Abstract
Acute kidney injury (AKI) continues to be a significant contributor to morbidity, mortality and healthcare expenditure. In the United States alone, it is estimated that over $10 billion is spent on AKI every year1. Currently, there are no available therapies to treat established AKI. The mitochondrion is positioned to be a critical player in AKI with its dual role as the primary source of energy for each cell and as a key regulator of cell death. This review aims to cover the current state of research on the role of mitochondria in AKI while also proposing potential therapeutic targets and future therapies.
Keywords: mitochondria, acute kidney injury, reactive oxygen species, metabolism
B. Historical Perspective
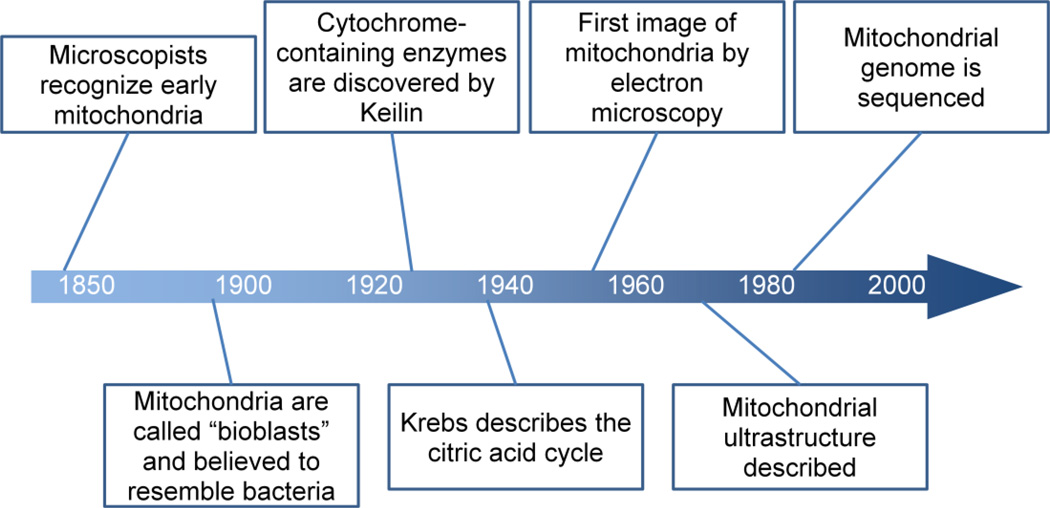
Intracellular structures that likely represented mitochondria were initially identified by microscopists starting in the 1840s, shortly after the discovery of the nucleus (reviewed in2). Altmann termed these structures “bioblasts” in 1890 and was perhaps the first to recognize their ubiquitous occurrence in eukaryotic cells, even noting a resemblance to intracellular bacteria (Figure 1). Functional evidence for a role in bioenergetic processes emerged more than two decades later. In 1913, Otto Warburg reported that respiration in live extracts of guinea pig liver was related to subcellular particles he termed “grana3.” And in 1925, Keilin described the cytochrome-containing enzymes, leading to the concept of a chain of catalysts that sequentially oxidize substrates4. Cellular fractionation studies in the ensuing years enabled a more detailed description of mitochondrial functional characteristics, including the description of the citric acid cycle by Hans Krebs in 19375. The first high-resolution electron photomicrograph of in situ mitochondria is attributed to George Palade in 1952.6
Figure 1.
The science of mitochondria in the kidney closely paralleled the overall development of the nascent field of nephrology. In Homer Smith’s seminal text “The Kidney,” Van Slyke and colleagues are credited with the first simultaneous determination of renal oxygen consumption and urea7. The greater density of mitochondria in the proximal tubule (and thick ascending limb) compared to other nephron segments led Krebs to identify fatty acids as the major fuel for respiration in the renal cortex 8. A group led by Benjamin Trump reported mitochondrial ultrastructure from the proximal tubule of healthy individuals in 19669, and later, evidence of mitochondrial swelling in the proximal tubule during circulatory shock10.
The strong correlation between respiration and solute transport led Franklin Epstein’s group and others to hypothesize that various forms of AKI may arise from a mismatch between local oxygen supply and the metabolic demands of moving solutes against chemical gradients. They showed, for example, that polyene antimicrobials such as amphotericin increase apical membrane permeability and thereby elevate the work of reabsorptive transport. When reabsorptive transport was chemically inhibited, tubular cell death was markedly reduced, suggesting that cell death arose from increased oxygen demand11. They later proposed that loop diuretics could attenuate hypoxic renal injury by reducing the work of active transport in the medullary thick ascending limb (mTAL)12. These and other indirect experiments assessing the role of tubular metabolism have informed the design and conduct of modern studies focusing on the molecular and cell biology of renal mitochondria in AKI.
C. Overview of the mitochondrion (Figure 2)
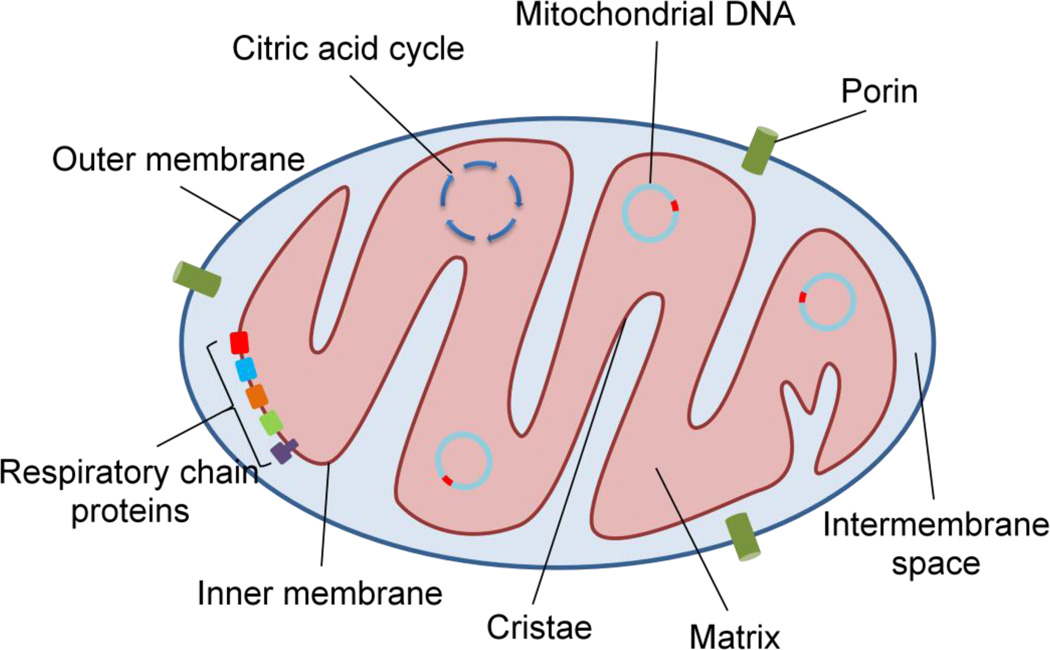
Figure 2.
