Abstract
Efficient reduction of CO2 into useful carbon resources particularly CO is an essential reaction for developing alternate sources of fuels and for reducing the greenhouse effect of CO2. The binuclear Ni, Fe−containing carbon monoxide dehydrogenase (CODHs) efficiently catalyzes the reduction of CO2 to CO. The location of Ni and Fe at proper positions allows their cooperation for CO2 to CO conversion through a push−pull mechanism. Bio−inspired from CODHs, we used several cofacial porphyrin dimers with different substituents as suitable ligands for holding two Fe ions with suitable Fe−Fe separation distance to efficiently and selectively promote CO2 to CO conversion with high turnover frequencies, TOFs. The substituents on the porphyrin rings greatly affect the catalysis process. By introducing electron-withdrawing/-donating groups, e.g. electron-withdrawing perfluorophenyl, at all meso positions of the porphyrin rings, the catalysis overpotential, η was minimized by ≈0.3 V compared to that obtained by introducing electron-donating mesityl groups. The Fe porphyrin dimers among reported catalysts are the most efficient ones for CO2 to CO conversion. Control experiments indicate that the high performance of the current CO2 to CO conversion catalysts is due to the presence of binuclear Fe centers at suitable Fe−Fe separation distance.
The efficient reduction of CO2 into useful carbon resources particularly CO is an essential reaction to overcome the limited supply of fossil fuels and the greenhouse effect of CO21,2,3. The CO2 to CO reduction is, for example, useful for generating syngas (CO + H2), which can be used to generate a wide variety of fuels with the current Fischer-Tropsch technologies4. Moreover, CO is a useful resource in methanol synthesis5 and in hydroformylation6. Plenty of homogeneous or heterogeneous CO2 to CO conversion catalysts have been reported. These include precious metal- (e.g. Re7,8,9,10,11, Ru12,13,14,15, Ir16, Au17,18, etc.) or non-precious metal- (e.g. Fe19,20,21,22,23, Ni24,25,26, Mn27,28 etc.) based catalysts. For large-scale fuel production, the development of base metal catalysts is essential29.
As base metal catalysts, Fe porphyrin monomers have been reported to efficiently catalyze the electrochemical reduction of CO2 to CO in the presence of external or localized phenol as a weak proton donor to act as a proton relay during CO2 to CO conversion. The electro-generated Fe0 porphyrin undergoes a concerted proton-electron transfer bond cleavage (CPETBC: electron transfer from the central Fe atom concerted with proton transfer and breaking of one C-O bond) rate-determining step to form an Fe−CO species that undergoes further reduction to release CO19,20,21,22,23.
In biological systems, several anaerobic bacteria and archaea utilize Ni, Fe−containing carbon monoxide dehydrogenases (CODHs) with a [NiFe4S4] cluster at the active site as highly efficient catalyst for CO2 to CO conversion with high turnover frequency, TOF (12 s−1) at a low overpotential, η < 100 mV30,31,32,33,34,35,36. Binding to Ni and Fe activates CO2 where Ni acts as a Lewis base to transfer a net electron to the antibonding lowest unoccupied molecular orbital of CO2 that increases the negative partial charges at the oxygen atoms which are stabilized by binding to a Lewis acid Fe center30,31,32,33,34,35,36. The biological CO2 to CO conversion indicates the importance of the binuclear metal ion centers with suitable separation distance in designing highly efficient catalysts for CO2 to CO conversion. Indeed, CO2 to CO conversion have been demonstrated with several binuclear- (e.g. Ni237,38,39, Cu240, Ir241, and Pd242) and polynuclear- (e.g. Ni343,44, Fe445, and iron-sulfur clusters; [Fe4S4(SR)4]2−, Ni−Fe4S4 and Co−Fe4S446,47,48) catalysts, however, with low TOF and/or high η-values.
Inspired from the Ni, Fe−containing CODHs, we recently reported a cofacial Fe tetraphenylporphyrin dimer, o-Fe2DTPP having binuclear Fe centers at a suitable Fe−Fe separation distance that efficiently and selectively catalyzes the electrochemical CO2 to CO conversion in a DMF/10% H2O solution with high Faradic efficiency (95%) and TOF (4,300 s−1) without use of any acids. However, its reaction showed a relatively high overpotential, η = 0.66 V. Control experiments with the mononuclear Fe porphyrin monomer, FeTPP and the 1,3-phenylene bridged binuclear Fe porphyrin dimer, m-Fe2DTPP indicate the importance of the binuclear Fe centers and the Fe−Fe separation distance for the CO2 to CO conversion49. Here we introduced electron-donating and electron-withdrawing substituents to the peripheral porphyrin rings (Fig. 1) that tuned η and the activity of the catalytic process. We also performed control experiments with Fe porphyrin monomers (Fig. 1) that show low activity for CO2 reduction compared to that obtained with the binuclear Fe porphyrin dimers that clearly demonstrate the importance of the binuclear metal centers for the high activity and stability in designing CO2 to CO conversion molecular catalysts. Benchmarking with other catalysts, the binuclear Fe porphyrin dimers are, to the best of our knowledge, the most efficient and stable homogeneous molecular catalysts for CO2 to CO conversion at present.
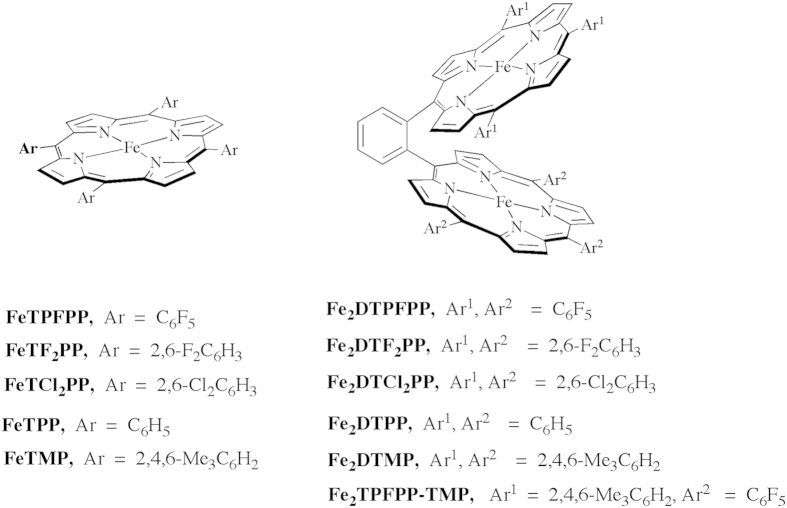
Figure 1. Chemical structures of the six Fe porphyrin dimers and their corresponding monomers.
Results
Six porphyrin dimer ligands linked by a 1,2-phenylene bridge and bearing different substituents at the porphyrin rings have been prepared according to stepwise methods outlined in Scheme S1 (SI). Fe was then inserted into the porphyrin cavities by similar reported procedures49. Typically excess FeBr2 was refluxed with the porphyrin dimer ligand in DMF overnight under Ar. The compounds were purified by column chromatography and characterized with ordinary spectroscopic methods including UV-vis., 1HNMR, and mass spectroscopy (Figure S1, SI). For control experiments, we also prepared their corresponding Fe porphyrin monomers.
