Abstract
Although, plant hormones play an important role in adjusting growth in response to environmental perturbation, the relative contributions of abscisic acid (ABA) and ethylene remain elusive. Using six spring wheat genotypes differing for stress tolerance, we show that young seedlings of the drought-tolerant (DT) group maintained or increased shoot dry weight (SDW) while the drought-susceptible (DS) group decreased SDW in response to mild drought. Both the DT and DS groups increased endogenous ABA and ethylene concentrations under mild drought compared to control. The DT and DS groups exhibited different SDW response trends, whereby the DS group decreased while the DT group increased SDW, to increased concentrations of ABA and ethylene under mild drought, although both groups decreased ABA/ethylene ratio under mild drought albeit at different levels. We concluded that SDW of the DT and DS groups might be distinctly regulated by specific ABA:ethylene ratio. Further, a foliar-spray of low concentrations (0.1 μM) of ABA increased shoot relative growth rate (RGR) in the DS group while ACC (1-aminocyclopropane-1-carboxylic acid, ethylene precursor) spray increased RGR in both groups compared to control. Furthermore, the DT group accumulated a significantly higher galactose while a significantly lower maltose in the shoot compared to the DS group. Taken all together, these results suggest an impact of ABA, ethylene, and ABA:ethylene ratio on SDW of wheat seedlings that may partly underlie a genotypic variability of different shoot growth sensitivities to drought among crop species under field conditions. We propose that phenotyping based on hormone accumulation, response and hormonal ratio would be a viable, rapid, and an early–stage selection tool aiding genotype selection for stress tolerance.
Keywords: abscisic acid, drought, ethylene, growth sensitivity, hormonal ratio, mild drought
Introduction
Drought is a major abiotic stress limiting plant growth and yield. While plant responses differ with drought intensity, timing, and duration (Claeys and Inzé, 2013), we now understand that traits that confer survival of severe stress episodes will not deliver sustained growth and yield under mild stress (Skirycz et al., 2011). From an agricultural viewpoint, severe growth reduction may result in significant yield loss even under mildly stressed field conditions. Most crop species exhibit a large genetic variability of expansion growth (Pereyra-Irujo et al., 2008; Parent et al., 2010; Welcker et al., 2011; Tardieu et al., 2014) and biomass growth (Wang et al., 2008; Boutraa et al., 2010; González, 2011) in response to drought. Therefore, identifying genotypes that maintain, or at least limit the reduction of, growth under stress might be a useful strategy to boost plant biomass (Skirycz et al., 2011; Hatier et al., 2014). Efficient translation of biomass into grains would also enhance yield productivity under stress conditions.
Plants have evolved some adaptive strategies to cope with mild and severe restriction in water availability (Davies and Zhang, 1991). In showing selectivity over the maintenance of either water balance and/or gas exchange, plant species favor either “survival” or “growth” behavior, respectively, when they encounter stress conditions (Tardieu et al., 2014). The latter strategy, which is an opportunistic risk-taking, is generally regarded as a stress-resistance trait (Sade et al., 2012) and plants with this behavior tend to occupy more mild to moderate drought-prone natural habitats (McDowell et al., 2008). This behavior may allow vegetative and reproductive growth under mild to moderate stress conditions but will confer no benefit under conditions of prolonged and severe stress in which plants with the former strategy may survive (Tardieu et al., 2014) but yield can be minimal. Hence, a survival vs. growth strategy of plants differs according to soil moisture. A homeostatic hydraulic regulation is known to partly drive this species specificity (Meinzer et al., 2014); however, some species, grapevine (Chaves et al., 2010), and poplar (Almeida-Rodriguez et al., 2010), can switch between “survival-growth” strategies in response to fluctuating soil moisture. The mechanistic basis of such a dual growth habit is yet to be fully understood, however, it could be regulated by an interaction of hydraulic and chemical signaling.
When drought stress develops, not all leaves respond similarly in stomatal closure (Blum, 2011). It was recently argued that drought insensitive stomata may favor carbon gain at the expense of expansive growth (Caldeira et al., 2014; Tardieu et al., 2014). Biomass accumulation and expansive growth may be controlled by independent environmental and genetic factors (Fatichi et al., 2014) and may govern yield under stress. The positive effect could be through enhancing carbon acquisition, in addition to specific adaptations that allow continued growth under drought (e.g., reprogrammed energy metabolism, osmotic adjustment and high cell wall extensibility; Claeys and Inzé, 2013). The negative effect of wide stomatal aperture on expansion growth could be a consequence of lower hydraulic conductivity (Caldeira et al., 2014). Though the role of abscisic acid (ABA) in plant hydraulics has been debated (Dodd, 2013), ABA can regulate hydraulic conductance (Jia and Davies, 2007; Pantin et al., 2012) via regulation of aquaporins (Sade et al., 2009; Prado et al., 2013). In addition, ethylene, under flooding, can promote (Kamaluddin and Zwiazek, 2002) or inhibit (Li et al., 2009) hydraulic conductivity under phosphorus deficiency depending on the environmental conditions. In addition, auxins and cytokinins closely regulate hydraulic conductivity, and thereby shoot growth, under stress conditions. The interaction between hydraulic and hormonal traits may therefore deliver differences in growth and yielding of crops under drought. We hypothesize that the subtle sensitivity of stomatal and growth traits to chemical regulators can be viewed as a model for species survival–growth behavioral plasticity (Soar et al., 2006; Rogiers et al., 2012) especially under drought.
Plant hormones are well-known to act as growth regulators and their concentrations change in response to numerous stresses (Hays et al., 2007; Ji et al., 2011). Both ABA and ethylene have been shown to exert dual effect on growth: stimulatory at low concentration (Ku et al., 1970; Suge, 1971; Nishizawa and Suge, 1995a,b; Lehman et al., 1996; Smalle et al., 1997) while inhibitory at high (Pratt and Goeschl, 1969; Guzmán and Ecker, 1990; Kieber et al., 1993; Tanaka et al., 2013), a “dose-growth” response phenomenon known as “hormesis” (Pierik et al., 2006; Gressel and Dodds, 2013). Relatively few studies have examined the stimulatory properties of low concentrations of ABA and ethylene (Suge, 1971; Takahashi, 1972; Neskovic et al., 1977; Pierik et al., 2006), suggesting that low concentrations (≤ 0.1 μl L−1 or ≤ 0.1 μM) of ethylene and ABA stimulate organ growth to the extent that, across planta, varies widely (0% to >100%) depending on the timing of application, level of organization (e.g., cell, organ), plant species, seedling age, and the physiological and growth conditions. The mechanisms controlling hormone dose-dependent growth response are largely unexplored. Nevertheless, hormetic growth response in general has been vigorously debated in ecotoxicology and medicine and its potential for increasing plant productivity has recently been discussed (Pierik et al., 2006; Gressel and Dodds, 2013).
In this study, we hypothesize that different genotypes may exhibit differential growth sensitivity to drought stress particularly via hormone responses that are normally induced by numerous stresses (Hays et al., 2007; Ji et al., 2011). We show that six spring wheat genotypes differing for stress-susceptibility (see below) exhibit a large genetic variability for early-stage growth sensitivity to very low concentrations of exogenous ABA and ethylene which reflects the yield performance of the genotype under mild stress and/or may indicate more general genotype-specific hormone responses that can benefit growth and yield later in plant development. Further, we show that drought-tolerant and drought-susceptible genotypes differ in their ABA and ethylene accumulation, which might be most likely to occur under mild drought-stressed natural habitats.
Materials and methods
Plant material and growth conditions
Six spring wheat (Triticum aestivum L.) genotypes were selected and designated as drought-tolerant (DT: Kea, Attila, Florkwa) or drought-sensitive (DS: SeriM32, Simorge, Barbet1) groups based on their stress susceptibility, biomass accumulation and yield potential in the field (Lopes et al., 2012). These groups were selected in such a way that both the DT and DS groups show contrasting yield susceptibility to stress and non-stress conditions (Figure 1). The DS group had higher yield under non-stress (1094 g/m2) while they maintain only 43% of non-stress yields (474 g/m2) under stress conditions. In contrast, DT group had lower grain yield under non-stress (829 g/m2) as compared to DS group but they maintain 73% (606 g/m2) of non-stress yields under stress conditions. Therefore, both groups have differential yield susceptibilities to stress and non-stress conditions. This atypical selection is at marked contrast to a widely accepted breeders conception that selection for high yield potential under non-stress conditions has also improved yield under stress especially for mild to moderate drought stress (Araus et al., 2002, 2008; Trethowan et al., 2002; Cattivelli et al., 2008). However, such genotype selection may be useful to understand the underlying mechanisms of growth and yield responses to the environment.
Figure 1.
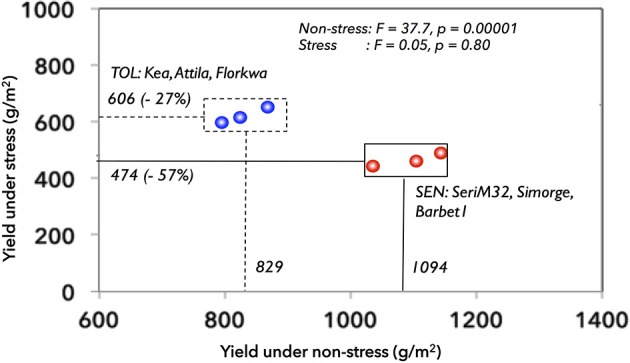
Grain yields of six different wheat genotypes grown under non-stress and stress conditions in the field. The selected wheat genotypes were: Tolerant: Kea, Attila, Florkwa; Sensitive: Simorge; Barbeti; SeriM82. SEN, sensitive group; TOL, tolerant group.
For all experiments, seeds were initially germinated on a wet-filter paper placed in the Petri dish at room temperature, and a 7-day-old seedlings were transplanted into 0.5 L plastic pots containing a well-prepared mixture of a soil-based compost (John Innes No. 2, UK). Plants were initially grown in a naturally lit glasshouse with supplementary artificial lighting of 200 μmol m−2 s−1 photosynthetically active radiation (PAR), and a photoperiod of 12 h with day/night temperatures of 25/18°C, respectively. When seedlings reached two-leaf stage, plants were shifted to growth cabinets with an average day/night temperature of 25/22°C, 12 h photoperiod with a relative humidity (RH) of 90%. All plants were well-watered daily and half-strength Hoagland nutrient solution was provided on alternative days. In chemical spray experiments, seedlings (two-leaf stage) were shifted to a modified hydroponic system (50 ml tube-system), in which nutrient solution was changed every 2-days and aeration was continuously provided with aquarium air-pump (BOYU, S-4000B, 3.2 L min−1).
