Abstract
We sought to determine if vitamin D supplementation, to target 25(OH)D concentrations of 30–40 ng/mL, improves endothelial function in Singapore’s multi-ethnic type 2 diabetes mellitus population. We randomised 64 type 2 diabetes mellitus patients with hypovitaminosis D to cholecalciferol 4000 International Unit/matching placebo [baseline 25(OH)D < 20 ng/mL] or cholecalciferol 2000 International Unit/matching placebo [baseline 25(OH)D: 20–30 ng/mL] daily for 16 weeks with a down titration at 8 weeks if 25(OH)D > 30 ng/mL. Endothelial function was assessed by peripheral tonometry (reactive hyperaemia index–endothelial peripheral arterial tonometry) and vascular biomarkers: E-selectin, von-Willebrand factor and high-sensitivity C-reactive protein. We compared the change from baseline parameters in the two groups using Student’s t-test or Kruskal–Wallis test. A log-normal multivariate regression analysis was used to adjust for relevant baseline variables. The median reactive hyperaemia index in the vitamin D group increased from 0.65 (interquartile range: 0.42) to 0.73 (interquartile range: 0.36), whereas it decreased from 0.73 (interquartile range: 0.65) to 0.65 (interquartile range: 0.38) (p = 0.02) in the placebo group. After adjustment for baseline variables, the change was not statistically significant for reactive hyperaemia index (p = 0.07) and for other vascular biomarkers (p > 0.05). Targeted vitamin D supplementation for 16 weeks resulted in a small but non-significant improvement in endothelial function in a type 2 diabetes mellitus cohort.
Keywords: Vitamin D, endothelial function, cholecalciferol, type 2 diabetes
Introduction
Patients with type 2 diabetes mellitus (T2DM) bear a disproportionately high burden for all cardiovascular events, and it has been seen that T2DM patients without a history of prior myocardial infarction have the same risk of cardiovascular deaths as non-diabetic subjects with a history of prior myocardial infarction.1 Hence, T2DM is considered a ‘coronary artery disease equivalent’ in terms of cardiovascular risk.1,2 Strict glycaemic control is associated with a significant reduction in microvascular complications, but it does not lead to a clear reduction in macrovascular complications.3,4 Hence, there is an unmet need to identify new therapeutic targets to reduce these complications.
Atherosclerosis classically starts as endothelial activation, and endothelial dysfunction has been postulated to be the ultimate common pathway by which multiple risk factors lead to vascular complications.5 The quantitative assessment of endothelial function (EF) by peripheral tonometry [reactive hyperaemia index (RHI)–endothelial peripheral arterial tonometry (EndoPAT)] is a powerful surrogate marker for cardiovascular health showing good correlations with the Framingham Risk Score,6 and is associated with late cardiovascular adverse effects such as cardiac death, myocardial infarction, revascularisation or cardiac hospitalisation.7 EF can also be assessed by vascular biomarkers such as von-Willebrand factor (vWF) and E-selectin.8 The multimeric glycoprotein, vWF, is produced almost exclusively by endothelial cells, and plasma levels are raised in different stages of endothelial damage9 so it is considered to be a useful marker. E-selectin is a group of adhesion molecules whose main role is the tethering of leukocytes to the endothelium. Plasma levels of soluble E-selectin are also considered to be markers of endothelial activation and raised levels are found in hypertension, diabetes and hyperlipidemia.10
Vitamin D protects the endothelium from the effects of advanced glycation end products seen in diabetes mellitus by reducing oxidative stress, modulating and attenuating the inflammatory response, attenuating platelet activation and reducing the expression of vascular adhesion markers on the endothelium as seen in in vitro and in vivo studies.11 Vitamin D is therefore essential in the maintenance of the quiescent endothelium, in the reversal of the activated endothelium and in repair of the damaged endothelium.11 Hypovitaminosis D has been associated with cardiovascular risk and endothelial dysfunction in patients with T2DM.11 Many randomised controlled trials (RCTs) have evaluated the effects of vitamin D supplementation on EF as reviewed in Dalan et al.11 However, all these studies have used a fixed dose of cholecalciferol11 and none used a dose titration regime to target 25-hydroxyvitamin D [25(OH)D] concentrations. We evaluated the effect of 16 weeks of cholecalciferol supplementation in a safe and effective dose on EF in a multi-ethnic group of patients with T2DM and hypovitaminosis D by targeting 25(OH)D concentrations to 30–40 ng/mL at a tertiary hospital in Singapore.
