Abstract
Importance
The clinical utility of monitoring behavioral changes during intraoperative testing of acute subcallosal cingulate deep brain stimulation (DBS) is unknown.
Objective
To characterize structural connectivity correlates of DBS evoked behavioral effects using probabilistic tractography.
Design
Categorization of acute behavioral effects was conducted during DBS implantation surgery for treatment-resistant depression in a randomized and blinded testing session. Post-hoc analyses of the structural tractography patterns mediating distinct categories of evoked behavioral effects were defined.
Setting
Intra-operative testing during DBS surgery for depression at Emory University.
Participants
9 adult participants with chronic treatment-resistant depression undergoing DBS surgery.
Main Outcomes and Measures
Categorization of stimulation-induced transient behavioral effects and delineation of the shared white matter tracts mediating response subtypes.
Results
Two stereotypical behavior patterns were identified: changes in interoceptive (noted changes in body state) and in exteroceptive awareness (shift in attention from patient to others). Structural connectivity showed that ‘best’ responses had a pattern of connections to bilateral ventromedial frontal cortex (via uncinate fasciculus and forceps minor) and cingulate cortex (via cingulum bundle) while ‘salient’ contacts had only cingulate involvement.
Conclusions and Relevance
This analysis of acute intraoperative behaviors in SCC DBS, and the subsequent identification of unique connectivity patterns may provide a potential biomarker to guide and optimize surgical implantation and to refine and optimize algorithms for selection of contacts in chronic stimulation
Keywords: DBS, depression, diffusion MRI, tractography, cingulate cortex, ventromedial frontal cortex, mood, biomarker
Introduction
Intraoperative stimulation during DBS implantation surgery offers a unique window on localized brain function beyond its critical role in determining optimal targeting and stimulation parameters. Intraoperative effects with acute stimulation has a well recognized role during movement disorder surgery where such testing is commonly used to both optimize clinically desirable changes in tremor, rigidity or bradykinesia as well as avoid negative side effects such as diplopia and paresthesias.1 While use of this strategy have been most systematically employed using contemporary DBS techniques, behavioral and emotional responses to acute stimulation were first described during the early studies of intracranial self-stimulation conducted in the 1960s.2 With development and testing of new clinical indications for DBS, these non-motor acute behavioral phenomena have now been increasingly observed with stimulation at various brain targets across multiple neuropsychiatric disorders: euphoria, involuntary smiles and laughter within the nucleus accumbens;3–5 despair,6 apathy, hypomania and aggressive behavior7 in and around the subthalamic nucleus, panic8,9 in the posterior hypothalamus, episodic memory recollections in the fornix10, among others.
DBS of the subcallosal cingulate white matter (SCC DBS) is an emerging strategy for treatment-resistant depression.11–19 In addition to growing evidence of long-term efficacy with chronic stimulation, transient changes in mood, attention, and social connectedness have been reported during intraoperative testing. These observations are not exclusive to the SCC target, as such effects have also been described with stimulation in other putative depression targets including the medial forebrain bundle and nucleus accumbens, currently being studied.20–23
To date, the experience of patients during SCC testing has been notable for certain stereotypical features. Patients commonly describe ‘a sudden calmness or lightness’, disappearance of a ‘void,’ a sense of ‘connectedness,’ increased interest, and even sudden brightening of the room.11 These responses occur with stimulation in either hemisphere, are contact and current-dose specific, and most importantly, occur with stimulation at some but not all contacts along the DBS lead. Consistently, these behavioral effects quickly fade with discontinuation of the stimulation. When present, they are unequivocal and reproducible for each subject; however, due to the idiosyncratic nature of the self-reports, quantification has yet to be standardized. Interestingly, theses acute SCC stimulation effects are rarely duplicated outside of the OR and if present, are considerably more subtle. As with acute stimulation effects seen with other DBS targets, it is unclear if these SCC stimulation effects reflect changes in local SCC or more widespread network function.6,24 Also unknown is if they are predictive of long-term antidepressant response to chronic DBS.
Structural connectivity analyses using diffusion MRI and patient-specific tractography maps to define the extent of the white matter network impacted by chronic SCC DBS have recently demonstrated that clinically effective SCC stimulation requires inclusion of four white matter bundles in each hemisphere: forceps minor of the anterior corpus callosum connecting the two ventromedial frontal cortices, the cingulum bundle connecting ipsilateral SCC to rostral and dorsal anterior cingulate cortices, the uncinate fasciculus connecting the SCC to the ventromedial frontal cortex and frontal-subcortical fibers connecting SCC to the basal ganglia, thalamus and brainstem.24 Identification of these white matter bundles in advance of DBS lead implantation is now being tested prospectively to determine if targeting and stimulation at this white matter ‘hub’ improves clinical outcomes over standard methods.25 With this foundation, this study examined similarly derived structural connectivity patterns of subcallosal cingulate stimulation mediating acute intraoperative behavior responses with the goal to identify an intraoperative biomarker of optimal SCC lead placement.
Methods
Participants
Between September 2011 and June 2013, nine consecutive patients with severe, chronic treatment-resistant major depressive disorder (TRD) were enrolled in a research protocol at Emory University testing the safety and efficacy of SCC DBS (clinicaltrials.gov NCT00367003). The protocol is approved by Emory University Institutional Review Board and the US Food and Drug Administration under a physician sponsored Investigational Device Exemption (G060028, HSM sponsor) and is monitored by the Emory University Department of Psychiatry and Behavioral Sciences Data and Safety Monitoring Board. All participants signed an informed consent to participate.