In a DMF/0.1 M nBut4NPF6 (nBut4NPF6 = tetra-n-butylammonium hexafluorophosphate) solution saturated with Ar, the Fe porphyrin dimers (0.5 mM) and their corresponding Fe porphyrin monomers (1 mM) depict cyclic voltammetric (CV) behaviors shown in Figure S2 (SI) at a 50 mV/s scan rate. The Fe porphyrin monomers show, as previously reported34,35,36,37,38, three successive reversible 1e− reductions/oxidations of the Fe centers, i.e. FeIII/II, FeII/1 and FeI/0 at standard redox potentials donated as E0(1), E0(2), and E0(3), respectively. The Fe porphyrin dimers, on the other hand, show three successive 2e− reductions/oxidations of the two Fe centers represented by equations (1)~(6) at standard redox potentials donated as E0(1), E0(2), and E0(3) for 2FeIII/II, 2FeII/1 and 2FeI/0, respectively. In some cases, the 2e− reductions/oxidations process of the dimers are divided into two successive 1e− reductions/oxidations with standard redox potentials donated as, for example, E0(1a) and E0(1b). The number of electrons was confirmed by controlled-potential electrolysis carried out at 100 mV negative potentials of the peak potentials. Table S1 (SI) summarizes the standard redox potentials, E0, of the Fe porphyrin dimers and their corresponding Fe porphyrin monomers vs. NHE (hereafter, all potentials are indicated against NHE except as noted). The standard redox potential of the 2FeIII/II reductions/oxidations of the dimers, E0(1) occur at significant negative potentials relative to that of the monomer possibly due to strong Cl− binding/association to the Fe centers between the two cofacial porphyrin bi-layers49. The peak currents of the Fe porphyrin dimers are significantly smaller than that of the corresponding Fe porphyrin monomers. This is consistent with their small diffusion coefficients resulting from their large molecular sizes relative to their corresponding Fe porphyrin monomers50. The reduction peak currents of the Fe porphyrin dimers change linearly with the square root of the scan rate,υ1/2 indicating diffusion controlled electron transfer processes50. The standard redox potentials, E0, reflect the different electronic environment around the Fe centers. For example, E0s are shifted to more positive potentials as the electron-withdrawing properties of the substituents on the peripheral porphyrin rings increase. Also, a clear separation among E0(1a) and E0(1b), E0(2a) and E0(2b), and E0(3a) and E0(3b) couples were observed for the Fe2TPFPP-TMP dimer that has a significant difference in the electronic environment around the two Fe centers.
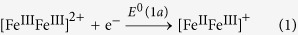 |
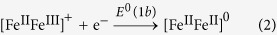 |
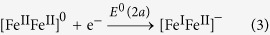 |
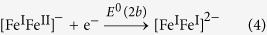 |
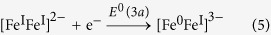 |
 |
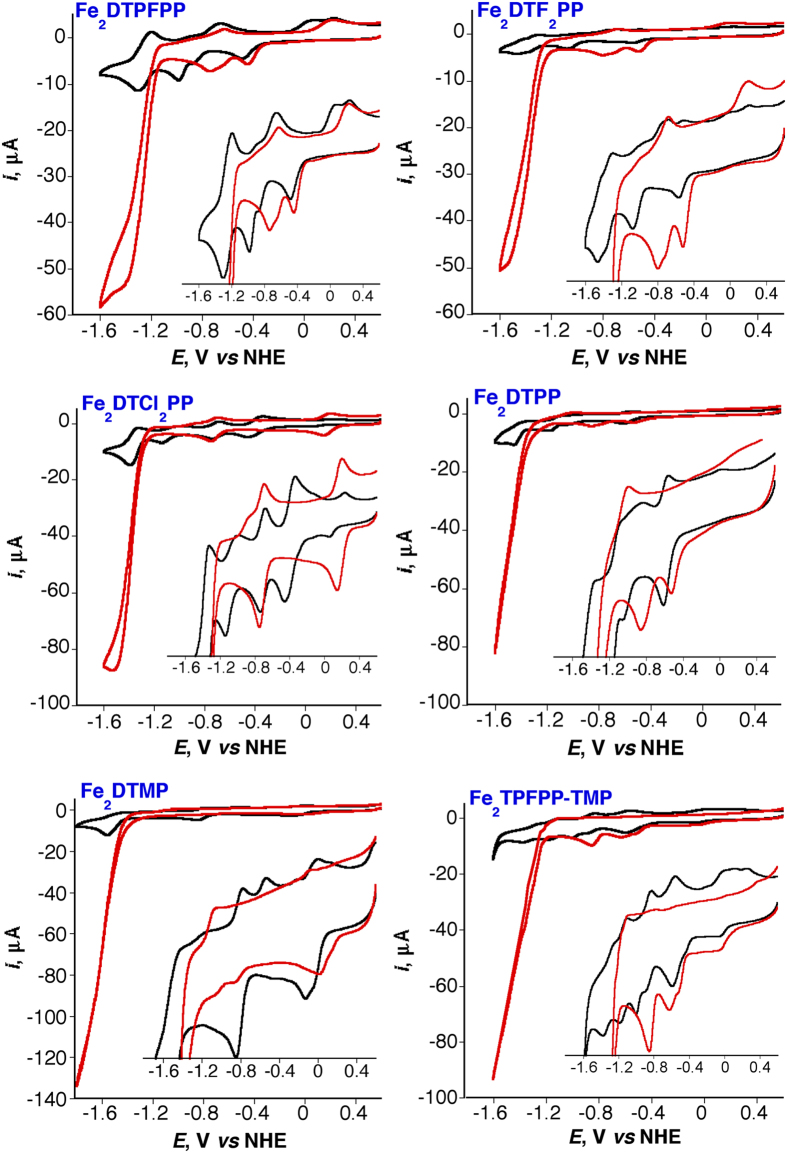
Under CO2, the CV behaviors of the Fe porphyrin dimers (0.5 mM) are depicted in Fig. 2 (red lines). The reversible 2FeIII/2FeII redox couple observed under Ar is replaced with a new reduction peak due, as we previously reported49, to the dissociation of the Cl− and the coordination of the CO2 molecule to the electro-generated 2FeII species inside the cofacial porphyrin cavity within the time scale of CV. The dissociation of the Cl− and coordination of CO2 is supported by the UV-vis spectra of the chemically reduced o-Fe2IIDTPP that shows the remarkable change of its Soret and Q-bands upon purging of CO2 gas at −30 °C, indicating the binding of CO2 to the FeII porphyrin species49. Indeed, the ability of CO2 to coordinate transition metal complexes is extensively investigated51. However, the coordination of CO2 to the electro-generated FeII species is not observed in the corresponding Fe porphyrin monomer under similar experimental conditions. Upon further scanning to more negative potential the electro-generated CO2-coordinated 2FeII species showed a reversible 2e− redox couple corresponding to the generation of CO2 coordinated 2FeII/2FeI species. The most interesting finding is the observation of a strong catalytic current in the presence of CO2 indicating electro-catalytic reduction of CO2 promoted by the six Fe porphyrin dimers. The appearance of the catalytic peak over the FeIFeI/FeIFe0 redox couple under Ar indicates the starting of the catalytic process once the FeIFe0 porphyrin species is electrochemically generated49. In general, the Fe porphyrin dimer with electron-withdrawing substituents shows electro-catalytic CO2 reduction behavior at more positive potential, i.e. at low η. In other words, the Fe porphyrin dimers are arranged in the following order with respect to their η (small to large) for the CO2 to CO conversion; Fe2DTPFPP < Fe2TPFPP-TMP < Fe2DTF2PP < Fe2DTCl2PP < Fe2DTPP < Fe2DTMP.
Figure 2. Cyclic voltammograms of the six Fe-porphyrin dimers (0.5 mM) in DMF/10% H2O at 50 mV/s scan rate under Ar (black lines) and CO2 (red lines).
Insets: magnified traces of CVs.
The effect of H2O content on the catalytic CO2 to CO conversion with Fe porphyrin dimers is tested by CVs at a 100 mV/s scan rate. Figure S3 (SI) shows the CVs of o-Fe2DTPP (0.5 mM) as a representative example at 100 mV/s scan rate in DMF containing 0.1 M nBut4NPF6 supporting electrolyte in the presence of different amounts of H2O under Ar or CO2. All the other Fe porphyrin dimers show similar behaviors. In H2O-free DMF solution, a large catalytic current was generated in a CO2-saturated solution. Increasing the H2O content to 10% in the medium induces the positive shift of the starting potential of the catalytic current and increases the catalytic peak current, Further addition of H2O above 10% decreases the catalytic peak current due to the decreasing solubility of the Fe porphyrin dimers.