Methods
Study population and setting
This study was conducted in a single tertiary centre in Singapore. Singapore is a tropical country and lies 137 km (1 degree) north of the equator. It has a tropical rainforest climate with no distinctive seasons and uniform temperature, and hours of daylight throughout the year. Between 17 October 2012 and 28 January 2014, we screened 104 consecutive patients with T2DM visiting the Diabetes and Endocrinology Clinic for hypovitaminosis D [25(OH)D < 30 ng/mL (75 nmol/L)]. The inclusion criteria were T2DM, glycated haemoglobin (HbA1c) of 6.0%–10.0%, hypovitaminosis D, and stable medications for glycaemic, blood pressure and lipid control. The exclusion criteria were baseline 25(OH)D > 30 ng/mL (75 nmol/L), HbA1c > 10.0%, eGFR<30mL/min/1.73m2 hypercalcaemia (Ca > 2.60 mmol/L), liver cirrhosis or transaminitis, sarcoidosis, renal calculi, malignancy, those on current treatment for tuberculosis, pregnancy or lactation, concomitant medications (including glucocorticoids, anti-retroviral agents, orlistat), bariatric surgery procedure or malabsorption disorders and any clinical indication for vitamin D supplementation, such as baseline bone mineral density (BMD), T-score of ⩽2.5 standard deviation (SD). Written informed consent was obtained from all patients. Ethics approval was obtained from the local Institutional Review Board [Domain Specific Review Board (DSRB Ref: 2012/00296)]. The study was conducted according to the principles of the Declaration of Helsinki. Clinical trials authorisation was obtained from the national regulator (HPRG/CTB 78:10/12-079). The trial was registered at clinicaltrials.gov, NCT01741181.
Study design and randomisation
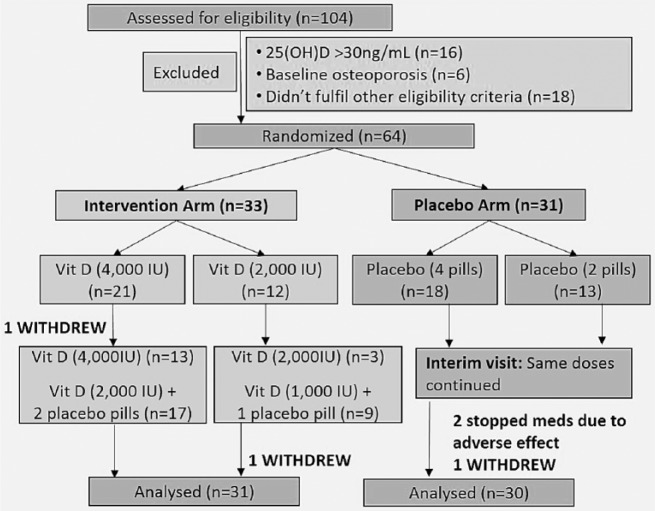
The study was a randomised, double-blind, placebo-controlled, parallel group trial. Figure 1 shows the patient participation flow chart: 104 patients were screened and 64 were randomly allocated into blocks of six to cholecalciferol or placebo. Patients were randomised in a 1:1 allocation ratio using a computer-generated random number sequence and allocated to either the vitamin D or placebo group using a centralised interactive password-protected web-based service, to ensure allocation concealment of patient enrollers. The study team and patients were blinded using placebo tablets (excipient without active ingredient) that appeared identical to the active tablets [each tablet 1000 International Unit (IU)]. Both types of tablets were manufactured, labelled and blinded in the same run in blocks of six, by D3 Pharmacy, Denmark (DK-9000 Aalborg), and imported by OneNine57 Pte Ltd. If the patient’s baseline 25(OH)D was ⩽20 ng/mL (50 nmol/L), they were started on four tablets of cholecalciferol (4000 IU) or matching placebo daily. If the baseline 25(OH)D was 21–30 ng/mL (51–75 nmol/L), they were started on two tablets of cholecalciferol (2000 IU) or matching placebo daily. All the patients had 25(OH)D concentrations measured at 8 weeks (interim visit). These were reviewed by an independent clinical research associate and the study dose was adjusted to half the initial dose (using matching placebo pills) if 25(OH)D was >30 ng/mL (75 nmol/L). Compliance was assessed by the percentage of prescribed pills ingested. A compliance rate of more than 80% was considered satisfactory. The trial ended upon completion of recruitment of 64 patients allowing for dropouts. The last patient was recruited on 28 January 2014 and completed follow-up on 20 May 2014.
Figure 1.
Flowchart for subject participation.
Measurements
The blinded study team performed all outcome measurements. At the initial and final visits, fasting venous blood was obtained from each patient to measure 25(OH)D, parathyroid hormone (PTH), HbA1c, total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides, high-sensitivity C-reactive protein (hsCRP), E-selectin and vWF. Clinical characteristics including body height, weight, waist and hip circumferences, and systolic and diastolic blood pressures (BPs) were measured. Data on concurrent complications, medications and clinical history were ascertained from the patient’s medical records or were self-reported. In addition, EF was measured by peripheral tonometry at the baseline and final visits. Fasting serum samples for 25(OH)D concentrations were measured on the same day by electro-chemiluminescence immunoassay (ECLIA). The lower limit of detection was 8 ng/mL (20 nmol/L) and patients with levels below 8 ng/mL (20 nmol/L) were assigned value of 8 ng/mL (20 nmol/L). Hypovitaminosis D was defined at 25(OH)D < 30 ng/mL. Bioactive intact PTH (iPTH) was measured by ECLIA. The reference range was 0.8–6.8 pmol/L. Calcium was measured by ion-selective electrode method and albumin by bromocresol purple. Adjusted serum calcium was calculated for each patient. The reference range for adjusted calcium was 2.15–2.58 mmol/L.