The study inclusion and exclusion criteria were identical to those previously published by Holtzheimer et al.14 In brief, patients were required to be depressed for at least 12 months in the current depressive episode with a minimum Hamilton Depression Rating Scale severity score of 20; to have failed at least 4 antidepressant treatments (including Electroconvulsive therapy); to have no significant psychiatric or medical comorbidities, and to be functionally disabled with a GAF (Global Assessment of Function) score <50 (Table 1).
Table 1.
Demographic and Clinical Characteristics of TRD Patients
| Characteristics | Patients with TRD |
|---|---|
| Age (years), mean (SD) | 46.11 (8.95) |
| Gender, Female/Male | 7/2 |
| HamD17 at surgery, mean (SD) | 22.53 (2.78) |
| Age onset of MDD, mean (SD) | 21.11 (11.61) |
| Lifetime number of MDD episode, mean (SD) | 3.67 (1.58) |
| Duration of current episode (months), mean (SD) | 36.67 (20) |
Abbreviations: HamD17 – 17 Item Hamilton Depression Rating Scale; MDD – Major Depressive Disorder
DBS Implantation Surgery
The surgical procedure for DBS lead and pulse generator implantation followed published methods.11,12,14 Extending a previously described frame-based, stereotaxic anatomical localization protocol whereby the grey-white matter junction at the mid-subcallosal cingulate region is identified on high resolution T1 MRI structural images,11,14 target selection for this patient cohort also utilized an individualized pre-operative deterministic tractography map identifying the location of the intersection of 4 white matter bundles recently shown necessary for effective antidepressant effects.26 The combined tractography and anatomical images guided standard localization of the DBS lead tip and trajectory using a surgical planning workstation (Stealth, Medtronic Inc). Bilateral DBS leads (Libra system, St Jude Medical, Plano, TX), each with 4 contacts (1.5 mm inter-contact spacing) were placed and secured and with the patient awake and alert, testing was initiated.
Intraoperative Behavior Response Assessments
The stimulation protocol consisted of 12 trials (one at each of the 8 available contacts; 4 left, 4 right plus 4 sham trials) of 3 minutes stim-on followed by 3 minutes stim-off using standard parameters employed for chronic SCC stimulation (monopolar stimulation, frequency=130 Hz, pulse width=90 μsec, current = 6mA). The order of active or ‘sham’ trials were randomized with both subject and the clinician rater blinded to the condition. The 3 minute On/3 minute Off design maximized the likelihood of adequate time to capture both acute (within the first minute) and sustained (maintained throughout the stimulation epoch) behavioral changes as well as to ensure that a new baseline was re-established prior to the subsequent trial. The procedure was videotaped to review, verify and catalogue patient comments outside of the OR. Patients were instructed to monitor themselves during each trial for any changes in sensation, feelings, mood or thought and to describe any changes when queried. Self-reports were recorded at fixed time points within each trial (1 minute after initiation of stimulation and again 1 minute following discontinuation of stimulation). At the conclusion of the protocol, responses for each of the 12 trials were reviewed and designated as either response present or absent. In positive response trials, features of the response were further classified into two categorical ‘types’ based on the salience, quality and magnitude of the self-report. Response Type 1 was defined by presence solely of a perceived change in body state (i.e., interoceptive awareness), or specific physical sensations. Response Type 2 was characterized by a more complex set of evoked thoughts and feelings commonly indicated by a shift in attention from themselves to others (exteroceptive awareness). The number of responses (either Type 1 or Type 2) was summed for each hemisphere and a ‘best’ contact was then selected reflecting the most robust combination of interoceptive (Type 1) and complex behavioral phenomena (Type 2) overall. Once rankings were completed, trials were unblinded and contacts (Left 1 – 4; Right 1 – 4) were matched to trial and response types. These classifications were subsequently used for structural connectivity analyses to define white matter tracts mediating the ‘Best’ versus any ‘Salient’ Responses (either Type 1 or Type 2 alone) as well as differences between right and left hemisphere stimulation effects.
Imaging Acquisition Protocol
One week prior to surgery, magnetic resonance imaging was performed using a Siemens 3.0-T Tim-Trio scanner (Siemens Medical Solutions, Malvern, PA, USA) at the Biomedical Imaging Technology Center (BITC) of Emory University. A high-resolution T1-weighted structural image was collected for each subject using 3D magnetization-prepared rapid gradient-echo (MPRAGE) sequence with following parameters: TR/TI/TE = 2600/900/3.02 ms; a flip angle of 8°, voxel resolution = 1×1×1 mm; number of slices = 176; matrix = 224×256. Sixty non-collinear diffusion weighted images were obtained using single-shot spin-echo echo-planar imaging with following parameters: generalized auto-calibration parallel acquisition (GRAPPA)27 R = 2; FOV = 256 × 256; b value = 1000 sec/mm2; voxel resolution = 2×2×2 mm; number of slices = 64; matrix = 128 × 128; TR/TE = 11300/104ms; four non-diffusion weighted images (b=0); two different phase encoding directions (anterior – posterior and posterior – anterior) to compensate susceptibility-induced distortion. Three weeks after surgery, a high-resolution computer tomography (CT) image was collected on a LightSpeed 16 (GE Medical System, 0.46×0.46×0.65 mm3 voxel size) to identify a lead and contact location.