The mitochondrion is uniquely structured to function as the powerhouse for the cell. Each mitochondrion is composed of an inner and an outer membrane, which are separated by the intermembrane space. The outer membrane contains a channel protein, called porin, which allows the passage of molecules less than ~5000 daltons to pass freely into the intermembrane space. In contrast, the inner membrane is highly impermeable to ions and small molecules and contains many folds, called cristae, to increase its surface area. The high content of the unique four-acyl chain phospholipid cardiolipin, helps to maintain the architecture of cristae and the proper anchoring of electron transport chain components. The impermeability of the inner membrane allows for a large electrochemical gradient to be established, which is necessary for the process of oxidative phosphorylation and the generation of ATP (Figure 2).
Each cell contains hundreds to thousands of mitochondria, with a larger number found in more metabolically active cells. Mitochondria travel along the cell cytoskeleton and can be localized to the areas with the highest energy requirements13. In contrast to older theories of mitochondria as distinct organelles, we now know that they form large inter-connected networks which are continuously fusing and dividing. These are tightly regulated processes and are controlled by a series of nuclear-encoded GTPase proteins. Fission is regulated by dynamin-related protein 1 (Drp1) which resides in the cytoplasm. When activated, Drp1 localizes to the mitochondrial outer membrane, where it forms multimers to create a physical ring that divides the mitochondrion into daughter organelles. Mitochondrial fusion involves mitofusin-1 (Mfn1) and mitofusin-2 (Mfn2), which are located on the outer membrane, as well as optic atrophy 1 (Opa1) on the inner membrane. These proteins create tight linkages between mitochondria and facilitate the fusion of the outer and inner membranes14.
As the powerhouse of the cell, mitochondria contain the components necessary for the citric acid cycle, fatty acid oxidation, and the electron transport chain. Pyruvate—the end product of glycolysis—and fatty acids are transported across the inner membrane and into the matrix where they are converted to acetyl CoA. In turn, acetyl CoA serves as the substrate for the citric acid cycle, which occurs exclusively inside the mitochondrial matrix. During the citric acid cycle, acetyl CoA is further oxidized and high energy electrons are stored in the carrier molecules NADH and FADH2. The electrons can then be transferred from these carrier molecules down the electron transport chain proteins, which are located on the inner membrane. The stepwise release of energy during this process results in the pumping of protons against their electrochemical gradient out of the matrix and into the intermembrane space. Subsequently, ATP synthase generates ATP while allowing protons to diffuse down their electrochemical gradient and back into the matrix15, 16. These complex pathways within the mitochondria allow the cell to harness over ten-fold more energy than with glycolysis alone, an adaptation that has enabled the evolution of sophisticated organ systems with high energy requirements.
Mitochondria are theorized to have originated as distinct organelles through an endosymbiotic relationship between ancient eukaryotic cells and bacteria17. The human mitochondrial genome, first sequenced in 1981, contains 16,569 base pairs and encodes 2 rRNAs, 22 tRNAs and 13 proteins which are needed for oxidative phosphorlyation18. Mitochondrial DNA (mtDNA) only encodes a small subset of the proteins required for the organelle’s function, obligating the mitochondria to rely heavily on nuclear-encoded proteins19. Most mitochondria contain several copies of mtDNA, with a range of 1 to 10 per organelle20.
Whereas replication of nuclear DNA is limited to the S phase of the cell cycle, mtDNA replication occurs continuously. However, there does appear to be some coordination of mtDNA replication with cell division, with peaks in mtDNA replication found in the G1 and late S phases21. Mitochondrial biogenesis is activated in response to increased cell demand for energy production, such as occurs with exercise training. Biogenesis is initiated by several transcription factors encoded by the nucleus, including nuclear respiratory factor 1 and 2 (NRF-1 and NRF-2) as well as PPARγ coactivator 1α (PGC1α)22, 23.
It has long been recognized that mtDNA is vertically transmitted in a maternal pattern. This property, along with its lack of recombination and high mutation rate, has been exploited to track the lineage of modern humans back to a common African ancestry 24. The lack of detectable paternal mtDNA was hypothesized to be due both to the relatively small amount of mtDNA in sperm compared to the oocyte, as well as by active degradation of sperm-derived mitochondria in the fertilized egg25, 26. However, exceptions to maternal inheritance have been described, as in the case of a young man with a mitochondrial myopathy which appeared to have been caused by mutations in paternal mtDNA27.
Mitochondria also play an important role in regulating cell death. A key mediator of this process is the mitochondrial permeability transition (MPT) pore. This structure is composed of several proteins on the inner and outer mitochondrial membranes, including the adenine nucleotide translocator (Ant), cyclophilin D (Cyp-D) and the voltage-dependent anion channel (Vdac)28. Opening of this pore allows the passage of molecules smaller than 1,500 daltons to travel between the matrix and cytoplasm. Mitochondrial permeability transition can be activated by several events inside the cell, including a rise in matrix calcium concentration, alkalinization of the matrix, increasingly negative voltage across the inner membrane, oxidative stress, and a high ratio of NAD+ to NADH29. Opening of the MPT pore allows the release of calcium out of the mitochondria, which can then activate the MPT pore in neighboring mitochondria. This event also leads to the release of several pro-apoptotic proteins from the mitochondrion into the cytoplasm, including cytochrome c and members of the Bcl-2/Bax family of proteins. Some of these proteins, including Bax and Bad, interact directly with the MPT pore complex to further promote opening. Lastly, opening of the MPT pore results in osmotic swelling of the mitochondrial matrix, which can result in rupture of the mitochondria. In sum, opening of the MPT pore occurs in response to diverse cell stressors, permits the efflux of calcium and pro-apoptotic proteins, and enables the influx of water and solutes that swell and rupture mitochondria.
Mitochondria are the main source of reactive oxygen species (ROS) which cause oxidative damage to mtDNA and proteins, necessitating an efficient system for removal of damaged organelles. Not surprisingly, mitochondria are subject to tight quality control systems, including the safe disposal of injured organelles through a process known as mitophagy. There are specific membrane-bound markers, such as MAP1 light chain 3, which serve as signals for destruction of the mitochondrion. Mitophagy involves sequestration of a mitochondrion inside a double membrane—known as the autophagosome—with subsequent degradation when the autophagosome fuses a lysosome30, 31.
