Abstract
In the frame of a Flemish wildlife surveillance in 2013, a serological screening was performed on sera from wild boar (Sus scrofa; n=238) in order to detect tick-borne encephalitis virus (TBEV)-specific antibodies. Neutralising antibodies were titrated with a seroneutralisation test (SNT), using two cut-off titres (1/10–1/15). Seven wild boars were found TBEV-seropositive and showed moderate (>1/15) to high (>1/125) SNT-titres; three individuals had borderline results (1/10–1/15). This study demonstrated the presence of TBEV-specific antibodies in wild boar and highlighted potential TBEV-foci in Flanders. Additional surveillance including direct virus testing is now recommended.
Keywords: tick-borne encephalitis (virus) TBE(V), wild boar, sentinel surveillance, seroneutralisation test SNT, endemic focus
To date, only six imported cases of human TBE from Scandinavia, Austria, Kyrgyzstan, and Slovenia were detected at the Belgian TBE National Reference Centre (NRC: WIV-ISP, Brussels, Belgium). However, seroconverted dogs, cattle, and roe deer have been found (1–4); therefore, the risk for emergence of tick-borne encephalitis virus (TBEV) in Belgium remains high.
TBEV-seropositivity has been observed in Eurasian wild boar (Sus scrofa) in several European countries. The seroprevalence in this species may exceed that of other hosts (5, 6) and at sufficient sample sizes wild boar studies allow spatial interpretations at the municipality level (6).
Methods
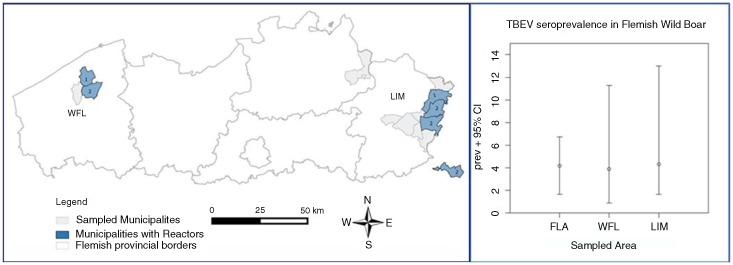
We used Flemish wild boar sera collected in 2013, within the frame of disease surveillance that was focused on Aujeszky's disease, Classical swine fever, and Brucellosis (7). The total target population size was estimated at roughly 3,000 heads (M. Vervaeke – ANB, pers. comm.). A total of 238 representative sera were obtained by veterinarians from the two Flemish wild boar subpopulations in different provinces: Limburg+Antwerp (LIM: n=161) and West Flanders (WFL: n=77). The study population and its seroprevalence (Fig. 1) were mapped using QGIS®2.2-Valmiera (www.qgis.org/), using a vector layer of Flanders in the Belgian Lambert 1972 EPSG-projection. Sample size calculations for disease detection and probability of freedom were performed in Survey Toolbox®1.04 (www.epitools.ausvet.com.au) and in WinEpiscope®2.0 (www.wageningenur.nl/).
Fig. 1.
Map of Wild boar sampling (left) and TBEV-seropositives (right) in Flanders. Left Part: Study population and positives per community; Right Part: Calculated wild boar TBEV-seroprevalence based on 10 SNT-reactors (positive/borderline – cut-off 1/10) out of 238 wild boar tested. FLA: Flanders total study population (n=238); WFL: West Flanders subpopulation (n=77); LIM: Limburg+Antwerp subpopulation (n=161).
A TBEV-specific retrospective serological screening was performed. Immunoglobulin of subtype G (IgG) antibodies were first detected by a commercial veterinary TBEV-ELISA (Immunozym FSME/TBE IgG All-Species-ELISA®, Progen Biotechnik GmbH, Germany). Then, neutralising TBEV-specific antibodies were titrated for all samples using a seroneutralisation test (SNT) and two cut-off titres (1/10–1/15) (3, 8). This test is considered to be the gold standard for medical and veterinary diagnosis of TBE (9, 10). Our final interpretation of the TBEV test results and the calculated prevalence were based on this test.
