Abstract
Moths and butterflies (Lepidoptera) have sex chromosome systems with female heterogamety (WZ/ZZ or derived variants). The maternally inherited W chromosome is known to determine female sex in the silkworm, Bombyx mori. However, little is known about the role of W chromosome in other lepidopteran species. Here we describe two forms of the W chromosome, W and neo-W, that are transmitted to both sexes in offspring of hybrids from reciprocal crosses between subspecies of wild silkmoths, Samia cynthia. We performed crosses between S. c. pryeri (2n=28, WZ/ZZ) and S. c. walkeri (2n=26, neo-Wneo-Z/neo-Zneo-Z) and examined fitness and sex chromosome constitution in their hybrids. The F1 hybrids of both reciprocal crosses had reduced fertility. Fluorescence in situ hybridization revealed not only the expected sex chromosome constitutions in the backcross and F2 hybrids of both sexes but also females without the W (or neo-W) chromosome and males carrying the W (or neo-W) chromosome. Furthermore, crosses between the F2 hybrids revealed no association between the presence or absence of W (or neo-W) chromosome and variations in the hatchability of their eggs. Our results clearly suggest that the W (or neo-W) chromosome of S. cynthia ssp. plays no role in sex determination and reproduction, and thus does not contribute to the formation of reproductive barriers between different subspecies.
Introduction
Sex chromosomes consist of two types: those appearing only in one sex (termed Y or W) and those appearing in both sexes in one or two copies (X or Z). It is believed that the sex chromosomes (XY or WZ) have evolved from a pair of originally recombining homologous chromosomes, which differentiated through the restriction of recombination after one member of the pair acquired a sex-determining function (Charlesworth et al., 2005). This model of sex chromosome evolution primarily concerns taxa where recombination is present in both sexes. To what extent is this model applicable to taxa where recombination is fully suppressed in the heterogametic sex, such as in males of flies and females of moths and butterflies (reviewed in Marec, 1996), remains to be clarified.
Sex-limited chromosomes (Y in males or W in females) genetically degenerate due to the lack of recombination and, therefore, their DNA compositions usually differ greatly from the other genomic regions. Although there are many mysteries about the function of these chromosomes, their importance for sex-specific trais has been revealed in various organisms. For example, in some vertebrates the Y or W chromosomes provide primary sex determination signals (Bachtrog et al., 2014). In contrast, the Y chromosome of Drosophila melanogaster has no effect on the sex determination cascade, which is controlled by the ratio between the number of X chromosomes and the number of sets of autosomes (Salz and Erickson, 2010). However, the Drosophila Y chromosome also plays an important role as it contributes significantly to fertility and male fitness (Brosseau, 1960; Lemos et al., 2008). In chicken, the DMRT1 gene located on the Z chromosome is considered a key player in the sex determination pathway (Smith et al., 2009), while the role of W chromosome is still unclear; at least recent data suggest that the W chromosome affects female fertility traits (Moghadam et al., 2012).
The sex chromosomes X or Z play an important role in fundamental evolutionary processes, such as speciation or adaptation (Qvarnström and Bailey, 2009). A large contribution of the X chromosome to reproductive isolation (the so-called large X-effect) has been well established in models with male heterogamety, especially in species of the Drosophila genus (Presgraves, 2008). A growing body of evidence suggests that also the Y chromosomes may play a role in the evolution of reproductive barriers (e.g., Sweigart, 2010; Campbell et al., 2012), in addition to their role in sex determination or sex-specific functions and traits (Dean and Mank, 2014).
Moths and butterflies (Lepidoptera) have holokinetic chromosomes and sex chromosome systems with female heterogamety. Besides the most common WZ/ZZ (female/male) system, numerical variations of the sex chromosomes such as Z0/ZZ, W1W2Z/ZZ and WZ1Z2/Z1Z1Z2Z2 occur in different branches of the lepidopteran phylogenetic tree (Marec et al., 2010). Basal groups (non-Ditrysia) share a Z0/ZZ system with Trichoptera (caddis-flies), the sister order of Lepidoptera, and available data suggest that the W chromosome arose later in the evolution of Lepidoptera in a common ancestor of the non-ditrysian Tischeriina plus advanced Lepidoptera, the Ditrysia (Lukhtanov, 2000).
Although it seems clear that the sex in Lepidoptera is determined by the sex chromosome constitution, the actual role of the W and Z chromosomes remains unknown except for the silkworm, Bombyx mori (see below). It has been proposed that the sexual development depends either on the presence of W chromosome encoding a female-determining signal (dominant W) or on the dosage of Z chromosomes carrying male-promoting genes and acting against female-promoting genes located on autosomes, the so-called Z-counting mechanism (Traut et al., 2007; Sahara et al., 2012). The ‘dominant W' sex determination mechanism has been well established in B. mori. In this species, the W chromosome carries a dominant female-determining factor (Fem) that promotes femaleness, irrespective the number of Z chromosomes present in the genome (Fujii and Shimada, 2007). Recently Kiuchi et al. (2014) made the surprising discovery that the feminizing factor in B. mori is not a protein-coding gene but a W-encoded small RNA named Fem piRNA. The authors also showed that the Fem piRNA downregulates the expression of a Z-linked gene, Masculinizer (Masc), which promotes male development in the absence of the W chromosome. However, it is not yet known (i) whether the Fem piRNA-Masc sex-determining pathway is conserved in other lepidopteran species with the W chromosome and (ii) whether Masc plays a role in species with a Z0/ZZ sex chromosome system that are thought to have the Z-counting mechanism of sex determination (Traut et al., 2007).
Based on the disproportionate association of sex-linked traits that distinguish closely related species, the lepidopteran Z chromosome is expected to have a large effect on species divergence (Sperling, 1994). Indeed, an increasing number of reports point to a major role of sex chromosomes in postzygotic and/or prezygotic reproductive barrier in some lepidopteran species (e.g., Naisbit et al., 2002; Dopman et al., 2005). Moreover, recent results suggest that structural rearrangements of sex chromosomes which involve autosomes, the so-called neo-sex chromosomes, can also contribute to reproductive isolation and speciation in Lepidoptera (Yoshido et al., 2011a; Nguyen et al., 2013). However, little is known about the actual impact of sex chromosomes and their meiotic behaviour on fitness of hybrids between distinct populations or closely related species.
