Abstract
Context
Phosgene’s primary mode of action is as a pulmonary irritant characterized by its early latent phase where life-threatening, non-cardiogenic pulmonary edema is typically observed 6–24 h post-exposure.
Objective
To develop an inhaled phosgene acute lung injury (ALI) model in C57BL/6 mice that can be used to screen potential medical countermeasures.
Methods
A Cannon style nose-only inhalation exposure tower was used to expose mice to phosgene (8 ppm) or air (sham). An inhalation lethality study was conducted to determine the 8 ppm median lethal exposure (LCt50) at 24 and 48 h post-exposure. The model was then developed at 1.2 times the 24 h LCt50. At predetermined serial sacrifice time points, survivors were euthanized, body and lung weights collected, and lung tissues processed for histopathology. Additionally, post-exposure clinical observations were used to assess quality of life.
Results and discussion
The 24-hour LCt50 was 226ppm*min (8 ppm for 28.2 min) and the 48-hour LCt50 was 215ppm*min (8 ppm for 26.9 min). The phosgene exposed animals had a distinct progression of clinical signs, histopathological changes and increased lung/body weight ratios. Early indicators of a 1.2 times the 24-hour LCt50 phosgene exposure were significant changes in the lung-to-body weight ratios by 4 h post-exposure. The progression of clinical signs and histopathological changes were important endpoints for characterizing phosgene-induced ALI for future countermeasure studies.
Conclusion
An 8 ppm phosgene exposure for 34 min (1.2 × LCt50) is the minimum challenge recommended for evaluating therapeutic interventions. The predicted higher mortality in the phosgene-only controls will help demonstrate efficacy of candidate treatments and increase the probability that a change in survival rate is statistically significant
Keywords: Inhalation exposure, LCt50, lung injury, model, mouse, pathology, phosgene
Introduction
Phosgene (COCl2) is a highly reactive chemical with numerous industrial applications (Currie et al., 1987) and is also known for its past use as a chemical warfare gas during World War I and II (International Program on Chemical Safety, 1997). Currently, phosgene is used as a chemical intermediate in the production of pesticides, plastics, dyes, polyurethanes and pharmaceuticals (NIOSH, 1993). Nearly, a million metric tons of phosgene are produced and used annually in the United States with a potential exposure of approximately 10 000 workers (NIOSH, 1993). As such, it is commonly referred to as a Toxic Industrial Chemical (TIC). Due to phosgene’s wide availability and use in both the chemical and pharmaceutical industries, it is a health concern from both accidental and deliberate releases.
Phosgene’s primary mode of action is as a pulmonary irritant characterized by an early latent phase, where life-threatening, non-cardiogenic pulmonary edema is typically observed 6–24 h post-exposure. Phosgene readily causes severe respiratory distress, hypoxia and even death due to respiratory failure. Phosgene is a weak irritant to the upper respiratory tract; however, it readily penetrates to the alveolar surface without evidence of hydrolysis (Diller, 1978; Diller et al., 1985; Duniho et al., 2002; Gordon et al., 2008; Ji et al., 2010; Li et al., 2005, 2006, 2011; Pauluhn, 2006a,b,c; Pauluhn et al., 2007; Sciuto & Hurt, 2004; Sciuto et al., 1995, 1996, 2003; Wang et al., 2013). Exposure to phosgene causes severe pathophysiological changes in the bronchoalveolar region of the lung, induces changes in energy metabolism, causes disruption of the glutathione redox cycle (GSH), and results in the release of reactive arachidonic acid metabolites (Currie et al., 1987; Gordon et al., 2008; Ji et al., 2010; Wang et al., 2013).
Using advanced modeling tools that account for factors, such as relative ease of production, availability, wide distribution and severity of the acute health effects, phosgene has been identified as a priority TIC by the United States Government (USG) (Blakey et al., 2013; Cox et al., 2010; Good et al., 2013). As such, the USG has dedicated resources to further understand the toxic effects of phosgene and to support the research and development of potential medical countermeasures to mitigate these toxicities (NIH Countermeasures Against Chemical Threats (CounterACT) program; www.ninds.nih.gov/counteract).
Current treatment strategies for phosgene-induced acute lung injury (ALI) are either focused on countermeasures, which target reactive oxygen species produced tissue damage and inflammation (Duniho et al., 2002; Ji et al., 2010; Sciuto & Hurt, 2004; Wang et al., 2013) or alleviating hypoxia and respiratory distress as a direct result of phosgene-induced pulmonary edema. When the damage to the alveolar epithelia and/or vascular endothelia has advanced to frank injury or apoptosis, supplemental oxygen or mechanical ventilation are the recommended treatment strategies (Gordon et al., 2008). No drug-related treatment strategy has been generally accepted for this type of ALI. Relevant animal models that closely mimic both the exposure and treatment route in humans are important when evaluating potential therapeutics against chemical insults. This leads to the development of a nose-only phosgene inhalation screening model in C57BL6 mice.
Duniho et al. (2002) demonstrated in mice that the desired degree of phosgene-induced acute lung injury (alveolar and interstitial edema in addition to epithelial damage in the terminal bronchioles) was induced using an 8 ppm exposure (Duniho et al., 2002; Li et al., 2013). The current studies were designed to develop a C57BL/6 mouse lung injury model for screening potential inhalation therapeutics following an acute nose-only inhalation phosgene exposure. This included the determination of the median lethal exposure level, clinical observations, lung and body weight data, and characterization of lung injury by histopathology evaluation. A lethality study was necessary due to the variability of published data (Cordier & Cordier, 1953; Diller & Zante, 1982; Diller et al., 1985; Duniho et al., 2002; Gordon et al., 2008; Gross et al., 1965; Li et al., 2005, 2006, 2011, 2013; Pauluhn, 2006a,b,c; Long & Hatch, 1961; Pauluhn et al., 2007; Sciuto & Hurt, 2004; Sciuto et al., 1995, 1996, 2003; Wang et al., 2013) and lack of available nose-only exposure data in mice (Pauluhn, 2006a,b,c).
Materials and methods
Animals
Male C57BL/6 mice, Mus musculus, were purchased from Charles River Laboratories (CRL; Saint Constant, QC, Canada). Mice placed on study had a mean body weight of 21 g and ranged from 18 to 25 g on day of challenge.