Cross-reactivity was excluded for those TBEV-SNT-/ELISA-positive samples that still had sufficient serum volume left (n=10), using a commercial indirect immunofluorescence test (immunofluorescence assay (IFA); Biochip Flavivirus-Mosaic-3: TBEV, West Nile virus (WNV), dengue virus (DENV) types 1–4, Japanese encephalitis virus (JEV), and yellow fever virus (YFV); Euroimmun®, Germany) adapted to detect porcine antibodies. Louping ill virus (LIV) was investigated by microtitre plate haemagglutination inhibition (HIT) (11) at Moredun Scientific Research Institute (Scotland, UK). In-house WNV–SNT and Usutu virus (USUV)-SNT tests were performed by Instituto Nacional de Investigación y Tecnologia Agraria y Alimentaria (INIA, Spain) (12, 13). Classical swine fever virus (CSFV)-E2 antibodies were investigated with HerdChek CSFV-ELISA (IDEXX®, The Netherlands).
Results
The sample size (n=238) was deemed sufficient for the purpose of detecting fairly low design seroprevalence 1.25% (95% confidence; 5% error; required: n=227). Provincial sample sizes for Limburg+Antwerp (LIM: sample: n=161; subpopulation: N~2,200) and West Flanders (WFL: sample: n=77; subpopulation: N~800) were suitable to detect design prevalence of 1.80% (required: n=159) and 3.50% (required: n=77), respectively.
Seven wild boars were TBEV-seropositive and showed moderate (>1/15) to high (>1/125) SNT-titres; three individuals had borderline results (1/10–1/15). Of these 10 reactors, 7 originated in LIM and 3 in WFL (Fig. 1). The overall Flemish SNT-seroprevalence was estimated around 2.9% (95% CI: 0.79–5.09%; n SNT+=7) or 4.20% (95% CI: 1.65–6.75%; n SNT+=10), using the 1/15 or 1/10 (Fig. 1) SNT cut-off, respectively.
The results of the TBEV-ELISA/SNT tests and of the cross-reactivity tests for 10 TBEV-reactor samples, that is, SNT-positive >1/15, doubtful >1/10 (n=7), or TBEV-ELISA-positive (n=3) are summarised in Table 1. Six of these samples selected for cross-reaction testing showed borderline (1/20) or positive (1/40–1/80) reactions in the LIV-HIT. Sample no. 8 tested positive in WNV/USUV-SNT and LIV-HIT. All samples were considered negative in CSFV-ELISA. Highly TBEV-SNT/ELISA-positive samples (no. 2, 3, and 4 in Table 1) reacted TBEV-IFA positive and negative for the other flaviviruses (WNV/JEV/YFV/DENV1-4), while low SNT-positives (sample no. 1, 9, and 10 in Table 1) and SNT-negatives but ELISA-positives (sample no. 5,6, and 7 in Table 1) did not.
Table 1.
Confirmation panel of 10 TBEV-reactors in SNT and/or ELISA.
| TBEV | TBEV | TBEV | LIV | CSFV | USUV | WNV | WNV | DENV | YFV | JEV | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample No. | IgG ELISA (VIEU/ml) | SNT (titre) | IFA (titre) | HIT (titre) | ELISA/SNT | 90%PRNT (titre) | 90%PRNT (titre) | IFA (titre) | 1–4 IFA (titre) | IFA (titre) | IFA (titre) |
| 1 | POS | POS | NEG | NEG | NEG | NEG | NEG | NEG | NEG | NEG | NEG |
| 230 | 1/33 | <1/10 | <1/20 | <1/20 | <1/20 | <1/10 | <1/10 | <1/10 | <1/10 | ||
| 2 | POS | POS | POS | border | NEG | NEG | NEG | NEG | NEG | NEG | NEG |
| >540 | 1/164 | 1/1,000 | 1/20 | <1/20 | <1/20 | <1/10 | <1/10 | <1/10 | <1/10 | ||
| 3 | POS | POS | POS | POS | NEG | / | / | NEG | NEG | NEG | NEG |
| 490 | 1/243 | 1/3,200 | 1/40 | <1/10 | <1/10 | <1/10 | <1/10 | ||||
| 4 | POS | POS | POS | POS | / | NEG | NEG | NEG | NEG | NEG | NEG |
| 520 | 1/127 | 1/3,200 | 1/40 | <1/20 | <1/20 | <1/10 | <1/10 | <1/10 | <1/10 | ||
| 5 | POS | NEG | NEG | NEG | NEG | NEG | NEG | NEG | NEG | NEG | NEG |
| >540 | <1/10 | <1/10 | <1/20 | <1/20 | <1/20 | <1/10 | <1/10 | <1/10 | <1/10 | ||
| 6 | POS | NEG | / | border | / | / | / | / | / | / | / |
| 430 | <1/10 | / | 1/20 | / | / | / | / | / | / | ||
| 7 | POS | NEG | NEG | NEG | / | NEG | NEG | NEG | NEG | NEG | NEG |