Mild drought stress under controlled conditions
Mild water deficit (MWD) was imposed as described previously (Deokar et al., 2011). When seedlings reached three-leaf stage, water was withheld from all pots to initiate a dry-down procedure. Weight of all individual pots was recorded daily in the morning at ~10.30 h to monitor soil moisture content in both treatments. Daily loss of water through evapotranspiration (ET) was calculated as the difference in pot weight on the current day from that of the previous day. After 7–8 days, when the soil moisture content reached target values of approximately 0.44 and 0.33 g per g−1 dry soil in WW and MWD treatments, respectively, (Supplementary Figures S1A–C) all plants were watered daily with the amount of water lost through ET of each pot daily. Thus, WW and MWD plants were maintained at ~94 and 70% of field capacity, reflecting a soil matric potential of ~−0.0048 and −0.08 MPa respectively, as determined from a moisture release curve of the same soil type (Dodd et al., 2006). Both treatments were maintained at the targeted soil moisture for 7 days and leaf samples were then collected before watering to determine endogenous ABA and ethylene accumulation. The remainder of the shoot was harvested separately. Shoot dry weight was determined after samples were oven-dried at 80°C for 72 h. Root growth was not measured in the study. Two experiments with four completely randomized replications for each genotype were conducted.
Determination of endogenous ABA and ethylene
Endogenous ABA concentrations and ethylene evolution were measured in MWD experiment. For ABA determination, leaf tissues (0.2–0.4 g fresh weight) were collected and immediately frozen in liquid nitrogen. Frozen leaf tissue was freeze-dried for 48 h, finely ground and then extracted in distilled deionized water with an extraction ratio of 1:40 (gram dry weight:mL water) at 4°C overnight. ABA concentrations of the extract were determined using a radioimmunoassay as described (Chen et al., 2013).
Endogenous ethylene emission from leaves was measured using a commercial laser-based ethylene detector (ETD-300, Sensor Sense B.V., Nijmegen, The Netherlands) in combination with a gas handling system (VC-6, Sensor Sense B.V.) as described previously (Wang et al., 2013). Leaf tissues (0.25–0.45 g fresh weight) of WW and MWD plants were sampled, weighed immediately, and placed in 50 mL glass tubes containing moistened filter paper and were allowed wound-induced ethylene to subside (Yang et al., 2006). Later, glass tubes were tightly capped with a double-bent rubber stopper and were further incubated for 5 h in the light at the room temperature. Using a 5-mL syringe, 4 mL gas was extracted through rubber stopper, and stored in 4 mL sealed glass vials. These vials were connected to inlet and outlet cuvettes of VC-6 system, which allow six cuvettes at once, and continuously flushed with air at a constant flow of 4 L h−1. Ethylene emission from each vial was monitored alternatively by ethylene detector in sample-mode for 10 min. To remove any traces of external ethylene or other hydrocarbons, the airflow was passed through a platinum-based catalyser before entering the cuvettes. A scrubber with KOH and CaCl2 was placed before ethylene detector to reduce the CO2 and water content in the gas flow, respectively. The ethylene emission was corrected for tissue fresh weight and the duration of incubation to determine ethylene emission rate.
Drought trials under field conditions
Four field trials were conducted during 2009–10 and 2010–11 under two different growth environments: two under well-irrigated conditions (controls, total crop water supply >700 mm), and two under drought (total crop water supply ≤ 300 mm). All trials were sown in alpha-lattice design with two replicates in the Yaqui Valley at CIMMYT's Obregon Experimental Station in North-Western Mexico (27°25′N 109°54′W, 38 m above sea level). Detailed trial procedures and meteorological data were described elsewhere (Lopes et al., 2012, 2015; Sukumaran et al., 2015). Briefly, the sowings were made in late November each year with either irrigation or drought. In drought trials, irrigation was at sowing with no further irrigation, making ~180 mm of water available to the crop. The experimental design was a randomized lattice with two replications in 2 m long and 0.8 m wide plots consisting of one raised bed with two rows per bed at seed rate of 120 kg/ha. Appropriate weed, fertilization, disease, and pest control were followed to avoid any yield limitations. When seedlings were at three-leaf stage (~23 days after sowing), normalized difference vegetation index (NDVI, a proxy for biomass) was measured along the length of the plot but avoided the boarders (0.25 m each side), averaged across trials and years and mean values were presented.
ABA and ACC spray experiment
When the seedlings reached three-fully emerged leaves, plants were foliar-sprayed with ABA and ACC (1-aminocyclopropane-1-carboxylic acid, ethylene precursor) as described previously (Chen et al., 2013). The optimal concentrations (at which shoot growth response is maximal) of ABA and ACC concentrations were determined in preliminary experiments (Supplementary Figure S1D). ACC, the endogenous ethylene precursor, was preferred as a source of ethylene to ethephon (a phosphonic acid), since non-ethylene generating phosphonic acids can have physiological effects on plants (Ernst et al., 1992; Chen et al., 2013). ACC was dissolved in water while ABA was dissolved in ethanol for stock solution preparation and a wetting agent Silwet (L-77, De Sangosse Ltd, Cambridge, UK) at 0.025% (v/v) was included in all solutions. Two-hours into the photoperiod, plants were foliar-sprayed (4-5 mL plant−1) either with water that contain ethanol and Silwet (controls), ABA (0.1 μM), or ACC (0.1 μM) assuming that a proportion of each chemical sprayed onto leaf surface will penetrate the leaf interior (Wilkinson and Davies, 2008). After spraying, plants were grown further for 7 days in the same hydroponic system and then harvested to determine shoot fresh weight. Shoot dry weight was determined after oven-drying at 80°C for 72 h. Shoot dry weight at the beginning (just before spray) and end of (7-days) treatment were used to calculate relative shoot growth rate (RGR) according to (Hoffmann and Poorter, 2002). The experiment was repeated twice, with four completely randomized replications for each genotype. We also measured RGR at six-leaf stage whereby plants were foliar-sprayed with ABA and ACC at the same concentration (0.1 μM) at the three-leaf stage.
Determination of carbohydrates
In the chemical spray experiment, sugars, and sugar alcohols (sucrose, glucose, fructose, raffinose, erlose, maltose, galactose, rhamnose, sorbitol) of the leaf tissue were quantified using high performance liquid chromatography (HPLC) method as described previously (O'Rourke et al., 2015). Grounded dry tissue samples (20–50 mg) were extracted two-times with 2.5 ml of 80% ethanol by boiling the samples in glass tubes in a 60°C water bath for 30 min each. After each extraction, the tubes were centrifuged at 4500 rpm for 10 min, and the extracts were then pooled and dried in a speedvac for ~3–4 h. From this, final extract 200 μL was further dried down to remove the ethanol and rediluted with 200 μL deionized water. HPLC with a Dionex IC-3000 system including electrochemical detection cell with gold electrode and temperature controlled column compartment at 30°C (Thermo Scientific, Hemel Hempsted, UK) was used. The column used was a Dionex CarboPac PA20 3 × 150 mm analytical column (Thermo Scientific, Hemel Hempsted, UK). Ten microliters of sample was injected into the sample loop connected to the ion exchange column. The peaks were identified by comparing retention times with those of standard sugar markers with Dionex Chromeleon software.
Data analyses
Statistical analyses were performed with R 3.0.1 (R Development Core Team, 2013). Data were averaged across genotypes, groups and treatments and mean values were reported. Two-way ANOVA considered treatments (WW and MWD) and groups (DT and DS) as explanatory variables while shoot dry weight was a response variable. An ANCOVA model was used considering SDW as the dependent variable with groups as the factor and hormones as the covariates. Principal component analysis (PCA) was performed on carbohydrate data to identify the carbohydrate response patterns between DT and DS groups as well as between the treatments. One-way ANOVA was used for the effects of exogenous hormones on the shoot growth rate, carbohydrates, and a Student's t-test was used to compute the pair-wise comparisons between group means with Bonferroni correction.
Results
Drought-tolerant and drought-sensitive genotypes show different shoot growth responses to mild drought
We studied the effect of mild drought on shoot growth response at the three-leaf stage of six wheat genotypes differing for drought sensitivity. Across all genotypes, average NDVI values measured at the three-leaf stage (23 DAS) in the field were comparable between WW and WD conditions (data not shown). When all genotypes were separated into drought-tolerant (DT, 3) and drought-sensitive (DS, 3) groups, the DS group showed a higher NDVI (13%) compared to DT group in WW conditions (Figure 2A). However, under mild drought, the DS group showed a reduction in NDVI (−7%) while the DT group had a slightly increased NDVI (+0.6%). Such NDVI responses were not significantly different between the DT and DS groups.
Figure 2.
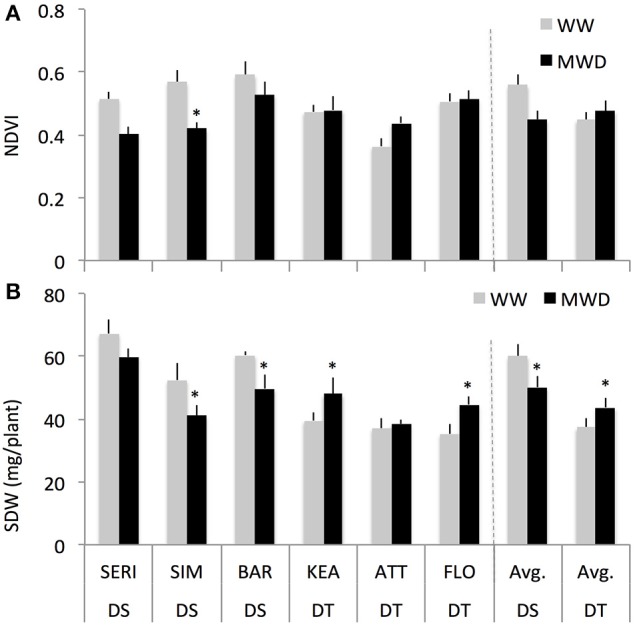
The normalized difference vegetation index (NDVI) values (A) and shoot dry weight [SDW, (B)] of drought-tolerant (DT) and drought-susceptible (DS) genotypes at 3rd leaf stage grown under field-drought (with two replications) and controlled drought conditions with four replications for each genotype, respectively. * indicates p < 0.05.
Consistent with the NDVI results (Figure 2A), both the DT and DS groups showed similar shoot dry weight (SDW) responses under controlled mild drought conditions albeit with a greater relative response (Figure 2B). The DS group had higher SDW (60%) than DT group under WW conditions. Under MWD, the DS group however showed a reduction in SDW (–16%) while the DT group had an increased SDW (+17%) relative to WW plants of the same group. A two-way ANOVA indicates that there was a significant interaction effect of groups and treatments on SDW (P = 0.008; for groups: P < 0.001; for treatments: P = 0.05). Further, treatments have significant effect on SDW within DS (P = 0.03) and DT (P = 0.03) groups. Similar shoot fresh weight responses of DT and DS groups in WW and MWD conditions were observed (Supplementary Figure S2A).
We examined whether differences in SDW of DT and DS groups could be related to plant water content. This seems unlikely, as both groups showed a tight association between FW and DW in both the conditions (Supplementary Figure S2B). DS plants have slightly more water content (< 1%) but both groups responded similarly to MWD (Supplementary Figure S2C). Overall, these results suggest that the two groups of wheat cultivars responded differently to mild drought.