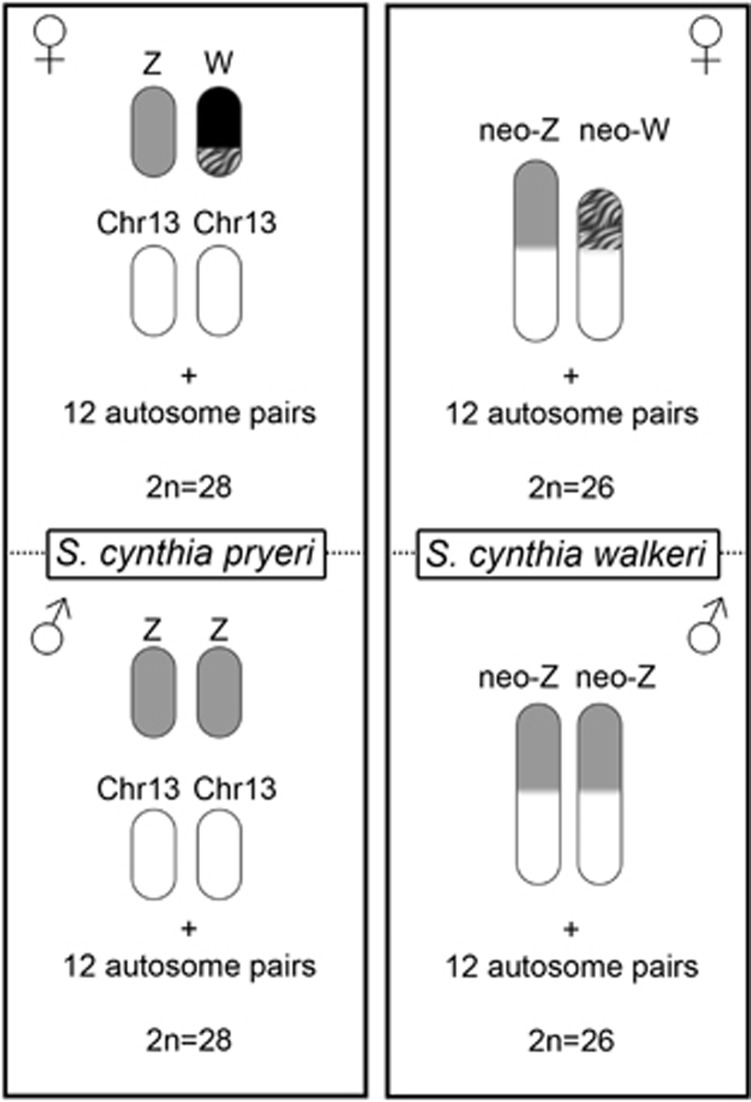
Our previous studies showed that different subspecies of wild silkmoths, Samia cynthia ssp. (Lepidoptera; Saturniidae), exhibit a unique sex chromosome polymorphism. Geographical populations of S. cynthia ssp. with allopatric distributions differ in chromosome numbers and have at least four different sex chromosome constitutions, predominant WZ/ZZ in S. c. pryeri (west Japan) with 2n=28♀/28♂, Z0/ZZ in S. c. ricini (Vietnam) and sporadically in S. c. pryeri (a part of Kagoshima population, Kyushu, Japan) with 2n=27♀/28♂, neo-WZ1Z2/Z1Z1Z2Z2 in S. cynthia subsp. indet. (Nagano, Japan) with 2n=25♀/26♂, and neo-Wneo-Z/neo-Zneo-Z in S. c. walkeri (Sapporo, Japan) with 2n=26♀/26♂ (Yoshido et al., 2005, 2011a, 2013). The variations in the constitution of sex chromosomes were derived from the loss of W chromosome and fusion between the sex chromosomes and autosomes, resulting in different chromosome numbers (Yoshido et al., 2011a, 2011b; Sahara et al., 2012). For example, the neo-Z chromosome in S. c. walkeri arose by fusion between the Z chromosome and chromosome 13 of S. c. pryeri (Figure 1). In addition, S. cynthia ssp. also exhibit unusual differences between W chromosomes. The W chromosome in S. c. pryeri consists of two parts, the highly heterochromatic part (black in Figure 1) and less heterochromatic part (striped grey in Figure 1). The former part was found only in S. c. pryeri while the latter part corresponds to the ancestral segment (striped grey in Figure 1) of the neo-W chromosome in S. c. walkeri (Yoshido et al., 2013). Hence S. cynthia ssp. provide a unique opportunity to address fundamental biological questions, such as the evolution of neo-sex chromosomes, their role in the formation of reproductive barriers between populations and closely related species and the role of multiple sex chromosome systems in sex determination.
Figure 1.
Schematic illustrations of sex chromosome constitutions in both sexes of Samia cynthia pryeri and Samia cynthia walkeri. S. c. pryeri has a WZ/ZZ sex chromosome system with 2n=28♀/28♂ and S. c. walkeri a neo-Wneo-Z/neo-Zneo-Z system with 2n=26♀/26♂. The neo-Z chromosome of S. c. walkeri arose by fusion of an ancestral Z chromosome (grey) and an autosome corresponding to chromosome 13 (Chr13) of S. c. pryeri. The W chromosome in S. c. pryeri consists of a highly heterochromatic part (black) and euchromatin-like part (striped grey), the latter corresponding to a part of the neo-W chromosome in S. c. walkeri.
Here we performed reciprocal crosses between S. cynthia ssp. (S. c. pryeri and S. c. walkeri) with distinct sex chromosome constitutions and different W chromosomes and examined egg hatch rates in F1, F2 and backcross hybrids. We also examined the constitution of sex chromosomes and their behaviour in the F2 and backcross hybrids using molecular cytogenetic markers. This detailed analysis of hybrids allowed us to test the role of different W chromosomes in sex determination of S. cynthia ssp. and whether they have any impact on fitness and contribute to the formation of reproductive barriers between the subspecies.
Materials and methods
Insects
We used specimens of two S. cynthia subspecies (Saturniidae), S. c. walkeri (Sapporo population, Hokkaido) and S. c. pryeri (Toyota population, Honshu). Two subspecies were collected at respective sites in 2005–2011 and reared on Alianthus altissima at the Field Science Center for Northern Biosphere (Hokkaido University, Sapporo, Japan; Yoshido et al., 2005, 2013).
Crosses
Mating experiments were carried out during the period from 2010 to 2012 in the Field Science Center for Northern Biosphere, Hokkaido University. After eclosion, virgin females and males were mated individually in mesh cages (24 × 80 × 25–50 cm) for 1–3 days. Behaviour of moths in cages was monitored at intervals of 6–10 h to check for mating. Mated females were kept separately in cages, where they laid eggs for 1–3 days. Eggs from individual females were collected and placed in plastic containers. About 10–15 days after oviposition, hatchability of the eggs was recorded. Scatterplots of fecundity (i.e., the number of eggs laid) and the egg hatchability were created using Excel templates for independent data, as shown in Weissgerber et al. (2015). In addition, the data obtained were statistically compared by one-way analysis of variance followed by Tukey's multiple comparisons test, using GraphPad Prism version 6 (GraphPad Software Inc., La Jolla, CA, USA). Bodies of the parents used for these crosses were stored at −30 °C to verify their genotype, if needed. Hatched larvae were reared on A. altissima.
Chromosome preparations
Mitotic chromosomes were obtained from wing discs of the last instar larvae as described in Yoshido et al. (2014). Spread preparations of pachytene complements were made from gonads of both sexes as given in Yoshido et al. (2013). Tissues of the last larvae were dissected in a saline solution, swollen for 10–15 min in a hypotonic solution (75 mm KCl) and then fixed for 10–15 min in Carnoy fixative (ethanol, chloroform, acetic acid, 6:3:1). Ovaries were fixed immediately after dissection, that is without hypotonic treatment. Cells were dissociated in 60% acetic acid and spread on a heating plate at 50 °C. Preparations were then passed through a graded ethanol series (70, 80, 98%, 30 s each) and stored in the freezer at −20 °C until use.
Fluorescence in situ hybridization (FISH)
For FISH with S. cynthia walkeri fosmid clones, we selected a Z chromosome marker, clone 45A6 and a chromosome 13 marker, clone 32B23 (for details, see Yoshido et al., 2011b). Fosmid probes were labelled using a Nick Translation Kit (Abbott Molecular Inc., Des Plaines, IL, USA) with Green-dUTP (Abbott Molecular Inc.) and Cy3-dUTP (GE Healthcare, Buckinghamshire, UK), respectively. For the detection of W chromosomes, we used FISH with W chromosome painting probes (the so-called type-2 W-probes), which highlighted the whole W chromosome in S. c. pryeri and a part of the neo-W chromosome in S. c. walkeri (Yoshido et al., 2013). The probes were prepared and then labelled with Cy3-dUTP (GE Healthcare) as described in Yoshido et al. (2013). FISH with W chromosome painting probes were carried out according to the reprobing protocol for Lepidoptera (Yoshido et al., 2014) after FISH with fosmid probes.