Soluble vascular markers, vWF and E-selectin were measured at baseline and at 16 weeks after intervention. E-selectin was measured by enzyme-linked immunosorbent assay (ELISA, IBL international kit). vWF was measured by immunoturbidometric assay (STA-Liatest vWF). HbA1c, total cholesterol, LDL-C, HDL-C, triglycerides, hsCRP and calcium concentrations were measured real time at baseline and at 16 weeks by a commercial laboratory.
The primary outcome measure was pre-specified as the change in EF between baseline and 16 weeks as quantified by the RHI. We used the EndoPAT 2000 (Itamar Medical Ltd, Israel) to measure the RHI. These measurements were performed at the same time as phlebotomy for fasting blood sampling. Patients were rested in a quiet room in a supine position at room temperature before initiation of the procedures. Two flexible probes were placed on the index fingers of the right (ischaemic) and left (control) hands. Measurements were made at baseline (6 min), occlusion (5 min) and reactive hyperaemia (5 min) phases. The EndoPAT software calculated the RHI as the ratio of the average pulse wave amplitude during baseline in the occluded arm divided by the same value in the control arm and then multiplied by the baseline correction factor.6,7 Augmentation index (AIx), defined as the difference between the first and second peaks of the arterial waveform expressed as a percentage of pulse pressure, in the EndoPAT is calculated from baseline resting pulse wave. It represents the relative contribution of augmented pressure due to wave reflection to the pressure waveform. As AIx is inversely related to heart rate, values were adjusted to represent arterial stiffness at a heart rate of 75 beats per minute.12 The EndoPAT derived augmentation index is known to correlate with radial artery tonometry.13
Statistical analyses
The primary endpoint was change from baseline to 16 weeks in RHI. The secondary endpoints were the change from baseline to 16 weeks in E-selectin, vWF, hsCRP and metabolic parameters including lipid profile and glycaemic control.
The required sample size of 60 patients (30 per intervention group) was based on the following assumptions: mean baseline RHI of 1.33 ± 0.08 (n = 20) in T2DM patients,14 1:1 allocation ratio, 5% type I error, 90% power of detecting an expected change in mean RHI after intervention of 0.4 units (30%) (SD = 0.4) using a two-sample t-test with equal variance and a dropout rate of 25%. Based on a previous study15 wherein a change in RHI of 0.38 ± 0.29 was seen, we used the expected change in mean RHI of 0.4 units. All analyses were performed based on the intention-to-treat principle. Data were summarised by intervention groups using mean (SD) or median [interquartile range (IQR)] for continuous variables as appropriate and frequency (proportions) for categorical variables. Intervention groups were compared using two-sample t-test and Mann–Whitney test for continuous variables and Fisher’s exact test for categorical variables. A log-normal multivariate regression analysis was also done to accommodate skewed outcomes and to adjust for relevant baseline variables. Significance level was set at 5% and all tests were two-sided. SAS version 9.3 software (SAS Institute, Cary, North Carolina) was used for the analyses.
Results
Of the 64 patients randomised, 39 patients (18 in the placebo group and 21 in the vitamin D group) had 25(OH)D < 20 ng/mL (50 nmol/L) (Figure 1). In the intervention arm, 21 patients were started on cholecalciferol 4000 IU and 12 patients were started on 2000 IU. At the 8-week visit, in the intervention arm, 16 patients (49%) had their dose reduced by half. No change was made in the placebo arm. A total of 61 patients completed the final follow-up at 16 weeks. The overall median compliance rate was 93% (IQR: 83–99) in the vitamin D group and 95% (IQR: 86–99) in the placebo group. In the vitamin D group, 70% attained 25(OH)D ⩾ 30 ng/mL (75 nmol/L), whereas in the placebo group, only one patient achieved the target 25(OH)D ⩾ 30 ng/mL (75 nmol/L).
The baseline characteristics of the study population are shown in Table 1. The mean age was 53.5 ± 9.5 years and 52% were male. Approximately 10% had a history of ischaemic heart disease (IHD) and 2% had a history of stroke. There was no significant difference in age, gender, ethnicity, body mass index (BMI), BPs, prevalence of IHD, stroke, use of oral hypoglycaemic agents, prandial insulin, statins, angiotensin-converting-enzyme (ACE)-inhibitors, calcium-channel blockers, beta blockers and diuretics, HbA1c and serum creatinine concentrations, between the two treatment groups (p > 0.05). Furthermore, baseline 25(OH)D concentrations, iPTH, lipid profile, hsCRP, vWF, E-selectin and RHI were similar between the two groups (p > 0.05). However, mean systolic BP in placebo group was 134.2 mmHg (19.4) when compared to vitamin D group of 143.8 mmHg (16.6) (p = 0.04). This is considered significant as this crosses the threshold for systolic hypertension at 140 mmHg, hence we adjusted for this in the multivariate analysis. The use of basal insulin and angiotensin receptor blockers (ARBs) were higher in the placebo group (p = 0.05). The use of basal insulin has been associated positively with EF,16 hence we adjusted for this in the multivariate analysis. The baseline RHI was slightly higher in the placebo group when compared to the vitamin D group; although this difference was not statistically significant, we adjusted for baseline RHI as well in the final analysis.
Table 1.