Image Processing
All image processing and analyses were performed using tools from the FMRIB Software Library (http://www.fmrib.ox.ac.uk/fsl).28,29 First, T1 image were normalized into MNI152 template using a combination of linear and nonlinear registration method (Flirt and Fnirt, FSL). Second, DTI data underwent simultaneous eddy-current and movement correction (Eddy, FSL), skull stripping (BET, FSL)30, and local tensor fitting (FDT, FSL).31 Then, DTI data were co-registered to T1 image using Boundary-Based Registration (BBR)32 method and normalized to MNI152 template using a previously calculated nonlinear warping field from T1 normalization step.
Leads Localization and Volume of Tissue Activated Modeling
The location of each of the eight contacts was identified using a post-operative CT image for each subject (Figure 1). First, the CT image was transferred to T1 space by linear transformation and each contact location was identified in T1 native space. Second, a patient specific Volume of Tissue Activated (VTA) was generated for each contact in T1 native space using DBS activation volume prediction modeling methods (Chaturvedi et al),33–35 and the following stimulation parameters: frequency = 130Hz, pulse width = 90us, and stimulation amplitude = 6mA and individual impedance measures. Lastly, the patient-specific VTAs were transformed to the MNI152 template for use as seeds for the probabilistic tractography analyses.
Figure 1.
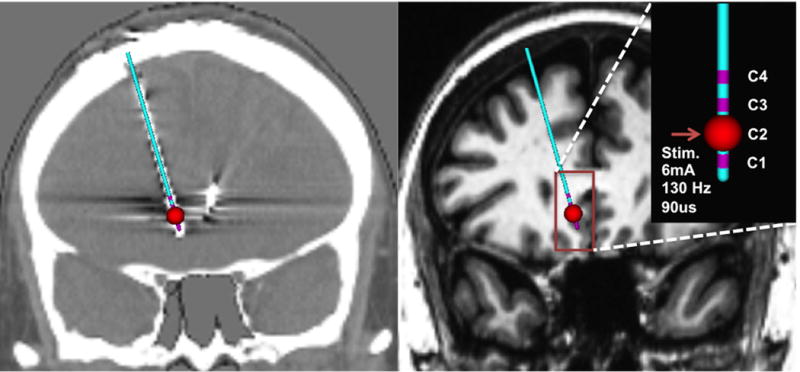
Identification of DBS lead in post-op CT (left) and Patient-specific volume of tissue activated modeling in T1 image (right) with given stimulation parameters frequency = 130Hz, pulse width = 90us, and stimulation amplitude = 6mA
Connectivity analysis of Acute Intraoperative Behavior
Whole-brain probabilistic fiber tractography was generated from each of the patient-specific VTA seeds to construct a structural connectivity map (Fdt, fsl).31 Eight different structural connectivity maps from patient-specific VTA seeds (four contacts in each hemisphere) were generated in each subject. Five thousand streamlines were sent out from each voxel within the VTA with masking of the CSF to reduce false positive connections. A streamline density map was generated by the number of connected streamlines divided by the total number of streamlines sent out.36 Next, the streamline density map was binarized at a threshold value of 0.2% following an optimization procedures testing various threshold values (0.01% ~ 1%).37 Lastly, the binarized maps were summed and divided by the total number of each specific response types to calculate the overlap map.
To identify the specific white matter bundles mediating the defined Response types, a common shared map (80% shared voxels; i.e., 7 of the 9 subjects for a best response) was generated for (1) the ‘Best’ contacts (regardless of hemisphere), (2) the non-best but otherwise ‘Salient’ contacts (left and right hemisphere contacts grouped separately) and (3) those with no response.
Results
Intraoperative Behavior Response Characteristics
Among the nine subjects, a total of 108 individual stimulation trials were recorded (72 active - 36 per hemisphere- and 36 sham). Thirty contacts generated a type I ‘salient’ response (17 on the left hemisphere, 13 on the right hemisphere) while 42 contacts evoked no response. Of the 36 sham trials, only 4 generated some mild Type 1 responses; none of Type 2. As previously observed, behavioral changes were apparent to subjects within the first minute of the initiation of the stimulation and effects were sustained while stimulation remained on; patients generally noted a clear fading of any effects within the first minute following discontinuation returning to their pre-trial baseline after about 2 minutes. The ‘Best’ contact for each of the 9 subjects was always in the left hemisphere. Self-reports from these ‘best’ contacts showed robust Type 1 and Type 2 responses: ‘lightening of mood’, ‘feeling warm’, ‘lighter’, ‘feeling more connected’, ‘I can get outside of myself to pay attention to you’, noticing objects, people and activities ongoing in the operating room, interest and perceived capacity to engage in various personally relevant activities if they were home (taking a shower, washing the dishes, walking the dog). ‘Salient’ (positive, but non-best) responses occurred with equal frequency with stimulation of contacts in either hemisphere. Self report statements included ‘lifting’, ‘less heaviness’, ‘less tension’, ‘increased air and ability to breathe’, ‘a feeling of relief’. These physical sensations were commonly accompanied by changes in facial expression observed by the rater (eyes widening, softening of corrugator muscle contractions) and increases in verbal fluency and speech output. Overall the ‘Salient’ responses involved primarily changes in interoceptive awareness (type 1 responses). In contrast, the ‘Best’ responses were consistently characterized by combination of interoceptive changes (type 1) and exteroceptive attention and engagement (type 2). Samples of spontaneous statements and clinical observations from the 9 patients are listed in Table 2.
Table 2.