D. Renal manifestations of genetic disorders of the mitochondrion
Mutations in mtDNA often disrupt oxidative phosphorylation and primarily affect organs with high rates of energy consumption, including skeletal muscle, the central nervous system, the heart and the kidneys. Signs that a clinical syndrome may be of mitochondrial origin include early age of onset, multiorgan system involvement, elevated lactic acid level, and a pattern of maternal inheritance. The proximal tubule is often affected given its high density of mitochondria; as a result, Fanconi syndrome is a common phenotype32, 33. Mutations can be inherited at birth or may arise de novo in an affected individual. Mitochondrial DNA has a mutation rate that is over 10-fold higher than nuclear DNA. Reasons for this include the proximity to ROS generated from oxidative phosphorylation, lack of protective histones, and less robust DNA repair and replication systems compared to the nuclear genome34.
Renal involvement in inherited mitochondrial diseases often presents within the first few years of life and is, therefore, less likely to be responsible for new onset renal disease in adults. Two of the better characterized mitochondrial cytopathies which can feature renal involvement are Kearns-Sayre syndrome and mitochondrial encephalomyelopathy, lactic acidosis and stroke-like episodes (MELAS) syndrome, a fuller discussion of which can be found in several reviews35, 36. These syndromes can have variable renal manifestations, but often involve proteinuria, metabolic acidosis and progressive renal failure.
Inherited mtDNA disorders can cause a variety of pathologic findings on histological examination. Renal biopsies from these patients typically show nonspecific abnormalities and atrophy of the tubules, with giant mitochondria being a frequent finding on electron microscopy37. Isolated glomerular involvement from a mitochondrial cytopathy has also been reported. Güçer, et al describe 2 pediatric patients who were found to have focal segmental glomerular sclerosis (FSGS) due to different mtDNA deletions38. Additionally, mitochondrial disease can demonstrate a pure tubulointerstitial nephritis without evidence of a proximal tubulopathy, as reported by Rotig, et al39.
Characterization of these genetic disorders is made difficult by the lack of a consistent genotype-phenotype relationship. The same mutation can have large variations in penetrance, affecting different organ systems and presenting over a wide age range. Conversely, many different mtDNA mutations can result in the same phenotype, as is the case with MELAS syndrome40. Some of this variability is due to the fact that cells contain a heterogenous population of mitochondria, a property known as heteroplasmy, and the ratio of wild-type to mutated mtDNA is an important determinant. The threshold of mtDNA that must be mutated to cause disease can be variable, but is typically thought to be on the order of 60–90%41.
E. Evidence for mitochondrial involvement in AKI
In addition to being the primary source of energy for maintaining cell function, mitochondria are also a source of many substances which can lead to cell death. These seemingly paradoxical roles of the mitochondrion are tightly regulated in healthy cells. In response to cell injury or hypoxia, further damage can be caused by the release of ROS or activation of the caspase system leading to apoptosis.
It is notable that after an insult, mitochondrial injury appears to precede the clinical manifestations of AKI. This argues against the idea that the mitochondrial changes seen during AKI are due to a drop in metabolic demand from decreased renal perfusion. Despite significantly impaired renal function during AKI, there is often a paucity of cell death seen on histopathological examination. Instead, one of the predominant findings is structural changes to the mitochondria and vacuole formation in affected tubular cells. Electron microscopy has shown that many of these vacuoles are swollen mitochondria as well as autophagosomes. This has been observed in patients who died from shock or trauma42 and well as sepsis43. Similar findings were obtained when renal tissue from patients undergoing partial nephrectomy was examined after the hilar vessels had been clamped for up to 60 minutes. Despite only a modest rise in creatinine and normal appearance on light microscopy, electron microscopy revealed several changes, including swollen mitochondria44. Studies such as these suggest that mitochondrial dysfunction, with or without cell death, play a critical role in the development of AKI.
Fragmentation of mitochondria appears to be an important early event in the development of AKI caused by a variety of insults. Brooks and colleagues showed that after proximal tubular cells were exposed to cisplatin or hypoxia/reoxygenation, there was marked fragmentation of mitochondria. This fragmentation preceded both the release of cytochrome c into the cytoplasm and apoptosis. Moreover, by inhibiting mitochondrial fragmentation with siRNA against the fission protein Drp1, there was a significant reduction in mitochondrial fragmentation, cytochrome c release, and apoptosis45. In mouse models of toxic and ischemic AKI, the authors showed renoprotection with a pharmacological inhibitor of fission, mdivi-1.
Work by our group has suggested that there is decreased oxygen consumption by renal tissue during sepsis. Using micro-ultrasound, there was a significant decrease in renal blood flow seen 18 hours after the administration of Gram negative endotoxin (lipopolysaccharides, LPS) to mice. Despite the decrease in blood flow, BOLD MRI showed that renal tissue oxygen levels remained normal. Mitochondrial dysfunction was proposed as the cause for decreased oxygen consumption in this model. Supporting this theory, renal tissue from LPS-treated animals was shown to have swollen mitochondria with significantly decreased staining for cytochrome c oxidase, suggesting decreased activity of the electron transport chain46.
In addition to ultrastructural changes within the renal tubular epithelium, there are a variety of metabolic changes that develop inside cells and in mitochondria after experiencing AKI. Funk and colleagues showed that expression of several mitochondrial respiratory proteins (ATP synthase β and cytochrome c oxidase subunits I and IV) was significantly attenuated in a model of myoglobinuric AKI. Expression of these proteins was also decreased after induction of ischemia-reperfusion injury47.
F. Approaches for mitochondrial targeting
As alluded to earlier, mitochondria are poised at the intersection of life and death for cells with high metabolic needs. Intense ATP production is necessary for cells of the proximal tubule and mTAL to reabsorb solutes through active transport. Moreover, ATP powers the electrogenic cell-surface ATPase that counteracts the constant threat of cell swelling from the passive entry of sodium ions and water. On the other hand, in response to various noxious stimuli, mitochondria become an important source of deleterious levels of ROS. Since the free radical reaction is auto-catalytic, multiple classes of macromolecules can rapidly become covalently modified. This impairs the function of enzymes and other proteins, weakening cell and organelle membranes through lipid peroxidation, and even alters the structure of nucleic acids. Finally, mitochondria play a key role in cell death, particularly via apoptosis48.
Mitochondria have been successfully targeted at multiple levels in various forms of experimental AKI. Interventions span the gamut from altering mitochondrial metabolism to modulating the network structure of mitochondria to pathways that drive clearance of injured mitochondria and replacement of mitochondrial mass through biogenesis. Rather than attempt a complete compendium, several of these broad classes will be introduced below with specific examples intended to illuminate concrete approaches. Moreover, these sections are organized to reflect a putative temporal sequence of changes in tubular mitochondria during AKI, with the implication that targeting earlier events may be more beneficial for prevention of AKI in high-risk settings whereas late events more be more amenable for development of treatments against established AKI (Table 1).