Chromosome preparations were removed from the freezer, passed through the graded ethanol series and air dried. Denaturation of chromosomes was carried out at 72 °C for 3.5 min in 70% formamide, 2 × saline sodium citrate (SSC) buffer. For one preparation, the probe cocktail contained 500 ng of each labelled DNA probe, 3.0–5.0 μg of unlabelled sonicated male genomic DNA and 25 μg of sonicated salmon sperm DNA (Sigma-Aldrich, Tokyo, Japan) in 10 μl hybridization solution (50% formamide, 10% dextran sulphate, 2 × SSC). The probe cocktail was denatured for 5 min at 90 °C and then spotted on denatured chromosomes. After incubation in a moist chamber at 37 °C for 3 days, slides were washed at 62 °C in 0.1 × SSC containing 1% Triton X-100. The slides were then counterstained and mounted in antifade based on DABCO (1,4-diazabicyclo (2.2.2)-octane), containing 0.5 μg ml−1 DAPI (4′,6-diamidino-2-phenylindole) (both Sigma-Aldrich, Tokyo, Japan). Preparations were observed in a Leica DMRE HC fluorescence microscope (Leica Microsystems Inc., Tokyo, Japan) or a Zeiss Axioplan 2 microscope (Carl Zeiss, Jena, Germany). Digital images were captured with a DFC350FX B&W CCD camera (Leica Microsystems Inc.) or an Olympus CCD monochrome camera XM10 equipped with cellSens 1.9 digital imaging software (Olympus Europa Holding, Hamburg, Germany) and processed with Adobe Photoshop CS4.
Cloning and Southern hybridization
DNA sequences derived from the W chromosome of S. c. pryeri (W-DNA) were obtained by laser microdissection (Yoshido et al., 2013). PCR products amplified from W-DNA of microdissected samples by WGA4 and WGA3 Kit (Sigma-Aldrich, St. Louis, MO, USA) were cloned into the pCR2.1-TOPO vector with the help of the TOPO TA Cloning Kit (Invitrogen Life Technologies, San Diego, CA, USA) using Escherichia coli DH5α competent cells as the recipient. More than 300 clones were screened by FISH whether they are derived from the W chromosome or not. Cloned inserts derived from the W chromosome were sequenced with an ABI PRISM 3100 Genetic Analyzer (Applied Biosystems, Tokyo, Japan).
Probes for Southern hybridization were generated from the cloned inserts and labelled with thermostable alkaline phosphatase using AlkPhos Direct Labeling and Detection System (GE Healthcare). Genomic DNAs were extracted separately from females and males by standard phenol–chloroform procedure. Samples of the extracted DNAs were digested each with three restriction enzymes, HindIII, HaeIII and AluI (Takara, Kyoto, Japan), respectively. Then 3.0 μg of each sample were loaded on 1.5% agarose gel in TAE buffer and blotted onto nylon membrane, Hybond-N+ (GE Healthcare), by capillary transfer in 20 × SSC. Hybridization and chemiluminescent detection with CDP-Star were carried out following the supplier's protocol. Membranes were exposed to Hyperfilm ECL (GE Healthcare).
Detection of W chromosomes in mated moths
Genomic DNAs of mated moth parents were extracted separately from fathers and mothers by DNAzol reagent (Invitrogen, Tokyo, Japan). For the detection of S. cynthia pryeri W chromosomes, we designed primers 5′-CGAAATTTCGATTACAACCC-3′ (forward; SCPW50-F) and 5′-TGTAGTTTTTCTGTATCCGG-3′ (reverse; SCPW50-R) derived from a sequence of the W chromosome, which was identified in this study (see Results). PCR reactions were performed using a reaction mixture composed of 5.0 ng of template genomic DNA, 10 pmol of each primers, 0.5 U of Ex-Taq polymerase and 1.0 μl of 10 × Ex-Taq PCR buffer (Takara). PCR amplifications were conducted under the following conditions: 94 °C for 5 min, 18 cycles of 94 °C for 10 s, 54 °C for 10 s, 72 °C for 20 s and a final extension step at 72 °C for 1 min. Three microliters of PCR products were loaded on 1.5% agarose gel in TAE buffer. Gels were stained with ethidium bromide and photographed under UV light.
For the detection of neo-W chromosomes in S. c. walkeri, we used the sequences of S. cynthia ortholog of a gene coding for a glycine-rich protein (GRP2), which were amplified from genomic DNA of respective individuals by PCR using two primers, 5′-GGCGCTCCCATCGTACAGAA-3′ (forward; GRP2-F) and 5′-TCCGTAGGAGGTGCTGACAC-3′ (reverse; GRP2-R). The primer sequences were obtained from the S. c. ricini EST database (http://silkbase.ab.a.u-tokyo.ac.jp/cgi-bin/index.cgi). This primer set was designed to amplify the exonic sequences (576 or 588 bp, see Results). PCR conditions were as follows: 94 °C for 5 min, 35 cycles of 94 °C for 30 s, 60 °C for 30 s, 72 °C for 30 s and a final extension step at 72 °C for 5 min. The PCR-generated fragments were sequenced with an ABI PRISM 3100 Genetic Analyzer (Applied Biosystems).
Results
Hatchability of eggs in crosses between S. c. pryeri and S. c. walkeri
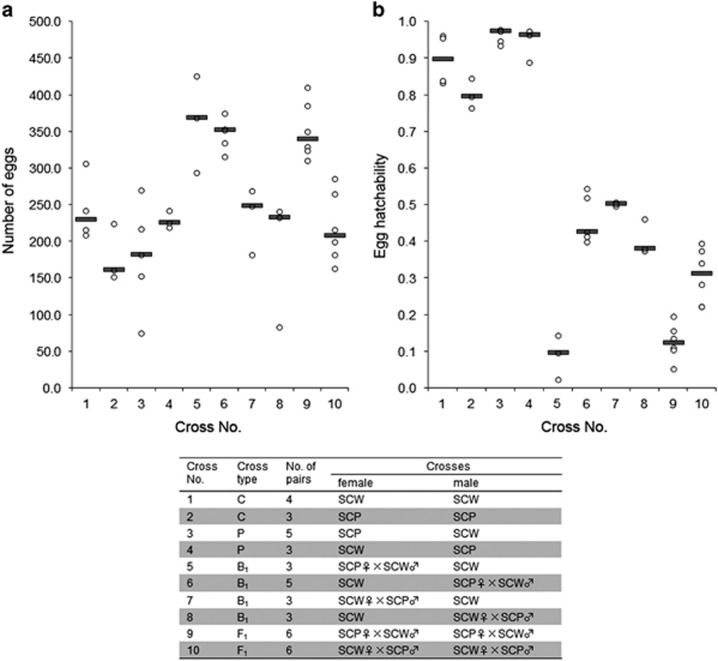
Parental crosses between S. c. pryeri (SCP) and S. c. walkeri (SCW) revealed no significant differences in reproductive capacity from control crosses within respective populations (cross types No. 1–4 in Figure 2 and Supplementary Table S1). Both reciprocal crosses showed comparable fecundity (i.e., the number of eggs laid; Figure 2a) and fertility, with the egg hatchability as high as about 95% (Figure 2b and Supplementary Table S1, cross types No. 3 and 4). Also backcrosses and F1 crosses showed no reduction of fecundity in comparison with both controls. Interestingly, females in all crosses of offspring derived from P crosses between SCP females and SCW males (cross type No. 3 in Figure 2 and Supplementary Table S1) laid a larger number of eggs than controls and all other crosses (Figure 2a and Supplementary Table S1, cross types No. 5, 6 and 9). However, the hatching rates in all four backcrosses and two reciprocal F1 crosses were considerably reduced to about 10–50% (cross types No. 5–10 in Figure 2b and Supplementary Table S1). Particularly drastic reduction of fertility was observed in one type of F1 females backcrossed to SCW males and in one type of crosses between F1 hybrids, where the hatchability of eggs decreased to 8.8 and 12.5% on average, respectively (crosses No. 5 and 9 in Figure 2b and Supplementary Table S1).
Figure 2.
Scatterplots of fecundity (a) and egg hatchability (b) in the control (C), parental (P) and F1 crosses, and backcrosses (B1) between Samia cynthia pryeri (SCP) and Samia cynthia walkeri (SCW). Grey bars represent median. Open circles show the number of eggs laid (a) and hatchability of eggs (b) for each mating.