Shoot growth sensitivity of drought-tolerant and drought-sensitive groups was closely associated with endogenous ABA and ethylene accumulation and responses
Previous studies have reported that wheat genotypes differ in the accumulation of, and their sensitivity to, ABA (Ji et al., 2011). We, therefore, measured endogenous ABA and ethylene in DT and DS genotypes grown under WW and MWD conditions. The DS and DT groups showed similar pattern of ABA and ethylene accumulation with both groups showing significantly higher ABA concentration (129% and 95%, respectively; P < 0.001) and ethylene production (160 and 138%, respectively; P = 0.001) in response to MWD (Figure 3, Supplementary Figures S3A,B). The main group effect on ABA was not significant (P > 0.05) but was significant for ethylene production (P = 0.01), indicating that both groups (DT and DS) significantly differed for ethylene production.
Figure 3.
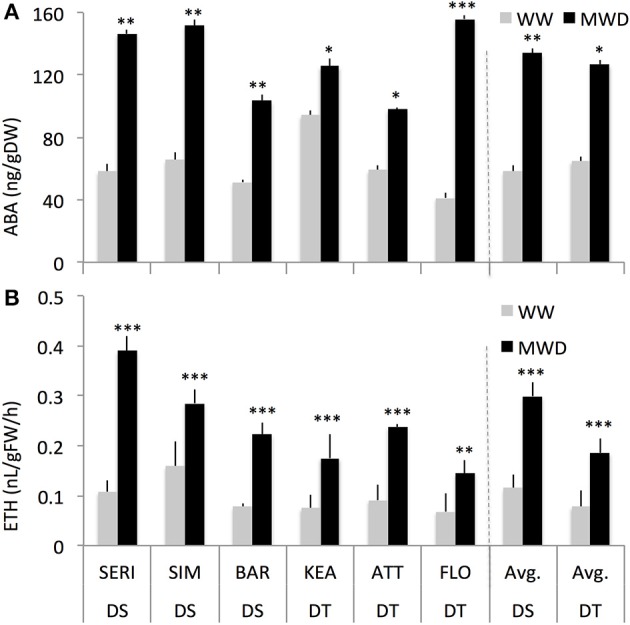
Shoot ABA concentration (A) and ethylene production (B) of drought-tolerant (DT) and drought-susceptible (DS) genotypes grown under well-watered (WW) or mild water-deficit (MWD) conditions with four replications for each genotype. *, **, *** Indicate p < 0.001, < 0.01, and < 0.05, respectively.
Across two treatments, SDW responses of the DT and DS groups to endogenous ABA and ethylene showed a tendency toward two response trends (Figures 4A,B, group effects for ABA: P < 0.0001; group effects for ethylene: P < 0.0001). The DT group showed an increased SDW with increasing concentrations of ABA (round circles, Figure 4A). In contrast, the DS group showed a decreased SDW with increasing concentrations of ABA (squares, Figure 4A). Such SDW responses of the DT and DS groups were consistent with ethylene whereby both the DT and DS groups showed an increased and a decreased SDW response to increasing levels of ethylene, respectively (Figure 4B). Such differential SDW response trends between DT and DS groups were largely driven by WW conditions, suggesting that hormone concentrations may regulate shoot growth even under optimal growing conditions.
Figure 4.
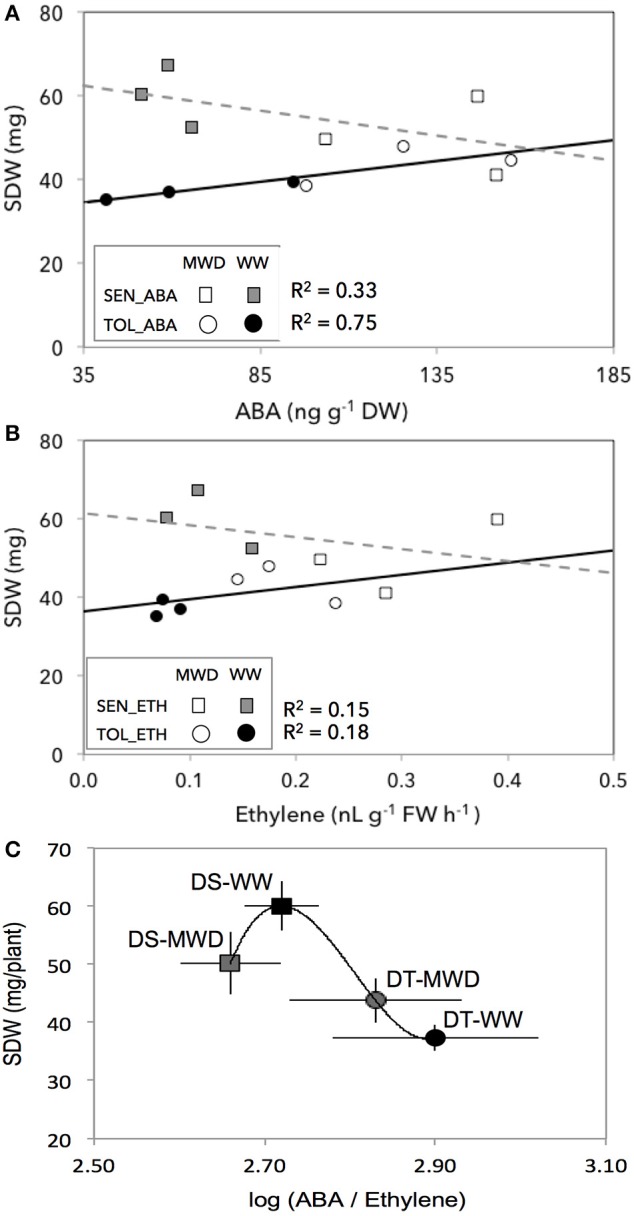
The association between shoot dry weight (SDW) and endogenous ABA (A), ethylene (B), and ABA:ethylene ratio (C) in drought-tolerant (circles, DT) and drought-susceptible (squares, DS) genotypes grown under well-watered (filled symbols) and mild drought (open symbols) with four replications for each genotype. SEN, drought-susceptible; TOL, drought-tolerant. ETH, ethylene; ABA, abscisic acid.
Across treatments (WW and MWD) and groups (DT and DS), SDW did not correlate with ABA (r2 = 0.003, P = 0.81; Supplementary Figure S3C) and ethylene (r2 = 0.15, P = 0.12; Supplementary Figure S3D). However, SDW responses of both groups followed ABA:ethylene ratio (Figure 4C) that fits well with their SDW responses to mild drought (Figure 2B). Among the four-subgroups (DS-WW, DS-MWD, DT-WW, and DT-MWD), DS-WW subgroup had a higher SDW with an ABA/ethylene ratio of 2.72 while DT-WW subgroup had a lower SDW with an ABA/ethylene ratio of 2.90. However, both groups reduced ABA:ethylene ratio in response to MWD albeit at different level (2.66 and 2.83, respectively) but were not significantly different between two groups. These results suggest that an appropriate ABA:ethylene ratio might be critical and the DT and DS groups exhibited a differential growth sensitivity to MWD by differential accumulation of ABA and ethylene.
Foliar-spray of exogenous ABA and ACC increase shoot relative growth rate of drought-tolerant and drought-sensitive groups under well-watered condition
Previous studies have often shown that very mild concentrations of exogenous ABA (Takahashi, 1972; Watanabe and Takahashi, 1997) and ethylene (Burg and Burg, 1966, 1968) stimulated growth of various organs of a range of plant species. We, therefore, examined whether low concentrations of exogenous ABA and ethylene could stimulate growth of DT and DS genotypes under WW condition. Both the DT and DS groups showed a significantly different shoot relative growth rate (RGR) response to exogenous ABA and ACC spray (Figure 5). ABA and ACC strongly promoted RGR of DS genotypes (131 and 130% respectively; P = 0.01) but had modest effect on RGR of DT genotypes (5 and 32% respectively; P = 0.03). Across all groups, ABA had an increased RGR by 50% (P = 0.04), while ACC was slightly more effective in stimulating shoot RGR (78%, P = 0.002). Such growth stimulation responses to exogenous ABA and ACC sprayed at the three-leaf stage were not significantly different from control at the 6th leaf-stage (Supplementary Figure S4), suggesting that low concentrations of ABA and ACC stimulated growth response may be dependent on the developmental stages.
Figure 5.
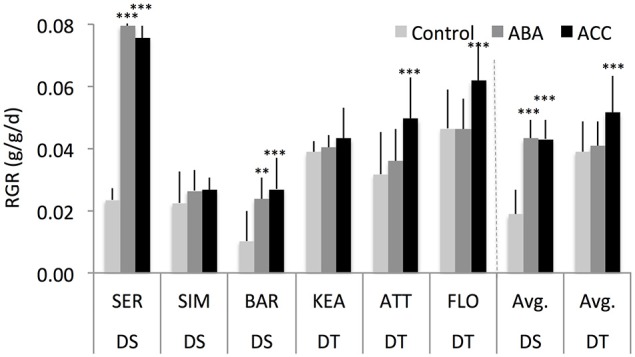
Shoot relative growth rate (RGR) of six wheat genotypes at 3rd leaf stage that were either sprayed with water (controls), abscisic acid (ABA, 0.1 μM), or the ethylene-precursor, 1-aminocyclopropane-1-carboxylic acid (ACC, 0.1 μM) with four replications for each genotype. **, *** indicate p < 0.01, and < 0.05, respectively.
Foliar-spray of exogenous ABA and ACC had differential effects on carbohydrates status of drought-tolerant and drought-sensitive groups under well-watered condition
We hypothesized that an increased RGR response to low concentrations of ABA and ACC could be related to an altered carbohydrate status in DT and DS groups. A two-way ANOVA indicates that there was a significant interaction effect of groups and treatments for carbohydrates such as rhamnose (P = 0.025), raffinose (P = 0.003), and maltose (P = 0.000) (Supplementary Table S1). Further, treatments and groups have significant effect on galactose (P = 0.000 and 0.000, respectively), glucose (P = 0.000 and 0.000, respectively), fructose (P = 0.004 and 0.054, respectively; group has marginal effect on fructose), and maltose (P = 0.004 and 0.000, respectively). However, there was no interaction effect of treatment and group on these carbohydrates (Supplementary Table S1).
Across two treatments, DS group had significantly lower galactose (–45%, P = 0.008) but had significantly higher maltose (+575%, P = 0.000) compared to the DT group (Figure 6) while both DS and DT groups do not show significant difference for sucrose, fructose, rhamnose, raffinose, erlose, and sorbitol (Supplementary Figure S5). Among the treatments, ACC had consistently significant effect on galactose (P = 0.000 and 0.001), and maltose (P = 0.000 and 0.025) in both the DS and DT groups, respectively (Figure 7). Although ABA increased these carbohydrates as well, it had significant effect only for maltose (P = 0.023) in DS group but not in DT group. Overall, these results indicate that both the DT and DS groups had altered carbohydrates status in response to foliar ABA and ACC spray (Figures 6, 7).
Figure 6.