Table 1.
| Category | Mechamism | Example | Reference |
|---|---|---|---|
| Metabolism | Augment fatty acid oxidation |
Overexpression of PPARα |
54 |
| Augment electron transport chain |
CoQ10 (ubiquinone) |
55 | |
| Reactive oxygen species |
Mitochondrial antioxidant |
MitoQ |
57, 58, 59 |
| SS-31 | 60, 61 | ||
| Membrane permeability transitionpore |
Inhibit Cyclophilin D (component of MPT) |
Cyclosporine | 65, 66 |
| Mitochondrial dynamics |
Inhibit Mitochondrial fission |
siRNA knowndown of Drp1 |
69 |
| Mitophagy | Activate mTOR to enhance autophagy |
Tersirolimus | 72 |
| Mitochondrial biogenesis |
Enhance nuclear transcription of mitochondrial proteins |
PPARγ- coactivator- 1α (PGC1α) |
46 |
| Activate β- Adrenergic receptors |
Formoterol | 79, 80 |
Mitochondrial metabolism
Both the high respiratory quotient of the renal cortex49 and the experiments of Hans Krebs8 have suggested that fatty acids are the chief fuel of respiration for the renal cortex. Fats accumulate in the proximal tubules of animals with AKI arising from toxic, ischemic, and inflammatory etiologies50. In a model of post-ischemic AKI using isolated rabbit proximal tubules subjected to hypoxia and reoxygenation, Weinberg and colleagues showed a persistent post-hypoxic defect in complex I of the electron transport chain51. Since this complex utilizes the electron carrier NADH generated from β-oxidation of fatty acids (in contrast to complex II that utilizes succinate from the citric acid cycle) the results suggest that inadequate utilization of fatty acids as a source of energy may be an early mitochondrial event in AKI.
Subsequent studies have suggested that the early energetic lesions of hypoxia-reoxygenation may involve both upstream and downstream components of energy metabolism, examples of which include the destruction of cellular and mitochondrial pools of NAD following reoxygenation52 and the identification of “free” fatty acid buildup leading to proton leaks across the inner mitochondrial membrane, thus uncoupling electron transport from ATP generation53. Using both pharmacological and genetic methods to augment fat oxidation, Portilla’s group has shown that the metabolic transcription factor PPARα may be an important target for toxic and post-ischemic AKI54. Finally, CoQ10 is a quinone-based molecule that transports electrons from complexes I and II to complex III. Its early supplementation in primary genetic CoQ10 deficiencies may prevent the onset of renal manifestations55. To our knowledge, CoQ10 has not been rigorously evaluated in clinical AKI.
Reactive oxygen species
Mitochondria become a major source of ROS during ischemia, which in turn, can affect the function of proteins and lipids56. During ischemia, the spike in ROS drives the peroxidation of cardiolipin, a change that is thought to distort cristae and thereby impair efficient oxidative phosphorylation. Attempts to target this process with generic antioxidants have been unsuccessful, but mitochondrially targeted antioxidants appear promising. One class of such molecules—e.g., mito Q—selectively accumulates in the mitochondrial matrix as a result of positive charge from a lipophilic cation conjugate. Mitochondrially targeted antioxidants have been shown to be renoprotective in cold storage injury, post-ischemic AKI, and cisplatin nephrotoxicity57–59. Another class of mitochondrially targeted antioxidants are the Szeto-Schiller (SS) peptides. These may be less dependent on intact mitochondrial membrane potential than mito Q-like compounds for efficient accumulation in the inner membrane60. SS-31 appears to exert potent renoprotection following ischemia/reperfusion61.
Membrane permeability transition (MPT) pore
Swollen mitochondria within tubular cells are an early and prominent feature of toxic, ischemic, and septic AKI, whether in animal models or in humans62–65. In a cell culture setting, opening of the MPT pore can be inhibited by applying cyclosporine, the target of which (cyclophilin D) is a major component of the MPT pore. Indeed, cyclosporine was shown to reduce infarct size when administered during cardiac revascularization, a clinical state of ischemia-reperfusion injury66, although a follow-up confirmatory study was negative67. The vasoconstrictive and nephrotoxic effects of cyclosporine diminish enthusiasm for AKI, but its actions on the mitochondrial pore warrant further consideration to explore alternative strategies to inhibit the MPT.
Mitochondrial dynamics
In the proximal tubule, elongated fusiform mitochondria are tightly arrayed in the basolateral infoldings. While ultrastructural images suggest a static packing of mitochondria, real-time imaging shows a dynamic and rapidly remodeling network of mitochondria, even in non-dividing cells. Individual organelles undergo fission (to create two daughter mitochondria from one “mother”) and fusion constantly, and the net balance between fission and fusion shifts according the cellular conditions68. Zheng’s group reported that mitochondrial fission in the tubules was upregulated in response to experimental cisplatin administration or ischemia reperfusion injury69. They further showed that genetic or pharmacological inhibition of fission, achieved by targeting dynamin-related protein-1, ameliorated renal injury in these models. Therapies to inhibit fission/fragmentation may warrant testing in toxic and post-ischemic renal injury.
Mitophagy
Autophagy, or “self-eating,” can be thought of as an intrinsic cellular process not only to remove damaged organelles and proteins, but also to recycle their basic components. Through incompletely understood pathways, autophagy is rapidly induced in during experimental AKI and may play an important role in renoprotection70. In experimental sepsis, the kidney exhibits a biphasic autophagic response, being elevated in the first 3 hours after cecal ligation puncture, but declining thereafter71. Induction of autophagy by administration of the mTOR inhibitor temsirolimus after the induction of sepsis still protected renal function72. Whether the beneficial effects of autophagy relate directly to mitophagy remains to be conclusively addressed73. However, since mitochondrial fission and loss of membrane potential are critical inducers of mitophagy, it is likely that mitochondrial dynamics and mitophagy act in a coordinated manner to maintain the cellular pool of healthy mitochondrial mass.
Mitochondrial biogenesis
As fissed mitochondria are broken down to fundamental building blocks by mitophagy, the cell faces a need to rebuild mitochondrial mass. This process, called mitochondrial biogenesis, is also regulated by the energetic environment of the cell (e.g., via AMP kinase), by cold temperature, by cell-surface signaling events (e.g., β-adrenergic signaling), and by the nutritional status of the cell (e.g., via sirtuin enzymes)74. Depending on the estimate, mitochondria are comprised of 1000–2000 proteins75. Most of this mitochondrial proteome is encoded in the nucleus, where gene expression is activated by an array of transcription factors such as PPARα, PPARγ, NRF1, ERRα, and others. A protein called the PPARγ -coactivator-1α (PGC1α) is a member of a family of proteins that bind transcription factors and augment their function. As a result, PGC1α has been called a mitochondrial biogenesis regulator.