Animals were quarantined for a minimum of 3 days prior to placement on study. The animals were maintained under Battelle’s animal care and use program accredited by the Association for Assessment and Accreditation for Laboratory Animal Care International. This care and use program was in accordance with guidelines set forth in the “Guide for the Care and Use of Laboratory Animals”, National Research Council, and/or the regulations and standards promulgated by the Agricultural Research Service, United States Department of Agriculture (USDA), pursuant to the Laboratory Animal Welfare Act of 26 August 1966, as amended. The experimental protocol was approved by the Battelle Institutional Animal Care and Use Committee (IACUC) at Battelle, Columbus, OH. Animals were maintained on a 12-h light/dark cycle with no twilight. Air temperature in the animal rooms was maintained within 17–26 °C range, with relative humidity maintained between 30 and 70%. The animals were fed commercially available rodent chow ad libitum (PMI Feeds, Inc., St. Louis, MO), and water was provided ad libitum. Mice were incrementally acclimated (10, 20 and 30 min) during the quarantine period to the open tipped restraint tubes used for the inhalation exposure over a period of three days.
Phosgene
The phosgene was purchased from Matheson Tri-Gas, Inc. (Montgomeryville, PA). The certified phosgene concentrations of the cylinders ranged from 498 to 501 ppm with balance nitrogen.
Exposure system
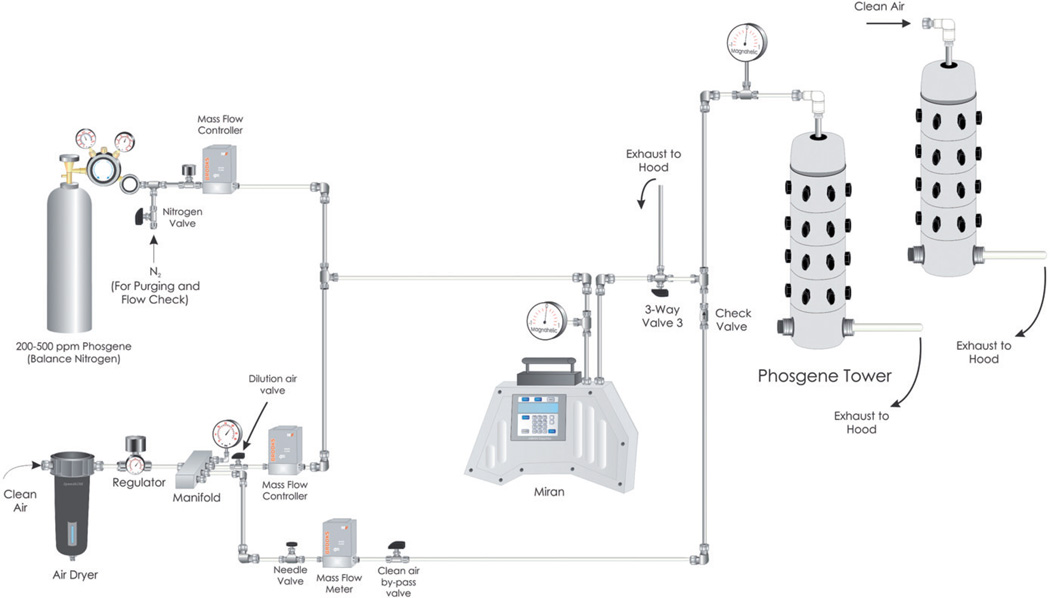
An in house cannon-style nose-only exposure tower was used to uniformly expose up to 30 mice at a time using a flow through design (Cannon et al., 1983). The system was custom designed utilizing Teflon tubing, Telflon or stainless fittings and the exposure tower was polyvinyl chloride (PVC) (Figure 1). The desired phosgene challenge concentration was produced by mixing the certified phosgene gas with dry air (<3% RH). The phosgene/air mixing ratio was precisely controlled by calibrated Smart Trak 100 premium digital mass flow controllers (MFC, Sierra Instruments, Monterey, CA). The MFC accuracy was verified before and after exposures. Air was supplied to the system by an in-house air system filtered through a High Efficiency Particulate Air (HEPA) capsule filter and dried using an inline desiccant chamber. Phosgene gas from the certified cylinders was diluted with air to achieve the desired 8 ppm phosgene concentration (32 mg/m3). All phosgene exposures were targeted at the 8 ppm level.
Figure 1.
Phosgene and sham exposure system diagram.
The phosgene challenge flowed through a Miran® Sapphire single beam infrared (IR) spectrometer (205B Series, Thermo Fisher Scientific, Pittsburgh, PA) to provide real-time verification of concentration stability. Once the phosgene challenge was stable, the restraint devices containing the mice were attached to the exposure tower, and the exposure was initiated by directing the phosgene challenge to the exposure tower. The phosgene entered the inner plenum of the exposure tower and traversed radially outward through the ports connected to the mouse restraint tubes where a “nose-only” challenge occurred. The atmosphere then entered the outer plenum, where it exited the exposure tower into the fume hood.
For the air-only (sham) exposures, a separate air flow control system and exposure tower was used. Since these components were not contaminated with phosgene, the sham exposures were not monitored with the Miran.
Exposure procedures
Prior to the animal exposure, the phosgene concentration was permitted to stabilize at the target concentration. The concentration was deemed stable when three consecutive Miran readings were within 2% of each other and were not trending high or low (e.g. three consecutive Miran AU readings that are not increasing or decreasing). The restraint tubes, containing the acclimated unanaesthetized mice, were then connected to a port in the exposure tower which provided clean breathing air. Upon stabilization of the phosgene concentration, the exposure was initiated by directing the phosgene to the exposure tower. Start time was defined as the time the three-way valve was switched, directing the phosgene laden flow to the exposure tower. Stop time was defined as the time the three-way valve was switched, directing the phosgene laden flow to the back of the fume hood and clean air was directed to the tower. All phosgene exposures were at the 8 ppm (32 mg/m3) exposure concentration. Daily mass flow controller (MFC) checks verified that the phosgene and dilution air flow rates were within 3% of the target levels. All exposures (including air-only shams) were followed by a 5-min clean air system flush before the animals were removed from the exposure tower. After removal from the restraint tubes, mice were returned to their home cages and clinically observed for signs of exposure.
Experimental design
Due to the variability of published lethality data and lack of available nose-only exposure data, a preliminary Lethality study was conducted. Exposure durations at 8 ppm ranged from 10 to 30 min during the Lethality study. A review of the data from the Lethality study leads to the conclusion that an exposure level of 8 ppm over 34 min would represent 1.2 times the 24-hour LCt50 level and would be used for the Model Development study. The complete experimental design is described in Table 1.
Table 1.