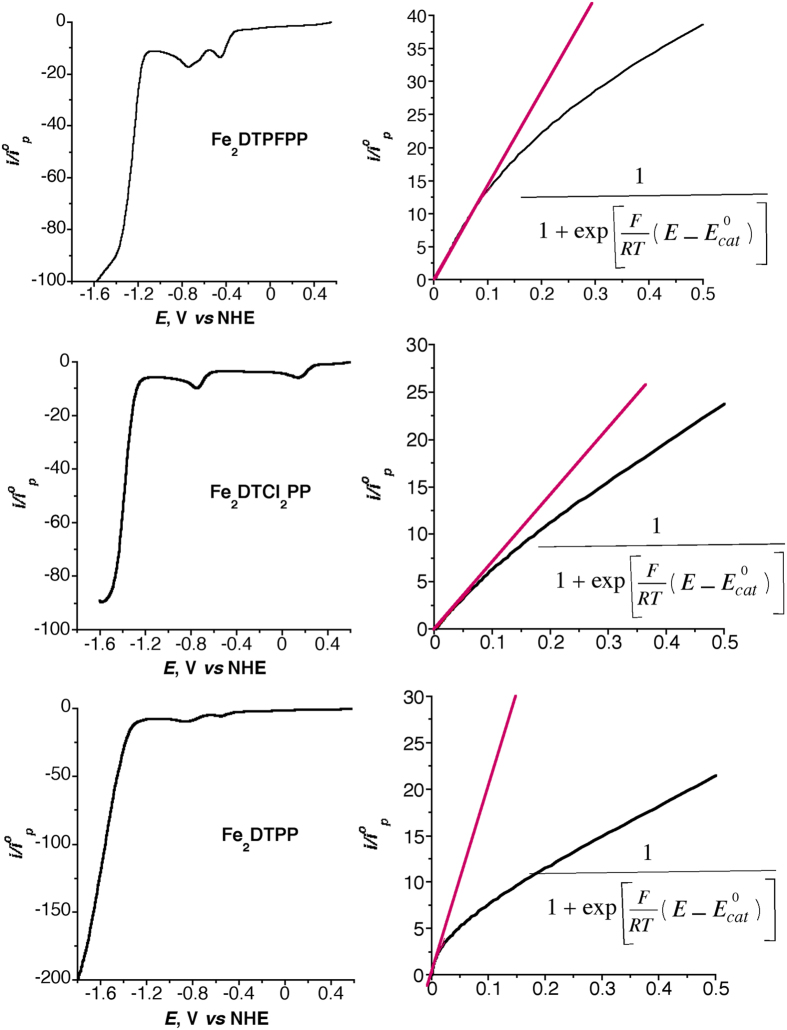
For fast catalytic process, the foot-of-the-wave analysis of the CVs has been reported to be a quick estimation of the catalysis rate constant, kcat, TON, and TOF of the catalysis reaction without the contribution of side phenomena such as substrate consumption, catalyst deactivation, and/or product inhibition19,23. The analysis is based on the linear correlation between i/i0p and 1/{1 + exp[F/RT(E − E0cat)]} “equation (7)”,where i is the catalytic current in the presence of CO2, i0p is the peak current in the absence of CO2, F, R, T, and E are the Faraday constant, gas constant, absolute temperature, and the electrode potential, respectively. Plotting i/i0p vs. 1/{1 + exp[F/RT(E − E0cat)]} gives rise to a straight line of slope 2.24(RT/Fν)1/2(kcat)1/2 (ν is the scan rate in V/s) from which the catalysis rate constant, kcat is calculated. The kcatis then used to calculate the TOF and the logTOF −η relationship according to equations (8) and (9), respectively, where TOF0 is the intrinsic turnover frequency (turnover frequency at zero η). The value of η is calculated according to equation (10) based on the reported thermodynamic redox potential of the CO2 to CO conversion in DMF/5% H2O solution containing 0.1 M nBut4NPF6 supporting electrolyte, E0(CO2/CO) = −0.69 V19. Figure 3 depicts the catalytic CVs responses (left) of three Fe porphyrin dimers (0.5 mM, 100 mV/s scan rate) and the corresponding foot-of-the-wave analysis (right). Other catalysts show similar behaviors. Figure 4 shows the catalytic CVs responses (a) of the six Fe porphyrin dimers and the corresponding logTOF −η relationship (b). Table 1 summarizes the catalysis parameters of the current Fe porphyrin dimers and that of the most efficient reported CO2/CO reduction molecular catalysts. The table clearly shows that η for the CO2 reduction decrease by increasing the electron-withdrawing substituents on the porphyrin rings for the Fe porphyrin dimers and their benchmarking superiority for the CO2 to CO conversion activity over the reported catalysts.
Figure 3. Catalytic CV responses (left, forward scan only shown for clarity) of three Fe porphyrin dimers (0.5 mM) at 100 mV/s scan rate in DMF/10% H2O saturated with CO2 and the corresponding foot-of-the-wave analysis (right).
Figure 4.
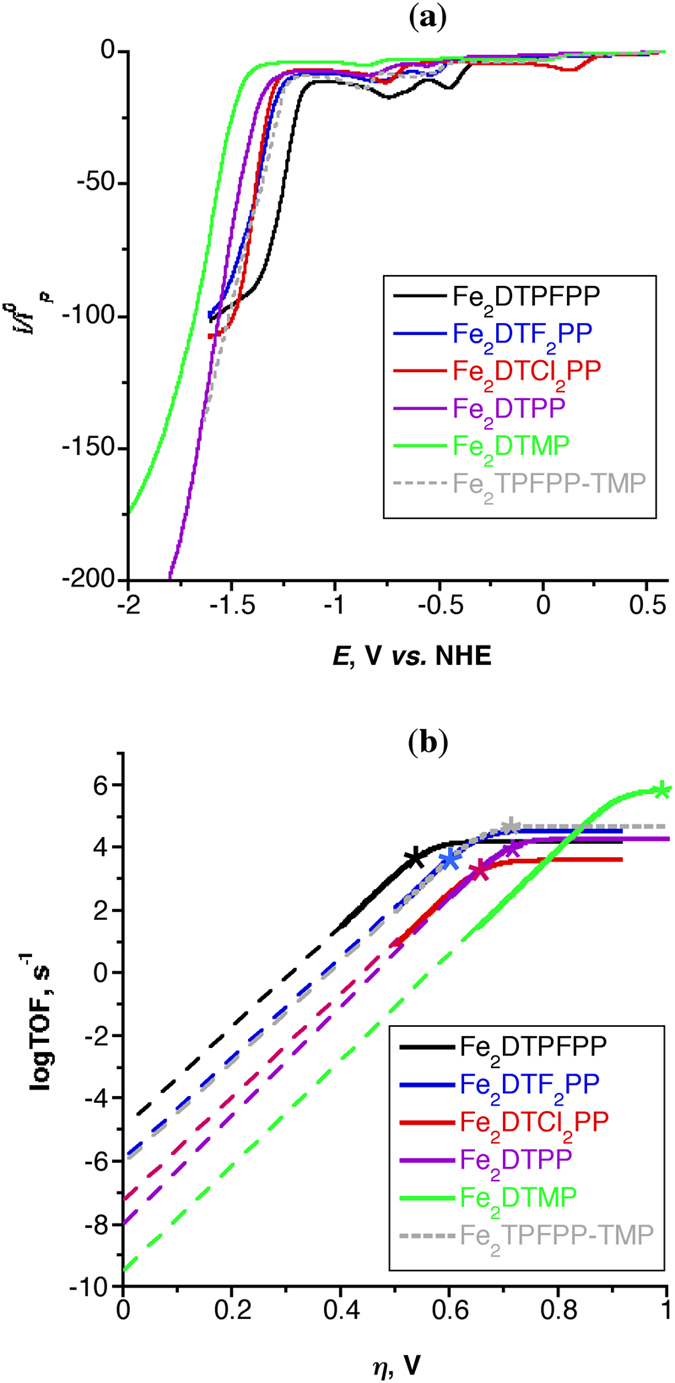
(a) Catalytic CVs responses (forward scan only shown for clarity) and (b) the Tafel plot [logTOF −η relationships] of the six Fe porphyrin dimers (0.5 mM) at 100 mV/s scan rate in DMF/10% H2O saturated with CO2. *logTOF resulted from bulk electrolysis experiments.