Among the sites of highest PGC1α expression in the mammalian body is the kidney76. The pattern of PGC1α expression, not surprisingly, closely overlaps the anatomical distribution of mitochondria throughout the kidney64. PGC1α expression is induced following oxidant injury to cultured tubular cells or different forms of in vivo renal injury, consistent with a role in functional recovery of tubular cells77, 78. Whereas global KO mice do not appear to have a strong renal phenotype, they are more susceptible to AKI following sepsis. PGC1α expressed in the proximal tubule was clearly important as conditional knockout mice (driven by the SGLT2 promoter) phenocopied the exacerbated AKI of global KO mice following endotoxemia64. Other studies in the work from Tran and colleagues showed that the septic kidney suffered markedly impaired oxygen delivery without developing hypoxia, a response consistent with reduced oxygen consumption. Indeed, tubular cells treated with inflammatory mediators such as TNFα developed a dose and time-sensitive reduction in oxygen consumption. This change was fully reversed when PGC1α expression was artificially maintained by genetic manipulation. Mitochondrial biogenesis may also be important in other forms of AKI. Seeking pharmacological inducers of mitochondrial biogenesis, Schnellmann’s group screened small molecule libraries for their ability to augment basal and uncoupled oxygen consumption in cultured cells and identified formoterol, a β-adrenergic agonist, as a candidate molecule that ameliorated IRI-related AKI, even when administered as a rescue therapy in the post-ischemic phase79, 80.
G. Implications for immune pathways during repair
Mitochondrial injury may be central to AKI pathogenesis. Moreover, mitochondrial biogenesis and mitophagy may be essential elements of recovery in tubular cells that have suffered sublethal injury. Relatively few studies have directly examined whether mitochondrial processes directly influence long-term outcomes following AKI, such as maladaptive repair leading to fibrosis.
Populations of both resident macrophages and blood-derived monocytes homing to injured renal tissue expand in numbers after ischemia-reperfusion. Chemokine and cytokines from an expanding range of pathways—e.g., CCR2, CX3CR1, CSF1, and IL-34—play important roles in recruiting and stimulating these cells during the repair phase81–84. Mitochondrial DNA and N-formyl-peptides found in mitochondria, when released following sterile injury, may also be potent stimulators of the innate immune response85. Depending on the function of these macrophage populations, the effects on renal function may be beneficial or detrimental. T lymphocytes are similar to macrophages in that they can promote injury but also signal repair after ischemia reperfusion injury86.
During normal repair following AKI, bone-marrow-derived cells such as mesenchymal stem cells appear to exert paracrine effects that reduce inflammation and stimulate growth87. Stem-cell derived microvesicles may even be responsible for directly transferring mRNAs, microRNAs, and organelles to parenchymal cells88. Whether mitochondrial transfer from stem cells important in AKI is unknown, but such “mitochondrial transplantation” from a healthy stem cell “donor” has been described in other, albeit in vitro, settings89, 90.
Tubular fibrosis is the final pathway from most forms of renal injury and tubular cell metabolism appears to play an important role in this process. Susztak and colleagues showed that expression of proteins involved in fatty acid oxidation is depressed in the setting of injury, resulting in lipid accumulation inside cells. By overexpressing key activators of fatty acid oxidation, they were able to reduce the degree of tubular fibrosis in response to experimental injury91.
H. Future horizons
Mitochondria may be a promising target both for the diagnosis and treatment of AKI. Given that mitochondrial injury appears to precede the clinical manifestcd envision non-invasive methods that assess kidney mitochondrial function as a marker of injury. Such information could be useful in several clinical scenarios, including the monitoring of patients with delayed graft function after renal transplant, determining the appropriate time to stop renal replacement therapy in patients with severe AKI, differentiating hepatorenal syndrome from intrinsic tubular injury, and titrating medical diuresis in cardiorenal patients. A more complete molecular understanding of the mitochondrial response to injury and recovery could facilitate the development of therapies aimed at hastening recovery and reducing the morbidity and mortality of AKI. We are only beginning to elucidate the complex role of mitochondria in AKI, and further research will hopefully lead to improved outcomes for patients.
Acknowledgments
Studies on mitochondria and metabolism in the Parikh Laboratory are supported in part by NIH/NIDDK R01 DK095072.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors have no financial interests to declare relevant to this work.
REFERENCES
- 1.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute Kidney Injury, Mortality, Length of Stay, and Costs in Hospitalized Patients. Journal of the American Society of Nephrology. 2005;16(11):3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 2.Ernster L, Schatz G. Mitochondria: a historical review. The Journal of cell biology. 1981;91(3 Pt 2):227s–255s. doi: 10.1083/jcb.91.3.227s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warburg O. Uber Sauerstoffatmende Koernchen aus Leberzellen und uber Sauerstoffatmung in Berekfeld-Filtralen Wassriger Leverextrakte. Arch Gesamte Physiol. 1913;154:599–617. [Google Scholar]
- 4.Keilin D. On cytochrome, a respiratory pigment, common to animals, yeast, and higher plants. Proc R Soc Lond B Biol Sci. 1925;98:312–399. [Google Scholar]
- 5.Krebs HA, Johnson WA. Metabolism of ketonic acids in animal tissues. The Biochemical journal. 1937;31(4):645–660. doi: 10.1042/bj0310645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palade GE. The fine structure of mitochondria. The Anatomical record. 1952;114(3):427–451. doi: 10.1002/ar.1091140304. [DOI] [PubMed] [Google Scholar]
- 7.Van Slyke DD, Rhoads CP, Hiller A, Alving AS. Relationships between urea extraction, renal blood flow renal oxygen consumption, and diuresis. The mechanism of urea excretion. Am J Physiol. 1934;109(2):336–374. [Google Scholar]