Sex chromosome constitution in F1 and F2 hybrids of crosses between S. c. pryeri and S. c. walkeri
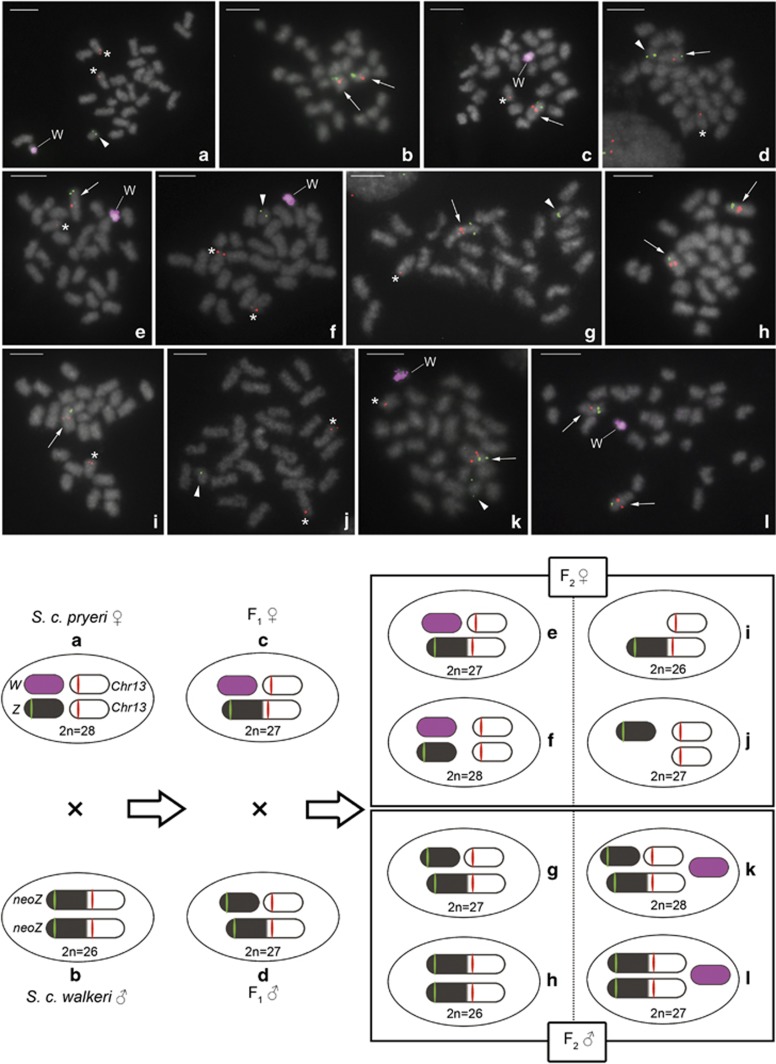
To determine the sex chromosome constitution in F1 and F2 hybrids of crosses between SCP females and SCW males (see Figure 1 for their sex chromosome constitution), we used the type-2 W-probe which painted the whole W chromosome of S. cynthia pryeri (Yoshido et al., 2013) and two fosmid probes, one derived from the Z chromosome (45A6 clone) and the other from chromosome 13 (32B23 clone) of S. cynthia ssp. (Yoshido et al., 2011b). FISH with these probes identified the Z chromosome, the W chromosome and two chromosome 13 in mitotic complements of SCP females with 2n=28 (Figure 3a) and two neo-Z chromosomes in mitotic complements of SCW males with 2n=26 (Figure 3b). With FISH we also confirmed that all studied F1 hybrids of crosses between SCP females and SCW males have the expected sex chromosome constitution, that is, W, neo-Z plus a chromosome 13 in F1 females with 2n =27 (Figure 3c) and Z, neo-Z plus a chromosome 13 in F1 males with 2n=27 (Figure 3d). However, in mitotic complements of F2 females, FISH mapping with the above probes showed not only two expected sex chromosome constitutions, that is, W, neo-Z plus a chromosome 13 with 2n=27 (Figure 3e) and W, Z plus a chromosome 13 pair with 2n=28 (Figure 3f), but also revealed two types of females without the W chromosome, having either neo-Z plus a chromosome 13 with 2n=26 (Figure 3i) or Z plus a chromosome 13 pair with 2n=27 (Figure 3j). Similarly in F2 males, we found two expected sex chromosome constitutions, that is, neo-Z, Z plus a chromosome 13 with 2n=27 (Figure 3g) and a neo-Z pair with 2n=26 (Figure 3h), and two unexpected types of males with the W chromosome, having either W, neo-Z, Z plus a chromosome 13 with 2n=28 (Figure 3k) or W plus a neo-Z pair with 2n=27 (Figure 3l). All sex chromosome constitutions identified by FISH mapping in F1 and F2 hybrids of crosses between SCP females and SCW males are schematically illustrated in Figure 3.
Figure 3.
Upper panel: FISH with sex chromosome derived probes in mitotic metaphase complements of parents (a, b) and F1 (c, d) and F2 (e–l) offspring of crosses between Samia cynthia pryeri females and Samia cynthia walkeri males. Chromosomes were stained with DAPI (grey). Cy3-labelled probe of the 32B23 fosmid clone (red signals) mapped to chromosome 13 or the corresponding autosomal part of the neo-Z chromosome and Green-labelled probe of the 45A6 fosmid clone (green signals) to the Z chromosome or the ancestral part of the neo-Z chromosome. Cy3-labelled W-painting probe (magenta signals) highlighted the S. c. pryeri W chromosome. Bar=5.0 μm. Arrows, arrowheads and asterisks indicate the neo-Z chromosome, Z chromosome, and chromosome 13, respectively. (a) S. c. pryeri female, (b) S. c. walkeri male, (c) F1 female, (d) F1 male, (e, f, i, j) F2 females and (g, h, k, l) F2 males. Mitotic metaphase complements of F2 hybrids were examined in eight males and eight females (Supplementary Table S2). Lower panel: schematic illustrations of sex chromosome constitutions in parents and F1 and F2 hybrids from matings between S. c. pryeri females and S. c. walkeri males, based on the FISH results.