Table 1. Catalysis parameters of Fe porphyrin dimers (this work) and other reported molecular CO2/CO reduction catalysts.
| Solvent E0(CO2/CO), V | Catalyst E0cat ,V | kcat, s−1 | η, V | logTOF, s−1 | logTOF0, s−1 | Ref. |
|---|---|---|---|---|---|---|
| DMF/10% H2O −0.69 | Fe2DTPFPP, −1.25 | 1.6 × 104 | 0.40 − 0.60 | 1.5 − 3.9, 4.2* | −5.0 | This work |
| DMF/10% H2O − 0.69 | Fe2DTF2PP, −1.34 | 3.7 × 104 | 0.50 − 0.65 | 2.0 − 4.3, 4.5* | −6.1 | This work |
| DMF/10% H2O − 0.69 | Fe2DTCl2PP, −1.35 | 4.1 × 103 | 0.55 − 0.70 | 1.8 − 3.5, 3.6* | −7.2 | This work |
| DMF/10% H2O − 0.69 | Fe2DTPP, −1.40 | 2.0 × 104 | 0.60 − 0.75 | 2.4 − 4.2, 4.3* | −8.1 | This work49 |
| DMF/10% H2O − 0.69 | Fe2DTMP, −1.60 | 4.1 × 103 | 0.70 − 0.85 | 2.3 − 4.8, 5.8* | −9.6 | This work |
| DMF/10% H2O − 0.69 | Fe2TPFPP, −TMP −1.35 | 7.3 × 105 | 0.55 − 0.70 | 2.8 − 4.3, 4.7* | −6.1 | This work |
| DMF/10% H2O − 0.69 | FeTPP, − 1.41 | 2.1 × 103 | 0.60 − 1.0 | 1.4 − 3.1, 3.3* | −8.4 | This work |
| DMF/5% H2O /3M PhOH −0.69 | CATa, − 1.35 | >5.0 × 106 | 0.45 − 0.70 | 1.8 − 3.2, 3.8* | −6.0 | 22 |
| DMF/5% H2O /3M PhOH −0.69 | FCATb, − 1.28 | >5.0 × 106 | 0.40 − 0.70 | 1.6 − 3.8, 4.0* | −5.5 | 22 |
| DMF/5% H2O /3M PhOH −0.69 | FeTPP, −1.43 | 3.5 × 104 | 0.60 − 1.00 | 2.5 − 4.3, 4.5* | −8.0 | 20 |
| CH3CN/0.8M CF3CH2OH −0.65 | Re(bpy)(py)(CO)3 , −1.30 | 875.0 | NA | 2.9* | −8.0 | 11 |
| CH3CN −0.65 | (bbpy)Mn(CO)3 , −1.28 | 5.0 × 103 | NA | 3.7* | −7.0 | 28 |
| CH3CN −0.65 | Ru(tpy)(Mebim-py), −1.34 | 59.0 | NA | 1.8* | −9.9 | 12 |
| CH3CN −0.65 | Ru(tpy)(bpy) −1.34 | 7.6 | NA | 0.9* | −10.8 | 12 |
| DMF/0.1M HBF4 −0.23 | m-(triphos)2Pd2 , −0.76 | 35.0 | NA | 1.5* | −7.4 | 42 |
aFe meso-tetra(2,6-dihydroxyphenyl)porphyrin.
bFe 5,15-di((2,6-dihydroxyphenyl)-10,20-di(pentafluorophenyl)porphyrin.
*logTOFmax, s−1.
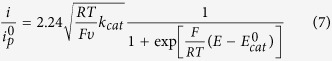 |
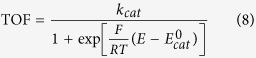 |
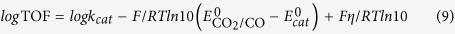 |
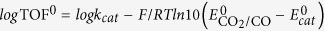 |
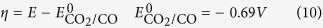 |
To test the activity, stability, and selectivity of the Fe porphyrin dimers for the CO2 reduction, a bulk electrolysis experiment was conducted in a gas-tight H shaped two-compartment electrochemical cell with a glass frit to separate the two compartments. The cell was filled with DMF/10% H2O solution (14 mL) containing 0.5 mM of the Fe porphyrin dimer and 0.1 M nBut4NPF6 as a supporting electrolyte. In one compartment, a glassy carbon working electrode (1 cm2) and a Ag/AgCl (3M NaCl) reference one were immersed close to each other (about 2 mm apart) in the solution. In the other compartment, a Pt foil (about 20 cm2) was immersed in the solution and used as a counter electrode. The solution in the two compartments was saturated with CO2 by bubbling CO2 for 30 min prior to the reaction. The products of the electro-catalytic reduction in the gas phase and in the solution phase were analyzed by gas chromatography and capillary electrophoreses, respectively. Figure 5a shows the current density-time profile of the electrolysis at −1.25 V vs. NHE (η = 0.56 V) in the presence and absence of the Fe2DTPFPP (0.5 mM) as a representative example of the Fe porphyrin dimers. The product analysis (Fig. 5b) of the headspace gas and solution shows the formation of CO gas in 92% Faradaic efficiency and H2 gas in 8% Faradaic efficiency. Only a very small amount of HCO2H detected in the solution, that means the Fe2DTPFPP dimer shows a high selectivity for CO2 reduction to CO. The other Fe porphyrin dimers show similar behavior for the CO2 to CO conversion however under slightly higher η. Based on the bulk electrolysis experiment, the catalysis parameters, kcat, TON, and TOF were calculated using equations (11)~(13);
Figure 5.
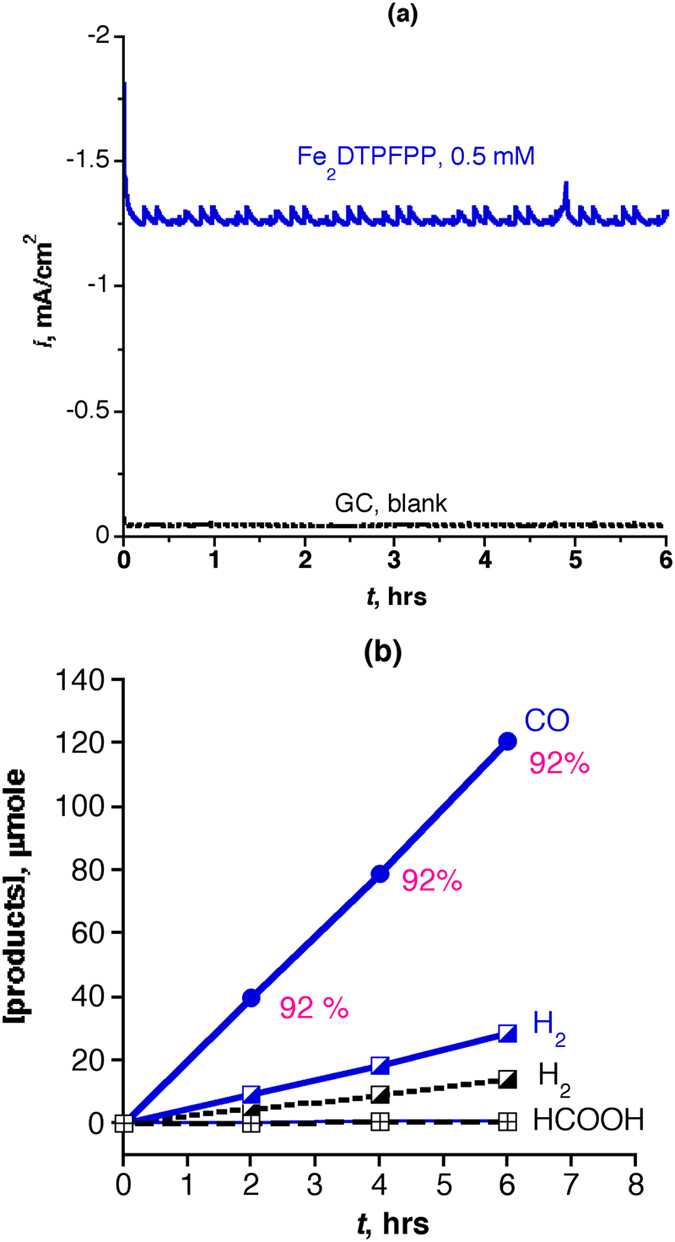
(a) Bulk electrolysis conducted for 6 hrs at −1.25 V vs. NHE (η = 0.56 V) and (b) products analysis of Fe2DTPFPP (0.5 mM) and GC blank in DMF/10% H2O under CO2.