Baseline characteristics of randomised participants.
| Characteristics | Vitamin D group (n = 33) | Placebo group (n = 31) | p-value |
|---|---|---|---|
| Age (years), mean (SD) | 52.2 (8.2) | 54.8 (10.8) | 0.28a |
| Male, n (%) | 14 (42.4) | 19 (61.3) | 0.14b |
| Ethnicity, n (%) | 0.87b | ||
| 1. Chinese | 19 (57.6) | 16 (51.6) | |
| 2. Malay | 3 (9.1) | 4 (12.9) | |
| 3. Indian | 11 (33.3) | 10 (32.3) | |
| 4. Others | 0 (0) | 1 (3.2) | |
| BMI, mean (SD) | 27.3 (5.8) | 28.9 (6.0) | 0.26a |
| IHD, no. (%) | 2 (6.1) | 5 (16.1) | 0.25b |
| Stroke or TIA (%) | 1 (3) | 0 | 1.00b |
| Sulfonylureas, no. (%) | 17 (51.5) | 12 (38.7) | 0.33b |
| Metformin, no. (%) | 32 (97) | 29 (93.5) | 0.61b |
| Basal insulin, no. (%) | 6 (18.2) | 13 (41.9) | 0.05b |
| Prandial insulin, no. (%) | 6 (18.2) | 6 (19.4) | 1.00b |
| Mixed regimes insulin, no. (%) | 4 (12.1) | 3 (9.7) | 1.00b |
| DPP-IV inhibitors, no. (%) | 3 (9.1) | 3 (9.7) | 1.00b |
| ACE-inhibitors, no. (%) | 20 (60.6) | 16 (51.6) | 0.61b |
| ARBs, no. (%) | 5 (15.2) | 12 (38.7) | 0.05b |
| Ca-channel blockers, no. (%) | 9 (27.3) | 13 (41.9) | 0.29b |
| Beta blockers, no. (%) | 5 (15.2) | 9 (29.0) | 0.23b |
| Diuretics, no. (%) | 3 (9.1) | 5 (16.1) | 0.47b |
| Statins, no. (%) | 29 (87.9) | 26 (83.9) | 0.73b |
| 25(OH)D (ng/mL), median (IQR) | 18.0 (7.0) | 17.0 (11.0) | 0.44c |
| 25(OH)D (nmol/L), median (IQR) | 45.0 (17.5) | 42.5 (27.5) | |
| PTH (pg/mL), mean (SD) | 4.6 (1.9) | 4.1 (1.8) | 0.29a |
| Serum adjusted Ca (mmol/L), mean (SD) | 2.41 (0.10) | 2.40 (0.09) | 0.51a |
| Creatinine (μmol) mean (SD) | 77.7 (24.7) | 81.0 (30.8) | 0.63a |
| Systolic BP (mmHg) | 134.2 (19.4) | 143.8 (16.6) | 0.04a |
| Diastolic BP (mmHg) | 75.5 (10.1) | 78.7 (10.4) | 0.22a |
| RHI, median (IQR) | 0.65 (0.42) | 0.73 (0.52) | 0.34a |
| AIx, median (IQR) | 0.20 (0.27) | 0.23 (0.34) | 0.65c |
| HbA1c, mean (SD) (%) | 7.9 (1.2) | 8.0 (1.0) | 0.80a |
| Total cholesterol (mmol/L) | 4.6 (1.2) | 4.5 (1.5) | 0.71a |
| LDL-C (mmol/L) | 2.7 (1.0) | 2.3 (0.5) | 0.07a |
| HDL-C (mmol/L) | 1.12 (0.27) | 1.14 (0.24) | 0.79a |
| Triglycerides (mmol/L) | 1.80 (1.23) | 2.36 (3.04) | 0.32a |
| hsCRP, median (IQR) | 1.6 (2.1) | 1.8 (3.2) | 0.85c |
| E-selectin, mean (SD) | 51.9 (23.9) | 49.1 (23) | 0.63a |
| v-WF, median | 124.3 (62.2) | 124.7 (55.4) | 0.98a |
SD: standard deviation; BMI: body mass index; IHD: ischaemic heart disease; TIA: transient ischaemic attack; ACE: angiotensin-converting-enzyme; ARBs: angiotensin receptor blockers; 25(OH)D: 25-hydroxyvitamin D; IQR: interquartile range; PTH: parathyroid hormone; BP: blood pressure; RHI: reactive hyperaemia index; AIx: augmentation index; HbA1c: glycated haemoglobin; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; hsCRP: high-sensitivity C-reactive protein; vWF: von-Willebrand factor.
Student’s t-test.
Fisher’s exact test.
Mann–Whitney test.