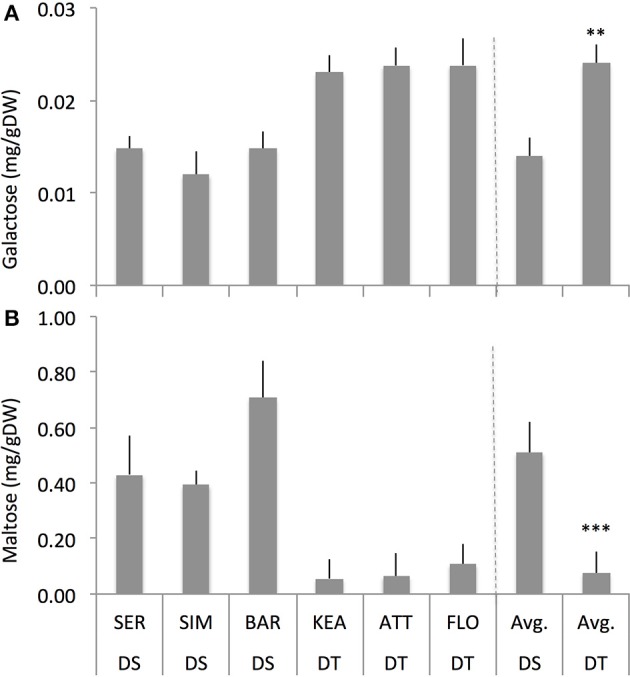
The concentrations of galactose (A) and maltose (B) of drought-susceptible (DS) and drought-tolerant (DT) genotypes across the treatments (control, ABA and ACC (1-aminocyclopropane-1-carboxylic acid) spray) with four replications for each genotype. **, *** indicate p < 0.01, and < 0.05, respectively.
Figure 7.
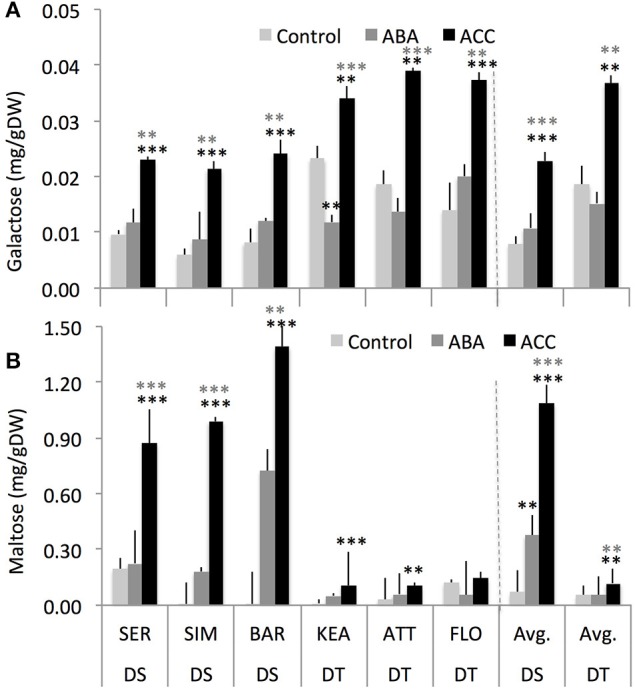
The effect of chemical spay on the concentrations of galactose (A), and maltose (B) between drought-susceptible (DS) and drought-tolerant (DT) genotypes at 3rd leaf stage that were either sprayed with water (controls), abscisic acid (ABA, 0.1 μM), or the ethylene-precursor, 1-aminocyclopropane-1-carboxylic acid (ACC, 0.1 μM) with four replications for each genotype. **, *** indicate p < 0.01, and < 0.05, respectively.
Discussion
Previous studies on shoot growth plasticity to varying soil moisture have provided valuable information leading to current understanding of growth control by multiple processes. We have further extended our understanding to assess the importance of individual physiological traits in the context of crop growth under stress conditions. First, we propose that a simple shoot biomass growth assay (Figure 2) can be used as a sensitive indicator of stress tolerance (Claeys et al., 2014). Second, an early seedling stage represents a suitable growing tissue for deducing precise drought adaptive mechanisms controlling growth since drought adaptive mechanisms differ between young growing- and mature-tissues (Harb et al., 2010; Skirycz et al., 2010). Third, many QTLs for several seedling-stage traits, including early shoot biomass, co-locate with QTLs linked with grain yields (Sandhu et al., 2015); hence, seedling responses can be relevant for crop yields under field conditions. This study encompasses the first two propositions and further suggests that an early seedling-stage can predict stress-adapted traits and reduces the time needed for genotype selections prior to time-consuming phenotypic evaluations under field conditions.
Mild drought stress enhanced shoot dry biomass in drought-tolerant genotypes but not in drought-susceptible genotypes
Our study showed that the DT group could enhance, or maintain, shoot biomass growth under mild drought conditions as compared to the DS group (Figures 2A,B). Such differential growth responses between DT and DS groups may be controlled by genotype-specific mechanisms (Hall et al., 1982; Chaves et al., 2002; Campos et al., 2004; An et al., 2014). Supporting, both DT and DS groups exhibited differential hormone responses (Figure 3) and ABA:ethylene ratios (Figure 4) when exposed to mild drought. Though marginally significant (P = 0.056), the DT group had a higher gs under MWD compared to WW conditions (Supplementary Figure S2D), suggesting a sustained gs may enhance carbon gain (Caldeira et al., 2014; Tardieu et al., 2014), and subsequently crop yield (Fischer et al., 1998; Lu et al., 1998), under mild stress conditions. Such differential stomatal responses between DT (small stomatal responses) and DS (large stomatal responses) genotypes have previously been reported and have also been shown to correlate with yield under stress conditions (Tardieu and Simonneau, 1998; Munns et al., 2010; Sade et al., 2012; Tardieu et al., 2014).
The enhanced shoot growth of DT group under MWD in our study might be related to the fact that we imposed a steady-state MWD on growing tissues of young seedlings, which greatly differ with mature tissues, for stress adaptive mechanisms (Lechner et al., 2008). We further propose that the positive growth response of young growing seedlings, such as in this study, might be easier to detect when not dominated by a negative or no growth response of mature tissues particularly at later developmental stages. While such differential growth responses between young and mature tissues at different developmental stages under MWD is worth for follow-up studied, it may partly explain a widely reported negative growth response of plants under drought that contain proportionately more mature tissue than young growing tissue. Such mechanisms may differ between the DT and DS genotypes (Ji et al., 2011).
Our results agree with previous studies that have reported enhanced shoot dry biomass under mild drought (Liu and Li, 2005; Boutraa et al., 2010). Additional evidence that mild-stresses can enhance shoot biomass growth comes from studies with two indica rice cultivars, where mild-salt stress (NaCl at 0.5% w/v) increased shoot dry weight in 4-weeks after treatment (Sripinyowanich et al., 2013; Tada et al., 2014). Taken all together, we postulate that mild stresses may enhance biomass growth at least in stress-tolerant genotypes although the precise underlying mechanisms can be debatable. Higher gs can be an obvious important stress-associated trait that can contribute to an increased carbon (C) gain in the DT genotypes during an initial stages of drought stress (Caldeira et al., 2014; Tardieu et al., 2014). In addition, mechanisms that involve lower energy costs, for example, lower root respiration could be important for growth regulation under MWD, as reported in drought-tolerant wheat genotype (Liu and Li, 2005).
ABA:ethylene ratio is an important trait in shoot growth regulation under mild drought that differs between drought-tolerant and drought-susceptible genotypes
It has long been known that plant hormones form a complex network to coordinate the regulation of numerous development processes. ABA and ethylene interactions in regulating numerous biological processes have been well-reported at the cell level (Tanaka et al., 2005; Beguerisse-Dıaz et al., 2012; Chen et al., 2013; Watkins et al., 2014). However, although the chemical control of growth by these hormones has been demonstrated in specific tissues (Sharp and LeNoble, 2002), our eco-physiological understanding of the regulation of these hormones in field crops at the whole plant/crop level is rather limited (Parent et al., 2009; Caldeira et al., 2014; Planes et al., 2015) as these hormones affect a very large number of processes and their interactions are complex. This study suggests a key role for an optimum threshold of ABA:ethylene ratio in regulating shoot biomass growth (Figure 4) whereby both the DT and DS groups had different ABA:ethylene ratios in response to mild drought. Such an ABA:ethylene ratio might be different for other crop species and physiological processes studied and may be specific to developmental stages, an issue worthy to be studied. We propose that genotypes and/or environmental conditions that lead to an optimum hormonal ratio under mild stress conditions—as was shown in this study—may allow greater shoot biomass gain as long as the hormonal ratio in other tissues is not detrimental, which may be different under more severe stress.
Our results complement several studies that have previously demonstrated the key roles of hormonal ratio sensing, for example, auxin:cytokinin ratio in shoot, root induction (Skoog and Miller, 1957; Mercier et al., 2003), and shoot vigor (Albacete et al., 2008), cytokinin:auxin ratio in shoot and inflorescence regeneration (Cheng et al., 2010), gibberellin:abscisic acid ratio in barley grains (Weier et al., 2014), and arabidopsis seed development (Yamaguchi, 2008), most likely through differential gene expressions (Weier et al., 2014). Knowing that ethylene is neither actively transported nor degraded, although ACC oxidase activity is constitutively present in most vegetative plant tissues, and that both DT and DS groups significantly differed for ethylene accumulation but not for ABA, strongly suggest that genetic variability in ethylene biosynthesis may play a crucial role in the changes of ABA:ethylene ratio and its effect on shoot dry biomass of plants. This is further supported by the fact that, across two groups, ethylene was increased by 149% while ABA was increased by 112% under mild drought, not inconsistent with previous studies reporting a higher (five-fold) and lower (two-five-fold) increases for ethylene and ABA, respectively, in a salt-stressed tomato (Albacete et al., 2008). Indeed, ethylene response transcription factors (ERF5/ERF6) have been proposed to act as molecular nodes in the stress-related network where growth control and stress tolerance diverge (Claeys and Inzé, 2013; Dubois et al., 2013). Because, wheat genotypes exhibit a large genetic variability in biosynthesis of, and sensitivity to, ABA and ethylene (Quarrie and Lister, 1983; Sridhar, 2003; Iehisa and Takumi, 2012; Valluru et al., 2014), a natural variation in ABA and ethylene biosynthesis, and consequently ABA:ethylene ratio, might reflect a genetic determinism that partly drive biomass accumulation among crop species under mild stress conditions. Therefore, hormonal ratio can be an invaluable candidate trait for the selection of genotypes for achieving higher biomass and yield under mild stress conditions (Wilkinson et al., 2012).