Narrative descriptions recorded by participants
| Subject | Best Response (Type 1 and 2 responses) | Salient Response (Type 1 responses) |
|---|---|---|
| 1 | Contact: Left 3 “I would choose this setting” “My head is getting clearer” “Thinking about making flower arrangements” “I Feel more connected” Speech is clearer, less retardation noted |
Contacts: Right 2, Right 3, Right 4 “My body seems more alive” Talking faster |
| 2 | Contact: Left 3 “There is a stronger sensation of being lighter. It is a mental sensation” “Lightness of my mood that came with the lightness of my feelings” “It would be easier to have a conversation with my partner” |
Contacts: Left 1, Right 2 “I feel lighter, not so surrounded” “It is a physical (not mental) sensation” “The blanket over me got lighter” |
| 3 | Contact: Left 3 “I imagine myself listening to a song I like” More connected with interviewer More animated voice |
Contacts: Left 2, Left 4, Right 4 “Lessened feeling of negative” |
| 4 | Contact: Left 4 “I feel a tingling of the upper body, good feeling” “I feel my heart beating faster” “Mental lightness…” “Easier to do things at home” “More connected with my partner” “I feel my heart beating faster” |
Contacts: Left 3, Right 1, Right3 Warmth, tingling of upper body |
| 5 | Contact: Left 3 “I feel like going dancing” “I am thinking happy thoughts” “I would like to listen to music” |
Contacts: Right 1, Right 3 smiling, mood a little better |
| 6 | Contact: Left 2 “Good change, lights are brighter, feel lighter emotionally” “I would like to hold my daughter” More awake and engaged in conversation |
Contact: Right 2 “I feel lighter, but not sure if I trust it” |
| 7 | Contact: Left 2 “I felt like laughing, I feel good” “I could be washing dishes in the kitchen, the dishes are done!” “Everything is lighter and easier“ “I would be walking my dog” Patient smiles |
Contacts: Left 3, Right 3 “I noticed the lights, brighter and nice” |
| 8 | Contact: Left 2 “I would like to go back to Cancun with my son” |
Contacts: Left 1, Left 4, Right 3 “Warm feelings…” “…in my own world” |
| 9 | Contact: Left 3 “I feel a mental clearing preceding physical sensation” |
Contacts: Left 1, Right 2 “I have a calm sensation” |
Behavioral Connectivity Analyses
Three common white matter bundles were impacted by stimulation of the 9 left-sided contacts mediating a ‘Best’ response: fibers connecting both ventromedial frontal cortices (ipsilateral (left) via the Uncinate Fasciculus and Forceps Minor and contralaterally through the Forceps Minor), as well as to the anterior cingulate cortex via the Cingulum Bundle (Figure 2).38,39 White matter bundles mediating the ‘Salient’ responses were limited to the ipsilateral cingulate bundle with a mirror pattern for right and left sided contacts (Figure 3). In contrast to ‘Best’ and ‘Salient’, the ‘No behavior’ contacts shared no common pathways regardless of hemisphere.
Figure 2.
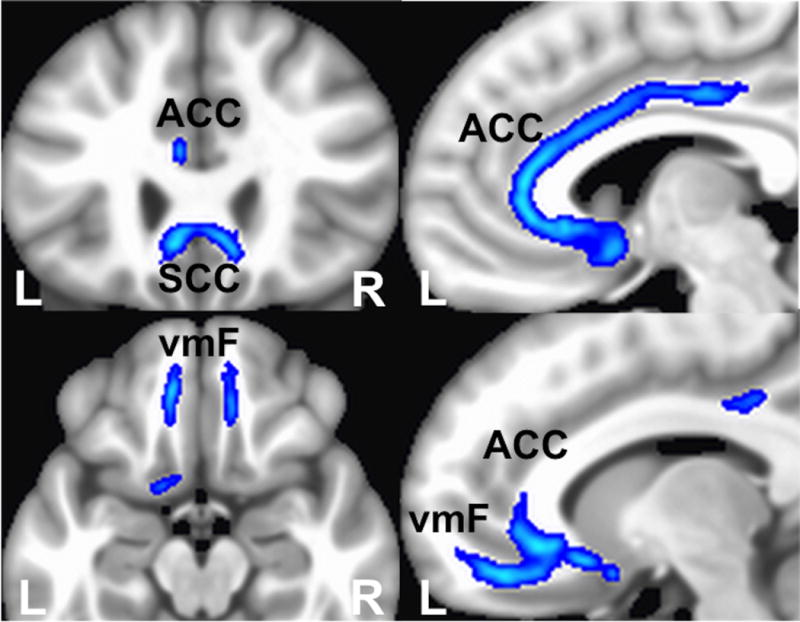
Whole-brain probabilistic tractography of shared fiber tract map of contacts with ‘Best’ intraoperative responses. Three common white matter bundles: Uncinate Fasciculus connecting to ipsilateral ventromedial frontal cortex, Forceps Minor connecting to bilateral ventromedial frontal cortices, and left cingulum bundle connecting to ipsilateral anterior cingulate cortex, Abbreviations: ACC - Anterior Cingulate Cortex, SCC – Subcallosal Cingulate Cortex, vmF – ventromedial frontal cortex
Figure 3.