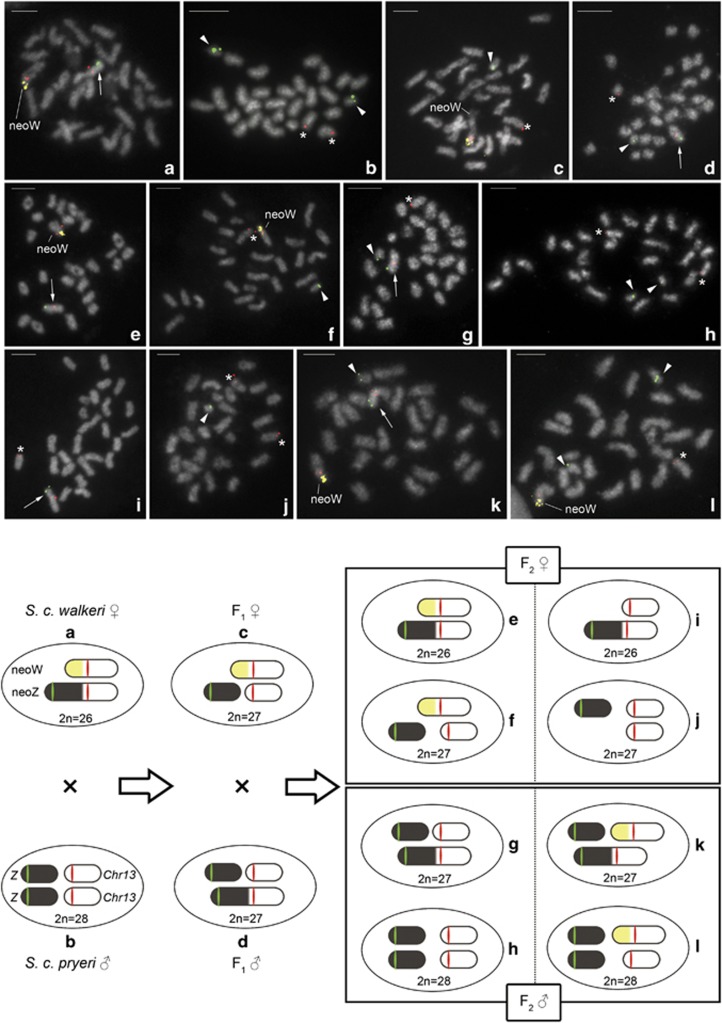
Because the type-2 W-probe can identify the original parts of the neo-W chromosome in S. c. walkeri (see Yoshido et al., 2013; Figure 4a in the present study), we also used this probe to determine the sex chromosome constitution by FISH in F1 and F2 hybrids originating from the reciprocal crosses between SCW females and SCP males (Figures 4c–l). FISH with the W-probe and fosmid probes (see above) confirmed that parents and F1 hybrids used in this cross have the expected sex chromosome constitution, that is, neo-W plus neo-Z in SCW females with 2n=26 (Figure 4a), a Z pair plus a chromosome 13 pair in SCP males with 2n=28 (Figure 4b), neo-W, a Z chromosome plus a chromosome 13 in F1 females with 2n=27 (Figure 4c) and neo-Z, a Z chromosome plus a chromosome 13 in F1 males with 2n=27 (Figure 4d). Also in this case, F2 females showed two expected sex chromosome constitutions, that is, neo-W plus neo-Z with 2n=26 (Figure 4e) and neo-W, Z plus a chromosome 13 with 2n=27 (Figure 4f), and two unexpected without neo-W, that is, neo-Z plus a chromosome 13 with 2n=26 (Figure 4i) and Z plus a chromosome 13 pair with 2n=27 (Figure 4j). In F2 males, FISH mapping also revealed two expected sex chromosome constitutions, neo-Z, Z plus a chromosome 13 with 2n=27 (Figure 4g) and a Z pair plus a chromosome 13 pair with 2n=28 (Figure 4h), and two unexpected with the neo-W chromosome, that is, neo-Z, Z plus a neo-W with 2n=27 (Figure 4k) and a Z pair, a chromosome 13 plus a neo-W with 2n=28 (Figure 4l). The sex chromosome constitutions identified in F1 and F2 hybrids of crosses between SCW females and SCP males are schematically illustrated in Figure 4.
Figure 4.
Upper panel: FISH with sex chromosome-derived probes in mitotic metaphase complements of parents (a, b) and F1 (c, d) and F2 (e-l) offspring of crosses between Samia cynthia walkeri females and Samia cynthia pryeri males. Chromosomes were stained with DAPI (grey). Cy3-labelled probe of the 32B23 fosmid clone (red signals) mapped to chromosome 13 or the corresponding autosomal part of the neo-Z and neo-W chromosomes, and Green-labelled probe of the 45A6 fosmid clone (green signals) to the Z chromosome or the ancestral part of the neo-Z chromosome. Cy3-labelled W-painting probe (yellow signals) highlighted the ancestral part of the neo-W chromosome of S. c. walkeri. Bar=5.0 μm. Arrows, arrowheads, and asterisks indicate the neo-Z chromosome, Z chromosome and chromosome 13, respectively. (a) S. c. walkeri female, (b) S. c. pryeri male, (c) F1 female, (d) F1 male, (e, f, i, j) F2 females and (g, h, k, l) F2 males. Mitotic metaphase complements of F2 hybrids were examined in eight males and eight females (Supplementary Table S2). Lower panel: schematic illustrations of sex chromosome constitutions in parents and F1 and F2 hybrids from matings between S. c. walkeri females and S. c. pryeri males, based on the FISH results.
In F2 hybrids of both reciprocal crosses, we did not observe any major bias in the sex ratio or proportion of individual types of sex chromosome constitutions. Both the expected and unexpected types (see Figures 3e–l and 4e–l) occurred in F2 hybrids in similar frequencies (Supplementary Table S2). To clarify this observation, we examined meiotic pairing of sex chromosomes in pachytene complements of F1 hybrids from both reciprocal crosses. Pachytene oocytes of F1 females (SCP females × SCW males; Figure 3c) mostly showed a sex chromosome trivalent, which consisted of the neo-Z and W sex chromosomes and a chromosome 13 (Supplementary Figure S1a and b; Yoshido et al., 2013). In the trivalent, the chromosomes were either fully synapsed (Supplementary Figure S1a) or only partially paired (Supplementary Figure S1b), presumably depending on the pachytene substage. While the chromosome 13 was always found either well synapsed or at least paired with the corresponding part of the neo-Z chromosome, in a few pachytene complements the W chromosome remained unpaired and formed a univalent (Supplementary Figure S1c). In F1 females (Figure 4c) from the reciprocal crosses (SCW females × SCP males), pachytene oocytes mostly showed a bivalent composed of the neo-W chromosome and a chromosome 13 and a Z chromosome univalent (Supplementary Figure S1e). Exceptionally, we observed a curious trivalent composed of the fully synapsed neo-W chromosome with a chromosome 13 and with a terminal segment of the Z chromosome (Supplementary Figure S1d). The analysis of meiotic pairing thus revealed considerable differences in the configuration of sex chromosomes between F1 females from both reciprocal crosses (Table 1). In F1 males from both crosses, all pachytene nuclei showed a sex chromosome trivalent, which consisted of the neo-Z and Z chromosomes and a chromosome 13 (not shown).
Table 1. Pairing configurations of sex chromosomes observed in pachytene nuclei of F1 females from reciprocal crosses between Samia cynthia pryeri (SCP) and Samia cynthia walkeri (SCW).
| P crosses | Total of nuclei (no.) | Trivalent (no.) | Bivalent+univalent (no.) | Incomplete pairing (%) |
|---|---|---|---|---|
| SCP♀ × SCW♂ | 150 | 144a | 6b | 4.0 |
| SCW♀ × SCP♂ | 177 | 11c | 166d | 93.8 |
Trivalent composed of neo-Z and W sex chromosomes and a chromosome 13 (see Supplementary Figure S1a and b).
Neo-Z chromosome and chromosome 13 formed a bivalent, W chromosome was a univalent (Supplementary Figure S1c).
Trivalent composed of the fully synapsed neo-W chromosome with chromosome 13 and a partially synapsed Z chromosome (Supplementary Figure S1d).
Neo-W chromosome and chromosome 13 formed a bivalent, Z chromosome was a univalent (Supplementary Figure S1e).
Development of a molecular marker for the identification of the S. c. pryeri W chromosome
To identify candidates for molecular markers, we cloned, sequenced and analysed DNA fragments derived from the microdissected samples of S. c. pryeri W chromosomes, amplified by PCR (Yoshido et al., 2013). From more than 300 sequences cloned, we obtained eight sequences, which were specific for the highly heterochromatic part of the W chromosome in S. c. pryeri, as proven by FISH (not shown). A sequence analysis showed that seven of eight clones contain homologous sequences. In total, we identified two different repetitive sequences, further referred to as major and minor W-repeats (Supplementary Table S3). Then one of the seven clones, SCPW50 containing 356 bp of the major W-repeats, was used in this study as a molecular marker of the S. c. pryeri W chromosome. Using FISH we confirmed that the SCPW50 probe highlights the whole highly heterochromatic part of the W chromosome in S. c. pryeri (Supplementary Figure S2a–d, arrows). In contrast, the SCPW67 clone (885 bp) of the minor W-repeats mapped to the end of the S. c. pryeri W chromosome (Supplementary Figure S2e and f, arrowheads).
We hybridized the SCPW50 and SCPW67 sequences to Southern blots of female and male genomic DNAs, digested separately with three different restriction enzymes. Southern hybridization clearly showed that SCPW50 sequences are a part of clustered tandem repeats, almost exclusively present in the female genomic DNA (Supplementary Figure S2g, left) and that SCPW67 sequences are also a part of clustered tandem repeats, preferentially present in the female genomic DNA but also occurring in the male genomic DNA (Supplementary Figure S2g, right). Based on the results of Southern hybridization and sequence analysis, we concluded that the major W-repeats and minor W-repeats are organized in tandem arrays of a 206 bp and a 490 bp sequence, respectively.