While our study emphasizes hormonal ratio influences on both plant growth and functioning, it does not throw light upon the mechanistic basis of the maintenance of an appropriate ABA:ethylene ratio in plants. We, however, propose that such an optimal ABA:ethylene ratio, rather than single hormone level, could be a sensitive regulator (or sensor) of, for instance, appropriate morphological development and physiological functioning (Weier et al., 2014; Zhang et al., 2014) providing a fitness advantage in complex natural environments. Plants may respond to environmental perturbations by synthesizing different hormone levels, thereby different hormonal ratio, enabling the communication and transduction of environmental cues into plastic responses (Pozo et al., 2015). It is now widely accepted that ethylene and ABA interact at multiple levels (Cheng et al., 2009; Krouk et al., 2011) and ABA induced stomatal closure has been widely shown to be antagonized by ethylene. An optimal ABA:ethylene ratio therefore keeps stomata partly open (higher gs) allowing enhanced gas exchange that indeed allow continued C gain in DT genotypes under mild drought. While expansive growth may be directly limited by hydraulic signals (Caldeira et al., 2014), continued C gain is important for attaining dry biomass gain when water status is re-established. However, modifying the hormonal ratio by attaining moderate levels of hormones through breeding remains a major challenge. Exploring phenotypic screens of large numbers of genotypes including landraces, wild relatives (Sridhar, 2003; Iehisa and Takumi, 2012; Valluru et al., 2014) and the use of molecular approaches targeted at specific tissues and growth stages (Habben et al., 2014) would facilitate the development of crop cultivars that are able to grow under numerous abiotic stress conditions with minimal yield losses (Peleg and Blumwald, 2011).
Low concentrations of ABA and ACC increase shoot relative growth and alter carbohydrate status that differ between drought-tolerant and drought-susceptible genotypes
Generally, the action of ABA and ethylene at the higher concentration has been related with the process of growth inhibition. However, there is recent evidence of their presence in developing tissues and also of being organ/tissue and development stage-specific where they may have a promoting action (Finkelstein and Rock, 2002; Sansberro et al., 2004; Peng et al., 2006; Skirycz et al., 2010; Duan et al., 2013). Our results demonstrate that low concentrations of ABA and ACC sprayed onto the seedlings favored vegetative growth, benefitting dry matter accumulation of wheat seedlings under optimal growing conditions particularly for DS genotypes (Figure 5). These results agree with previous studies that have reported that field-grown wheat plants treated with ABA (300 mg L−1) under water stress showed higher shoot biomass accumulation (Travaglia et al., 2007, 2010). Further, exogenous ABA (10 mg L−1) application at anthesis stage increased dry matter accumulation 7 days after anthesis in a field-grown stay-green wheat line (Yang et al., 2014). In addition, ABA (300 mg L−1) sprayed onto the leaves of soybean plants showed an enhanced dry matter accumulation under field conditions (Travaglia et al., 2009). Moreover, ABA and ACC spray lead to the accumulation of specific carbohydrates in leaves (Figure 7). Overall, these results suggest that both ABA and ethylene at low concentration may be important regulators of shoot biomass likely due to improved physiological parameters such as chlorophyll, green leaf area and duration, photosynthesis, and carbohydrate status (source effects), as reported previously (Khan, 2004; Travaglia et al., 2007, 2010; Khan et al., 2008; Iqbal et al., 2011, 2012).
Again, both DT and DS groups showed differences in the accumulation of specific carbohydrates (Figure 6). The DS group had significantly lower galactose but had significantly higher maltose contents compared to the DT group. This suggest that the DS group had more utilization of sugars such as galactose (galactose is directly converted to glucose, for example, in wheat seedlings; Hassid et al., 1956), and maltose levels (Figure 6) compared to the DT group. Higher maltose levels indicate high turnover of starch. Although galactose at higher concentration has often been shown to be detrimental to organ growth, lower concentrations of galactose can transiently increase the sink demand for carbon, and therefore, enhances carbon unloading from the phloem (Thorpe et al., 1999). Because galactose is known as a unique sugar that increase carbon import and phloem unloading, it may offer avenues to examine possible sugar signals resulting in phloem unloading in sink tissues and consequent biomass development (Seifert et al., 2002) especially when compared the DT and DS genotypes.
In addition to growth stimulation, low-concentrations of ABA and ethylene may condition the crop plants that, in essence, would provide competence for adaptation to stresses of similar or others (Bartels et al., 1990). Since these hormones have knock-on effects on several growth processes that can also be measured, this study therefore suggests that phenotyping for low-concentrations of ABA- and ethylene-induced growth per se would potentially represent a positive contribution to crop biomass and yield under field conditions (Figure 5, Cai et al., 2014), and may also lead to novel germplasm being made available to breeders for the development of high yielding and stress adapted crop cultivars.
In conclusion, the hormone interaction presented here may deliver benefits in terms of dry biomass gain under mild stress conditions. In environments with optimal to sub-optimal growing conditions, which induce slightly elevated concentrations of both hormones, the ABA and ethylene ratio presented here may underlie a part of genetic determinism that control shoot dry biomass gain in wheat. This is supported by our results that (1) both the DT and DS groups exhibited different SDW responses to mild drought (Figure 2; Liu and Li, 2005; Boutraa et al., 2010), (2) mild drought induced low concentrations of ABA and ethylene (Figure 3 Wright, 1977; Ali et al., 1999; Dodd et al., 2010), and (3) low concentrations of ABA and ACC stimulated SDW of wheat seedlings (Figure 5; Takahashi, 1972; Watanabe and Takahashi, 1997) likely through altered carbohydrates status of the plants (Figures 6, 7).
Author contributions
RV, WD: Designed, conducted and oversee the glasshouse experiments; MR: designed and oversee the field experiments; RV, WD, MR and ID: wrote the paper. All authors read and approved the final manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work is supported by the Consultative Group on International Agricultural Research (CGIAR) Research Program (CRP-WHEAT, SI-6) through the CIMMYT. Authors thank Xiaoqing Li and Guofeng Hu for their assistance in the glasshouse experiments and Timothy Gregson for the sugars analysis.
Supplementary material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2016.00461
(A), Soil moisture content (g H2O g−1 dry soil) in both well-watered and mild drought stress treatments; (B), Soil water potential (MPa) of both well-watered and mild-drought stressed plants; (C), Temperature and humidity levels during the experiment, and (D), Primary leaf growth response to different concentrations of ABA and ACC.
(A) Shoot fresh weight of six wheat genotypes that were either grown under well-watered (WW) or mild water-deficit (MWD) conditions. (B), the relationship between log values of fresh weight (FW) and dry weight (DW) in WW and MWD treatments; (C), plant water content (%) of drought-susceptible and drought-tolerant wheat genotypes in WW and MWD treatments; (D), stomatal conductance of drought-susceptible and drought-tolerant wheat genotypes in WW and MWD treatments.
ABA concentration (A) and ethylene evolution (B) in well-watered (WW) and mild-drought stress (MWD) treatments across all genotypes. Linear correlations between shoot dry weight (SDW) and endogenous ABA (C) and ethylene (D) across all groups and treatments. ** indicates p < 0.01.
Shoot relative growth rate in drought-susceptible (A) and drought-tolerant (B) wheat genotypes that were either sprayed with water (controls), abscisic acid (ABA, 0.1 μM) or the ethylene-precursor, 1-aminocyclopropane-1-carboxylic acid (ACC, 0.1 μM) at the 6th leaf stage.
Sucrose (A), fructose (B), rhamnose (C), raffinose (D), erlose (E), and sorbitol (F) concentrations of drought-susceptible (DS) and drought-tolerant (DT) wheat genotypes across the treatments [control, ABA and ACC (1-aminocyclopropane-1-carboxylic acid) spray].
Two-way ANOVA for the effects of treatments (well-watered and mild drought) and stress group (drought-tolerant and drought-susceptible) and their interaction on several carbohydrate concentrations of six wheat genotypes that were either sprayed with water (controls), abscisic acid (ABA, 0.1 μM) or the ethylene-precursor, 1-aminocyclopropane-1-carboxylic acid (ACC, 0.1 μM) at the 3rd leaf stage. *, **, *** indicate p < 0.001, < 0.01, and < 0.05, respectively.
References
- Albacete A., Ghanem M. E., Martínez-Andújar C., Acosta M., Sánchez-Bravo J., Martínez V., et al. (2008). Hormonal changes in relation to biomass partitioning and shoot growth impairment in salinized tomato (Solanum lycopersicum L.) plants. J. Exp. Bot. 59, 4119–4131. 10.1093/jxb/ern251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M., Jensen C. R., Mogensen V. O., Andersen M. N., Henson I. E. (1999). Root signalling and osmotic adjustment during intermittent soil drying sustain grain yield of field grown wheat. Field Crops Res. 62, 35–52. 10.1016/S0378-4290(99)00003-9 [DOI] [Google Scholar]
- Almeida-Rodriguez A. M., Cooke J. E. K., Yeh F., Zwiazek J. J. (2010). Functional characterization of drought-responsive aquaporins in Populus balsamifera and Populus simonii × balsamifera clones with different drought resistance strategies. Physiol. Plant. 140, 321–333. 10.1111/j.1399-3054.2010.01405.x [DOI] [PubMed] [Google Scholar]
- An Y., Zhou P., Liang J. (2014). Effects of exogenous application of abscisic acid on membrane stability, osmotic adjustment, photosynthesis and hormonal status of two lucerne (Medicago sativa L.) genotypes under high temperature stress and drought stress. Crop Pasture Sci. 65, 274–286. 10.1071/CP13162 [DOI] [Google Scholar]
- Araus J. L., Slafer G. A., Reynolds M. P., Royo C. (2002). Plant Breeding and drought in C3 cereals: what should we breed for? Ann. Bot. 89, 925–940. 10.1093/aob/mcf049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araus J. L., Slafer G. A., Royo C., Serret M. D. (2008). Breeding for yield potential and stress adaptation in cereals. Crit. Rev. Plant Sci. 27, 377–412. 10.1080/07352680802467736 [DOI] [Google Scholar]
- Bartels D., Schneider K., Terstappen G., Piatkowski D., Salamini F. (1990). Molecular cloning of abscisic acid-modulated genes which are induced during desiccation of the resurrection plant Craterostigma plantagineum. Planta 181, 27–34. 10.1007/BF00202321 [DOI] [PubMed] [Google Scholar]