Effects of vitamin D supplementation
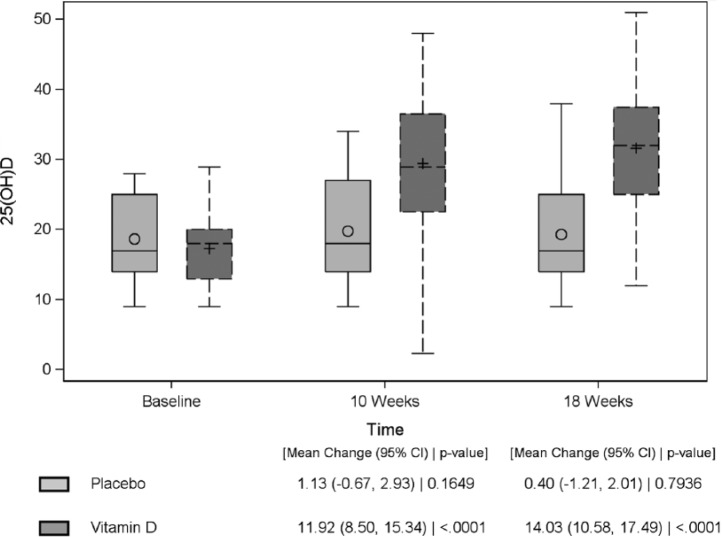
In the intervention group, the mean 25(OH)D at baseline was 17.3 ± 5.2 ng/mL, mean adjusted calcium was 2.41 ± 0.10 mmol/L and iPTH was 4.6 ± 1.9 mmol/L. At the 8-week visit, the mean 25(OH)D was 31.3 ± 9.0 ng/mL (change of 14.0 ng/mL; p < 0.0001). No patient developed hypercalcaemia. At 8 weeks, 16 out of 33 [49%; 95% confidence interval (CI): 33%–65%] patients achieved 25(OH)D > 30 ng/mL (75 nmol/L) and had a dose reduction to half their initial dose at the interim visit (Figure 1). At the final 16-week visit, the mean 25(OH)D was 31.6 ± 9.5 ng/mL (change of 14.3 ng/mL; p < 0.0001), mean adjusted calcium was 2.41 ± 0.10 mmol/L and iPTH was 4.5 ± 2.1 mmol/L (Figure 2). At 16 weeks, 23 out of 33 (70%; 95% CI: 53%–83%) patients achieved 25(OH)D > 30 ng/mL (75 nmol/L).
Figure 2.
Box plot of 25(OH)D concentrations (ng/mL) in the vitamin D and placebo group over time.
The change from baseline in RHI was significantly different between the intervention group and the placebo group in the unadjusted analysis. Median RHI increased from 0.65 (IQR: 0.42) to 0.73 (IQR: 0.36) [median difference of 0.09 (IQR: 0.25)] in the vitamin D group, whereas the median RHI decreased from 0.73 (IQR: 0.65) to 0.65 (IQR: 0.38) [median difference of −0.09 (IQR: 0.24) (p = 0.02)] in the placebo group (Table 2). However, after adjustment for baseline variables (systolic BP, baseline RHI and use of basal insulin), the difference was not significant (p = 0.07) (Table 3). Changes in vWF, E-selectin and hsCRP were not significant (p > 0.05). Vitamin D supplementation did not have a significant impact on BMI, HbA1c or lipid profile as well. Despite a significant increase in 25(OH)D concentration, there was no significant impact on calcium and iPTH concentrations (Table 2). After adjustments, the AIx, a marker of arterial stiffness as measured by the EndoPAT, increased significantly in the intervention group [median change of 0.01 (IQR: 0.18)] in comparison to the control group [median decrease of 0.05 (IQR: 0.15)] (p = 0.02) (Table 3).
Table 2.
Treatment effect of 16 weeks in vascular parameters and metabolic profile.
| Characteristics | Intervention arm, pre- and post-treatment levels | Intervention arm, change between final and baseline values | Placebo arm, pre- and post-treatment levels | Placebo arm, change between final and baseline values | Unadjusted analyses (Student’s t-test or Kruskal–Wallis test), p-value |
|---|---|---|---|---|---|
| 25(OH)D (ng/mL), mean | 17.3–31.6 | 14.0 ± 9.6 | 18.6–19.3 | 0.4 ± 4.3 | <0.0001a |
| 25(OH)D (nmol/L), median | 43.25–79.0 | 35.0 ± 24 | 46.5–48.25 | 1 ± 10.75 | |
| RHI, median | 0.65–0.73 | 0.09 (IQR: 0.25) | 0.73–0.65 | −0.09 (IQR: 0.24) | 0.02b |
| % change in RHI, median | – | 16.62 (IQR: 48.02) | – | −14.21 (IQR: 16.62) | 0.01b |
| AIx, median | 0.20–0.21 | 0.01 (IQR: 0.18) | 0.23–0.25 | −0.05 (IQR: 0.15) | 0.07b |
| hsCRP, median | 1.6–1.9 | 0.2 (IQR: 0.8) | 1.8–2.3 | 0.1 (IQR: 1.8) | 0.62b |
| vWF, mean | 124.3–130.2 | 6.0 ± 23.5 | 124.7–128.7 | 4.0 ± 17.8 | 0.70a |
| E-selectin, mean | 51.9–50.7 | −1.4 ± 19.6 | 49.1–42.0 | −6.1 ± 16.5 | 0.32a |
| BMI (kg/m2) | 27.3–27.7 | 0.3 ± 1.4 | 28.9–28.5 | −0.1 ± 2.5 | 0.45a |
| Systolic BP (mmHg), mean | 134.2–134.6 | 0.0 ± 20.1 | 143.8–136 | −6.9 ± 15.9 | 0.12a |
| Diastolic BP (mmHg), mean | 75.5–75.5 | 0.2 ± 9.2 | 78.7–74.3 | −3.7 ± 11.7 | 0.15a |
| HbA1c (%) | 7.9–8.0 | 0.1 ± 0.8 | 8.0–8.0 | 0.1 ± 1.0 | 0.92a |
| Total cholesterol (mmol/L) | 4.6–4.8 | 0.2 ± 0.5 | 4.5–4.3 | −0.2 ± 1.1 | 0.04a |