Experimental design.
| Study | Exposure duration (min) |
Number of phosgene exposed animals |
Number of sham animals |
Clinical observation time points (h) |
Quality of life scores (Y/N) |
Scheduled sacrifices (time post-challenge) |
Body and wet lung weights (Y/N) |
Gross necropsy and histopathology (Y/N) (n) |
|---|---|---|---|---|---|---|---|---|
| Lethality | 10–30 | 249 | 214 | 1, 2, 4, 6, 8, 24 and 48 | N | 0, 4, 6, 8, 12, 24, and 48 h | Y | Y (84) |
| Model Development | 34 | 185 | 55 | 1, 2, 4, 6, 8, 12, 15, 18, 21, 24, 36 and 48 | Y | 0, 2, 4, 6, 8, 12, 18, 24, 48 h, 5 and 7 days | Y | Y (156) |
Clinical observations and quality of life scores
For the Lethality study, clinical observations were collected immediately following the 5-min washout period and at 1, 2, 4, 6, 8 12 h, then twice daily through 48 h. Mice were pair housed and the group observations were recorded for posture changes, activity level, respiratory distress and death at each time point.
For the Model Development study, clinical observations were collected individually following the washout period at 1, 2, 4, 6, 8, 12, 15, 18, 21, 24, 36 and 48 h post-exposure and twice daily out to 7 days post-challenge. These individual animal observations were used to calculate quality of life (QOL) scores as described in Table 2. In this assessment, a QOL score of 0 was assigned to a normal animal, while a score of five represented an animal that had died. Scores of 1–4 corresponded to clinical signs that ranged from mild to severe. Body weights were collected immediately prior to exposure for all animals and immediately following euthanasia, for animals that survived to their sacrifice time point.
Table 2.
Quality of life scoring definitions: model development study.
| Classification of signs | Signs | QOL score |
|---|---|---|
| Normal | NA | 0 |
| Mild | Hunched posture | 1 |
| Lethargic | ||
| Rough hair coat | ||
| Moderate | Labored breathing | 2 |
| Severe | Respiratory distress | 3 |
| Moribund | 4 | |
| Terminal | Found dead | 5 |
Pathology
A gross necropsy and histopathologic evaluation of the lungs were performed on selected mice during both the Lethality and Model Development studies. At predetermined serial sacrifice time points (0, 2, 4, 6, 8, 12, 18, 24 and 48 h, 5 and 7 days post-exposure), surviving animals were euthanized. Wet lung weights were collected from sacrificed animals and from selected animals that died on study to assess pulmonary edema. After weighing, the intact lung was infused with 10% neutral buffered formalin then submerged into neutral buffered formalin until processed for histopathology. Histopathology (routine processing to 4–6 micron H&E stained sections) included parameter severity scoring assessments for cellular accumulation, degeneration, edema, fibrin exudation, epithelial hyperplasia, bronchiolar/alveolar infiltrates, interstitial infiltrates and inflammation. Microscopic findings were graded semi-quantitatively using the following numerical score to calculate the average severity grades for each lesion by group. Minimal (Grade 1) represented the least detectible lesion; mild (Grade 2) represented an easily discernible lesion; moderate (Grade 3) represented a change affecting a large area of the represented tissue; and marked (Grade 4) represented a lesion that approached maximal. Pathology data were recorded in the Xybion Next Generation PATH/TOX SYSTEM version 1.7.2 (Xybion Medical Systems Corporation) for tabulation and analysis.
Statistical approach
Probit dose-response models were fit to the lethality data to determine the exposure duration for the 24 and 48 h LCt50. Fisher’s exact test was used to compare mortality proportions between phosgene and sham exposed groups with the same scheduled sacrifice times, as well as sham animals that were pooled over a subset of sacrifice times. The median times to death and 95% confidence intervals were calculated for each challenge group using the product-limit method. The Kaplan-Meier survival curves were plotted together on the same axis to characterize the distributions of survival times for the exposure and sacrifice times.
For the analysis of QOL data, animals found dead before their scheduled sacrifice time were assigned a score of “5”, up to and including, their scheduled sacrifice time. For each exposure group (phosgene or sham), available data were pooled together across sacrifice groups for each post-exposure time. A set of two-sample Wilcoxon exact test (one-sided) comparisons were performed to compare QOL scores for phosgene and sham exposed groups at each post-exposure time point.
For statistical analysis of body and lung weight data, two endpoints were calculated for statistical analysis: the percent change in body weight and the lung to terminal body weight ratio. For each weight endpoint, a one-way analysis of variance (ANOVA) model was fitted to the data for all groups, and specific contrasts were performed to conduct pairwise comparisons of the endpoints between pairs of exposure groups with the same sacrifice times. Descriptive statistics were computed for the histopathology severity scores for each parameter and analysis group. For each parameter, one-sided Wilcoxon rank sum tests were conducted to perform all pairwise comparisons between phosgene- and sham-exposed groups with the same scheduled sacrifice times.
All statistical analyses were performed using SAS (version 9.3, Cary, NC) or Stata (version 12, College Station, TX). All tests were conducted at the 0.05 level of significance.
Results
Lethality study
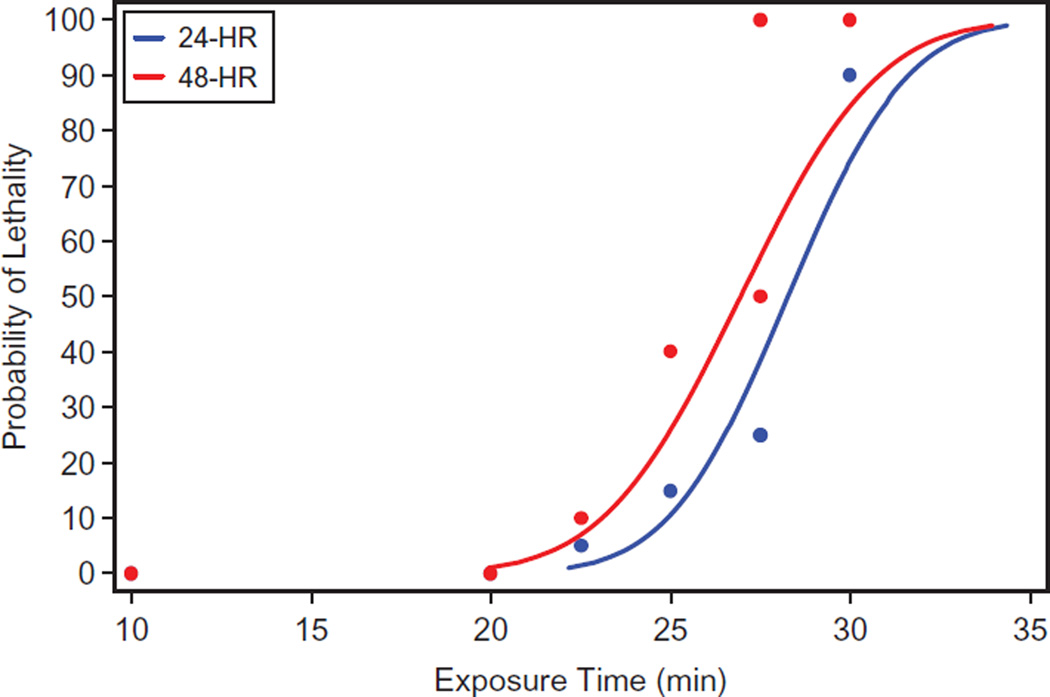
Probit dose-response models were fitted to the mortality data from the Lethality study to calculate the exposure duration that resulted in 50% mortality at 24 and 48 h post-exposure (Table 3). The exposure duration that resulted in 50% mortality at 24 h was 28.3 min with 95% confidence interval of 27.5 to 29.4 min. The exposure duration that produced 50% mortality at 48 h was 26.9 min with a 95% confidence interval of 25.7 to 28.4 min (Figure 2). The calculated 24 and 48 h LCt50 levels were 226 and 216 ppm*minute, respectively (Table 3).