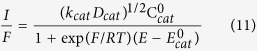 |
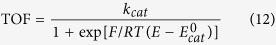 |
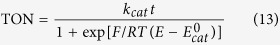 |
where, I is the net current density (the current density obtained from the bulk electrolysis corrected for the background current density and Faradic efficiency), Dcat is the diffusion coefficient of the catalyst calculated based on Randles-Sevcik equation50, and C0cat is the bulk catalyst concentration, 5 × 10−7 mole/cm3 (0.5 mM). Table S2 (SI) summarizes the catalysis parameters of the six Fe porphyrin dimers obtained from the bulk electrolysis experiment conducted for 6 hrs in DMF/10% H2O saturated with CO2. The results of the bulk electrolysis experiments conducted at a single potential are presented as stars in Fig. 4b that demonstrate the validity of the foot-of-the-wave analysis for estimating the catalysis parameters19.
To clarify the indispensableness of the dimer structure for the high catalytic activity as CO2 to CO conversion catalysts as well as to compare the catalytic performance between the dimers and the monomers, we prepared the corresponding Fe porphyrin monomers, FeTPFPP, FeTF2PP, FeTCl2PP, FeTPP and FeTMP (Fig. 1) as controls and measured their CV behaviors under Ar or CO2 in DMF/10% H2O solution. To normalize for the concentration of their iron centers, the Fe porphyrin monomers and dimers were tested at 1 mM and 0.5 mM concentration, respectively. Under Ar (Figure S2, SI), the Fe porphyrin monomers show three reversible 1e− redox couples assigned for the FeIII/II, FeII/I, and FeI/0 at standard redox potentials depicted in Table S1. Under CO2, the Fe porphyrin monomers show catalytic current peaks for the CO2 reduction, however, lower than those shown by the corresponding Fe porphyrin dimers. This indicates the importance of the binuclear Fe centers for the high activity of the current Fe porphyrin dimers as CO2 to CO conversion catalysts. Figure S4 (SI) shows the CVs of the Fe2DTPFPP and FeTPFPP under Ar and CO2 as, a representative example, which clearly shows the high activity of the Fe porphyrin dimer compared to the corresponding monomer for CO2 to CO conversion without use of any acids.
For a clear kinetic comparison between the Fe porphyrin dimers and their corresponding monomers for CO2 to CO conversion, the catalytic peak current, i is normalized to the peak current of the FeII/I, i0p. Figure S5 (SI) compares the activity of the six Fe porphyrin dimers with their corresponding monomers. Table S3 (SI) summarizes the catalysis parameters of the Fe porphyrin monomers, derived from the foot-of-the-wave analysis of the CVs. These results clearly elucidate that the binuclear structure is essential for highly efficient CO2 reduction. We tentatively propose that the cofacial dimer structure will provide a suitable way for the cooperation of the two Fe centers during the CO2 electro-catalytic reduction similar to that observed in CODHs. Consistent with this, the 1,3-phenylene bridged Fe porphyrin dimer, m-Fe2DTPP that has a large Fe-Fe separation distance (≈10.9 Å) showed very low activity (Table S3) for CO2 to CO conversion compared with the current cofacial Fe porphyrin dimers under the same conditions49.
Discussion
In biological systems, CODHs use a [NiFe4S4] cluster, termed cluster C, to reversibly reduce CO2 to CO with high TON and TOF at low η. The crystallographic studies, at the atomic resolution (≤1.1 Å) of CODH in complex with CO2 revealed a CO2 ligand bridged between Ni and Fe in a μ2η2 coordination geometry that clearly indicates the cooperation between the binuclear Ni and Fe centers in the activation of CO2 reduction36. The presence of binuclear Ni and Fe centers in CODHs inspired us to utilize molecular inorganic catalysts containing binuclear centers for CO2 to CO conversion keeping in mind that the binuclear centers should be at a suitable separation distance to allow the cooperation between them. We previously utilized several cofacial porphyrin dimers as suitable ligands for holding two Mn ions at suitable Mn−Mn separation distances (3.7–6.2 Å) to promote water oxidation to O2 or H2O2 disproportionation52,53,54. As a first generation of molecular catalysts containing binuclear centers, we utilized six cofacial porphyrin dimers ligands with different electron donating and withdrawing substituents at the peripheral porphyrin rings and holding two Fe ions as bio-inspired catalysts for CO2 to CO conversion. Assuming similar Fe−Fe separation distances in the current catalysts, this will fit CO2 bridging to the Fe ions and allowing their cooperation for the CO2 to CO conversion. The results obtained demonstrate that, the Fe porphyrin dimers have the greatest activity, stability, and selectivity for CO2 electro-catalytic reduction to CO among the reported precious and non-precious CO2 to CO reduction molecular catalysts (Table 1). Control experiments with Fe porphyrin monomers (Figure S2, Table S3) and a 1,3-phenylene bridged Fe porphyrin dimer (Table S3) indicate the importance of the binuclear Fe centers and the Fe−Fe separation distance for the high catalytic performance.
On the time scale of CV, the data show that the cofacial Fe porphyrin dimers under Ar are reduced stepwisely as in equations (1)~(6). Under CO2, the catalytic current for the CO2 to CO conversion is observed on the peaks corresponding to [FeIFeI] to [FeIFe0] and [FeIFe0] to [Fe0Fe0] conversions as in equations (5) and (6) (Fig. 2). This means the active species for the CO2 reduction is either [FeIFe0] or [Fe0Fe0] species. The differentiation between the two species is unclear in the five symmetrical Fe porphyrin dimers where the two Fe centers have similar electronic environments. The hetero-dimer, Fe2TPFPP-TMP that has quite different electronic environment around the two Fe centers, on the other hand, showed clear separation of the stepwise reductions of the Fe centers under Ar (Table S1) and showed only strong catalytic current over the [FeIFe0] species (Fig. 2) under CO2. This observation indicates that Fe0 and FeI possibly act as a Lewis base and a Lewis acid, respectively, in the catalysis process. Through such roles of the binuclear centers, these Fe porphyrin dimers would promote the CO2 reduction without any acids49. At its lower reduced state, FeIIFeII, these catalysts can capture CO2, judging from the appearance of a new CV peak under CO2 atmosphere (Fig. 2)49. This association of substrate CO2 and the catalysts at the lower reduced state will contribute to the increase of TOF in the dimer catalysts, because it is ready to do the rapid reduction of the bound CO2 at the catalyst Fe0FeI state.
The results obtained by the current cofacial Fe porphyrin dimers also indicate that η and TOF of the catalysis process can be tuned by the introduction of electronically different substituents to the porphyrin peripheral positions (Fig. 4 and Table 1). For example, introduction of electron-withdrawing perfluorophenyl substituents to the meso positions of the dimer reduces η ≈ 0.3 V compared to that obtained with electron-donating mesityl group. In general, the introduction of electron-withdrawing groups on catalysts promotes the positive shifts of their reduction potentials, resultantly decrease of η of the reduction reaction. However, this electron-withdrawing effect also causes the decrease of the electron density at the active center, which sacrifices their nucleophilic activities to lead the decrease of TOF. On the other hand, electron-donating groups such as mesityl on the porphyrin rings leads high TOFs. In general, low reaction η and high TOF by electronic tuning is not compatible22, although TOFs for the dimer catalysts are kept within the moderate range even the catalysts bearing electron-withdrawing groups.
This work will turn the intention for designing new generations of highly efficient binuclear molecular catalysts for CO2 to CO conversion, working under neutral conditions. We are currently trying to obtain hetero-binuclear dimers to elucidate the catalytic mechanism.
Methods
Materials, Instruments, CV measurements, Bulk electrolysis, and products analysis of CO2 reduction are presented in the supporting information.
Preparation of Fe porphyrin dimers
The cofacial Fe porphyrin dimers were prepared as previously reported by stepwise procedures according to Scheme S1 (SI)49,55. We will discuss the preparation of [Fe2DTMP]Cl2 as a representative example in details. The other dimers were synthesized similarly.