- 8.Weidemann MJ, Krebs HA. The fuel of respiration of rat kidney cortex. The Biochemical journal. 1969;112(2):149–166. doi: 10.1042/bj1120149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tisher CC, Bulger RE, Trump BF. Human renal ultrastructure. I. Proximal tubule of healthy individuals. Laboratory investigation; a journal of technical methods and pathology. 1966;15(8):1357–1394. [PubMed] [Google Scholar]
- 10.Trump BF, Valigorsky JM, Jones RT, Mergner WJ, Garcia JH, Cowley RA. The application of electron microscopy and cellular biochemistry to the autopsy. Observations on cellular changes in human shock. Human pathology. 1975;6(4):499–516. doi: 10.1016/s0046-8177(75)80068-2. [DOI] [PubMed] [Google Scholar]
- 11.Brezis M, Rosen S, Silva P, Spokes K, Epstein FH. Polyene toxicity in renal medulla: injury mediated by transport activity. Science. 1984;224(4644):1666–1668. doi: 10.1126/science.6322305. [DOI] [PubMed] [Google Scholar]
- 12.Heyman SN, Rosen S, Epstein FH, Spokes K, Brezis ML. Loop diuretics reduce hypoxic damage to proximal tubules of the isolated perfused rat kidney. Kidney Int. 1994;45(4):981–985. doi: 10.1038/ki.1994.132. [DOI] [PubMed] [Google Scholar]
- 13.Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nature Reviews Molecular Cell Biology. 2007;8(11):870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- 14.Longo DL, Archer SL. Mitochondrial dynamics—mitochondrial fission and fusion in human diseases. New England Journal of Medicine. 2013;369(23):2236–2251. doi: 10.1056/NEJMra1215233. [DOI] [PubMed] [Google Scholar]
- 15.Boyer PD, Chance B, Ernster L, Mitchell P, Racker E, Slater EC. Oxidative phosphorylation and photophosphorylation. Annual review of biochemistry. 1977;46(1):955–966. doi: 10.1146/annurev.bi.46.070177.004515. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191(4784):4144–4148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- 17.Gray MW, Burger G, Lang BF. Mitochondrial evolution. Science. 1999;283(5407):1476–1481. doi: 10.1126/science.283.5407.1476. [DOI] [PubMed] [Google Scholar]
- 18.Anderson S, Bankier AT, Barrell BG, De Bruijn M, Coulson AR, Drouin J, et al. Sequence and organization of the human mitochondrial genome. 1981 doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 19.Taanman J-W. The mitochondrial genome: structure, transcription, translation and replication. Biochimica et Biophysica Acta (BBA)-Bioenergetics. 1999;1410(2):103–123. doi: 10.1016/s0005-2728(98)00161-3. [DOI] [PubMed] [Google Scholar]
- 20.Satoh M, Kuroiwa T. Organization of multiple nucleoids and DNA molecules in mitochondria of a human cell. Experimental cell research. 1991;196(1):137–140. doi: 10.1016/0014-4827(91)90467-9. [DOI] [PubMed] [Google Scholar]
- 21.Chatre L, Ricchetti M. Prevalent coordination of mitochondrial DNA transcription and initiation of replication with the cell cycle. Nucleic Acids Res. 2013;41(5):3068–3078. doi: 10.1093/nar/gkt015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes & development. 2004;18(4):357–368. doi: 10.1101/gad.1177604. [DOI] [PubMed] [Google Scholar]
- 23.Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, et al. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. The FASEB Journal. 2002;16(14):1879–1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- 24.Watson E, Forster P, Richards M, Bandelt H-J. Mitochondrial footprints of human expansions in Africa. The American Journal of Human Genetics. 1997;61(3):691–704. doi: 10.1086/515503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaneda H, Hayashi J, Takahama S, Taya C, Lindahl KF, Yonekawa H. Elimination of paternal mitochondrial DNA in intraspecific crosses during early mouse embryogenesis. Proceedings of the National Academy of Sciences. 1995;92(10):4542–4546. doi: 10.1073/pnas.92.10.4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shitara H, Hayashi J-I, Takahama S, Kaneda H, Yonekawa H. Maternal inheritance of mouse mtDNA in interspecific hybrids: segregation of the leaked paternal mtDNA followed by the prevention of subsequent paternal leakage. Genetics. 1998;148(2):851–857. doi: 10.1093/genetics/148.2.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz M, Vissing J. Paternal inheritance of mitochondrial DNA. New England Journal of Medicine. 2002;347(8):576–580. doi: 10.1056/NEJMoa020350. [DOI] [PubMed] [Google Scholar]
- 28.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem j. 1999;341:233–349. [PMC free article] [PubMed] [Google Scholar]
- 29.Zamzami N, Kroemer G. The mitochondrion in apoptosis: how Pandora’s box opens. Nat Rev Mol Cell Biol. 2001;2(1):67–71. doi: 10.1038/35048073. [DOI] [PubMed] [Google Scholar]
- 30.Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Archives of biochemistry and biophysics. 2007;462(2):245–253. doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nature reviews Molecular cell biology. 2011;12(1):9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris AA, Taylor RW, Birch-Machin MA, Jackson MJ, Coulthard MG, Bindoff LA, et al. Neonatal Fanconi syndrome due to deficiency of complex III of the respiratory chain. Pediatric Nephrology. 1995;9(4):407–411. doi: 10.1007/BF00866711. [DOI] [PubMed] [Google Scholar]
- 33.Sperl W, Ruitenbeek W, Trijbels J, Sengers R, Stadhouders A, Guggenbichler J. Mitochondrial myopathy with lactic acidaemia, Fanconi-De Toni-Debré syndrome and a disturbed succinate: cytochrome c oxidoreductase activity. European journal of pediatrics. 1988;147(4):418–421. doi: 10.1007/BF00496424. [DOI] [PubMed] [Google Scholar]
- 34.McFarland R, Taylor R, Turnbull D. Mitochondrial disease—its impact, etiology, and pathology. Current topics in developmental biology. 2007;77:113–155. doi: 10.1016/S0070-2153(06)77005-3. [DOI] [PubMed] [Google Scholar]
- 35.Zeviani M, Moraes C, DiMauro S, Nakase H, Bonilla E, Schon E, et al. Deletions of mitochondrial DNA in Kearns-Sayre syndrome. Neurology. 1998;51(6):1525--a. doi: 10.1212/wnl.51.6.1525-a. [DOI] [PubMed] [Google Scholar]
- 36.DiMauro S, Davidzon G. Mitochondrial DNA and disease. Annals of medicine. 2005;37(3):222–232. doi: 10.1080/07853890510007368. [DOI] [PubMed] [Google Scholar]
- 37.Niaudet P, Rotig A. The kidney in mitochondrial cytopathies. Kidney international. 1997;51(4):1000–1007. doi: 10.1038/ki.1997.140. [DOI] [PubMed] [Google Scholar]
- 38.Güçer Ş, Talim B, Aşan E, Korkusuz P, Özen S, Ünal Ş, et al. Focal segmental glomerulosclerosis associated with mitochondrial cytopathy: report of two cases with special emphasis on podocytes. Pediatric and Developmental pathology. 2005;8(6):710–717. doi: 10.1007/s10024-005-0058-z. [DOI] [PubMed] [Google Scholar]