- Beguerisse-Dıaz M., Hernández-Gómez M. C., Lizzul A. M., Barahona M., Desikan R. (2012). Compound stress response in stomatal closure: a mathematical model of ABA and ethylene interaction in guard cells. BMC Syst. Biol. 6:146. 10.1186/1752-0509-6-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum A. (2011). Plant water relations, plant stress and plant production, in Plant Breeding for Water-Limited Environments, (New York, NY: Springer; ), 11–52. Available online at: http://link.springer.com/chapter/10.1007/978-1-4419-7491-4_2 (Accessed on October 8, 2014). [Google Scholar]
- Boutraa T., Akhkha A., Al-Shoaibi A. A., Alhejeli A. M. (2010). Effect of water stress on growth and water use efficiency (WUE) of some wheat cultivars (Triticum durum) grown in Saudi Arabia. J. Taibah Univ. Sci. 3, 39–48. 10.1016/S1658-3655(12)60019-3 [DOI] [Google Scholar]
- Burg S. P., Burg E. A. (1966). The interaction between auxin and ethylene and its role in plant growth. Proc. Natl. Acad. Sci. U.S.A. 55, 262–269. 10.1073/pnas.55.2.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. (1968). Ethylene formation in pea seedlings; its relation to the inhibition of bud growth caused by Indole-3-Acetic acid. Plant Physiol. 43, 1069–1074. 10.1104/pp.43.7.1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai T., Xu H., Peng D., Yin Y., Yang W., Ni Y., et al. (2014). Exogenous hormonal application improves grain yield of wheat by optimizing tiller productivity. Field Crops Res. 155, 172–183. 10.1016/j.fcr.2013.09.008 [DOI] [Google Scholar]
- Caldeira C. F., Bosio M., Parent B., Jeanguenin L., Chaumont F., Tardieu F. (2014). A hydraulic model is compatible with rapid changes in leaf elongation under fluctuating evaporative demand and soil water status. Plant Physiol. 164, 1718–1730. 10.1104/pp.113.228379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos H., Cooper M., Habben J. E., Edmeades G. O., Schussler J. R. (2004). Improving drought tolerance in maize: a view from industry. Field Crops Res. 90, 19–34. 10.1016/j.fcr.2004.07.003 [DOI] [Google Scholar]
- Cattivelli L., Rizza F., Badeck F.-W., Mazzucotelli E., Mastrangelo A. M., Francia E., et al. (2008). Drought tolerance improvement in crop plants: an integrated view from breeding to genomics. Field Crops Res. 105, 1–14. 10.1016/j.fcr.2007.07.004 [DOI] [Google Scholar]
- Chaves M. M., Pereira J. S., Maroco J., Rodrigues M. L., Ricardo C. P., Osório M. L., et al. (2002). How plants cope with water stress in the field? Photosynthesis and growth. Ann. Bot. 89, 907–916. 10.1093/aob/mcf105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves M. M., Zarrouk O., Francisco R., Costa J. M., Santos T., Regalado A. P., et al. (2010). Grapevine under deficit irrigation: hints from physiological and molecular data. Ann. Bot. 105, 661–676. 10.1093/aob/mcq030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Dodd I. C., Davies W. J., Wilkinson S. (2013). Ethylene limits abscisic acid- or soil drying-induced stomatal closure in aged wheat leaves. Plant Cell Environ. 36, 1850–1859. 10.1111/pce.12094 [DOI] [PubMed] [Google Scholar]
- Cheng W.-H., Chiang M.-H., Hwang S.-G., Lin P.-C. (2009). Antagonism between abscisic acid and ethylene in Arabidopsis acts in parallel with the reciprocal regulation of their metabolism and signaling pathways. Plant Mol. Biol. 71, 61–80. 10.1007/s11103-009-9509-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z. J., Zhu S. S., Gao X. Q., Zhang X. S. (2010). Cytokinin and auxin regulates WUS induction and inflorescence regeneration in vitro in Arabidopsis. Plant Cell Rep. 29, 927–933. 10.1007/s00299-010-0879-8 [DOI] [PubMed] [Google Scholar]
- Claeys H., Inzé D. (2013). The agony of choice: how plants balance growth and survival under water-limiting conditions. Plant Physiol. 162, 1768–1779. 10.1104/pp.113.220921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claeys H., Landeghem S. V., Dubois M., Maleux K., Inzé D. (2014). What is stress? dose-response effects in commonly used in vitro stress assays. Plant Physiol. 165, 519–527. 10.1104/pp.113.234641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies W. J., Zhang J. (1991). Root signals and the regulation of growth and development of plants in drying soil. Annu. Rev. Plant Physiol. Plant Mol. Biol. 42, 55–76. 10.1146/annurev.pp.42.060191.000415 [DOI] [Google Scholar]
- Deokar A. A., Kondawar V., Jain P. K., Karuppayil S. M., Raju N. L., Vadez V., et al. (2011). Comparative analysis of expressed sequence tags (ESTs) between drought-tolerant and -susceptible genotypes of chickpea under terminal drought stress. BMC Plant Biol. 11:70. 10.1186/1471-2229-11-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd I. C., Egea G., Watts C. W., Whalley W. R. (2010). Root water potential integrates discrete soil physical properties to influence ABA signalling during partial rootzone drying. J. Exp. Bot. 61, 3543–3551. 10.1093/jxb/erq195 [DOI] [PubMed] [Google Scholar]
- Dodd I. C., Theobald J. C., Bacon M. A., Davies W. J. (2006). Alternation of wet and dry sides during partial rootzone drying irrigation alters root-to-shoot signalling of abscisic acid. Funct. Plant Biol. 33, 1081–1089. 10.1071/FP06203 [DOI] [PubMed] [Google Scholar]
- Dodd I. C. (2013). Abscisic acid and stomatal closure: a hydraulic conductance conundrum. New Phytol 197, 6–8. 10.1111/nph.12052 [DOI] [PubMed] [Google Scholar]
- Duan L., Dietrich D., Ng C. H., Chan P. M. Y., Bhalerao R., Bennett M. J., et al. (2013). Endodermal ABA signaling promotes lateral root quiescence during salt stress in Arabidopsis seedlings. Plant Cell Online 25, 324–341. 10.1105/tpc.112.107227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois M., Skirycz A., Claeys H., Maleux K., Dhondt S., De Bodt S., et al. (2013). ETHYLENE RESPONSE FACTOR6 acts as a central regulator of leaf growth under water-limiting conditions in Arabidopsis. Plant Physiol. 162, 319–332. 10.1104/pp.113.216341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst R., Bjornsen J. E., Arditti J. (1992). Effects of ethephon, its nonethylene-generating analog ethylphosphonic acid, and phosphorous acid in aseptic culture of orchid seedlings. Am. J. Bot. 79, 275–278. 10.2307/2445015 [DOI] [Google Scholar]
- Fatichi S., Leuzinger S., Körner C. (2014). Moving beyond photosynthesis: from carbon source to sink-driven vegetation modeling. New Phytol. 201, 1086–1095. 10.1111/nph.12614 [DOI] [PubMed] [Google Scholar]
- Finkelstein R. R., Rock C. D. (2002). Abscisic acid biosynthesis and response. Arabidopsis Book 1:e0058. 10.1199/tab.0058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer R. A., Rees D., Sayre K. D., Lu Z.-M., Condon A. G., Saavedra A. L. (1998). Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Sci. 38, 1467 10.2135/cropsci1998.0011183X003800060011x [DOI] [Google Scholar]
- González Á. (2011). Response of coleoptiles to water deficit: growth, turgor maintenance and osmotic adjustment in barley plants (Hordeum vulgare L.). Agric. Sci. 2, 159–166. 10.4236/as.2011.23022 [DOI] [Google Scholar]
- Gressel J., Dodds J. (2013). Commentary: hormesis can be used in enhancing plant productivity and health; but not as previously envisaged. Plant Sci. 213, 123–127. 10.1016/j.plantsci.2013.09.007 [DOI] [PubMed] [Google Scholar]
- Guzmán P., Ecker J. R. (1990). Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell Online 2, 513–523. 10.1105/tpc.2.6.513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habben J. E., Bao X., Bate N. J., DeBruin J. L., Dolan D., Hasegawa D., et al. (2014). Transgenic alteration of ethylene biosynthesis increases grain yield in maize under field drought-stress conditions. Plant Biotechnol. J. 12, 685–693. 10.1111/pbi.12172 [DOI] [PubMed] [Google Scholar]
- Hall A. J., Vilella F., Trapani N., Chimenti C. (1982). The effects of water stress and genotype on the dynamics of pollen-shedding and silking in maize. Field Crops Res. 5, 349–363. 10.1016/0378-4290(82)90036-3 [DOI] [Google Scholar]
- Harb A., Krishnan A., Ambavaram M. M. R., Pereira A. (2010). Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiol. 154, 1254–1271. 10.1104/pp.110.161752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassid W. Z., Putman E. W., Ginsburg V. (1956). Metabolism of galactose in canna leaves and wheat seedlings. Biochim. Biophys. Acta 20, 17–22. 10.1016/0006-3002(56)90256-6 [DOI] [PubMed] [Google Scholar]
- Hatier J.-H. B., Faville M. J., Hickey M. J., Koolaard J. P., Schmidt J., Carey B.-L., et al. (2014). Plant vigour at establishment and following defoliation are both associated with responses to drought in perennial ryegrass (Lolium perenne L.). J. Exp. Bot. 65, 5823–5834. 10.1093/jxb/eru318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays D. B., Do J. H., Mason R. E., Morgan G., Finlayson S. A. (2007). Heat stress induced ethylene production in developing wheat grains induces kernel abortion and increased maturation in a susceptible cultivar. Plant Sci. 172, 1113–1123. 10.1016/j.plantsci.2007.03.004 [DOI] [Google Scholar]
- Hoffmann W. A., Poorter H. (2002). Avoiding bias in calculations of relative growth rate. Ann. Bot. 90, 37–42. 10.1093/aob/mcf140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iehisa J. C. M., Takumi S. (2012). Variation in abscisic acid responsiveness of Aegilops tauschii and hexaploid wheat synthetics due to the D-genome diversity. Genes Genet. Syst. 87, 9–18. 10.1266/ggs.87.9 [DOI] [PubMed] [Google Scholar]
- Iqbal N., Khan N. A., Nazar R., da Silva J. A. T. (2012). Ethylene-stimulated photosynthesis results from increased nitrogen and sulfur assimilation in mustard types that differ in photosynthetic capacity. Environ. Exp. Bot. 78, 84–90. 10.1016/j.envexpbot.2011.12.025 [DOI] [Google Scholar]
- Iqbal N., Nazar R., Syeed S., Masood A., Khan N. A. (2011). Exogenously-sourced ethylene increases stomatal conductance, photosynthesis, and growth under optimal and deficient nitrogen fertilization in mustard. J. Exp. Bot. 62, 4955 –4963. 10.1093/jxb/err204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X., Dong B., Shiran B., Talbot M. J., Edlington J. E., Hughes T., et al. (2011). Control of abscisic acid catabolism and abscisic acid homeostasis is important for reproductive stage stress tolerance in cereals. Plant Physiol. 156, 647–662. 10.1104/pp.111.176164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia W., Davies W. J. (2007). Modification of leaf apoplastic pH in relation to stomatal sensitivity to root-sourced abscisic acid signals. Plant Physiol. 143, 68–77. 10.1104/pp.106.089110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamaluddin M., Zwiazek J. J. (2002). Ethylene enhances water transport in hypoxic aspen. Plant Physiol. 128, 962 –969. 10.1104/pp.010791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N. A., Mir M. R., Nazar R., Singh S. (2008). The application of ethephon (an ethylene releaser) increases growth, photosynthesis and nitrogen accumulation in mustard (Brassica juncea L.) under high nitrogen levels. Plant Biol. 10, 534–538. 10.1111/j.1438-8677.2008.00054.x [DOI] [PubMed] [Google Scholar]
- Khan N. A. (2004). An evaluation of the effects of exogenous ethephon, an ethylene releasing compound, on photosynthesis of mustard (Brassica juncea) cultivars that differ in photosynthetic capacity. BMC Plant Biol. 4:21. 10.1186/1471-2229-4-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieber J. J., Rothenberg M., Roman G., Feldmann K. A., Ecker J. R. (1993). CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell 72, 427–441. 10.1016/0092-8674(93)90119-B [DOI] [PubMed] [Google Scholar]