Preparation of 5-(2′-methylbenzoate)-10,15,20-trismesityl-porphyrin (compound b, Ar1 = 2,4,6-Me3C6H2)
A 2 L three-neck round-bottomed flask was charged with Methyl-2-formylbenzoate (a) (1.94 mL 11.8 mmol), mesitylaldehyde (4.5 mL, 36 mmol), pyrrole (3.2 mL, 48 mmol) and CHCl3 (600 mL). The colorless solution was purged with nitrogen for about 15 min. Then, boron trifluoride diethyl etherate (BF3·Et2O, 3.2 mL) was added via a syringe, accordingly the color of the solution changed immediately to dark red. The mixture was stirred at room temperature under nitrogen and TLC used to monitor the reaction progress. At the end of 5-hrs. reaction, DDQ (DDQ = 2,3-dichloro-5,6-dicyano-p-benzoquinone) solution (8 g/150 mL benzene, 36 mmol) was added to the reaction mixture, whose color changed to dark green. The mixture was stirred at room temperature for 1.5 hours. After solvent removal, the residue was dissolved into a small amount of CHCl3 and loaded into Al2O3 column then eluted with CHCl3. The first broad reddish violet band was collected. The TLC of this band showed the presence of three bands; a light red band on the top followed by strong reddish violet band then green and black bands on the bottom. The mixture was separated by a silica-gel column. The first red band eluted with n-hexane-benzene mixture (1:1 v/v) was collected and characterized as 0.2 g TMPH2 byproduct. The second reddish violet band contained 1.6 g of the desired compound b. The compound was further purified with a silica-gel column eluted with n-haxane- CHCl3 (1:1 v/v) to give the pure one (1.4 g, 14.8% yield). 1H NMR (CDCl3, 400 MHz): δ 8.65 (d, 8H, pyrrole β-H), 8.39 (m, 1H, Ar-H), 8.09 (m, 1H, Ar-H), 7.85 (m, 2H, Ar-H), 2.86 (s, 3H, OCH3), 2.64 (s, 9H, Ar-CH3), 2.00 (s, 3H, Ar-CH3), 1.88 (s, 3H, Ar-CH3), 1.86 (s, 9H, Ar-CH3), 1.80 (s, 3H, Ar-CH3), −2.46 (s, 2H, pyrrole N-H). MALDI-TOF-MS m/z = 798.25 (found), 799.02 (calcd.).
Preparation of 5-(2′-Benzomethanol)-10,15,20-mesitylporphyrin (compound c)
A 500 mL flask was charged with compound b (0.5 g, 0.626 mmol) and dry THF (15 mL). The solution was cooled to 0 °C then LDBBA (LDBBA = lithium diisobutyl-t-butoxyaluminum hydride) reducing agent (12.5 ml, 4 mmol, 0.33 M solution) was added drop-wise. The reaction mixture was stirred for 3 hrs at 0 °C under N2 while its progress was monitored with TLC. After completed, the reaction quenched by adding 2M HCl and the product was extracted three times with CH2Cl2. The solvent was removed and the residue was purified with a silica-gel column using CH2Cl2 as an eluent to give a pure violet powder of the desired compound (0.47 g, 97.5% yield). 1HNMR (CDCl3, 400 MHz): δ 8.62 (d, 8H, pyrrole β-H), 8.02 (m, 1H, Ar-H), 7.93 (m, 1H, Ar-H), 7.82 (m, 1H, Ar-H), 7.63 (m, 1H, Ar-H), 4.38 (s, 2H, -CH2OH), 4.05 (s, 1H, -OH), 2.57 (s, 9H, Ar-CH3), 2.04 (s, 3H, Ar-CH3), 1.96 (s, 3H, Ar-CH3), 1.84 (s, 9H, Ar-CH3), 1.53 (s, 3H, Ar-CH3), −2.47 (s, 2H, pyrrole N-H). MALDI-TOF-MS m/z = 771.1 (found), 771.0 (calcd.).
Preparation of 5-(2′-Benzaldehyde)-10,15,20-trismesityl-porphyrin (compound d)
Compound c (3.5 g, 4.5 mol) was dissolved into dry CH2Cl2 (500 mL) then excess active MnO2 (~15 g) was added. The solution was stirred under N2 for 2 hrs. The TLC showed complete conversion of the compound to d. The MnO2 was isolated by filtration. The solvent was removed under vacuum and the residue was purified with Silica-gel column using CH2Cl2 as eluent to give a pure violet powder of the desired compound (3.2 g, 91.6% yield). 1H NMR (CDCl3, 400 MHz): δ 8.66 (d, 8H, pyrrole β-H), 8.41 (m, 1H, Ar-H), 8.20 (m, 1H, Ar-H), 7.91 (m, 2H, Ar-H), 5.3 (s, 1H, -CHO), 2.62 (s, 9H, Ar-CH3), 1.87 (s, 18H, Ar-CH3), −2.52 (s, 2H, pyrrole N-H). MALDI-TOF-MS m/z = 768.5 (found), 768.99 (calcd.).
Preparation of 1′,2′-bis[10,15,20-trimesitylporphyrin]-benzene (compound e)
A 1L three-necked flask was charged with compound d (2.0 g, 2.6 mmol), pyrrole (1.4 g, 20.8 mmol), mesitylaldehyde (3.05 g, 15.5 mmol), and dry CH2Cl2 (400 mL). The solution was stirred at room temperature for 30 min. under N2. Then, BF3·Et2O catalyst (5 mL) was added. After 4-hrs stirring, DDQ solution (5 g in 100 mL benzene) was added and the stirring is continued for 2 more hrs. The solvent was reduced by the rotary evaporator and loaded to Al2O3 column and the porphyrins mixture was collected using CHCl3 as eluent. The porphyrins mixture (TMPH2 and the dimmer) was separated by using silica-gel column eluted with CHCl3:hexane (1:3 v/v). The TMPH2 came first then the desired compound. The compound e was isolated as pure violet powder (0.85 g, 21% yield). 1H NMR (CDCl3, 400 MHz): δ 9.04 (d, 4H, pyrrole β-H), 8.73 (dd, 2H, Ar-H), 8.19 (d, 4H, pyrrole β-H), 8.19–8.15 (m, 2H, Ar-H), 8.11 (d, 4H, pyrrole β-H), 7.93 (d, 4H, pyrrole β-H), 7.02 (s, 2H, Ar-H), 7.01 (s, 4H, Ar-H), 6.99 (s, 2H, Ar-H), 6.76 (s, 4H, Ar-H), 2.55 (s, 18H, Ar-CH3), 1.47 (s, 36H, Ar-CH3), −3.52 (s, 4H, por-pyrrole NH). MALDI-TOF-MS m/z = 1547.2 (found), 1547.46 (calcd.).
Preparation of diiron 1′-[10,15,20-trispentafluorophenyl-porphyrin]-2′-[10,15,20-trismesitylporphyrin]-benzene (Fe2DTMP, Compound f)
A solution of compound e (200 mg, 0.13 mmol) in dry DMF (20 mL) was refluxed overnight with excess FeBr2 (150 mg, 0.69 mmol). The solvent was then removed and the desired compound was extracted with CHCl3/1M HCl three times then with CHCl3/NaHCO3 (saturated) in three times and finally with the CHCl3/H2O in three times. The organic layer containing the desired compound was dried over anhydrous sodium sulfate. The compound was further purified with a silica-gel column using 5% MeOH-CHCl3 as an eluent. The reddish brown band was collected to give reddish brown powder (197 mg, 88.3% yield). MALDI-TOF-MS m/z = 1511, 1546, 1583 corresponding to Fe2DTMP (calcd 1511.5), [Fe2DTMP]Cl (calcd. 1546.95) and [Fe2DTMP]Cl2 (calcd. 1582.4), respectively.