- 39.Rötig A, Goutières F, Niaudet P, Rustin P, Chretien D, Guest G, et al. Deletion of mitochondrial DNA in patient with chronic tubulointerstitial nephritis. The Journal of pediatrics. 1995;126(4):597–601. doi: 10.1016/s0022-3476(95)70359-4. [DOI] [PubMed] [Google Scholar]
- 40.Kirby D, McFarland R, Ohtake A, Dunning C, Ryan M, Wilson C, et al. Mutations of the mitochondrial ND1 gene as a cause of MELAS. Journal of medical genetics. 2004;41(10):784–789. doi: 10.1136/jmg.2004.020537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tuppen HA, Blakely EL, Turnbull DM, Taylor RW. Mitochondrial DNA mutations and human disease. Biochimica et Biophysica Acta (BBA)-Bioenergetics. 2010;1797(2):113–128. doi: 10.1016/j.bbabio.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 42.Trump BF, Valigorsky JM, Jones RT, Mergner WJ, Garcia JH, Cowley RA. The application of electron microscopy and cellular biochemistry to the autopsy: Observations on cellular changes in human shock. Human pathology. 1975;6(4):499–516. doi: 10.1016/s0046-8177(75)80068-2. [DOI] [PubMed] [Google Scholar]
- 43.Takasu O, Gaut JP, Watanabe E, To K, Fagley RE, Sato B, et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. American journal of respiratory and critical care medicine. 2013;187(5):509–517. doi: 10.1164/rccm.201211-1983OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parekh DJ, Weinberg JM, Ercole B, Torkko KC, Hilton W, Bennett M, et al. Tolerance of the human kidney to isolated controlled ischemia. Journal of the American Society of Nephrology. 2013 doi: 10.1681/ASN.2012080786. ASN. 2012080786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brooks C, Wei Q, Cho S-G, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. The Journal of clinical investigation. 2009;119(5):1275. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tran M, Tam D, Bardia A, Bhasin M, Rowe GC, Kher A, et al. PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. The Journal of clinical investigation. 2011;121(10):4003. doi: 10.1172/JCI58662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Funk JA, Schnellmann RG. Persistent disruption of mitochondrial homeostasis after acute kidney injury. American Journal of Physiology-Renal Physiology. 2012;302(7):F853–F864. doi: 10.1152/ajprenal.00035.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang C, Youle RJ. The role of mitochondria in apoptosis*. Annual review of genetics. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dickens F, Simer F. The metabolism of normal and tumour tissue: The respiratory quotient, and the relationship of respiration to glycolysis. The Biochemical journal. 1930;24(5):1301–1326. doi: 10.1042/bj0241301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zager RA, Johnson AC, Hanson SY. Renal tubular triglyercide accumulation following endotoxic, toxic, and ischemic injury. Kidney Int. 2005;67(1):111–121. doi: 10.1111/j.1523-1755.2005.00061.x. [DOI] [PubMed] [Google Scholar]
- 51.Weinberg JM, Venkatachalam MA, Roeser NF, Nissim I. Mitochondrial dysfunction during hypoxia/reoxygenation and its correction by anaerobic metabolism of citric acid cycle intermediates. Proc Natl Acad Sci U S A. 2000;97(6):2826–2831. doi: 10.1073/pnas.97.6.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feldkamp T, Kribben A, Roeser NF, Senter RA, Kemner S, Venkatachalam MA, et al. Preservation of complex I function during hypoxia-reoxygenation-induced mitochondrial injury in proximal tubules. American journal of physiology Renal physiology. 2004;286(4):F749–F759. doi: 10.1152/ajprenal.00276.2003. [DOI] [PubMed] [Google Scholar]
- 53.Feldkamp T, Kribben A, Roeser NF, Senter RA, Weinberg JM. Accumulation of nonesterified fatty acids causes the sustained energetic deficit in kidney proximal tubules after hypoxia-reoxygenation. American journal of physiology Renal physiology. 2006;290(2):F465–F477. doi: 10.1152/ajprenal.00305.2005. [DOI] [PubMed] [Google Scholar]
- 54.Li S, Nagothu KK, Desai V, Lee T, Branham W, Moland C, et al. Transgenic expression of proximal tubule peroxisome proliferator-activated receptor-alpha in mice confers protection during acute kidney injury. Kidney Int. 2009;76(10):1049–1062. doi: 10.1038/ki.2009.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ozaltin F. Primary coenzyme Q10 (CoQ 10) deficiencies and related nephropathies. Pediatr Nephrol. 2014;29(6):961–969. doi: 10.1007/s00467-013-2482-z. [DOI] [PubMed] [Google Scholar]
- 56.Plotnikov EY, Kazachenko AV, Vyssokikh MY, Vasileva AK, Tcvirkun DV, Isaev NK, et al. The role of mitochondria in oxidative and nitrosative stress during ischemia/reperfusion in the rat kidney. Kidney Int. 2007;72(12):1493–1502. doi: 10.1038/sj.ki.5002568. [DOI] [PubMed] [Google Scholar]
- 57.Mitchell T, Rotaru D, Saba H, Smith RA, Murphy MP, MacMillan-Crow LA. The mitochondria-targeted antioxidant mitoquinone protects against cold storage injury of renal tubular cells and rat kidneys. The Journal of pharmacology and experimental therapeutics. 2011;336(3):682–692. doi: 10.1124/jpet.110.176743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mukhopadhyay P, Horvath B, Zsengeller Z, Zielonka J, Tanchian G, Holovac E, et al. Mitochondrial-targeted antioxidants represent a promising approach for prevention of cisplatin-induced nephropathy. Free Radic Biol Med. 2012;52(2):497–506. doi: 10.1016/j.freeradbiomed.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Plotnikov EY, Chupyrkina AA, Jankauskas SS, Pevzner IB, Silachev DN, Skulachev VP, et al. Mechanisms of nephroprotective effect of mitochondria-targeted antioxidants under rhabdomyolysis and ischemia/reperfusion. Biochimica et biophysica acta. 2011;1812(1):77–86. doi: 10.1016/j.bbadis.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 60.Szeto HH, Schiller PW. Novel therapies targeting inner mitochondrial membrane--from discovery to clinical development. Pharmaceutical research. 2011;28(11):2669–2679. doi: 10.1007/s11095-011-0476-8. [DOI] [PubMed] [Google Scholar]