In the next step, we designed a pair of PCR primers (SCPW50-F/SCPW50-R; see Materials and methods), specific for the major W-repeats. Using the primers, two distinct DNA fragments (approx. 200 and 400 bp) were amplified exclusively from genomic DNAs of female progeny of S. c. pryeri mothers (Supplementary Figure S2h). The results showed that the S. c. pryeri W chromosome is specifically detected with the designed PCR primers.
Development of a molecular marker for the identification of the S. c. walkeri neo-W chromosome
We could not identify any sequence specific for part of the neo-W chromosome in S. c. walkeri that was highlighted by the type-2 W-probe used in cytogenetic experiments. Thus a molecular marker for the neo-W chromosome identification has been developed with the help of sequence polymorphism of the GRP2 (glycine-rich protein 2) gene (Supplementary Table S3), which is located in the autosomal parts of the neo-sex chromosomes in S. c. walkeri and in chromosome 13 of S. c. pryeri (Supplementary Figure S3; see also Figure 1 in Yoshido et al., 2011b). We amplified DNA fragments of S. cynthia ortholog of GRP2 by PCR using specific primers (GRP2-F/GRP2-R; see Materials and methods) from four females and four males in S. c. walkeri and S. c. pryeri, respectively. The fragments generated from S. c. walkeri DNA were 588 bp long and those from S. c. pryeri 576 bp long (Supplementary Table S3). A sequence analysis of the GRP2 fragments revealed several synonymous substitutions and an insertion/deletion in the exonic region between the subspecies. In addition, a non-synonymous substitution was found only in all S. c. wakeri females but not in males and in all S. c. pryeri specimens examined, indicating that the non-synonymous substitution is fixed only in S. c. walkeri females and can be used as a neo-W chromosome marker. Overall, we distinguished the neo-W, neo-Z and chromosome 13 according to different amino acids, derived from the nucleotide sequences of respective fragments of S. cynthia GRP2 orthologs (Supplementary Figure S3).
Hatchability of eggs in crosses between F2 hybrids with and without the W/neo-W chromosome
We did not find any remarkable phenotype differences between F2 males with and without the W (or neo-W) chromosome. Similarly, no obvious differences between F2 females with different sex chromosome constitutions were found. Almost every F2 hybrid irrespective of the presence or absence of the W (or neo-W) chromosome could mate with the opposite sex.
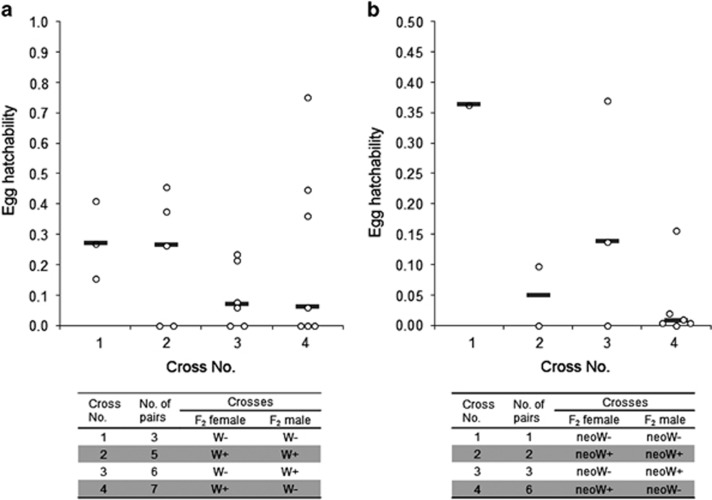
To find out whether the presence or absence of the W (or neo-W) chromosome affects fertility of F2 hybrid, we performed crosses between each type of F2 hybrids and examined the relationship between fertility of these crosses and the presence or absence of the W (or neo-W) chromosome (Figure 5). In crosses between F2 hybrids from the SCP female × SCW male, individual F2 females laid varying proportions of fertile (hatched) and sterile eggs in respective pairs (Figure 5a and Supplementary Table S4). After egg laying, we extracted genomic DNAs from respective parents and performed PCR tests for the presence or absence of the SCP W chromosome using the W-specific molecular marker. In 21 pairs of F2 hybrids, the PCR tests revealed crosses between females (W−) and males (W−), females (W+) and males (W+), females (W−) and males (W+), and females (W+) and males (W−) in the numbers of 3, 5, 6, and 7 pairs, respectively (Supplementary Figure S4 and Table S4). The analysis of egg hatchability in crosses between F2 hybrids showed no apparent association between the presence or absence of the W chromosome and the level of their fertility (Figure 5a). Crosses between F2 hybrids from the SCW female × SCP male showed lower egg hatchability than those from the reciprocal crosses, with a high proportion of sterile eggs (Figure 5b). In 12 pairs of F2 hybrids, sequencing for the detection of the neo-W molecular marker (Supplementary Figure S3) showed all combinations of F2 parents with respect to the presence (neo-W+) or absence (neo-W−) of the neo-W chromosome in numbers of 1, 2, 3 and 6 pairs, respectively (Supplementary Table S4). Similar to F2 hybrids originating from the reciprocal crosses, there was no clear association between the presence or absence of the neo-W chromosome and sterility/fertility of pairs established (Figure 5b). Overall, the results suggest that neither the W nor the neo-W chromosomes, by themselves, affect the level of fertility in S. cynthia ssp.
Figure 5.
Scatterplots of the egg hatchability in crosses between F2 hybrids of Samia cynthia ssp. (a) Hatchability of eggs in 21 pairs of F2 hybrids from crosses between S. c. pryeri females and S. c. walkeri males. W+ and W− indicate F2 hybrids with and without the W chromosome, respectively. (b) Hatchability of eggs in 12 pairs of F2 hybrids from crosses between S. c. walkeri females and S. c. pryeri males. neo-W+ and neo-W− indicate F2 hybrids with and without the neo-W chromosome, respectively. Grey bars represent median. Open circles show hatchability of eggs for each mating.
Discussion
In this work, we clearly showed that the female-specific W chromosome (or neo-W) is transmitted not only to females but also to males in the progenies of hybrids from crosses between geographical subspecies of S. cynthia with distinct sex chromosome constitutions. We observed this phenomenon also in the progenies of backcrosses (data not shown). In S. cynthia ssp., the W (neo-W) chromosome is generally inherited only by females of the respective geographical populations with the exception of two cases, a part of the S. c. pryeri Kagoshima population that lost the W chromosome (Yoshido et al., 2013) and S. c. ricini, which has a Z0/ZZ sex chromosome system (Yoshido et al., 2011a). To our knowledge, transmission of the W (or neo-W) chromosomes to males is limited to hybrids produced by F1 females of crosses between S. cynthia ssp. How could it happen that the F1 females transmitted the W (or neo-W) chromosomes to the male progeny? In F1 females from crosses between S. c. pryeri females and S. c. walkeri males, we observed only very few pachytene oocytes with a W chromosome univalent which would result in random segregation of the chromosome (Supplementary Figure S1c and Table 1). In the vast majority of pachytene oocytes, the W chromosome formed a sex chromosome trivalent with the neo-Z chromosome derived from father and a chromosome 13 derived from mother (see Supplementary Figure S1a and b in this study and Figure 3c in Yoshido et al., 2013). Since in this trivalent, the W plus chromosome 13 were both paired with the neo-Z chromosome, regular segregation of the W chromosome to female offspring was expected. Thus, the event that enabled the W chromosome transmission to both sexes had to occur at later stages of meiosis, that is, after pachytene. In the achiasmatic meiosis of lepidopteran females, paired chromosomes remain associated through a modified synaptonemal complex (SC) beyond the pachytene stage until metaphase I (reviewed in Marec, 1996). Since the short W chromosome showed a weaker affinity to the neo-Z chromosome than chromosome 13 due to the absence of homology (Supplementary Figure S1b), it could be often released from the trivalent, even when the trivalent is still held together by the modified SC. On the contrary, in F1 females originating from the reciprocal crosses, the neo-W chromosome mostly formed a pachytene bivalent with a chromosome 13 inherited from the father (Supplementary Figure S1e and Table 1), while a trivalent was observed rarely (Supplementary Figure S1d and Table 1). Thus, the unpaired Z chromosome inherited from the father could randomly segregate either with the neo-W chromosome or with chromosome 13. The observed pairing patterns in F1 females of oth reciprocal crosses suggest that a weaker synapsis of the W chromosome with the ancestral part of the neo-Z chromosome and the absence of pairing of the Z chromosome with the ancestral part of the neo-W chromosome result in a partially random segregation of the W and Z chromosomes, respectively. Thus, the failure of meiotic pairing seems to be responsible for the occurrence of unexpected sex chromosome constitutions in both sexes of F2 hybrids.