Similarly, the other Fe porphyrin dimers were prepared and characterized by MALDI-TOF mass and UV-vis spectra. Fe2DTPFPP dimer shows peaks at m/z values of 1798.4 corresponding to Fe2DTPFPP (calcd. 1798.73) and 1834.0 corresponding to [Fe2DTPFPP]Cl (calcd. 1834.18). Fe2DTF2PP dimer shows peaks at m/z values of 1474.5 corresponding to Fe2DTF2PP (calcd. 1474.9). Fe2DTCl2PP dimer shows only one peak of m/z value of 1672.2 corresponding to Fe2DTCl2PP (calcd. 1672.36). The Fe2DTPP dimer shows peaks at m/z values of 1294.1 corresponding to [Fe2DTPP]Cl (calcd. 1294.47) and 1259.0 corresponding to Fe2DTPP (calcd. 1259.02). Fe2TPFPP-TMP shows peaks at m/z = 1690.1 and 1655.0 corresponding to [Fe2TPFPP-TMP]Cl (calcd. 1690.57) and Fe2(TPFPP-TMP) (calcd. 1655.1). Figure S1 (SI) depicts the MALDI-TOF mass spectra of the six Fe-porphyrin dimers and their UV-vis spectra (15 μM) in DMF.
Additional Information
How to cite this article: Zahran, Z. N. et al. Bio-inspired cofacial Fe porphyrin dimers for efficient electrocatalytic CO2 to CO conversion: Overpotential tuning by substituents at the porphyrin rings. Sci. Rep. 6, 24533; doi: 10.1038/srep24533 (2016).
Supplementary Material
Acknowledgments
Y.N. acknowledges the financial supports from JST ACT-C, JSPS KAKENHI Grant Numbers 23245035, 15K12720, and a Chubu University Research Grant.
Footnotes
Author Contributions Z.N.Z. and E.A.M. prepared and characterized the catalysts. Z.N.Z. did the electrocatalytic investigation and wrote the manuscript. Y.N. supervised the work.
References
- Qiao J., Liu Y. Y., Hong F. & Zhang J. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem. Soc. Rev. 43, 631–675 (2014). [DOI] [PubMed] [Google Scholar]
- Costentin C., Robert M. & Saveant J.-M. Catalysis of the electrochemical reduction of carbon dioxide. Chem. Soc. Rev. 42, 2423–2436 (2013). [DOI] [PubMed] [Google Scholar]
- Lackner K. S. A guide to CO2 sequestration. Science 300, 1677–1678 (2003). [DOI] [PubMed] [Google Scholar]
- Glasser D., Hildebrandt D., Liu X., lu X. & Masuku C. M. Recent advances in understanding the Fischer–Tropsch synthesis (FTS) reaction. Curr. Opin. Chem. Eng. 1, 296–302 (2012). [Google Scholar]
- Martin O. & Perez-Ramirez J. New and revisited insights into the promotion of methanol synthesis catalysts by CO2. Catl. Sci. Technol. 3, 3343–3352 (2013). [Google Scholar]
- Franke R., Selent D. & Börner A. Applied hydroformylation. Chem. Rev. 112, 5675–5732 (2012). [DOI] [PubMed] [Google Scholar]
- Hawecker J., Lehn J. M. & Ziessel R. Electrocatalytic reduction of carbon dioxide mediated by Re(bipy)(CO)3Cl (bipy = 2,2′-bipyridine). J. Chem. Soc., Chem. Commun. 328–330 (1984). [Google Scholar]
- Machan C. W. et al. Supramolecular assembly promotes the electrocatalytic reduction of carbon dioxide by Re(I) bipyridine catalysts at a lower overpotential. J. Am. Chem. Soc. 136, 14598–14607 (2014). [DOI] [PubMed] [Google Scholar]
- Riplinger C., Sampson M. D., Ritzmann A. M., Kubiak C. P. & Carter E. A. Mechanistic contrasts between manganese and rhenium bipyridine electrocatalysts for the reduction of carbon dioxide. J. Am. Chem. Soc. 136, 16285–16298 (2014). [DOI] [PubMed] [Google Scholar]
- Vollmer M. V. et al. Synthesis, spectroscopy, and electrochemistry of (α-diimine)M(CO)3Br, M = Mn, Re, complexes: Ligands isoelectronic to bipyridyl show differences in CO2 reduction. Organometallics 34, 3–12 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K.-Y., Chung W.-H. & Lau C.-P. The effect of weak Brönsted acids on the electrocatalytic reduction of carbon dioxide by a rhenium tricarbonyl bipyridyl complex. J. Electroanal. Chem. 453, 161–170 (1998). [Google Scholar]
- Chen Z. et al. Electrocatalytic reduction of CO2 to CO by polypyridyl ruthenium complexes. Chem. Commun. 47, 12607–12609 (2011). [DOI] [PubMed] [Google Scholar]
- Tamaki Y., Morimoto T., Koike K. & Ishitani O. Photocatalytic CO2 reduction with high turnover frequency and selectivity of formic acid formation using Ru(II) multinuclear complexes. Proc. Natl. Acad. Sci. USA 109, 15673–15678 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsu H. & Tanaka K. An organic hydride transfer reaction of a ruthenium NAD model complex leading to carbon dioxide reduction. Angew. Chem. Int. Ed. 51, 9792–9795 (2012). [DOI] [PubMed] [Google Scholar]
- Tanaka K. Metal-catalyzed reversible conversion between chemical and electrical energy designed towards a sustainable society. Chem. Rec. 9, 169–186 (2009). [DOI] [PubMed] [Google Scholar]
- Kang P. et al. Selective electrocatalytic reduction of CO2 to formate by water-stable iridium dihydride pincer complexes. J. Am. Chem. Soc. 134, 5500–5503 (2012). [DOI] [PubMed] [Google Scholar]
- Chen Y., Li C. W. & Kanan M. W. Aqueous CO2 reduction at very low overpotential on oxide-derived Au nanoparticles. J. Am. Chem. Soc. 134, 19969–19972 (2012). [DOI] [PubMed] [Google Scholar]
- Zhu W. et al. Monodisperse Au nanoparticles for selective electrocatalytic reduction of CO2 to CO. J. Am. Chem. Soc. 135, 16833–16836 (2013). [DOI] [PubMed] [Google Scholar]
- Costentin C., Drouet S., Robert M. & Savéant J.-M. A local proton source enhances CO2 electroreduction to CO by a molecular Fe catalyst. Science 338, 90–94 (2012). [DOI] [PubMed] [Google Scholar]
- Costentin C., Drouet S., Passard G., Robert M. & Savéant J.-M. Proton-coupled electron transfer cleavage of heavy-atom bonds in electrocatalytic processes. Cleavage of a C-O bond in the catalyzed electrochemical reduction of CO2. J. Am. Chem. Soc. 135, 9023–9031 (2013). [DOI] [PubMed] [Google Scholar]
- Costentin C., Passard G., Robert M. & Savéant J.-M. Pendant acid–base groups in molecular catalysts: H-bond promoters or proton relays? mechanisms of the conversion of CO2 to CO by electrogenerated iron(0)porphyrins bearing prepositioned phenol functionalities. J. Am. Chem. Soc. 136, 11821–11829 (2014). [DOI] [PubMed] [Google Scholar]
- Costentin C., Passard G., Robert M. & Savéant J.-M. Ultraefficient homogeneous catalyst for the CO2-to-CO electrochemical conversion. Proc. Natl. Acad. Sci. USA 111, 14990–14994 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costentin C., Drouet S., Robert M. & Savéant J.-M. Turnover numbers, turnover frequencies, and overpotential in molecular catalysis of electrochemical reactions. Cyclic voltammetry and preparative-scale electrolysis. J. Am. Chem. Soc. 134, 11235–11242 (2012). [DOI] [PubMed] [Google Scholar]
- Beley M., Collin J. P., Ruppert R. & Sauvage J. P. Nickel(II)-cyclam: an extremely selective electrocatalyst for reduction of CO2 in water. J. Chem. Soc., Chem. Commun. 1315–1316 (1984). [Google Scholar]
