1932–1940. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 6.Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol. 2012;9:88–96. doi: 10.1038/nrgastro.2011.244. [DOI] [PubMed] [Google Scholar]
- 7.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 8.Drekonja D, Reich J, Gezahegn S, et al. Fecal Microbiota Transplantation for Clostridium difficile Infection: A Systematic Review. Ann Intern Med. 2015;162:630–638. doi: 10.7326/M14-2693. [DOI] [PubMed] [Google Scholar]
- 9.Fuentes S, van Nood E, Tims S, et al. Reset of a critically disturbed microbial ecosystem: faecal transplant in recurrent Clostridium difficile infection. ISME J. 2014;8:1621–1633. doi: 10.1038/ismej.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weingarden AR, Chen C, Bobr A, et al. Microbiota transplantation restores normal fecal bile acid composition in recurrent Clostridium difficile infection. Am J Physiol Gastrointest Liver Physiol. 2014;306:G310–319. doi: 10.1152/ajpgi.00282.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weingarden A, Gonzalez A, Vazquez-Baeza Y, et al. Dynamic changes in short- and long-term bacterial composition following fecal microbiota transplantation for recurrent Clostridium difficile infection. Microbiome. 2015;3:10. doi: 10.1186/s40168-015-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seril DN, Shen B. Clostridium difficile infection in patients with ileal pouches. Am J Gastroenterol. 2014;109:941–947. doi: 10.1038/ajg.2014.22. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton MJ, Weingarden AR, Sadowsky MJ, et al. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107:761–767. doi: 10.1038/ajg.2011.482. [DOI] [PubMed] [Google Scholar]
- 14.Borody TJ, Leis S, Pang G, et al. Fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection. In: Rutgeerts P, ed. Fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection. 2014 UpToDate. [Google Scholar]
- 15.Petrof EO, Khoruts A. From stool transplants to next-generation microbiota therapeutics. Gastroenterology. 2014;146:1573–1582. doi: 10.1053/j.gastro.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson KH. Efficiency of various bile salt preparations for stimulation of Clostridium difficile spore germination. J Clin Microbiol. 1983;18:1017–1019. doi: 10.1128/jcm.18.4.1017-1019.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arroyo LG, Rousseau J, Willey BM, et al. Use of a selective enrichment broth to recover Clostridium difficile from stool swabs stored under different conditions. J Clin Microbiol. 2005;43:5341–5343. doi: 10.1128/JCM.43.10.5341-5343.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorg JA, Sonenshein AL. Bile salts and glycine as cogerminants for Clostridium difficile spores. J Bacteriol. 2008;190:2505–2512. doi: 10.1128/JB.01765-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sorg JA, Sonenshein AL. Chenodeoxycholate is an inhibitor of Clostridium difficile spore germination. J Bacteriol. 2009;191:1115–1117. doi: 10.1128/JB.01260-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorg JA, Sonenshein AL. Inhibiting the initiation of Clostridium difficile spore germination using analogs of chenodeoxycholic acid, a bile acid. J Bacteriol. 2010;192:4983–4990. doi: 10.1128/JB.00610-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofmann AF, Hagey LR. Bile acids: chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell Mol Life Sci. 2008;65:2461–2483. doi: 10.1007/s00018-008-7568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bliss DZ, Johnson S, Clabots CR, et al. Comparison of cycloserine-cefoxitinfructose agar (CCFA) and taurocholate-CCFA for recovery of Clostridium difficile during surveillance of hospitalized patients. Diagn Microbiol Infect Dis. 1997;29:1–4. doi: 10.1016/s0732-8893(97)00113-2. [DOI] [PubMed] [Google Scholar]
- 23.Caporaso JG, Lauber CL, Walters WA, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schloss PD, Westcott SL, Ryabin T, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edgar RC, Haas BJ, Clemente JC, et al. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cole JR, Wang Q, Cardenas E, et al. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009;37:D141–145. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heeg D, Burns DA, Cartman ST, et al. Spores of Clostridium difficile clinical isolates display a diverse germination response to bile salts. PLoS One. 2012;7:e32381. doi: 10.1371/journal.pone.0032381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamilton MJ, Weingarden AR, Unno T, et al. High-throughput DNA sequence analysis reveals stable engraftment of gut microbiota following transplantation of previously frozen fecal bacteria. Gut Microbes. 2013;4:125–135. doi: 10.4161/gmic.23571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reshef L, Kovacs A, Ofer A, et al. Pouch Inflammation is Associated with a Decrease in Specific Bacterial Taxa. Gastroenterology. 2015 doi: 10.1053/j.gastro.2015.05.041. [DOI] [PubMed] [Google Scholar]
- 30.Francis MB, Allen CA, Shrestha R, et al. Bile acid recognition by the Clostridium difficile germinant receptor, CspC, is important for establishing infection. PLoS Pathog. 2013;9:e1003356. doi: 10.1371/journal.ppat.1003356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giel JL, Sorg JA, Sonenshein AL, et al. Metabolism of bile salts in mice influences spore germination in Clostridium difficile. PLoS One. 2010;5:e8740. doi: 10.1371/journal.pone.0008740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Theriot CM, Koenigsknecht MJ, Carlson PE, Jr., et al. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat Commun. 2014;5:3114. doi: 10.1038/ncomms4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buffie CG, Bucci V, Stein RR, et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature. 2014 doi: 10.1038/nature13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koenigsknecht MJ, Theriot CM, Bergin IL, et al. Dynamics and Establishment of Clostridium difficile Infection in the Murine Gastrointestinal Tract. Infect Immun. 2014 doi: 10.1128/IAI.02768-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel LN, Schairer J, Shen B. Fecal transplantation therapy for Clostridium difficile-associated pouchitis. Int J Colorectal Dis. 2014;29:263–264. doi: 10.1007/s00384-013-1771-0. [DOI] [PubMed] [Google Scholar]
- 36.Hofmann AF. Biliary secretion and excretion in health and disease: current concepts. Ann Hepatol. 2007;6:15–27. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.