Table 3.
Probit dose-response model fit to mortality data from the lethality study at 24 h and 48 h.
| Group | Intercept | Slope | p value | Median exposure time associated with 50% lethality |
LCt50 (ppm*min) |
95% Confidence interval |
|---|---|---|---|---|---|---|
| 24-Hour | −10.77 | 0.38 | <0.001 | 28.27 | 226 | (27.49, 29.37) |
| 48-Hour | −8.94 | 0.33 | <0.001 | 26.94 | 216 | (25.71, 28.43) |
Figure 2.
Probit dose-response model fit lethality study data at 24 and 48 h.
The first clinical signs did not present until 4 h after exposure when several mice became lethargic with a hunched posture. By 6 h post-exposure, 32% of the animals experienced progressive clinical signs including lethargy, hunched posture and early signs of respiratory dysfunction. By 12 h post-exposure, all mice exposed to 30 min of phosgene were experiencing severe clinical signs to include respiratory distress. All mice in this 30-min exposure group had succumbed by 48 h.
Pre-exposure and terminal body weights were collected from all animals in order to evaluate the percent change in body weight. There was a significantly different decrease in body weight between the phosgene exposed and sham exposed animals at the 24 h sacrifice time point (p = 0.0009). Significant increases in the lung to body weight ratios were reported at the 4 h (p = 0.0003), 6 h (p = 0.0001), 8 h (p<0.0001), 12 h (p<0.0001) and 24 h (p<0.0001) sacrifice time points for animals exposed to 27.5 min phosgene.
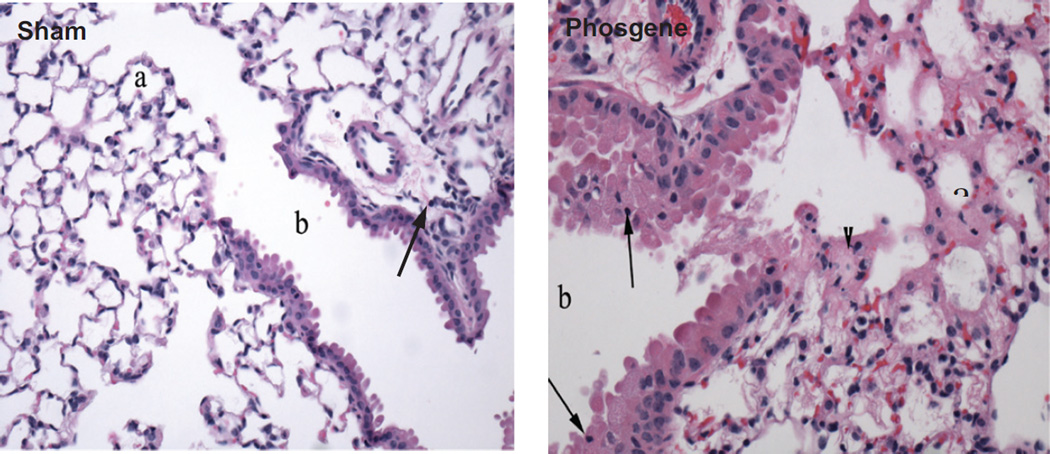
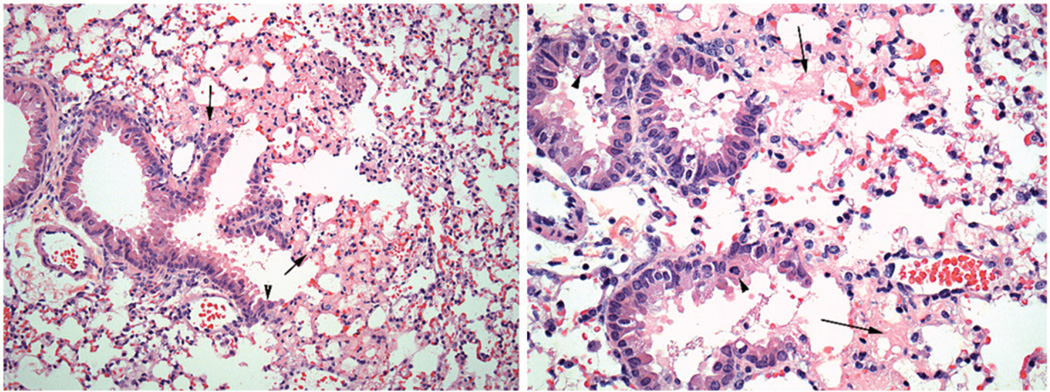
Eighty-four mice were necropsied and submitted for lung histopathology at pre-determined post-exposure sacrifice time points. No gross lesions in any of the animals were noted at the time of necropsy. At the 8 and 12 h post-exposure time points, lesions were subtle and consisted of edema near the bronchiolar-alveolar junction and occasional scattered alveolar macrophages. Mice exposed to 8 ppm phosgene for 27.5 min and surviving to scheduled sacrifice at 24 h post-exposure, developed extensive lesions at the bronchiolar-alveolar junction. These lesions included alveolar edema, accumulation of alveolar macrophages, fibrin in alveoli, neutrophilic inflammation and epithelial changes that include degeneration (cell swelling or apical rounding, vacuolization or cell flattening) of bronchiolar epithelium and/or hyperplasia of Type II pneumocytes extending from the terminal bronchioles into adjacent alveoli (Figure 3). These lesions were consistent with those previously reported in mice following phosgene exposure (Duniho et al., 2002; Li et al., 2006, 2011; Pauluhn et al., 2007; Sciuto & Hurt, 2004; Sciuto et al., 1996, 2003). Scant interstitial infiltrates were also noted in mice from both the sham- and phosgene-exposed groups. This finding was consistent with spontaneous background infiltrate commonly seen in laboratory mice, where small number of migratory cells, including monocytes, lymphocytes, neutrophils and pulmonary interstitial macrophages, are often seen. Overall, mild-to-moderate lung pathology findings were observed, but the only statistically significant difference in severity scores for the Lethality Study was for degeneration at the 24 h sacrifice time (p = 0.0242).