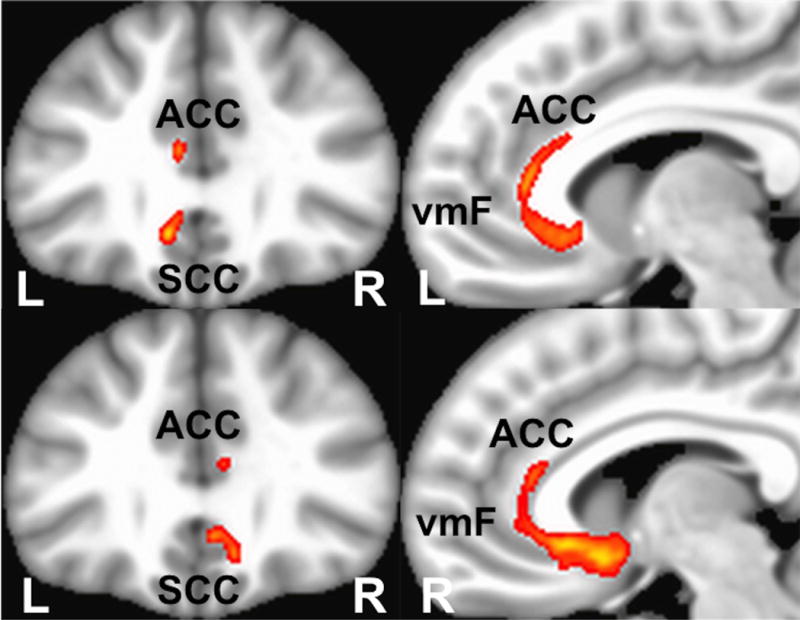
Whole-brain probabilistic tractography of shared fiber tract map of contacts with ‘Salient’ intraoperative responses. The common white matter bundle: cingulum bundle connecting to ipsilateral anterior cingulate cortex. Top two images are left contacts with ‘Salient’ intraoperative response, Bottom two images are right hemisphere contacts, Abbreviations: ACC - Anterior Cingulate Cortex, SCC – Subcallosal Cingulate Cortex, vmF – ventromedial frontal cortex
Discussion
This study characterized the tractography patterns mediating transient, stereotypical behavioral changes evoked by acute high frequency SCC stimulation performed during DBS lead implantation surgery in patients with TRD. Double-blinded evaluations during the acute stimulation test of each contact revealed two behavioral response patterns. Type 1, a change in interoceptive state (less tension, lighter, warmth), was reported with stimulation at more than one contact in either the right or left hemisphere for most patients. Type 2 responses were more multifaceted, with complex shifts in attentional focus, social connectedness and interest occurring only with stimulation of left contacts. Notably, contacts eliciting a robust Type 2 response also showed a Type 1 response immediately preceding the self-referential and action oriented declarations. These two part responses were consistently the contact categorized as ‘Best’ for every subject. Structural connectivity analyses further demonstrated that these distinct response ‘types’ were mediated by differential impact on the ventromedial frontal cortex and cingulate with bilateral frontal white matter tracts distinguishing ‘Best’ from merely ‘Salient’ contacts where only the cingulate was involved. Interestingly, the responses were mostly described as a relief and lessening of a negative state rather than the sudden appearance of positive mood as described in other DBS targets like nucleus accumbens, medial forebrain bundle or ventral capsule.
Interoceptive awareness has been operationally defined as perception of physiological changes in bodily states,40–42 with quantitative measures of autonomic reactivity often used as a physiological surrogate. The role of the ventral, anterior and mid-cingulate in these behaviors is well established. Functional imaging studies have demonstrated activity changes in both the SCC and the mid-cingulate to correlate with the blood pressure, heart rate and skin conductance changes during a variety of mental and emotional tasks.43–54 Activation of these regions has been similarly reported in studies of provoked visceral, somatic and emotional pain.55–60 These regions have been further classified as key components in the so-called resting-state salience network61,62 that mediates shifts between personally relevant internal and external stimuli.63 Changes in both the SCC and the dorsal anterior and mid-cingulate activity are repeated reported across antidepressant treatment trials using various interventions including SCC DBS.64 Similarly changes in these regions are among the most robust with acute sad mood induction.65,66 Across studies, both left and right sided activity changes have been reported, consistent with the lack of lateralized effects seen here with acute stimulation. While other regions, including the anterior insula and frontal cortex are clearly critical to a full interoceptive experience and a complete recovery from depression,67 the common involvement of the cingulate in both the Type 1 ‘Salient’ and Type 2 ‘Best’ response types is nonetheless consistent with this principal role of the cingulate in interoceptive processing.
Exteroceptive awareness in contrast, is defined as the perception of external stimuli and the shifting of attention away from the self towards the environment.68 Imaging studies have repeatedly demonstrated a role of the ventromedial frontal cortex in activities utilizing exteroceptive engagement such as mentalizing, self-knowledge and outcome monitoring.69 Ventromedial frontal cortex activity has specifically been demonstrated during self-referential processing of emotional words and pictures,70–72 in generating external versus internal emotional states,73 in recognizing and imagining the experience of others.74,75 Particularly relevant to depression, medial frontal cortex has an important role in protecting the execution of long-term mental plans from immediate environmental or internal demands.76 Patients with depression commonly show hypoactivity of these regions and inappropriate deactivation during negative mood challenge.77,78 Not surprisingly, the medial frontal cortex has a critical role in the resting state default mode network and has shown hyperconnectivity to the SCC in TRD.79 Failure of this system to shift towards external stimuli and remain stuck inside one’s ‘self’ is the very definition of the TRD state. The common involvement of bilateral medial frontal fibers linking directly to the SCC as well as passing fibers connecting the medial frontal to the amygdala via the uncinate fasciculus during stimulation of the ‘Best’ contacts suggests a mechanisms for the observed transient depression ‘switch’ from a pervasive ‘stuck negative’ state to sensing sudden capacity to get outside of themselves.
The significant overlap of the tract patterns seen with the ‘best’ contacts with those mediating long-term antidepressant response26 further suggests that these transient behavioral effects may provide a behavioral biomarker of the optimal site for long-term DBS, particularly at the left contact. In support of this hypothesis, 7 of the 9 patients using the Type 2 ‘best’ left contact were responders at 6 months.