- Krouk G., Ruffel S., Gutiérrez R. A., Gojon A., Crawford N. M., Coruzzi G. M., et al. (2011). A framework integrating plant growth with hormones and nutrients. Trends Plant Sci. 16, 178–182. 10.1016/j.tplants.2011.02.004 [DOI] [PubMed] [Google Scholar]
- Ku H. S., Suge H., Rappaport L., Pratt H. K. (1970). Stimulation of rice coleoptile growth by ethylene. Planta 90, 333–339. 10.1007/BF00386385 [DOI] [PubMed] [Google Scholar]
- Lechner L., Pereyra-Irujo G. A., Granier C., Aguirrezábal L. A. N. (2008). Rewatering plants after a long water-deficit treatment reveals that leaf epidermal cells retain their ability to expand after the leaf has apparently reached its final size. Ann. Bot. (Lond.) 101, 1007–1015. 10.1093/aob/mcn029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman A., Black R., Ecker J. R. (1996). HOOKLESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyl. Cell 85, 183–194. 10.1016/S0092-8674(00)81095-8 [DOI] [PubMed] [Google Scholar]
- Li Y.-S., Mao X.-T., Tian Q.-Y., Li L.-H., Zhang W.-H. (2009). Phosphorus deficiency-induced reduction in root hydraulic conductivity in Medicago falcata is associated with ethylene production. Environ. Exp. Bot. 67, 172–177. 10.1016/j.envexpbot.2009.05.013 [DOI] [Google Scholar]
- Liu H. S., Li F. M. (2005). Root Respiration, Photosynthesis and grain yield of two spring wheat in response to soil drying. Plant Growth Regul. 46, 233–240. 10.1007/s10725-005-8806-7 [DOI] [Google Scholar]
- Lopes M. S., Dreisigacker S., Peña R. J., Sukumaran S., Reynolds M. P. (2015). Genetic characterization of the wheat association mapping initiative (WAMI) panel for dissection of complex traits in spring wheat. Theor. Appl. Genet. 128, 453–464. 10.1007/s00122-014-2444-2 [DOI] [PubMed] [Google Scholar]
- Lopes M. S., Reynolds M. P., Jalal-Kamali M. R., Moussa M., Feltaous Y., Tahir I. S. A., et al. (2012). The yield correlations of selectable physiological traits in a population of advanced spring wheat lines grown in warm and drought environments. Field Crops Res. 128, 129–136. 10.1016/j.fcr.2011.12.017 [DOI] [Google Scholar]
- Lu Z., Percy R. G., Qualset C. O., Zeiger E. (1998). Stomatal conductance predicts yields in irrigated Pima cotton and bread wheat grown at high temperatures. J. Exp. Bot. 49, 453–460. 10.1093/jxb/49.Special_Issue.453 [DOI] [Google Scholar]
- McDowell N., Pockman W. T., Allen C. D., Breshears D. D., Cobb N., Kolb T., et al. (2008). Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol. 178, 719–739. 10.1111/j.1469-8137.2008.02436.x [DOI] [PubMed] [Google Scholar]
- Meinzer F. C., Woodruff D. R., Marias D. E., Mcculloh K. A., Sevanto S. (2014). Dynamics of leaf water relations components in co-occurring iso- and anisohydric conifer species. Plant Cell Environ. 37, 2577–2586. 10.1111/pce.12327 [DOI] [PubMed] [Google Scholar]
- Mercier H., Souza B. M., Kraus J. E., Hamasaki R. M., Sotta B. (2003). Endogenous auxin and cytokinin contents associated with shoot formation in leaves of pineapple cultured in vitro. Braz. J. Plant Physiol. 15, 107–112. 10.1590/S1677-04202003000200006 [DOI] [Google Scholar]
- Munns R., James R. A., Sirault X. R. R., Furbank R. T., Jones H. G. (2010). New phenotyping methods for screening wheat and barley for beneficial responses to water deficit. J. Exp. Bot. 61, 3499–3507. 10.1093/jxb/erq199 [DOI] [PubMed] [Google Scholar]
- Neskovic M., Petrović J., Radojević L., Vujičić R. (1977). Stimulation of growth and nucleic acid biosynthesis at low concentration of abscisic acid in tissue culture of spinacia oleracea. Physiol. Plant. 39, 148–154. 10.1111/j.1399-3054.1977.tb04027.x [DOI] [Google Scholar]
- Nishizawa T., Suge H. (1995a). Ethylene and carbon dioxide: regulation of oat mesocotyl growth. Plant Cell Environ. 18, 197–203. [Google Scholar]
- Nishizawa T., Suge H. (1995b). The regulation of maize mesocotyl growth by ethylene and carbon dioxide. Jpn. J. Crop Sci. 64, 794–800. [Google Scholar]
- O'Rourke C., Gregson T., Murray L., Sadler I. H, Fry S. C. (2015). Sugar composition of the pectic polysaccharides of charophytes, the closest algal relatives of land-plants: presence of 3-O-methyl-D-galactose residues. Ann. Bot. 116, 225–236. 10.1093/aob/mcv089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantin F., Monnet F., Jannaud D., Costa J. M., Renaud J., Muller B., et al. (2012). The dual effect of abscisic acid on stomata. New Phytol. 197, 65–72. 10.1111/nph.12013 [DOI] [PubMed] [Google Scholar]
- Parent B., Hachez C., Redondo E., Simonneau T., Chaumont F., Tardieu F. (2009). Drought and abscisic acid effects on aquaporin content translate into changes in hydraulic conductivity and leaf growth rate: a trans-scale approach. Plant Physiol. 149, 2000–2012. 10.1104/pp.108.130682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent B., Suard B., Serraj R., Tardieu F. (2010). Rice leaf growth and water potential are resilient to evaporative demand and soil water deficit once the effects of root system are neutralized. Plant Cell Environ. 33, 1256–1267. 10.1111/j.1365-3040.2010.02145.x [DOI] [PubMed] [Google Scholar]
- Peleg Z., Blumwald E. (2011). Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 14, 290–295. 10.1016/j.pbi.2011.02.001 [DOI] [PubMed] [Google Scholar]
- Peng Y.-B., Zou C., Wang D.-H., Gong H.-Q., Xu Z.-H., Bai S.-N. (2006). Preferential localization of abscisic acid in primordial and nursing cells of reproductive organs of Arabidopsis and cucumber. New Phytol. 170, 459–466. 10.1111/j.1469-8137.2006.01683.x [DOI] [PubMed] [Google Scholar]
- Pereyra-Irujo G. A., Velázquez L., Lechner L., Aguirrezábal L. A. N. (2008). Genetic variability for leaf growth rate and duration under water deficit in sunflower: analysis of responses at cell, organ, and plant level. J. Exp. Bot. 59, 2221–2232. 10.1093/jxb/ern087 [DOI] [PubMed] [Google Scholar]
- Pierik R., Tholen D., Poorter H., Visser E. J. W., Voesenek L. A. C. J. (2006). The Janus face of ethylene: growth inhibition and stimulation. Trends Plant Sci. 11, 176–183. 10.1016/j.tplants.2006.02.006 [DOI] [PubMed] [Google Scholar]
- Planes M. D., Niñoles R., Rubio L., Bissoli G., Bueso E., García-Sánchez M. J., et al. (2015). A mechanism of growth inhibition by abscisic acid in germinating seeds of Arabidopsis thaliana based on inhibition of plasma membrane H+-ATPase and decreased cytosolic pH, K+, and anions. J. Exp. Bot. 66, 813–825. 10.1093/jxb/eru442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo M. J., López-Ráez J. A., Azcón-Aguilar C., García-Garrido J. M. (2015). Phytohormones as integrators of environmental signals in the regulation of mycorrhizal symbioses. New Phytol. 205, 1431–1436. 10.1111/nph.13252 [DOI] [PubMed] [Google Scholar]
- Prado K., Boursiac Y., Tournaire-Roux C., Monneuse J.-M., Postaire O., Ines O. D., et al. (2013). Regulation of Arabidopsis leaf hydraulics involves light-dependent phosphorylation of aquaporins in veins. Plant Cell Online 25, 1029–1039. 10.1105/tpc.112.108456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt H. K., Goeschl J. D. (1969). Physiological roles of ethylene in plants. Annu. Rev. Plant Physiol. 20, 541–584. 10.1146/annurev.pp.20.060169.002545 [DOI] [Google Scholar]
- Quarrie S. A., Lister P. G. (1983). Characterization pf Spring wheat genotypes differing in drought-induced abscisic acid accumulation I. Drought-stressed abscisic acid production. J. Exp. Bot. 34, 1260–1270. 10.1093/jxb/34.10.1260 [DOI] [Google Scholar]
- R Development Core Team (2013). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; Available online at: http://www.R-project.org/ [Google Scholar]
- Rogiers S. Y., Greer D. H., Hatfield J. M., Hutton R. J., Clarke S. J., Hutchinson P. A., et al. (2012). Stomatal response of an anisohydric grapevine cultivar to evaporative demand, available soil moisture and abscisic acid. Tree Physiol. 32, 249–261. 10.1093/treephys/tpr131 [DOI] [PubMed] [Google Scholar]
- Sade N., Gebremedhin A., Moshelion M. (2012). Risk-taking plants: anisohydric behavior as a stress-resistance trait. Plant Signal. Behav. 7, 767–770. 10.4161/psb.20505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sade N., Vinocur B. J., Diber A., Shatil A., Ronen G., Nissan H., et al. (2009). Improving plant stress tolerance and yield production: is the tonoplast aquaporin SlTIP2;2 a key to isohydric to anisohydric conversion? New Phytol. 181, 651–661. 10.1111/j.1469-8137.2008.02689.x [DOI] [PubMed] [Google Scholar]
- Sandhu N., Torres R. O., Sta Cruz M. T., Maturan P. C., Jain R., Kumar A., et al. (2015). Traits and QTLs for development of dry direct-seeded rainfed rice varieties. J. Exp. Bot. 66, 225–244. 10.1093/jxb/eru413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansberro P. A., Mroginski L. A., Bottini R. (2004). Foliar sprays with ABA promote growth of Ilex paraguariensis by alleviating diurnal water stress. Plant Growth Regul. 42, 105–111. 10.1023/B:GROW.0000017476.12491.02 [DOI] [Google Scholar]
- Seifert G. J., Barber C., Wells B., Dolan L., Roberts K. (2002). Galactose biosynthesis in Arabidopsis: genetic evidence for substrate channeling from UDP-D-Galactose into cell wall polymers. Curr. Biol. 12, 1840–1845. 10.1016/S0960-9822(02)01260-5 [DOI] [PubMed] [Google Scholar]
- Sharp R. E., LeNoble M. E. (2002). ABA, ethylene and the control of shoot and root growth under water stress. J. Exp. Bot. 53, 33–37. 10.1093/jexbot/53.366.33 [DOI] [PubMed] [Google Scholar]
- Skirycz A., Bodt S. D., Obata T., Clercq I. D., Claeys H., Rycke R. D., et al. (2010). Developmental stage specificity and the role of mitochondrial metabolism in the response of Arabidopsis leaves to prolonged mild osmotic stress. Plant Physiol. 152, 226–244. 10.1104/pp.109.148965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirycz A., Vandenbroucke K., Clauw P., Maleux K., De Meyer B., Dhondt S., et al. (2011). Survival and growth of Arabidopsis plants given limited water are not equal. Nat. Biotechnol. 29, 212–214. 10.1038/nbt.1800 [DOI] [PubMed] [Google Scholar]
- Skoog F., Miller C. O. (1957). Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp. Soc. Exp. Biol. 11, 118–130. [PubMed] [Google Scholar]
- Smalle J., Haegman M., Kurepa J., Montagu M. V., Straeten D. V. D. (1997). Ethylene can stimulate Arabidopsis hypocotyl elongation in the light. Proc. Natl. Acad. Sci. U.SA. 94, 2756–2761. 10.1073/pnas.94.6.2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soar C. J., Speirs J., Maffei S. M., Penrose A. B., Mccarthy M. G., Loveys B. R. (2006). Grape vine varieties Shiraz and Grenache differ in their stomatal response to VPD: apparent links with ABA physiology and gene expression in leaf tissue. Aust. J. Grape Wine Res. 12, 2–12. 10.1111/j.1755-0238.2006.tb00038.x [DOI] [Google Scholar]
- Sridhar G. (2003). Studies on Endogenous Hormonal Changes during Grain Development in Wheat Genotypes. Available online at: https://www.researchgate.net/publication/46093009_Studies_on_Endogenous_Hormonal_Changes_during_Grain_Development_in_Wheat_Genotypes_(PhD_Thesis) (Accessed on June 10, 2014).