Figure 3.
Representative lung pathology in the 27.5 min duration sham exposed and the 27.5 min phosgene exposed animals 24 h after challenge: lethality study.
Model development study
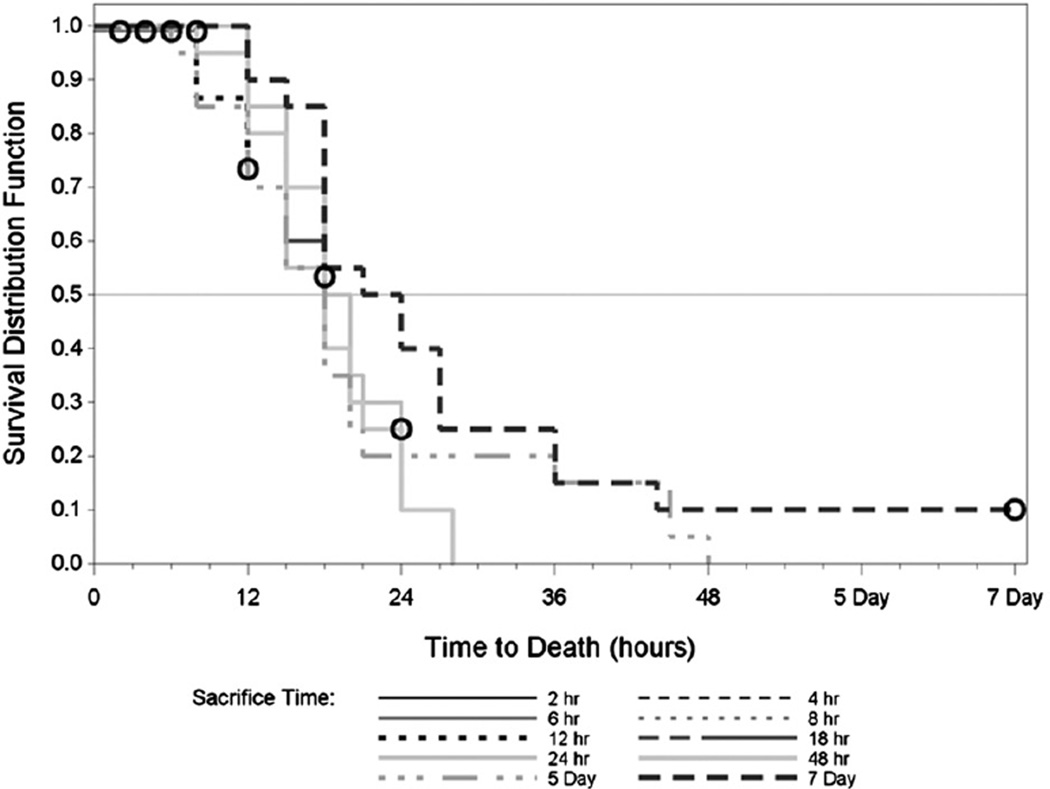
Figure 4 presents the Kaplan-Meier survival curves associated with time-to-death, calculated based on the exposure end time, in hours for each exposure duration. The first phosgene death occurred 6 h post-challenge in the Model Development study. At 18 h post-exposure, 50% lethality was observed, and by 36 h, lethality had increased to 90%. All sham exposed animals survived for the duration of the experiment. The predicted median time to death for the phosgene animals was 18 h with a confidence bound of 18 to 20 h.
Figure 4.
Kaplan-Meier survival curves for phosgene exposure groups: model development study.
At 4 h post-challenge, 81% of the animals presented with hunched posture and by 6 h post-challenge, hunched posture was observed in all animals. This was the most prevalent clinical observation through the end of the study. Labored breathing was observed in 17% of the animal at 8 h and increased to 60% by 21 h. Respiratory distress was present in 37% of the mice at 24 h. Due to the high mortality, clinical observations beyond the 24 h post-challenge time point provided very limited information.
The observed clinical signs during the Model Development study were assigned a numerical value, which was used to calculate a QOL Score. For phosgene-exposed animals, mean scores were significantly higher (p value <0.0001) than corresponding sham exposed animals beginning at 4 h after exposure. All sham exposed animals had QOL scores of 0 (normal) for the duration of the experiment. The mean QOL score reached mild classification (0.83 to 1.12) 4 to 6 h post-challenge, moderate (1.95) by 12 h, and severe (4.04) by 21 h. Mean QOL scores reached 4.87 by the 48 h observation time point.
Body weight losses occurred in surviving animals at all observed time points until Day 5 post-exposure. On Day 5, the average changes in body weight reflected a gain in weight for the survivors. The only significant difference in percent change in body weight was noted for the 18-h sacrifice groups, where the weight loss in the phosgene group was significantly greater than that in the sham exposed group (p<0.0001). At the 2-h through 24-h sacrifice time points, the average lung/body weight ratio was significantly greater in the phosgene-exposed group compared to the corresponding sham-exposed group; potentially due to combined factors of reduced body weight as well as increased absolute lung weight attributable to edema, fibrin exudation and/or inflammation. For the 48-h and Day 5 groups, no comparisons could be performed since there were no survivors in the phosgene exposed groups. For the Day 7 groups, only two survivors remained in the phosgene group; the ratios were not significantly different.
In the Model Development study, gross necropsies and histopathology were performed on 156 mice. Fibrin was found in the alveoli in the bronchiolar-alveolar junction (centriacinar region) beginning 4 h post-exposure and affecting the majority of mice at each time point from 6 to 24 h post-exposure. Degeneration (cell swelling and cytoplasmic vacuolization) and necrosis of terminal bronchiolar epithelium were noted beginning at the 6 h post-exposure time point, affecting the majority of mice through the 24 h time point. Inflammation, consisting of a few neutrophils in the alveolar septae near the bronchiolar-alveolar junction or associated with fibrin in the alveolus, was initially noted at the 6 h time point and remained apparent in several mice at each time point through Day 7 post-exposure. Edema of the alveolus or interstitium was only occasionally found in this study. Hypertrophy/hyperplasia of Type II pneumocytes at the bronchiolar-alveolar junction was occasionally found beginning 6 to 8 h post-exposure and affected most of the mice at the 24 h and Day 7 time points. By Day 7, septae lined by these pneumocytes were noticeably thickened. These lesions were consistent with those previously reported in mice following phosgene exposure (Duniho et al., 2002; Li et al., 2005, 2006, 2011; Pauluhn et al., 2007; Sciuto et al., 2003). Five sham-exposed mice per time point (0, 2, 4, 6, 8, 12, 18, 24 h, 5 days, and 7 days) had histopathology performed during the Model Development study. All observations in sham-exposed animals were graded as minimal and were typical of background findings in laboratory mice.