In Lepidoptera, little is known about the role of maternally inherited W chromosomes in sex determination except for the silkworm (B. mori). In this species, sex determination depends on the presence or absence of the W chromosome, which carries a dominant female-determining factor, a small PIWI-interacting RNA named Fem piRNA. The Fem piRNA controls female-specific splicing of the B. mori doublesex (Bmdsx) gene by downregulating expression of the Z-linked Masculinizer (Masc) gene (Kiuchi et al., 2014). However, our study clearly showed that the W (or neo-W) chromosome in S. cynthia ssp. does not determine femaleness. Furthermore, our results suggest that the sex in S. cynthia ssp. could be determined by the number of Z chromosomes, that is, by a Z-counting mechanism (see Traut et al., 2007), irrespective of the presence or absence of the W (neo-W) chromosome. What is then the molecular mechanism that controls the sexual development in S. cynthia ssp.? It is now generally accepted that the doublesex (dsx) acts as a double switch gene at the bottom of sex-determining pathway in insects (Suzuki, 2010; Gempe and Beye, 2011), and we can assume that the dsx gene plays this role also in S. cynthia ssp. However, upstream components of the sex-determining pathway in S. cynthia ssp. are not yet known. The Z-counting mechanism of sex determination assumes the existence of a male-promoting gene such as Masc on the Z chromosome acting against a female-promoting gene on an autosome (Traut et al., 2007). The occurrence of Masc orthologs in several other lepidopteran species may indicate an evolutionarily conserved role for Masc in sex determination (Kiuchi et al., 2014).
Data obtained in model lepidopteran species suggest that the W chromosome is important for fitness of females. In B. mori, W-linked mutations have been isolated that cause masculinization of the external genitalia in females and abnormalities in the ovaries (Fujii et al., 2010; Hara et al., 2012). In Ephestia kuehniella, inheritance of several radiation-induced Z-chromosome fragments containing a piece of the W chromosome exclusively by females along with female-biased sex ratio in the progeny suggested the presence of a male-killing factor on the W chromosome (Marec et al., 2001). However, unlike the above findings, the W (neo-W) chromosome in S. cynthia ssp. appears to have no effect on fertility or viability. In both sexes of F2 hybrids, we did not find any remarkable phenotype differences between specimens with and without the W (or neo-W) chromosome. Almost every F2 hybrid regardless the presence or absence of the W (or neo-W) chromosome could mate with the opposite sex. Furthermore, in crosses between F2 hybrids, no association between the presence or absence of the W (or neo-W) chromosome and variations in hatch rates was found (Figure 5). These findings suggest that the W (or neo-W) chromosome has no influence on fitness of females in S. cynthia ssp.
Hybrid sterility or inviability of the heterogametic sex has been demonstrated in many animals including a number of lepidopteran species (Qvarnström and Bailey, 2009). For example, hybridization experiments between species and/or populations of Heliconius butterflies showed sterility in female hybrids, following thus the Haldane's rule in the heterogametic sex (Naisbit et al., 2002). Two S. cynthia ssp. used in our study, S. c. walkeri and S. c. pryeri with distinct sex chromosome constitutions, can be defined as allopatric clades (Yoshido et al., 2013). It is unknown whether they hybridize in nature or not (Peigler and Naumann, 2003). Our hybridization experiments in laboratory conditions showed no evidence of prezygotic reproductive barriers, with the exception of their geographical distributions. Both reciprocal crosses were fully fertile (Figure 2). However, F1 hybrids (both sexes) showed significantly reduced fertility (see the egg hatchability in backcrosses and F1 crosses in Figure 2b and Supplementary Table S1). In addition, more than half of crosses between F2 hybrids were almost sterile (Figure 5). These results suggest the existence of postzygotic reproductive barriers between the two geographically isolated S. cynthia ssp.
It is well known that hybrids produced by crosses between species or populations with distinct karyotypes, such as in the house mouse or Drosophila fruit flies, have heterozygous karyotypes and typically show reduced fertility to germ cell death for abnormalities of meiotic pairing, recombination failure and chromosome missegregation (Forejt, 1996; Kulathinal and Singh, 1998). However, our results of crosses between S. cynthia ssp. with distinct sex chromosome constitutions revealed that the sex chromosome (W or Z chromosome) missegregation has little effect on their hybrids and therefore may not necessarily contribute to the reproductive barrier between the two S. cynthia ssp.
Hybrid incompatibility can cause reproductive barriers between closely related species (Coyne and Orr, 2004). Genes that cause hybrid incompatibility within and between species have been identified in several model organisms (Presgraves, 2010). Among plants, recent results of cross-breeding analysis between two Silene species (S. latifolia and S. diclinis) with distinct sex chromosome constitutions suggest that aberrant phenotypes in the hybrids occur because of hybrid incompatibility rather than improper inheritance of neo-sex chromosomes (Weingartner and Delph, 2014). Our results also point to hybrid incompatibility rather than sex chromosome missegregation as a cause of the postzygotic reproductive barrier between S. cynthia subspecies. Although the mechanism of hybrid incompatibility cannot be inferred from our data, we can at least conclude that the turnover of sex chromosomes alone is not responsible for the remarkable sterility of F2 hybrids between geographical subspecies of S. cynthia.
Sex-specific Y or W chromosomes are known to play an important role in sex determination, fertility, fitness or speciation. However, our results suggest that the W (neo-W) chromosome in S. cynthia ssp. has no significant effect on any of the above functions. The sex chromosome constitutions could thus become diverse in different populations or subspecies. It has been proposed that the lepidopteran W chromosome arose in a common ancestor of Tischeriina plus Ditrysia as an evolutionary novelty from a Z0 sex chromosome system and that it was lost occasionally in different groups of advanced Lepidoptera (Lukhtanov, 2000; see Figure 3.4 in Marec et al., 2010). In this respect, geographical subspecies of S. cynthia represent unique models for tracking the evolutionary process of rise and fall of the W chromosome within one species complex. Further research of S. cynthia ssp. will provide important insights not only about the evolution of sex chromosomes and their actual role in the formation of reproductive barriers, but also about the evolution of sex determination in lepidopteran species.
Data archiving
Sequence data have been submitted to GenBank: accession numbers LC033561–LC033565. Data available from Dryad repository: http://dx.doi.org/10.5061/dryad.2752g.