- Sripinyowanich S., Klomsakul P., Boonburapong B., Bangyeekhun T., Asami T., Gu H., et al. (2013). Exogenous ABA induces salt tolerance in indica rice (Oryza sativa L.): the role of OsP5CS1 and OsP5CR gene expression during salt stress. Environ. Exp. Bot. 86, 94–105. 10.1016/j.envexpbot.2010.01.009 [DOI] [Google Scholar]
- Suge H. (1971). Stimulation of oat and rice mesocotyl growth by ethylene. Plant Cell Physiol. 12, 831–837. [Google Scholar]
- Sukumaran S., Dreisigacker S., Lopes M., Chavez P., Reynolds M. P. (2015). Genome-wide association study for grain yield and related traits in an elite spring wheat population grown in temperate irrigated environments. Theor. Appl. Genet. 128, 353–363. 10.1007/s00122-014-2435-3 [DOI] [PubMed] [Google Scholar]
- Tada Y., Komatsubara S., Kurusu T. (2014). Growth and physiological adaptation of whole plants and cultured cells from a halophyte turf grass under salt stress. AoB Plants 10.1093/aobpla/plu041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K. (1972). Abscisic acid as a stimulator for rice mesocotyl growth. Nature 238, 92–93. 10.1038/newbio238092a0 [DOI] [Google Scholar]
- Tanaka Y., Nose T., Jikumaru Y., Kamiya Y. (2013). ABA inhibits entry into stomatal-lineage development in Arabidopsis leaves. Plant J. 74, 448–457. 10.1111/tpj.12136 [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Sano T., Tamaoki M., Nakajima N., Kondo N., Hasezawa S. (2005). Ethylene inhibits abscisic acid-induced stomatal closure in Arabidopsis. Plant Physiol. 138, 2337–2343. 10.1104/pp.105.063503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu F., Parent B., Caldeira C. F., Welcker C. (2014). Genetic and physiological controls of growth under water deficit. Plant Physiol. 164, 1628–1635. 10.1104/pp.113.233353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu F., Simonneau T. (1998). Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modelling isohydric and anisohydric behaviours. J. Exp. Bot. 49, 419–432. 10.1093/jxb/49.Special_Issue.419 [DOI] [Google Scholar]
- Thorpe M. R., MacRae E. A., Minchin P. E. H., Edwards C. M. (1999). Galactose stimulation of carbon import into roots is confined to the Poaceae. J. Exp. Bot. 50, 1613–1618. 10.1093/jxb/50.339.1613 [DOI] [Google Scholar]
- Travaglia C., Cohen A. C., Reinoso H., Castillo C., Bottini R. (2007). Exogenous abscisic acid increases carbohydrate accumulation and redistribution to the grains in wheat grown under field conditions of soil water restriction. J. Plant Growth Regul. 26, 285–289. 10.1007/s00344-007-9018-3 [DOI] [Google Scholar]
- Travaglia C., Reinoso H., Bottini R. (2009). Application of abscisic acid promotes yield in field-cultured soybean by enhancing production of carbohydrates and their allocation in seed. Crop Pasture Sci. 60, 1131–1136. 10.1071/CP08396 [DOI] [Google Scholar]
- Travaglia C., Reinoso H., Cohen A., Luna C., Tommasino E., Castillo C., et al. (2010). Exogenous ABA increases yield in field-grown wheat with moderate water restriction. J. Plant Growth Regul. 29, 366–374. 10.1007/s00344-010-9147-y [DOI] [Google Scholar]
- Trethowan R. M., van Ginkel M., Rajaram S. (2002). Progress in breeding wheat for yield and adaptation in global drought affected environments. Crop Sci. 42, 1441 10.2135/cropsci2002.1441 [DOI] [Google Scholar]
- Valluru R., Thiry A., Wilkinson S., Davies W., Reynolds M. (2014). Phenotypic selection for wheat spike-ethylene, in Proceedings of the 4th International Workshop of the Wheat Yield Consortium (Ciudad Obregon), 109–113. [Google Scholar]
- Wang H., Stier G., Lin J., Liu G., Zhang Z., Chang Y., et al. (2013). Transcriptome changes associated with delayed flower senescence on transgenic petunia by inducing expression of etr1-1, a mutant ethylene receptor. PLoS ONE 8:e65800. 10.1371/journal.pone.0065800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.-Y., Li F.-M., Xiong Y.-C., Xu B.-C. (2008). Soil-water threshold range of chemical signals and drought tolerance was mediated by ROS homeostasis in winter wheat during progressive soil drying. J. Plant Growth Regul. 27, 309–319. 10.1007/s00344-008-9057-4 [DOI] [Google Scholar]
- Watanabe H., Takahashi K. (1997). Effects of abscisic acid, fusicoccin, and potassium on growth and morphogenesis of leaves and internodes in dark-grown rice seedlings. Plant Growth Regul. 21, 109–114. 10.1023/A:1005783425363 [DOI] [Google Scholar]
- Watkins J. M., Hechler P. J., Muday G. K. (2014). Ethylene-induced flavonol accumulation in guard cells suppresses reactive oxygen species and moderates stomatal aperture. Plant Physiol. 164, 1707–1717. 10.1104/pp.113.233528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weier D., Thiel J., Kohl S., Tarkowská D., Strnad M., Schaarschmidt S., et al. (2014). Gibberellin-to-abscisic acid balances govern development and differentiation of the nucellar projection of barley grains. J. Exp. Bot. 65, 5291–5304. 10.1093/jxb/eru289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welcker C., Sadok W., Dignat G., Renault M., Salvi S., Charcosset A., et al. (2011). A common genetic determinism for sensitivities to soil water deficit and evaporative demand: meta-analysis of quantitative trait loci and introgression lines of maize. Plant Physiol. 157, 718–729. 10.1104/pp.111.176479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S., Davies W. J. (2008). Manipulation of the apoplastic pH of intact plants mimics stomatal and growth responses to water availability and microclimatic variation. J. Exp. Bot. 59, 619–631. 10.1093/jxb/erm338 [DOI] [PubMed] [Google Scholar]
- Wilkinson S., Kudoyarova G. R., Veselov D. S., Arkhipova T. N., Davies W. J. (2012). Plant hormone interactions: innovative targets for crop breeding and management. J. Exp. Bot. 63, 3499–3509. 10.1093/jxb/ers148 [DOI] [PubMed] [Google Scholar]
- Wright S. T. C. (1977). The relationship between leaf water potential ψ leaf and the levels of abscisic acid and ethylene in excised wheat leaves. Planta 134, 183–189. 10.1007/BF00384969 [DOI] [PubMed] [Google Scholar]
- Yamaguchi S. (2008). Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 59, 225–251. 10.1146/annurev.arplant.59.032607.092804 [DOI] [PubMed] [Google Scholar]
- Yang D., Luo Y., Ni Y., Yin Y., Yang W., Peng D., et al. (2014). Effects of exogenous ABA application on post-anthesis dry matter redistribution and grain starch accumulation of winter wheat with different staygreen characteristics. Crop J. 2, 144–153. 10.1016/j.cj.2014.02.004 [DOI] [Google Scholar]
- Yang J., Zhang J., Wang Z., Liu K., Wang P. (2006). Post-anthesis development of inferior and superior spikelets in rice in relation to abscisic acid and ethylene. J. Exp. Bot. 57, 149–160. 10.1093/jxb/erj018 [DOI] [PubMed] [Google Scholar]
- Zhang G.-B., Yi H.-Y., Gong J.-M. (2014). The Arabidopsis ethylene/jasmonic Acid-NRT signaling module coordinates nitrate reallocation and the trade-off between growth and environmental adaptation. Plant Cell 26, 3984–3998. 10.1105/tpc.114.129296 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A), Soil moisture content (g H2O g−1 dry soil) in both well-watered and mild drought stress treatments; (B), Soil water potential (MPa) of both well-watered and mild-drought stressed plants; (C), Temperature and humidity levels during the experiment, and (D), Primary leaf growth response to different concentrations of ABA and ACC.
(A) Shoot fresh weight of six wheat genotypes that were either grown under well-watered (WW) or mild water-deficit (MWD) conditions. (B), the relationship between log values of fresh weight (FW) and dry weight (DW) in WW and MWD treatments; (C), plant water content (%) of drought-susceptible and drought-tolerant wheat genotypes in WW and MWD treatments; (D), stomatal conductance of drought-susceptible and drought-tolerant wheat genotypes in WW and MWD treatments.
ABA concentration (A) and ethylene evolution (B) in well-watered (WW) and mild-drought stress (MWD) treatments across all genotypes. Linear correlations between shoot dry weight (SDW) and endogenous ABA (C) and ethylene (D) across all groups and treatments. ** indicates p < 0.01.
Shoot relative growth rate in drought-susceptible (A) and drought-tolerant (B) wheat genotypes that were either sprayed with water (controls), abscisic acid (ABA, 0.1 μM) or the ethylene-precursor, 1-aminocyclopropane-1-carboxylic acid (ACC, 0.1 μM) at the 6th leaf stage.
Sucrose (A), fructose (B), rhamnose (C), raffinose (D), erlose (E), and sorbitol (F) concentrations of drought-susceptible (DS) and drought-tolerant (DT) wheat genotypes across the treatments [control, ABA and ACC (1-aminocyclopropane-1-carboxylic acid) spray].
Two-way ANOVA for the effects of treatments (well-watered and mild drought) and stress group (drought-tolerant and drought-susceptible) and their interaction on several carbohydrate concentrations of six wheat genotypes that were either sprayed with water (controls), abscisic acid (ABA, 0.1 μM) or the ethylene-precursor, 1-aminocyclopropane-1-carboxylic acid (ACC, 0.1 μM) at the 3rd leaf stage. *, **, *** indicate p < 0.001, < 0.01, and < 0.05, respectively.