This study has a number of limitations. Intraoperative testing is a strenuous task for patients, and some important responses may have been missed due to patient’s pain or fatigue. Therefore, lack of response to a particular contact does not mean that a particular contact in not the desired target or impacting a certain circuit. Clinical ratings, although done in a double blinded fashion, can still misinterpret some observations that are unique to individuals; further not all ‘salient’ responses are of the same intensity. It may be preferable to utilize continuous measures, such as heart rate, skin conductance, facial or speech recognition technology, that may capture more nuanced phenomena than the categorical metrics utilized here. Current work is focused on concurrent or antecedent changes in autonomic reactivity, facial expression and speech output, as well as electrophysiologic changes in SCC and frontal cortex. In regards to the tractography technique, there are resolution limits set by the acquisition protocol and analytic methods. It is therefore possible that other shared bundles, particularly small or more variable subcortical tracts may have been missed.
It is as yet, untested if left sided stimulation alone would be adequate to achieve a full antidepressant response as to date, only bilateral stimulation has been evaluated. However, current tractography studies of effective bilateral stimulation demonstrate that bilateral cingulum and uncinate fasciculus in addition to crossing fibers within the forceps minor are needed. Explicit comparisons of right and left unilateral stimulation await future investigation.
In treatment-resistant depression, pervasive negative mood and mental anguish, disinterest and social disengagement as well as paucity of thought and action are prominent clinical features. We posit that acute stimulation in the requisite combination of fibers impacting bilateral ventral frontal cortex plus the anterior and mid cingulate has immediate but transient effects on these core depression features and represents the first stage of depression network engagement required for long-term antidepressant effects of SCC DBS.
Acknowledgments
We thank the following individuals for their contributions to the study from which these data were derived: psychiatrists Steve Garlow, MD PhD, Andrea Crowell, MD, image and data analyst Justin Rajendra and research coordinator Sinead Quinn. Grant Support: Dana Foundation and the Hope for Depression Research Foundation, NIH R01 NS059736. Device Donation: St Jude Medical, Inc. Ki Sueng Choi, PhD, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Abbreviations
- DBS
deep brain stimulation
- SCC
subcallosal cingulate
Footnotes
Financial Disclosures. Dr. Mayberg has received consulting and intellectual licensing fees from St. Jude Medical Inc. Robert E. Gross has received consulting fees from Boston Scientific Neuromodulation, St. Jude Medical Neuromodulation, and Medtronic/Lilly. All other authors report no commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Rezai AR, Kopell BH, Gross RE, et al. Deep brain stimulation for Parkinson’s disease: surgical issues. Mov Disord. 2006;21(Suppl 14):S197–218. doi: 10.1002/mds.20956. [DOI] [PubMed] [Google Scholar]
- 2.Bishop MP, Elder ST, Heath RG. Intracranial self-stimulation in man. Science. 1963;140(3565):394–396. doi: 10.1126/science.140.3565.394. [DOI] [PubMed] [Google Scholar]
- 3.Nuttin BJ, Gabriels LA, Cosyns PR, et al. Long-term electrical capsular stimulation in patients with obsessive-compulsive disorder. Neurosurgery. 2003;52(6):1263–1272. doi: 10.1227/01.neu.0000064565.49299.9a. discussion 1272-1264. [DOI] [PubMed] [Google Scholar]
- 4.Okun MS, Foote KD. A mnemonic for Parkinson disease patients considering DBS: a tool to improve perceived outcome of surgery. Neurologist. 2004;10(5):290. doi: 10.1097/01.nrl.0000138737.97544.7c. [DOI] [PubMed] [Google Scholar]
- 5.Krack P, Vercueil L. Review of the functional surgical treatment of dystonia. Eur J Neurol. 2001;8(5):389–399. doi: 10.1046/j.1468-1331.2001.00231.x. [DOI] [PubMed] [Google Scholar]
- 6.Bejjani BP, Damier P, Arnulf I, et al. Transient acute depression induced by high-frequency deep-brain stimulation. N Engl J Med. 1999;340(19):1476–1480. doi: 10.1056/NEJM199905133401905. [DOI] [PubMed] [Google Scholar]
- 7.Bejjani BP, Houeto JL, Hariz M, et al. Aggressive behavior induced by intraoperative stimulation in the triangle of Sano. Neurology. 2002;59(9):1425–1427. doi: 10.1212/01.wnl.0000031428.31861.23. [DOI] [PubMed] [Google Scholar]
- 8.Rasche D, Rinaldi PC, Young RF, Tronnier VM. Deep brain stimulation for the treatment of various chronic pain syndromes. Neurosurg Focus. 2006;21(6):E8. doi: 10.3171/foc.2006.21.6.10. [DOI] [PubMed] [Google Scholar]
- 9.Shapira NA, Okun MS, Wint D, et al. Panic and fear induced by deep brain stimulation. J Neurol Neurosurg Psychiatry. 2006;77(3):410–412. doi: 10.1136/jnnp.2005.069906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamani C, McAndrews MP, Cohn M, et al. Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann Neurol. 2008;63(1):119–123. doi: 10.1002/ana.21295. [DOI] [PubMed] [Google Scholar]
- 11.Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64(6):461–467. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 13.Lozano AM, Giacobbe P, Hamani C, et al. A multicenter pilot study of subcallosal cingulate area deep brain stimulation for treatment-resistant depression. J Neurosurg. 2012;116(2):315–322. doi: 10.3171/2011.10.JNS102122. [DOI] [PubMed] [Google Scholar]
- 14.Holtzheimer PE, Kelley ME, Gross RE, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry. 2012;69(2):150–158. doi: 10.1001/archgenpsychiatry.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puigdemont D, Perez-Egea R, Portella MJ, et al. Deep brain stimulation of the subcallosal cingulate gyrus: further evidence in treatment-resistant major depression. Int J Neuropsychopharmacol. 2012;15(1):121–133. doi: 10.1017/S1461145711001088. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy SH, Giacobbe P, Rizvi SJ, et al. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am J Psychiatry. 2011;168(5):502–510. doi: 10.1176/appi.ajp.2010.10081187. [DOI] [PubMed] [Google Scholar]