- 61.Szeto HH, Liu S, Soong Y, Wu D, Darrah SF, Cheng FY, et al. Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. Journal of the American Society of Nephrology : JASN. 2011;22(6):1041–1052. doi: 10.1681/ASN.2010080808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parekh DJ, Weinberg JM, Ercole B, Torkko KC, Hilton W, Bennett M, et al. Tolerance of the human kidney to isolated controlled ischemia. J Am Soc Nephrol. 2013;24(3):506–517. doi: 10.1681/ASN.2012080786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takasu O, Gaut JP, Watanabe E, To K, Fagley RE, Sato B, et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med. 2013;187(5):509–517. doi: 10.1164/rccm.201211-1983OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tran M, Tam D, Bardia A, Bhasin M, Rowe GC, Kher A, et al. PGC-1alpha promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest. 2011;121(10):4003–4014. doi: 10.1172/JCI58662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zsengeller ZK, Ellezian L, Brown D, Horvath B, Mukhopadhyay P, Kalyanaraman B, et al. Cisplatin nephrotoxicity involves mitochondrial injury with impaired tubular mitochondrial enzyme activity. J Histochem Cytochem. 2012;60(7):521–529. doi: 10.1369/0022155412446227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. The New England journal of medicine. 2008;359(5):473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 67.Mewton N, Cung TT, Morel O, Cayla G, Bonnefoy-Cudraz E, Rioufol G, et al. Rationale and design of the Cyclosporine to ImpRove Clinical oUtcome in ST-elevation myocardial infarction patients (the CIRCUS trial) American heart journal. 2015;169(6):758–766. doi: 10.1016/j.ahj.2015.02.020. e6. [DOI] [PubMed] [Google Scholar]
- 68.Roy M, Reddy PH, Iijima M, Sesaki H. Mitochondrial division and fusion in metabolism. Current opinion in cell biology. 2015;33:111–118. doi: 10.1016/j.ceb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest. 2009;119(5):1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Livingston MJ, Dong Z. Autophagy in acute kidney injury. Semin Nephrol. 2014;34(1):17–26. doi: 10.1016/j.semnephrol.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hsiao HW, Tsai KL, Wang LF, Chen YH, Chiang PC, Chuang SM, et al. The decline of autophagy contributes to proximal tubular dysfunction during sepsis. Shock. 2012;37(3):289–296. doi: 10.1097/SHK.0b013e318240b52a. [DOI] [PubMed] [Google Scholar]
- 72.Howell GM, Gomez H, Collage RD, Loughran P, Zhang X, Escobar DA, et al. Augmenting autophagy to treat acute kidney injury during endotoxemia in mice. PloS one. 2013;8(7):e69520. doi: 10.1371/journal.pone.0069520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ishihara M, Urushido M, Hamada K, Matsumoto T, Shimamura Y, Ogata K, et al. Sestrin-2 and BNIP3 regulate autophagy and mitophagy in renal tubular cells in acute kidney injury. American journal of physiology Renal physiology. 2013;305(4):F495–F509. doi: 10.1152/ajprenal.00642.2012. [DOI] [PubMed] [Google Scholar]
- 74.Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27(7):728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- 75.Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong SE, et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134(1):112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92(6):829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 77.Funk JA, Schnellmann RG. Persistent disruption of mitochondrial homeostasis after acute kidney injury. Am J Physiol Renal Physiol. 2012;302(7):F853–F864. doi: 10.1152/ajprenal.00035.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rasbach KA, Schnellmann RG. Signaling of mitochondrial biogenesis following oxidant injury. J Biol Chem. 2007;282(4):2355–2362. doi: 10.1074/jbc.M608009200. [DOI] [PubMed] [Google Scholar]
- 79.Jesinkey SR, Funk JA, Stallons LJ, Wills LP, Megyesi JK, Beeson CC, et al. Formoterol restores mitochondrial and renal function after ischemia-reperfusion injury. Journal of the American Society of Nephrology : JASN. 2014;25(6):1157–1162. doi: 10.1681/ASN.2013090952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wills LP, Trager RE, Beeson GC, Lindsey CC, Peterson YK, Beeson CC, et al. The beta2-adrenoceptor agonist formoterol stimulates mitochondrial biogenesis. The Journal of pharmacology and experimental therapeutics. 2012;342(1):106–118. doi: 10.1124/jpet.112.191528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baek JH, Zeng R, Weinmann-Menke J, Valerius MT, Wada Y, Ajay AK, et al. IL-34 mediates acute kidney injury and worsens subsequent chronic kidney disease. J Clin Invest. 2015 doi: 10.1172/JCI81166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang MZ, Yao B, Yang S, Jiang L, Wang S, Fan X, et al. CSF-1 signaling mediates recovery from acute kidney injury. J Clin Invest. 2012;122(12):4519–4532. doi: 10.1172/JCI60363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li L, Huang L, Sung SS, Vergis AL, Rosin DL, Rose CE, Jr, et al. The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury. Kidney Int. 2008;74(12):1526–1537. doi: 10.1038/ki.2008.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oh DJ, Dursun B, He Z, Lu L, Hoke TS, Ljubanovic D, et al. Fractalkine receptor (CX3CR1) inhibition is protective against ischemic acute renal failure in mice. American journal of physiology Renal physiology. 2008;294(1):F264–F271. doi: 10.1152/ajprenal.00204.2007. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Linfert D, Chowdhry T, Rabb H. Lymphocytes and ischemia-reperfusion injury. Transplant Rev (Orlando) 2009;23(1):1–10. doi: 10.1016/j.trre.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Humphreys BD, Bonventre JV. Mesenchymal stem cells in acute kidney injury. Annual review of medicine. 2008;59:311–325. doi: 10.1146/annurev.med.59.061506.154239. [DOI] [PubMed] [Google Scholar]
- 88.Camussi G, Cantaluppi V, Deregibus MC, Gatti E, Tetta C. Role of microvesicles in acute kidney injury. Contributions to nephrology. 2011;174:191–199. doi: 10.1159/000329397. [DOI] [PubMed] [Google Scholar]
- 89.Cho YM, Kim JH, Kim M, Park SJ, Koh SH, Ahn HS, et al. Mesenchymal stem cells transfer mitochondria to the cells with virtually no mitochondrial function but not with pathogenic mtDNA mutations. PloS one. 2012;7(3):e32778. doi: 10.1371/journal.pone.0032778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lin HY, Liou CW, Chen SD, Hsu TY, Chuang JH, Wang PW, et al. Mitochondrial transfer from Wharton’s jelly-derived mesenchymal stem cells to mitochondria-defective cells recaptures impaired mitochondrial function. Mitochondrion. 2015;22:31–44. doi: 10.1016/j.mito.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 91.Kang HM, Ahn SH, Choi P, Ko Y-A, Han SH, Chinga F, et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nature medicine. 2015;21(1):37–46. doi: 10.1038/nm.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gigliotti JC, Huang L, Ye H, Bajwa A, Chattrabhuti K, Lee S, et al. Ultrasound prevents renal ischemia-reperfusion injury by stimulating the splenic cholinergic anti-inflammatory pathway. Journal of the American Society of Nephrology. 2013;24(9):1451–1460. doi: 10.1681/ASN.2013010084. [DOI] [PMC free article] [PubMed] [Google Scholar]