Acknowledgments
We are grateful to Y Yamada (Field Science Center for Northern Biosphere, Hokkaido University, Sapporo, Japan) for enabling us to rear S. cynthia subspecies on Ailanthus altissima trees. Our thanks go to Y Banno, Y Sakamaki, T Mano, K Yamagishi, I Miura and H Nagata for providing the specimens of S. cynthia populations. This work was funded by grants 19-1114 (AY), 23380030 (KS) and a research fellowship 21-7147 (KS) from the Japan Society for the Promotion of Science. AY acknowledges additional funding from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 316304. FM was supported by Grant 14-22765S of the Czech Science Foundation.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Heredity website (http://www.nature.com/hdy)
Supplementary Material
References
- Bachtrog D, Mank JE, Peichel CL, Kirkpatrick M, Otto SP et al. (2014). Sex determination: why so many ways of doing it? PLoS Biol 12: e1001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosseau GE. (1960). Genetic analysis of the male fertility factors on the Y chromosome of Drosophila melanogaster. Genetics 45: 257–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P, Good JM, Dean MD, Tucker PK, Nachman MW. (2012). The contribution of the Y chromosome to hybrid male sterility in house mice. Genetics 191: 1271–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth D, Charlesworth B, Marais G. (2005). Steps in the evolution of heteromorphic sex chromosomes. Heredity 95: 118–128. [DOI] [PubMed] [Google Scholar]
- Coyne JA, Orr HA. (2004) Speciation. Sinauer Associates: Sunderland, MA. [Google Scholar]
- Dean R, Mank JE. (2014). The role of sex chromosomes in sexual dimorphism: discordance between molecular and phenotypic data. J Evol Biol 27: 1443–1453. [DOI] [PubMed] [Google Scholar]
- Dopman EB, Perez L, Bogdanowicz SM, Harrison RG. (2005). Consequences of reproductive barriers for genealogical discordance in the European corn borer. Proc Natl Acad Sci USA 102: 14706–14711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forejt J. (1996). Hybrid sterility in the mouse. Trends Genet 12: 412–417. [DOI] [PubMed] [Google Scholar]
- Fujii T, Shimada T. (2007). Sex determination in the silkworm, Bombyx mori: A female determinant on the W chromosome and the sex-determining gene cascade. Semin Cell Dev Biol 18: 379–388. [DOI] [PubMed] [Google Scholar]
- Fujii T, Abe H, Shimada T. (2010). Molecular analysis of sex chromosome-linked mutants in the silkworm Bombyx mori. J Genet 89: 365–374. [DOI] [PubMed] [Google Scholar]
- Gempe T, Beye M. (2011). Function and evolution of sex determination mechanisms, genes and pathways in insects. Bioessays 33: 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Fujii T, Suzuki Y, Sugano S, Shimada T, Katsuma S et al. (2012). Altered expression of testis-specific genes, piRNAs, and transposons in the silkworm ovary masculinized by a W chromosome mutation. BMC Genomics 13: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiuchi T, Koga H, Kawamoto M, Shoji K, Sakai H, Arai Y et al. (2014). A single female-specific piRNA is the primary determiner of sex in the silkworm. Nature 509: 633–636. [DOI] [PubMed] [Google Scholar]
- Kulathinal R, Singh RS. (1998). Cytological characterization of premeiotic versus postmeiotic defects producing hybrid male sterility among sibling species of the Drosophila melanogaster complex. Evolution 52: 1067–1079. [DOI] [PubMed] [Google Scholar]
- Lemos B, Araripe LO, Hartl DL. (2008). Polymorphic Y chromosomes harbor cryptic variation with manifold functional consequences. Science 319: 91–93. [DOI] [PubMed] [Google Scholar]
- Lukhtanov VA. (2000). Sex chromatin and sex chromosome systems in nonditrysian Lepidoptera (Insecta). J Zoolog Syst Evol Res 38: 73–79. [Google Scholar]
- Marec F. (1996). Synaptonemal complexes in insects. Int J Insect Morphol Embryol 25: 205–233. [Google Scholar]
- Marec F, Sahara K, Traut W. (2010) Rise and fall of the W chromosome in Lepidoptera. In: Goldsmith MR, Marec F (eds). Molecular Biology and Genetics of the Lepidoptera. CRC Press: Boca Raton, FL, USA, pp 49–63. [Google Scholar]
- Marec F, Tothová A, Sahara K, Traut W. (2001). Meiotic pairing of sex chromosome fragments and its relation to atypical transmission of a sex-linked marker in Ephestia kuehniella (Insecta: Lepidoptera). Heredity 87: 659–671. [DOI] [PubMed] [Google Scholar]
- Moghadam HK, Pointer MA, Wright AE, Berlin S, Mank JE. (2012). W chromosome expression responds to female-specific selection. Proc Natl Acad Sci USA 109: 8207–8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naisbit RE, Jiggins CD, Linares M, Salazar C, Mallet J. (2002). Hybrid sterility, Haldane's rule and speciation in Heliconius cydno and H. melpomene. Genetics 161: 1517–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen P, Sýkorová M, Šíchová J, Kůta V, Dalíková M, Čapková Frydrychová R et al. (2013). Neo-sex chromosomes and adaptive potential in tortricid pests. Proc Natl Acad Sci USA 110: 6931–6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peigler RS, Naumann S. (2003) A Revision of the Silkmoth Genus Samia. University of the Incarnate Word: San Antonio, TX, USA. [Google Scholar]
- Presgraves DC. (2008). Sex chromosomes and speciation in Drosophila. Trends Genet 24: 336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presgraves DC. (2010). The molecular evolutionary basis of species formation. Nat Rev Genet 11: 175–180. [DOI] [PubMed] [Google Scholar]
- Qvarnström A, Bailey RI. (2009). Speciation through evolution of sex-linked genes. Heredity 102: 4–15. [DOI] [PubMed] [Google Scholar]
- Sahara K, Yoshido A, Traut W. (2012). Sex chromosome evolution in moths and butterflies. Chromosome Res 20: 83–94. [DOI] [PubMed] [Google Scholar]
- Salz HK, Erickson JM. (2010). Sex determination in Drosophila. Fly 4: 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, Roeszler KN, Ohnesorg T, Cummins DM, Farlie PG, Doran TJ et al. (2009). The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature 461: 267–271. [DOI] [PubMed] [Google Scholar]
- Sperling FAH. (1994). Sex-linked genes and species differences in Lepidoptera. Can Entomol 126: 807–818. [Google Scholar]
- Suzuki MG. (2010). Sex determination: insights from the silkworm. J Genet 89: 357–363. [DOI] [PubMed] [Google Scholar]
- Sweigart AL. (2010). Simple Y-autosomal incompatibilities cause hybrid male sterility in reciprocal cross between Drosophila virilis and D. americana. Genetics 184: 779–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traut W, Sahara K, Marec F. (2007). Sex chromosomes and sex determination in Lepidoptera. Sex Dev 1: 332–346. [DOI] [PubMed] [Google Scholar]
- Weingartner LA, Delph LF. (2014). Neo-sex chromosome inheritance across species in Silene hybrids. J Evol Biol 27: 1491–1499. [DOI] [PubMed] [Google Scholar]
- Weissgerber TL, Milic NM, Winham SJ, Garovic VD. (2015). Beyond bar and line graphs: time for a new data presentation paradigm. PLoS Biol 13: e1002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshido A, Marec F, Sahara K. (2005). Resolution of sex chromosome constitution by genomic in situ hybridization and fluorescence in situ hybridization with (TTAGG)n telomeric probe in some species of Lepidoptera. Chromosoma 114: 193–202. [DOI] [PubMed] [Google Scholar]
- Yoshido A, Sahara K, Marec F, Matsuda Y. (2011. a). Step-by-step evolution of neo-sex chromosomes in geographical populations of wild silkmoths, Samia cynthia ssp. Heredity 106: 614–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshido A, Sahara K, Yasukochi Y. (2014) Silk moths (Lepidoptera). In: Sharakhov IV (ed.). Protocols for Cytogenetic Mapping of Arthropod Genomes. CRC Press: Boca Raton, FL, USA, pp 219–256. [Google Scholar]
- Yoshido A, Šíchová J, Kubíčková S, Marec F, Sahara K. (2013). Rapid turnover of the W chromosome in geographical populations of wild silkmoths, Samia cynthia ssp. Chromosome Res 21: 149–164. [DOI] [PubMed] [Google Scholar]
- Yoshido A, Yasukochi Y, Sahara K. (2011. b). Samia cynthia versus Bombyx mori: Comparative gene mapping between a species with a low-number karyotype and the model species of Lepidoptera. Insect Biochem Mol Biol 41: 370–377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.