| HDL-C (mmol/L) | 1.12–1.15 | 0.02 ± 0.36 | 1.14–1.21 | 0.07 ± 0.50 | 0.62a |
| LDL-C (mmol/L) | 2.7–2.7 | 0.1 ± 0.5 | 2.3–2.2 | −0.1 ± 0.6 | 0.23a |
| Triglycerides (mmol/L) | 1.80–2.11 | 0.36 ± 0.90 | 2.36–2.01 | −0.33 ± 1.83 | 0.06a |
| Calcium adjusted (mmol/L) | 2.41–2.41 | −0.01 ± 0.11 | 2.40–2.37 | −0.03 ± 0.10 | 0.44a |
| PTH (pmol/L) | 4.6–4.5 | −0.1 ± 1.7 | 4.1–3.9 | −0.2 ± 1.3 | 0.49a |
25(OH)D: 25-hydroxyvitamin D; RHI: reactive hyperaemia index; IQR: interquartile range; AIx: augmentation index; hsCRP: high-sensitivity C-reactive protein; vWF: von-Willebrand factor; BMI: body mass index; BP: blood pressure; HbA1c: glycated haemoglobin; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; PTH: parathyroid hormone.
Student’s t-test.
Mann–Whitney test.
Table 3.
Multivariate adjusted analysis (ANCOVA) of 16 weeks’ treatment effect for change in vascular parameters and metabolic profile.
| Characteristics | Estimate (standard error) | p-value |
|---|---|---|
| 1. Reactive hyperaemia index (RHI) | 0.12 | 0.07 |
| Treatment effect | 0.12 (0.07) | 0.07 |
| Basal insulin | 0.03 (0.07) | 0.66 |
| Systolic blood pressure | 0.00 (0.00) | 0.84 |
| Baseline RHI | −0.57 (0.11) | <0.0001 |
| 2. Augmentation index (Aix) | 0.20 | 0.02 |
| Treatment effect | 0.20 (0.09) | 0.02 |
| Basal insulin | 0.09 (0.09) | 0.33 |
| Systolic blood pressure | 0.01 (0.00) | 0.01 |
| Baseline AIx | −1.21 (0.25) | <0.0001 |
| 3. hsCRP | −0.21 | 0.20 |
| Treatment effect | −0.21 (0.16) | 0.20 |
| Basal insulin | −0.21 (0.23) | 0.37 |
| Systolic blood pressure | −0.00 (0.00) | 0.49 |
| Baseline hsCRP | 0.00 (0.02) | 0.85 |
| 4. vWF | −0.00 | 0.10 |
| Treatment effect | 0.00 (0.08) | 0.10 |
| Basal insulin | −0.18 (0.10) | 0.06 |
| Systolic blood pressure | 0.00 (0.00) | 0.93 |
| Baseline vWF | −0.00 (0.00) | 0.41 |
| 5. E-selectin | 0.09 | 0.11 |
| Treatment effect | 0.09 (0.06) | 0.11 |
| Basal insulin | −0.12 (0.07) | 0.10 |
| Systolic blood pressure | −0.00 (0.00) | 0.58 |
| Baseline E-selectin | −0.01 (0.00) | <0.0001 |
ANCOVA: analyses of covariance; hsCRP: high-sensitivity C-reactive protein; vWF: von-Willebrand factor.
Discussion
This study shows a high prevalence of hypovitaminosis D in this subset of T2DM population of Singapore. Only 16 of the 104 (15%) patients screened had 25(OH)D concentrations of >30 ng/mL. This was significantly higher than another study conducted in Singapore among the healthy middle aged and elderly population, wherein approximately 32% of the patients had 25(OH)D > 75 nmol/L (30 ng/mL).17 Hypovitaminosis D has been associated with T2DM and an inverse association between 25(OH)D concentrations and incident T2DM has been reported in diverse populations.18 The increased prevalence in the T2DM population may be partly explained by this association although a cause and effect relationship remains to be proven.
The target approach taken in this study was found to be safe; daily oral supplementation of cholecalciferol at 2000–4000 IU was safe and effective in improving the vitamin D status in T2DM patients with no significant adverse effect or sign of hypercalcaemia.
We found that after 16 weeks of cholecalciferol supplementation, T2DM patients revealed a non-significant improvement in vascular function parameters despite a significant change in vitamin D status and increase in 25(OH)D concentrations. Vitamin D treatment also did not have a significant effect on traditional cardiovascular risk factors including BP, lipid profile and glycaemic control.