| 265 | <1/10 | <1/10 | <1/20 | <1/20 | <1/20 | <1/10 | <1/10 | <1/10 | <1/10 | ||
| 8 | NEG | POS | NEG | POS | NEG | POS | POS | NEG | NEG | NEG | NEG |
| 15 | 1/140 | <1/10 | 1/80 | 1/320 | 1/390 | <1/10 | <1/10 | <1/10 | <1/10 | ||
| 9 | NEG | POS | NEG | border | NEG | NEG | NEG | NEG | NEG | NEG | NEG |
| 35 | 1/17 | <1/10 | 1/20 | <1/20 | <1/20 | <1/10 | <1/10 | <1/10 | <1/10 | ||
| 10 | NEG | POS | NEG | NEG | / | NEG | NEG | NEG | NEG | NEG | NEG |
| 15 | 1/25 | <1/10 | <1/20 | <1/20 | <1/20 | <1/10 | <1/10 | <1/10 | <1/10 | ||
TBEV-reactors in TBEV-SNT (n=7/10) and reactors in TBEV-ELISA (n=3); n=7 samples reacted. TBEV: tick-borne encephalitis virus; LIV: louping ill virus; CSFV: classical swine fever virus; USUV: Usutu virus; WNV: West Nile virus; DENV: dengue virus; YFV: yellow fever virus; JEV: Japanese encephalitis virus; IgG: immunoglobulin of subtype G; ELISA: enzyme-linked immunosorbent assay; SNT: seroneutralisation test; IFA: immunofluorescence assay; HIT: haemagglutination inhibition test; PRNT: plaque reduction neutralisation test; Bold=positive result, not bold=negative or borderline result, NEG: negative result; /: no result due to bad quality or lack of volume sample.
Discussion
In a cross-sectional serological screening of a Flemish wild boar population, the TBEV-seroprevalence ranged between 2.90 and 4.20%. We could not substantiate freedom of TBEV (probability of not being infected=0.000), not even in the conservative case using only the SNT-positives (n=7 with titre>1/15). However, the true prevalence can be expected to be below 2.5%. The seropositive and borderline reactors were clustered in a few municipalities in the two subpopulations (Fig. 1).
Three reactors presented with very high anti-TBEV SNT-titres (>1/125), supporting classification as true positives. The specificity of the SNT-results was also assessed using a panel of tests. The IFA results confirmed the high specificity of the TBEV-SNT: highly SNT-positive sera also tested TBEV-positive in IFA, whereas SNT-negative/low positive sera did not (samples 8, 9, and 10 in Table 1). This may be due to a superior accuracy (specificity/sensitivity) of the SNT (14, 15). The TBEV-ELISA, on the contrary, did not seem very accurate or robust (see false positives and negatives in Table 1), but the IFA confirmed several of the positive SNT-results. LIV-HIT revealed several positive reactions; however, the titres were markedly lower than the associated TBEV-titres, and most samples were negative in all other tests. Hence, following worldwide accepted specificity criteria, these reactions were mostly TBEV-specific. For sample 8, flaviviral specificity could not be definitively assessed, although this did not seem to be an aspecific or toxic reaction. Although the titres were highest for USUV-/WNV-specificity, TBEV or LIV seem a priori equally likely to emerge in Belgium, and one SNT could not be considered more sensitive than another.
Conclusion
This study has demonstrated the presence of TBEV-specific antibodies in wild boar and potential TBEV-foci at the community level in Flanders. Within its range, a wild boar population can thus effectively be used for local TBEV-sentinel surveillance in low-prevalence areas. Additional active veterinary surveillance and direct virus testing (especially in rodents) are now recommended to attempt TBE-virus detection and to further determine the characteristics of potential endemic foci, while continued passive medical and veterinary surveillance is indicated to monitor the potential risk for Belgian public health.
Conflict of interest and funding
This study was generously granted by the Flemish Agency of Nature and Forests (ANB). The authors have not received any funding or benefits from industry to conduct this study.