Severity scores from the phosgene-exposed group in the Model Development study were significantly higher (p <0.05) than those for the comparable sham group at the 6-h, 8-h, 12-h and 18-h sacrifice times, for both degeneration/necrosis and fibrin parameters. For the 24-h and Day 7 groups, the small group sizes of surviving animals in the phosgene groups resulted in a loss of statistical power and non-significant comparisons. For the 48-h and Day 5 groups, the comparisons could not be performed because there were no surviving animals remaining in the phosgene groups.
A comparative assessment of histopathology results was performed across both the Lethality study and the larger Model Development study. Both studies involved inhalation exposure to 8 ppm phosgene for various exposure durations (25 min, 27.5 min or 30 min in the Lethality study; 34 min in the Model Development study) with scheduled sacrifice at several time points post-exposure and subsequent histopathologic examination of the lungs from selected mice.
The majority of acute lesions across both studies involved damage to pulmonary microvasculature, as evidenced by edema and fibrin accumulation near the broncho-alveolar junction. The shorter exposure times (25, 27.5 or 30 min) were primarily associated with edema, at the earliest time points (6–24 h), while fibrin, at a lower incidence, was largely confined to the 24–48 h sacrifice time points. The longer phosgene exposure time (34 min) was associated with a higher incidence of fibrin exudation (Figure 5), largely noted from 4 to 24 h post-exposure, while edema had a lower incidence and was mostly present from 12 to 24 h post-exposure. The slight difference in time of onset or incidence of lesions from the different exposure times was presumably related to the degree of vascular wall damage and the subsequent leakage of low protein fluid (edema) versus fibrin into the interstitium or alveoli.
Figure 5.
Fibrin accumulation at 12 h post phosgene exposure (34 min): model development study.
Epithelial degeneration (cell swelling and cytoplasmic vacuolization) and/or necrosis involving terminal bronchioles were common across both studies. The onset of microscopically visible bronchiolar epithelial damage occurred earlier (6 h time point) in the mice exposed to phosgene for 34 min (Model Development study) than in mice exposed for 25 min or 27.5 min in the lethality study (24 h time point). This bronchiolar epithelial lesion affected the majority of mice following 34 min of phosgene exposure.
Damage to alveoli near the bronchiolar-alveolar junction resulted in reactive Type II pneumocyte hypertrophy (increased cell size) and hyperplasia (proliferation). Reactive Type II pneumocyte hypertrophy/hyperplasia was somewhat variable in onset but was seen in some mice beginning 8–24 h post-exposure and in the majority of mice by 48 h to Day 7 post-exposure (the time point with the greatest severity).
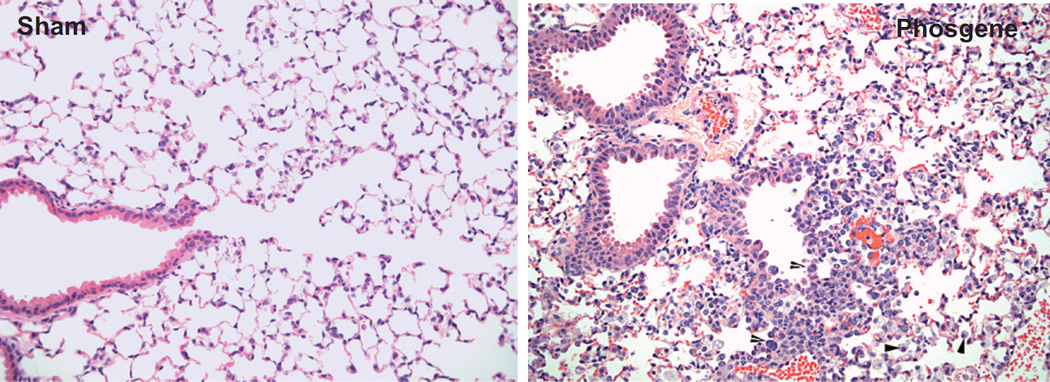
Neutrophilic inflammation and alveolar macrophage accumulation (or, equivalently, alveolar histiocytic infiltrate) developed in response to phosgene-induced tissue damage. Neutrophilic inflammation near the bronchiolar-alveolar junction was noted as early as 6 h post-exposure and generally affected the majority of mice beyond 24 h post-exposure. Alveolar macrophage accumulation was somewhat variable in onset and incidence but tended to develop 24 h or more post-exposure, being most severe in one out of two surviving mice at Day 7 post-exposure (Figure 6).
Figure 6.
Alveolar macrophage accumulation at 7 days post-phosgene and sham exposure: model development study.
Discussion
Lethality study
The Lethality study was designed to determine 24 and 48 h LCt50 in a nose-only phosgene exposure scenario. Additionally, the Lethality study characterized the phosgene- induced ALI using clinical signs, body and lung weight data, and lung histopathology at scheduled sacrifice times or death. Phosgene-induced histopathologic changes centered on the bronchiolar-alveolar junction included fibrin exudation, edema, epithelial degeneration/necrosis, inflammation, Type II pneumocyte hyperplasia and alveolar macrophage accumulation. These findings are consistent with the findings described by Pauluhn et al. (2007) for a high-level acute phosgene exposure in rodents.
By comparing histopathological changes with the lung to body weight ratios and clinical observations, we are able to correlate early indicators of phosgene exposure in the C57BL/6 mouse. The earliest indication was the significant change in the lung to body weight ratios in the phosgene exposed mice by the 4 h post-exposure time point. This coincided with the onset of clinical symptoms at 4 h, when two out of 50 animals showed lethargy. By 6 h post-exposure, 32% of the animals experienced progressive clinical signs in addition to the significant changes in lung to body weight ratios. The clinical signs, time frames and progression of clinical signs, are important for characterizing ALI in future countermeasure studies.