- Beley M., Collin J. P., Ruppert R. & Sauvage J. P. Electrocatalytic reduction of carbon dioxide by by nickel cyclam2+ in water: study of the factors affecting the efficiency and the selectivity of the process. J. Am. Chem. Soc. 108, 7461–7467 (1986). [DOI] [PubMed] [Google Scholar]
- Froehlich J. D. & Kubiak C. P. Homogeneous CO2 reduction by Ni(cyclam) at a glassy carbon electrode. Inorg. Chem. 51, 3932–3934 (2012). [DOI] [PubMed] [Google Scholar]
- Bouttez M., Molton F., Chardon-Noblat S. & Deronzier A. [Mn(bipyridyl)(CO)3Br]: an abundant metal carbonyl complex as efficient electrocatalyst for CO2 reduction. Angew. Chem. Int. Ed. 50, 9903–9906 (2011). [DOI] [PubMed] [Google Scholar]
- Sampson M. D. et al. Manganese catalysts with bulky bipyridine ligands for the electrocatalytic reduction of carbon dioxide: eliminating dimerization and altering catalysis. J. Am. Chem. Soc. 136, 5460–5471 (2014). [DOI] [PubMed] [Google Scholar]
- Thoi V. S. & Chang C. J. Nickel N-heterocyclic carbene–pyridine complexes that exhibit selectivity for electrocatalytic reduction of carbon dioxide over water. Chem. Commun. 47, 6578–6580 (2011). [DOI] [PubMed] [Google Scholar]
- Appel A. M. et al. Frontiers, opportunities, and challenges in biochemical and chemical catalysis of CO2 fixation. Chem. Rev. 113, 6621–6658 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can M., Armstrong F. A. & Ragsdale S. W. Structure, function, and mechanism of the nickel metalloenzymes, CO dehydrogenase, and acetyl-CoA synthase. Chem. Rev. 114, 4149–4174 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeoung J. H., Fesseler J., Goetzl S. & Dobbek H. Carbon monoxide. toxic gas and fuel for anaerobes and aerobes: carbon monoxide dehydrogenases. In Metal Ions in Life Sciences Eds.: kroneck P. M. H. & Sosa Torres M. E., Springer, Heidelberg, 14, 37–69 (2014). [DOI] [PubMed] [Google Scholar]
- Ensign S. A. Reactivity of carbon monoxide dehydrogenase from rhodospirillum rubrum with carbon dioxide, carbonyl sulfide, and carbon disulfide. Biochemistry 34, 5372–5381 (1995). [DOI] [PubMed] [Google Scholar]
- Svetlitchnyi V., Peschel C., Acker G. & Meyer O. Two membrane-associated NiFeS-carbon monoxide dehydrogenases from the anaerobic carbon-monoxide-utilizing eubacterium Carboxydothermus hydrogenoformans. J. Bacteriol. 183, 5134–5144 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeoung J. H. & Dobbek H. Carbon dioxide activation at the Ni,Fe-cluster of anaerobic carbon monoxide dehydrogenase. Science 318, 1461–1464 (2007). [DOI] [PubMed] [Google Scholar]
- Fesseler J., Jeoung J.-H. & Dobbek H. How the [NiFe4S4] cluster of CO dehydrogenase activates CO2 and NCO. Angew. Chem. Int. Ed. 54, 8560–8564 (2015). [DOI] [PubMed] [Google Scholar]
- Collin J.-P., Jouaiti A. & Sauvage J.-P. Electrocatalytic properties of (tetraazacyclotetradecane)nickel(2+) and Ni2(biscyclam)4+ with respect to carbon dioxide and water reduction. Inorg. Chem. 27, 1986–1990 (1988). [Google Scholar]
- de Alwis C. et al. Cyclic voltammetry study of the electrocatalysis of carbon dioxide reduction by bis(polyazamacrocyclic) nickel complexes. Electrochimica Acta 45, 2061–2074 (2000). [Google Scholar]
- Simon-Manso E. & Kubiak C. P. Dinuclear nickel complexes as catalysts for electrochemical reduction of carbon dioxide. Organomettalics 24, 96–102 (2005). [Google Scholar]
- Haines R. J., Wittrig R. E. & Kubiak C. P. Electrocatalytic reduction of carbon dioxide by the binuclear copper complex [Cu2(6-(diphenylphosphino-2,2′-bipyridyl)2(MeCN)2][PF6]2. Inorg. Chem. 33, 4723–4728 (1994). [Google Scholar]
- Cheng S. C., Blaine C. A., Hill M. G. & Mann K. R. Electrochemical and IR spectroelectrochemical studies of the electrocatalytic reduction of carbon dioxide by [Ir2(dimen)4]2+ (dimen = 1,8-Diisocyanomenthane). Inorg. Chem. 35, 7704–7708 (1996). [Google Scholar]
- Raebiger J. W. et al. Electrochemical reduction of CO2 to CO catalyzed by a bimetallic palladium complex. Organomettalics 54, 3345–3351 (2006). [Google Scholar]
- Ratliff K. S., Lentz R. E. & Kubiak C. P. Carbon dioxide chemistry of the trinuclear complex [Ni3(μ3-CNMe)(μ3-I)(dppm)3][PF6]. Electrocatalytic reduction of carbon dioxide. Organometallics 11, 1986–1988 (1992). [Google Scholar]
- Lee E. Y., Hong D., Park W. H. & Suh M. P. Synthesis, properties, and reactions of trinuclear macrocyclic nickel(II) and nickel(I) Complexes: electrocatalytic reduction of CO2 by nickel(II) Complex. Eur. J. Inorg. Chem. 3242–3249 (2003). [Google Scholar]
- Rail M. D. & Berben L. A. Directing the reactivity of [HFe4N(CO)12]− toward H+ or CO2 reduction by understanding the electrocatalytic mechanism. J. Am. Chem. Soc. 133, 18577–18579 (2011). [DOI] [PubMed] [Google Scholar]
- Tezuka M. et al. Electroreduction of carbon dioxide catalyzed by iron-sulfur cluster compounds [Fe4S4(SR)4]2−. J. Am. Chem. Soc. 104, 6834–6836 (1982). [Google Scholar]
- Nakazawa M. et al. Electrochemical reduction of carbon dioxide using iron–sulfur clusters as catalyst precursors. Bull. Chem. Soc. Jpn., 59, 809–814 (1986). [Google Scholar]
- Yuhas B. D., Prasittichai C., Hupp J. T. & Kanatzidis M. G. Enhanced electrocatalytic reduction of CO2 with ternary Ni-Fe4S4 and Co-Fe4S4-based biomimetic chalcogels. J. Am. Chem. Soc. 133, 15854–15857 (2011). [DOI] [PubMed] [Google Scholar]
- Mohamed E. A., Zahran Z. N. & Naruta Y. Efficient electrocatalytic CO2 reduction with a molecular cofacial iron porphyrin dimer. Chem. Commun. 51, 16900–16903 (2015). [DOI] [PubMed] [Google Scholar]
- Andrieux C. P. & Savéant J. M. Electrochemical reactions, In Investigation of Rates and Mechanisms of Reactions, Bernasconi C. F. Ed. Techniques of Chemistry, Vol. 6, 41E, Part 2, pp. 305–390, Wiley: New York (1986). [Google Scholar]
- Leitner W. The coordination chemistry of carbon dioxide and its relevance for catalysis: a critical survey. Coord. Chem. Rev. 153, 257–284 (1996). [Google Scholar]
- Naruta Y., Sasayama M.-A. & Sasaki T. Oxygen Evolution by Oxidation of Water with Manganese Porphyrin Dimers. Angew. Chem. Int. Ed. 33, 1839–1841 (1994). [Google Scholar]
- Shimazaki Y. et al. Characterization of a dinuclear MnV = O complex and its efficient evolution of O2 in the presence of water. Angew. Chem. Int. Ed. 43, 98–99 (2004). [DOI] [PubMed] [Google Scholar]
- Naruta Y. & Sasayama M. Importance of Mn–Mn separation and their relative arrangement on the development of high catalase activity in manganese porphyrin dimer catalysts. J. Chem. Soc., Chem. Commun. 2667–2668 (1994). [Google Scholar]
- Osuka A., Nakajima S., Nagata T., Maruyama K. & Toriumi K. A 1,2-Phenylene-Bridged Porphyrin Dimer-Synthesis, Properties, and Molecular Structure. Angew. Chem. Int. Ed. 30, 582–584 (1991). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.