References
- 1.Roelandt S, Heyman P, De Filette M, Vene S, Van der Stede Y, Caij AB, et al. Tick-borne encephalitis virus seropositive dog detected in Belgium: screening of the canine population as sentinels for public health. Vector Borne Zoonotic Dis. 2011;11:1371–6. doi: 10.1089/vbz.2011.0647. [DOI] [PubMed] [Google Scholar]
- 2.Linden A, Wirtgen M, Nahayo A, Heyman P, Niedrig M, Schulze Y. Tick-borne encephalitis virus antibodies in wild cervids in Belgium. Vet Rec. 2012;170:108. doi: 10.1136/vr.e646. [DOI] [PubMed] [Google Scholar]
- 3.Roelandt S, Suin V, Riocreux F, Lamoral S, Van der Heyden S, Van der Stede Y, et al. Autochthonous tick-borne encephalitis virus-seropositive cattle in Belgium: a risk-based targeted serological survey. Vector Borne Zoonotic Dis. 2014;14:640–7. doi: 10.1089/vbz.2014.1576. [DOI] [PubMed] [Google Scholar]
- 4.Tavernier P, Sys SU, De Clerck K, De Leeuw I, Caij AB, De Baere M, et al. Serologic screening for thirteen infectious agents in roe deer (Capreolus capreolus) in Flanders. Infect Ecol Epidemiol. 2015;5:12. doi: 10.3402/iee.v5.29862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomez Martinez C. Role of cervids and wild boar on the presence of tick-borne encephalitis virus in Sweden. Master degree thesis in Biology at the Department of Wildlife, Fish, and Environmental Studies, Sweden, Umea. 2014. p. 17. Available from: http://stud.epsilon.slu.se [cited 17 November 2014]
- 6.Cisak E, Wójcik-Fatla A, Sroka J, Zajac V, Bilska-Zajac E, Chmurzynska E, et al. Prevalence of tick-borne encephalitis virus antibodies in domestic and game animals from Eastern Poland. Bull Vet Inst Pulawy. 2012;56:275–8. [Google Scholar]
- 7.Vervaeke M. Agentschap voor Natuur en Bos; 2012. Zijn de everzwijnen in Vlaanderen gezond? Overzicht van de surveillance van Klassieke varkenspest, Brucellose en ziekte van Aujeszky bij in het wild levende everzwijnen in het Vlaamse Gewest in de periode 2010–2012; p. 7. (Report in Dutch). Available from: http://www.natuurenbos.be/nl-BE/natuurbeleid/soortenbeleid/ziekten [cited 11 November 2015] [Google Scholar]
- 8.Vene S, Haglund M, Vapalahti O, Lundkvist Å. A rapid fluorescent focus inhibition test for detection of neutralizing antibodies to tick-borne encephalitis virus. J Virol Methods. 1998;73:71–5. doi: 10.1016/s0166-0934(98)00041-x. [DOI] [PubMed] [Google Scholar]
- 9.Holzmann H, Kundi M, Stiasny K, Clement J, McKenna P, Kunz C, et al. Correlation between ELISA, hemagglutination inhibition, and neutralization tests after vaccination against tick-borne encephalitis. J Med Virol. 1996;48:102–7. doi: 10.1002/(SICI)1096-9071(199601)48:1<102::AID-JMV16>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 10.Klaus C, Beer M, Saier R, Schubert H, Bischoff S, Suss J. Evaluation of serological tests for detecting tick-borne encephalitis virus (TBEV) antibodies in animals. Berl Munch Tierarztl Wochenschr. 2011;124:443–9. [PubMed] [Google Scholar]
- 11.Clarke DH, Casals J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958;7:561–73. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- 12.Escribano-Romero E, Lupulović D, Merino-Ramos T, Blázquez AB, Lazić G, Lazić S, et al. West Nile virus serosurveillance in pigs, wild boars, and roe deer in Serbia. Vet Microbiol. 2015;176:365–9. doi: 10.1016/j.vetmic.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Alonso-Padilla J, Loza-Rubio E, Escribano-Romero E, Córdoba L, Cuevas S, Mejía F, et al. The continuous spread of West Nile virus (WNV): seroprevalence in asymptomatic horses. Epidemiol Infect. 2009;137:1163–8. doi: 10.1017/S0950268809002325. [DOI] [PubMed] [Google Scholar]
- 14.Holzmann H. Diagnosis of tick-borne encephalitis. Vaccine. 2003;21(Suppl 1):S36–40. doi: 10.1016/s0264-410x(02)00819-8. [DOI] [PubMed] [Google Scholar]
- 15.Niedrig M, Avsic T, Aberle SW, Ferenczi E, Labuda M, Rozentale B, et al. Quality control assessment for the serological diagnosis of tick borne encephalitis virus infections. J Clin Virol. 2007;38:260–4. doi: 10.1016/j.jcv.2006.12.013. [DOI] [PubMed] [Google Scholar]