Several RCTs have been conducted in the last decade to evaluate the effects of vitamin D supplementation on EF.11 In a similar trial done in Hong Kong in patients with T2DM, no significant improvement was seen in EF after a fixed dose supplementation of 5000 IU daily.19 In another RCT using a single dose of 100,000 vitamin D2, there was an improvement in EF by 2.5% as measured by brachial artery flow mediated dilatation at 8 weeks.20 Recent studies have been done in diverse populations including patients with a recent episode of myocardial infarction, patients undergoing coronary angiogram studies for IHD, patients with T2DM, hypertension and patients with underlying HIV infection. All have failed to show an improvement in EF in the short term. The mode of vitamin D supplementation was different in all these studies, ranging from regular replacements with vitamin D3 at 3000–5000 IU daily to single or multiple large doses of vitamin D3 (100,000 IU).11 The duration of treatment also ranged from 12 to 16 weeks in most trials, with only two trials lasting for 6 months to a year. It has recently been suggested that there is a reverse J-shaped association between serum 25(OH)D concentrations and cardiovascular risk and mortality, with the lowest risk being at 20.0–40.0 ng/mL (50–100 nmol/L). This is conjectured to be due to increased calcium absorption, which in high concentrations may accelerate intravascular calcification.21 In three studies done in healthy adults of different ethnic groups with hypovitaminosis D, replacement of vitamin D to target levels did show a small but significant improvement in EF.15,22,23
In this study, a targeted approach was specifically designed to keep 25(OH)D concentrations <40 ng/mL (100 nmol/L). Although in the original unadjusted analyses a significant improvement was seen, adjustments for baseline RHI, systolic BP and basal insulin led to non-significant results.
The exact physiological reason for the lack of a positive effect in our study and in several other short term studies mentioned above is not known. In our study, we observed a significant increase in AIx in the vitamin D group although there was no significant change in serum calcium concentrations. Even if there was a small increment in tissue calcium concentrations, it is unlikely to have led to a significant effect in the short duration of 16 weeks. Moreover, it has been seen that in patients with diabetes, AIx is not correlated to arterial compliance and cannot be used as an indicator for systemic arterial stiffness.24 The acute effects of vitamin D supplementation on the vascular dynamics need to be studied further.
Vitamin D supplementation might not improve EF in patients who have relatively advanced diabetes mellitus and significant endothelial dysfunction as was the case in this patient group. Perhaps earlier intervention in patients with impaired glucose tolerance or early diabetes might have a greater impact.
The intervention period of 16 weeks might be too short. A significant difference was seen in the unadjusted analysis. Hence, it is possible that if the study was continued for a longer duration, a significant difference in RHI between the placebo and the intervention arms would have been observed. In an open label study of 42 healthy adults with vitamin D insufficiency, targeted replacement with an aim to achieve 25(OH)D ⩾ 30 ng/mL for a period of 6 months did result in a significant increase in RHI (0.38 ± 0.14; p = 0.009).15 Most other studies in the literature using duration of 6 months to 1 year have not used daily oral supplementation as the mode of vitamin D replacement.11
Vitamin D deficiency might be exacerbated in patients with low vitamin D binding protein concentrations leading to a greater bioavailability at lower 25(OH)D concentrations.25 Vitamin D binding proteins concentrations are largely determined by the ethnicity and genotype. We did not measure vitamin D binding proteins or perform genotyping in our study. In our study, there was no observable difference in effect between ethnic groups.
Finally, only some surrogate biomarkers of vascular function and cardiovascular diseases were studied. A more comprehensive assessment of vascular function and cardiovascular outcomes might demonstrate some benefits.
Our study showed that short term vitamin D supplementation leads to a small and almost significant (p = 0.07) improvement in vascular function in patients with T2DM. If vitamin D concentrations are maintained for a longer duration this may translate into a clinically significant reduction in cardiovascular risk. This indicates the need for large scale, long duration trials with vitamin D supplementation in patients with T2DM to clarify the effects of vitamin D supplementation on cardiovascular health. Future trials need to incorporate baseline levels of vitamin D, the effect of vitamin D metabolism and genetic polymorphisms on the biological effects as well as longer follow-up periods.
Acknowledgments
The authors are grateful to the following people: Drs CK Chew, WH Hoi, HT Khor, M Jong, YC Kon, CJ Seow, A Shakoor and S Lam for helping in patient recruitment; clinical coordinators Ms Jiao Hong Li and Ms Xu Weiru, and independent clinical research associate Ms Noriza Mustapa for patient recruitment and study procedure coordination. They also thank Ms Amanda Tay for formatting and referencing this manuscript. They wish to thank D3 Pharmacy, Denmark DK-9000 Aalborg for manufacturing both cholecalciferol and placebo, and OneNine57 Pte Ltd for importing the medications. Clinical Trial Registration Information-ClinicalTrials.gov Identifier: NCT01741181.
Footnotes
Author contribution: R.D. conceived this study, conducted the study, wrote the manuscript and is overall in charge of the study and takes responsibility for the integrity of the data and accuracy of the data analysis. H.L., A.W.K.T. and D.E.K.C. conducted the study and contributed towards writing of the manuscript. E.C., P.N.A. and F.S. contributed towards initial study design, data collection, final analyses and writing of the manuscript. M.K.S.L. contributed towards conceiving this study, conduct of the study, analyses and the final critical review of this manuscript. B.O.B. contributed towards analysis, writing of the manuscript and the final critical review of the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from Duke-NUS Graduate Medical School and funded by the Tanoto Initiative of Diabetes Research (Duke-NUS-TIDR/2012/0005R), National Healthcare Group Clinician Scientist Scheme research grants as well as the Ministry of Health, National Medical Research Council, Centre Grant (NMRC/CG/017/2013) allocated to Tan Tock Seng Hospital for manpower support.