There is a wealth of acute phosgene inhalation data in numerous laboratory species including mice, rats, guinea pigs, rabbits cats, dogs, sheep and pigs (Cordier & Cordier, 1953; Diller, 1978; Diller & Zante, 1982; Diller et al., 1985; Duniho et al., 2002; Gordon et al., 2008; Ji et al., 2010; Li et al., 2005, 2006, 2011, 2013; Pauluhn, 2006a,b,c; Pauluhn et al., 2007; Sciuto & Hurt, 2004; Sciuto et al., 1995, 1996, 2003; Wang et al., 2013). Historical data show a variation in the reported toxicity of phosgene with the older studies showing higher values (Pauluhn et al., 2007). For the development of a new in vivo mouse screening model for potential therapeutics, it was important to refine the LCt50 values prior to conducting any screening.
Model development study at 1.2 LCt50
This study further developed the nose-only inhaled phosgene mouse lung injury model using lung histopathology at predetermined intervals (up to 7 days post-exposure) in combination with secondary endpoints including clinical observations, quality of life, wet lung weights and lung weight to body weight ratios at 1.2 times the 24 h LCt50. The phosgene-exposed animals (8 ppm for 34 min) in the Model Development study had a distinct progression of clinical signs, histopathologic observations, and increase in lung/body weight ratio very similar to the endpoints observed in the Lethality study. The majority of the phosgene-exposed mice exhibited hunched posture by 4 h post-exposure. Animals then become progressively lethargic from 6 to 18 h post-exposure. The percentage of animals exhibiting adverse respiratory signs (labored breathing and respiratory distress) increased with time, but did not exceed 25% until the 18 h post-exposure time point. Histopathologic findings included fibrin exudation and degeneration/necrosis of bronchiolar epithelium by 6 h post-exposure, while bronchiolar epithelial degeneration/necrosis (increasing in incidence and average severity), edema, fibrin exudation, neutrophilic inflammation and/or Type II pneumocyte hyperplasia were seen in mice terminated between 6 and 18 h post-exposure. There was also a significant increase in lung weight from 4 h to the median time to death (18 h), which could be related to fibrin exudation, inflammation and/or vascular changes (i.e. congestion).
Lung lesions in all mice were qualitatively very similar to those previously reported in the scientific literature (Duniho et a., 2002; Li et al., 2005, 2006, 2011; Pauluhn et al., 2007; Sciuto et al., 1996, 2003) and followed a similar temporal pattern of vascular damage followed by airway epithelial damage and reactive epithelial and interstitial changes. Based on photomicrographs and severity scores provided by Duniho et al. (2002), it appears that lesions reported in that particular study had somewhat greater severity, which is interesting given the shorter exposure time (20 min), compared to Battelle studies, and lack of unscheduled deaths. However, it is possible that mouse strain-related differences were a factor (Leikauf et al., 2013).
Current treatment strategies for phosgene-induced ALI include alleviating hypoxia and respiratory distress as a direct result of phosgene-induced pulmonary edema. In many moderate to severe cases, supplemental oxygen or mechanical ventilation are the primary interventions. In order to develop a more robust treatment protocol for humans receiving an acute inhalation phosgene exposure, there is a need to develop a drug-related treatment strategy. A desirable animal model for testing candidate treatments is one that closely mimics both the exposure and treatment route in humans. This need for a treatment screening model led to the development of our nose-only inhalation model in C57BL6 mice.
Based on the 1.2 times the median lethal phosgene exposure level, there was a short time interval between the first clinical signs and mortality. The onset of clinical signs occurred 4 h post-exposure, while the median time to death was 18 h post-exposure. Potential future treatments would have to demonstrate efficacy within this limited window of opportunity. The lower exposure levels that were used in the Lethality study (8 ppm over 22.5, 25, 27.5 and 30 min) had a similar time to onset of clinical signs, but the median time to death increased with the shorter exposure durations. This typical dose-response indicates that the lower exposure levels should afford more time to mitigate the phosgene-induced injuries. Treatments that are shown as efficacious in this rodent screening model can be pushed forward into a more rigorous efficacy test and into a large animal efficacy model.
Conclusion
The 24 h LCt50 was 226 ppm*min (8 ppm for 28.2 min) and the 48 h LCt50 was 215 ppm*min (8 ppm for 26.9 min). Exposure of C57BL/6 mice to 1.2 times the LCt50 of phosgene had a distinct progression of clinical signs, histopathology observations and increase in lung/body weight ratio.
Lung lesions in this nose-only exposure model were qualitatively very similar to those previously reported in the scientific literature and followed a similar temporal pattern of vascular damage followed by airway epithelial damage and reactive epithelial and interstitial changes; although lesion severity was lower and mortality was higher in the C57BL/6 mice compared to the CD-1 mice used in the Duniho study.
Acknowledgments
Declaration of interest
This work was supported by the National Institutes of Health (NIH) Office of the Director through an interagency agreement (OD#: Y1-OD-0387-01) between the National Institute of Allergy and Infectious Diseases (NIAID) and Department of Defense (DoD) and prepared under the auspices of the National Institutes of Health, NIAID, National Institute of Neurological Disorders and Stroke (NINDS), and the DoD Defense Technical Information Center (DTIC) under the Chemical, Biological, Radiological & Nuclear Defense Information Analysis Center (CBRNIAC) program, Contract No. SP0700-00-D-3180, Delivery Order Number 0687, CBRNIAC Task 832/CB-IO-OOI2.
The views expressed in this article are those of the authors and do not reflect the official policy of the NIH, Department of Health and Human Services (HHS), or the USG. No official support or endorsement of this article by the NIAID, NINDS, NIH, HHS or DoD is intended or should be inferred.