- 17.Merkl A, Schneider GH, Schonecker T, et al. Antidepressant effects after short-term and chronic stimulation of the subgenual cingulate gyrus in treatment-resistant depression. Exp Neurol. 2013;249:160–168. doi: 10.1016/j.expneurol.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 18.Ramasubbu R, Anderson S, Haffenden A, Chavda S, Kiss ZH. Double-blind optimization of subcallosal cingulate deep brain stimulation for treatment-resistant depression: a pilot study. J Psychiatry Neurosci. 2013;38(5):325–332. doi: 10.1503/jpn.120160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guinjoan SM, Mayberg HS, Costanzo EY, et al. Asymmetrical contribution of brain structures to treatment-resistant depression as illustrated by effects of right subgenual cingulum stimulation. J Neuropsychiatry Clin Neurosci. 2010;22(3):265–277. doi: 10.1176/jnp.2010.22.3.265. [DOI] [PubMed] [Google Scholar]
- 20.Schlaepfer TE, Cohen MX, Frick C, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33(2):368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- 21.Schlaepfer TE, Bewernick BH, Kayser S, Madler B, Coenen VA. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry. 2013;73(12):1204–1212. doi: 10.1016/j.biopsych.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 22.Bewernick BH, Hurlemann R, Matusch A, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67(2):110–116. doi: 10.1016/j.biopsych.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Bewernick BH, Kayser S, Sturm V, Schlaepfer TE. Long-term effects of nucleus accumbens deep brain stimulation in treatment-resistant depression: evidence for sustained efficacy. Neuropsychopharmacology. 2012;37(9):1975–1985. doi: 10.1038/npp.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stefurak T, Mikulis D, Mayberg H, et al. Deep brain stimulation for Parkinson’s disease dissociates mood and motor circuits: a functional MRI case study. Mov Disord. 2003;18(12):1508–1516. doi: 10.1002/mds.10593. [DOI] [PubMed] [Google Scholar]
- 25.Riva-Posse P. Identification of Critical White Matter Pathways involved in Efficacious Subcallosal Cingulate Deep Brain Stimulation: Implications for Prospective Target Selection. Biological Psychiatry. 2014;7S(9):S15. doi: 10.1016/j.biopsych.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riva-Posse P, Choi KS, Holtzheimer PE, et al. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2014;76(12):963–969. doi: 10.1016/j.biopsych.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griswold MA, Jakob PM, Heidemann RM, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA) Magn Reson Med. 2002;47(6):1202–1210. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- 28.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 29.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Behrens TE, Woolrich MW, Jenkinson M, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50(5):1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- 32.Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48(1):63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaturvedi A, Lujan JL, McIntyre CC. Artificial neural network based characterization of the volume of tissue activated during deep brain stimulation. J Neural Eng. 2013;10(5):056023. doi: 10.1088/1741-2560/10/5/056023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lujan JL, Chaturvedi A, Malone DA, Rezai AR, Machado AG, McIntyre CC. Axonal pathways linked to therapeutic and nontherapeutic outcomes during psychiatric deep brain stimulation. Hum Brain Mapp. 2012;33(4):958–968. doi: 10.1002/hbm.21262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McIntyre CC, Miocinovic S, Butson CR. Computational analysis of deep brain stimulation. Expert Rev Med Devices. 2007;4(5):615–622. doi: 10.1586/17434440.4.5.615. [DOI] [PubMed] [Google Scholar]
- 36.Hagmann P, Cammoun L, Gigandet X, et al. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6(7):e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L, Rilling JK, Preuss TM, Glasser MF, Hu X. The effects of connection reconstruction method on the interregional connectivity of brain networks via diffusion tractography. Hum Brain Mapp. 2012;33(8):1894–1913. doi: 10.1002/hbm.21332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wakana S, Caprihan A, Panzenboeck MM, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36(3):630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hua K, Zhang J, Wakana S, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39(1):336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiens S. Interoception in emotional experience. Curr Opin Neurol. 2005;18(4):442–447. doi: 10.1097/01.wco.0000168079.92106.99. [DOI] [PubMed] [Google Scholar]
- 41.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 42.Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13(4):500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 43.Critchley HD, Elliott R, Mathias CJ, Dolan RJ. Neural activity relating to generation and representation of galvanic skin conductance responses: a functional magnetic resonance imaging study. J Neurosci. 2000;20(8):3033–3040. doi: 10.1523/JNEUROSCI.20-08-03033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Critchley HD, Mathias CJ, Josephs O, et al. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126(Pt 10):2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- 45.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7(2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 46.Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493(1):154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- 47.Critchley HD, Eccles J, Garfinkel SN. Interaction between cognition, emotion, and the autonomic nervous system. Handb Clin Neurol. 2013;117:59–77. doi: 10.1016/B978-0-444-53491-0.00006-7. [DOI] [PubMed] [Google Scholar]