References
- 1. Haffner SM, Lehto S, Rönnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in non-diabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339: 229–234. [DOI] [PubMed] [Google Scholar]
- 2. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106: 3143–3421. [PubMed] [Google Scholar]
- 3. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 4. ACCORD Study Group, Gerstein HC, Miller ME, et al. Long term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med 2011; 364: 818–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cohn JN, Quyyumi AA, Hollenberg NK, et al. Surrogate markers for cardiovascular disease: functional markers. Circulation 2004; 109: IV31–V46. [DOI] [PubMed] [Google Scholar]
- 6. Hamburg NM, Keyes MJ, Larson MG, et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation 2008; 117: 2467–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rubinshtein R, Kuvin JT, Soffler M, et al. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J 2010; 31: 1142–1148. [DOI] [PubMed] [Google Scholar]
- 8. Conway DS, Pearce LA, Chin BS, et al. Prognostic value of plasma von Willebrand factor and soluble P-selectin as indices of endothelial damage and platelet activation in 994 patients with nonvalvular atrial fibrillation. Circulation 2003; 107: 3141–3145. [DOI] [PubMed] [Google Scholar]
- 9. Blann A. Von Willebrand factor and the endothelium in vascular disease. Br J Biomed Sci 1993; 50: 125–134. [PubMed] [Google Scholar]
- 10. Roldan V, Marin F, Lip GY, et al. Soluble E-selectin in cardiovascular disease and its risk factors: a review of the literature. Thromb Haemost 2003; 90: 1007–1020. [DOI] [PubMed] [Google Scholar]
- 11. Dalan R, Liew H, Tan AWK, et al. Vitamin D and the endothelium: basic, translational and clinical research updates. IJC Metab Endocr 2014; 4: 4–17. [Google Scholar]
- 12. McCrea CE, Skulas-Ray AC, Chow M, et al. Test-retest reliability of pulse amplitude tonometry measures of vascular endothelial function: implications for a clinical trial design. Vasc Med 2012; 17: 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haller MJ, Silverstein JH, Shuster JJ. Correlation between radial artery tonometry- and fingertip tonometry-derived augmentation index in children with type 1 diabetes. Diab Vasc Dis Res 2007; 4: 66. [DOI] [PubMed] [Google Scholar]
- 14. Aversa A, Vitale C, Volterrani M, et al. Chronic administration of sildenafil improves markers of endothelial function in men with Type 2 diabetes. Diabetic Med 2008; 25: 37–44. [DOI] [PubMed] [Google Scholar]
- 15. Al Mheid I, Patel R, Murrow J, et al. Vitamin D status is associated with arterial stiffness and vascular dysfunction in healthy humans. J Am Coll Cardiol 2011; 58: 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Makino H, Hishida A, Koezuka R, et al. The effect of basal insulin therapy on endothelial function in type 2 diabetic patients. Diabetes 2014; 63: A123. [Google Scholar]
- 17. Robien K, Butler LM, Wang R, et al. Genetic and environmental predictors of serum 25(OH)D concentrations among middle-aged and elderly Chinese in Singapore. Br J Nutr 2013; 109: 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Song Y, Wang L, Pittas AG, et al. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care 2013; 36: 1422–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yiu YF, Yiu KH, Siu CW, et al. Randomised controlled trial of vitamin D supplement on endothelial function in patients with type 2 diabetes. Atherosclerosis 2013; 227: 140–146. [DOI] [PubMed] [Google Scholar]
- 20. Sugden JA, Davies JL, Witham MD, et al. Vitamin D improves endothelial function in patients with type 2 diabetes mellitus and low vitamin D levels. Diabetic Med 2008; 25: 320–325. [DOI] [PubMed] [Google Scholar]
- 21. Lomashvili K, Wang X, O’Neill WC. Role of local versus systemic vitamin D receptors in vascular calcification. Arterioscler Thromb Vasc Biol 2014; 34: 146–151. [DOI] [PubMed] [Google Scholar]
- 22. Harris A, Pederson-White J, Guo DH, et al. Vitamin D3 supplementation for 16 weeks improves flow mediated dilatation in overweight African-American adults. Am J Hypertens 2011; 24: 557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tarcin O, Yavuz DG, Ozben B, et al. Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. J Clin Endocrinol Metab 2009; 94: 4023–4030. [DOI] [PubMed] [Google Scholar]
- 24. Red C, Nikolic SB, Otahal P, et al. Augmentation index and arterial stiffness in patients with type 2 diabetes mellitus. Artery Res 2013; 7: 194–200. [Google Scholar]
- 25. Powe CE, Evans MK, Wenger J, et al. Vitamin D binding protein and vitamin D status of Black Americans and White Americans. N Engl J Med 2013; 369: 1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]