References
- Blakey DH, Lafontaine M, Lavigne J, et al. A screening tool to prioritize public health risk associated with accidental or deliberate release of chemicals into the atmosphere. BMC Public Health. 2013;13:253. doi: 10.1186/1471-2458-13-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon WC, Blanton EF, McDonald KE. The flow-past chamber: an improved nose-only exposure system for rodents. Am Ind Hyg Assoc J. 1983;44:923–928. doi: 10.1080/15298668391405959. [DOI] [PubMed] [Google Scholar]
- Cordier D, Cordier G. Toxicity of weak concentrations of phosgene in repeated inhalations. J Physiol (Paris) 1953;45:421–428. [PubMed] [Google Scholar]
- Cox JA, Roszell LE, Whitmire M. Chemical terrorism risk assessment: a biennial assessment of risk to the nation. Chemical Security Analysis Center, United States Department of Homeland Security. 2010 [Google Scholar]
- Currie WD, Hatch GE, Frosolono MF. Changes in lung ATP concentration in the rat after low-level phosgene exposure. J Biochem Toxicol. 1987;2:105–114. doi: 10.1002/jbt.2570020204. [DOI] [PubMed] [Google Scholar]
- Diller WF. Medical phosgene problems and their possible solution. J Occup Med. 1978;20:189–193. doi: 10.1097/00043764-197803000-00007. [DOI] [PubMed] [Google Scholar]
- Diller WF, Bruch J, Dehnen W. Pulmonary changes in the rat following low phosgene exposure. Arch Toxicol. 1985;57:184–190. doi: 10.1007/BF00290885. [DOI] [PubMed] [Google Scholar]
- Diller WF, Zante R. Dose-response relations in the phosgene effect on humans and animals (literature study) Zentralbl Arbeitsmed Arbeitsschutz Prophyl Ergonomie. 1982;32:360–368. [PubMed] [Google Scholar]
- Duniho SM, Martin J, Forster JS, et al. Acute changes in lung histopathology and bronchoalveolar lavage parameters in mice exposed to the choking agent gas phosgene. Toxicol Pathol. 2002;30:339–349. doi: 10.1080/01926230252929918. [DOI] [PubMed] [Google Scholar]
- Good K, Winkel D, VonNiederhausern M, et al. Medical mitigation model: quantifying the benefits of the public health response to a chemical terrorism attack. J Med Toxicol. 2013;9:125–132. doi: 10.1007/s13181-012-0244-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon CJ, Spencer PJ, Hotchkiss J, et al. Thermoregulation and its influence on toxicity assessment. Toxicology. 2008;244:87–97. doi: 10.1016/j.tox.2007.10.030. [DOI] [PubMed] [Google Scholar]
- Gross P, Rinehart WE, Hatch T. Chronic pneumonitis caused by phosgene. An experimental study. Arch Environ Health. 1965;10:768–775. doi: 10.1080/00039896.1965.10664088. [DOI] [PubMed] [Google Scholar]
- International Program on Chemical Safety. Environmental health criteria 193: phosgene. Geneva, Switzerland: International Program on Chemical Safety; 1997. [Google Scholar]
- Ji L, Liu R, Zhang XD, et al. Nacetylcysteine attenuates phosgene-induced acute lung injury via up-regulation of Nrf2 expression. Inhal Toxicol. 2010;22:535–542. doi: 10.3109/08958370903525183. [DOI] [PubMed] [Google Scholar]
- Leikauf G, Concel V, Bein K, et al. Functional genomic assessment of phosgene-induced acute lung injury in mice. Am J Resp Cell Molec Biol. 2013;49:368–383. doi: 10.1165/rcmb.2012-0337OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Liu F, Wang C, et al. Novel insights into phosgene-induced acute lung injury in rats: role of dysregulated cardiopulmonary reflexes and nitric oxide in lung edema pathogenesis. Toxicol Sci. 2013;131:612–628. doi: 10.1093/toxsci/kfs317. [DOI] [PubMed] [Google Scholar]
- Li WL, Hai CX, Liang X, et al. Apoptosis of ATII cells in mice induced by phosgene. Inhal Toxicol. 2006;18:71–77. doi: 10.1080/08958370500282936. [DOI] [PubMed] [Google Scholar]
- Li WL, Hai CX, Pauluhn J. Inhaled nitric oxide aggravates phosgene model of acute lung injury. Inhal Toxicol. 2011;23:842–852. doi: 10.3109/08958378.2011.618849. [DOI] [PubMed] [Google Scholar]
- Li WL, Hai CX, Yang C, et al. Apoptosis of pulmonary epithelial cells and endothelial cells in mice exposed to phosgene. Di Yi Jun Yi Da Xue Xue Bao. 2005;25:983–985. 990. [PubMed] [Google Scholar]
- Long JE, Hatch TF. A method for assessing the physiological impairment produced by low-level exposure to pulmonary irritants. Am Ind Hyg Assoc J. 1961;22:6–13. doi: 10.1080/00028896109344126. [DOI] [PubMed] [Google Scholar]
- NIOSH. Criteria for a recommended standard: occupational exposure to phosgene. Washington, DC: CDC Publication; 1993. [Google Scholar]
- Pauluhn J. Acute nose-only exposure of rats to phosgene. Part I. Concentration × time dependence of LC50s, nonlethal-threshold concentrations, and analysis of breathing patterns. Inhal Toxicol. 2006a;18:423–435. doi: 10.1080/08958370600563680. [DOI] [PubMed] [Google Scholar]
- Pauluhn J. Acute nose-only exposure of rats to phosgene. Part II. Concentration × time dependence of changes in bronchoalveolar lavage during a follow-up period of 3 months. Inhal Toxicol. 2006b;18:595–607. doi: 10.1080/08958370600742771. [DOI] [PubMed] [Google Scholar]
- Pauluhn J. Acute head-only exposure of dogs to phosgene. Part III. Comparison of indicators of lung injury in dogs and rats. Inhal Toxicol. 2006c;18:609–621. doi: 10.1080/08958370600742797. [DOI] [PubMed] [Google Scholar]
- Pauluhn J, Carson A, Costa DL, et al. Workshop summary: phosgene-induced pulmonary toxicity revisited: appraisal of early and late markers of pulmonary injury from animal models with emphasis on human significance. Inhal Toxicol. 2007;19:789–810. doi: 10.1080/08958370701479133. [DOI] [PubMed] [Google Scholar]
- Sciuto AM, Cascio MB, Moran TS, Forster JS. The fate of antioxidant enzymes in bronchoalveolar lavage fluid over 7 days in mice with acute lung injury. Inhal Toxicol. 2003;15:675–685. doi: 10.1080/08958370390197245. [DOI] [PubMed] [Google Scholar]
- Sciuto AM, Hurt HH. Therapeutic treatments of phosgene-induced lung injury. Inhal Toxicol. 2004;16:565–580. doi: 10.1080/08958370490442584. [DOI] [PubMed] [Google Scholar]
- Sciuto AM, Strickland PT, Kennedy TP, Gurtner GH. Protective effects of N-acetylcysteine treatment after phosgene exposure in rabbits. Am J Respir Crit Care Med. 1995;151:768–772. doi: 10.1164/ajrccm.151.3.7881668. [DOI] [PubMed] [Google Scholar]
- Sciuto AM, Strickland PT, Kennedy TP, et al. Intratracheal administration of DBcAMP attenuates edema formation in phosgene-induced acute lung injury. J Appl Physiol. 1996;80:149–157. doi: 10.1152/jappl.1996.80.1.149. [DOI] [PubMed] [Google Scholar]
- Wang P, Ye XL, Liu R, et al. Mechanism of acute lung injury due to phosgene exposition and its protection by cafeic acid phenethyl ester in the rat. Exp Toxicol Pathol. 2013;65:311–318. doi: 10.1016/j.etp.2011.10.001. [DOI] [PubMed] [Google Scholar]