- 48.Critchley HD, Harrison NA. Visceral influences on brain and behavior. Neuron. 2013;77(4):624–638. doi: 10.1016/j.neuron.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 49.Fredrickson BL. What Good Are Positive Emotions? Rev Gen Psychol. 1998;2(3):300–319. doi: 10.1037/1089-2680.2.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pereira EA, Wang S, Paterson DJ, Stein JF, Aziz TZ, Green AL. Sustained reduction of hypertension by deep brain stimulation. J Clin Neurosci. 2010;17(1):124–127. doi: 10.1016/j.jocn.2009.02.041. [DOI] [PubMed] [Google Scholar]
- 51.Pollatos O, Kirsch W, Schandry R. Brain structures involved in interoceptive awareness and cardioafferent signal processing: a dipole source localization study. Hum Brain Mapp. 2005;26(1):54–64. doi: 10.1002/hbm.20121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barrett LF, Simmons WK. Interoceptive predictions in the brain. Nat Rev Neurosci. 2015;16(7):419–429. doi: 10.1038/nrn3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lane RD, Fink GR, Chau PM, Dolan RJ. Neural activation during selective attention to subjective emotional responses. Neuroreport. 1997;8(18):3969–3972. doi: 10.1097/00001756-199712220-00024. [DOI] [PubMed] [Google Scholar]
- 54.Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey: II. Cortical afferents. J Comp Neurol. 1987;262(2):271–289. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- 55.Vogt BA, Sikes RW. The medial pain system, cingulate cortex, and parallel processing of nociceptive information. Prog Brain Res. 2000;122:223–235. doi: 10.1016/s0079-6123(08)62141-x. [DOI] [PubMed] [Google Scholar]
- 56.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6(7):533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vogt BA. Submodalities of emotion in the context of cingulate subregions. Cortex. 2014;59:197–202. doi: 10.1016/j.cortex.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 58.Wager TD, van Ast VA, Hughes BL, Davidson ML, Lindquist MA, Ochsner KN. Brain mediators of cardiovascular responses to social threat, part II: Prefrontal-subcortical pathways and relationship with anxiety. Neuroimage. 2009;47(3):836–851. doi: 10.1016/j.neuroimage.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12(8):453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mayer EA, Gupta A, Kilpatrick LA, Hong JY. Imaging brain mechanisms in chronic visceral pain. Pain. 2015;156(Suppl 1):S50–63. doi: 10.1097/j.pain.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21(4):424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- 62.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mayberg HS. Targeted electrode-based modulation of neural circuits for depression. J Clin Invest. 2009;119(4):717–725. doi: 10.1172/JCI38454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156(5):675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 66.Liotti M, Mayberg HS, Brannan SK, McGinnis S, Jerabek P, Fox PT. Differential limbic–cortical correlates of sadness and anxiety in healthy subjects: implications for affective disorders. Biol Psychiatry. 2000;48(1):30–42. doi: 10.1016/s0006-3223(00)00874-x. [DOI] [PubMed] [Google Scholar]
- 67.Jones CL, Minati L, Nagai Y, et al. Neuroanatomical substrates for the volitional regulation of heart rate. Front Psychol. 2015;6:300. doi: 10.3389/fpsyg.2015.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351(1346):1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- 69.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 70.Fossati P. Neural correlates of emotion processing: from emotional to social brain. Eur Neuropsychopharmacol. 2012;22(Suppl 3):S487–491. doi: 10.1016/j.euroneuro.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 71.Smith R, Braden BB, Chen K, Ponce FA, Lane RD, Baxter LC. The neural basis of attaining conscious awareness of sad mood. Brain Imaging Behav. 2014 doi: 10.1007/s11682-014-9318-8. [DOI] [PubMed] [Google Scholar]
- 72.Simpson JR, Jr, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: I. During cognitive task performance. Proc Natl Acad Sci U S A. 2001;98(2):683–687. doi: 10.1073/pnas.98.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reiman EM, Lane RD, Ahern GL, et al. Neuroanatomical correlates of externally and internally generated human emotion. Am J Psychiatry. 1997;154(7):918–925. doi: 10.1176/ajp.154.7.918. [DOI] [PubMed] [Google Scholar]
- 74.Nyberg L, Kim AS, Habib R, Levine B, Tulving E. Consciousness of subjective time in the brain. Proc Natl Acad Sci U S A. 2010;107(51):22356–22359. doi: 10.1073/pnas.1016823108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Addis DR, Pan L, Vu MA, Laiser N, Schacter DL. Constructive episodic simulation of the future and the past: distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia. 2009;47(11):2222–2238. doi: 10.1016/j.neuropsychologia.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 76.Koechlin E, Hyafil A. Anterior prefrontal function and the limits of human decision-making. Science. 2007;318(5850):594–598. doi: 10.1126/science.1142995. [DOI] [PubMed] [Google Scholar]
- 77.Liotti M, Mayberg HS. The role of functional neuroimaging in the neuropsychology of depression. J Clin Exp Neuropsychol. 2001;23(1):121–136. doi: 10.1076/jcen.23.1.121.1223. [DOI] [PubMed] [Google Scholar]
- 78.Kruger S, Seminowicz D, Goldapple K, Kennedy SH, Mayberg HS. State and trait influences on mood regulation in bipolar disorder: blood flow differences with an acute mood challenge. Biol Psychiatry. 2003;54(11):1274–1283. doi: 10.1016/s0006-3223(03)00691-7. [DOI] [PubMed] [Google Scholar]
- 79.Greicius MD, Flores BH, Menon V, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62(5):429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]


