Abstract
Endotoxins are the major components of the outer membrane of most Gram-negative bacteria and are one of the main targets in inflammatory diseases. The presence of endotoxins in blood can provoke septic shock in case of pronounced immune response. Here we show in vitro inactivation of endotoxins by polymyxin B (PMB). The inflammatory activity of the LPS–PMB complex in blood was examined in vitro in freshly drawn blood samples. Plasma protein binding of PMB was determined by ultracentrifugation using membranes with different molecular cut-offs, and PMB clearance during dialysis was calculated after in vitro experiments using the AV1000S filter. The formed LPS–PMB complex has lower inflammatory activity in blood, which results in highly reduced cytokine secretion. According to in vitro measurements, the appropriate plasma level of PMB for LPS inactivation is between 100 and 200 ng/ml. Furthermore, the combination of cytokine removal by adsorbent treatment with LPS inactivation by PMB dosage leads to strong suppression of inflammatory effects in blood in an in vitro model. Inactivation of endotoxins by low-dose intravenous PMB infusion or infusion into the extracorporeal circuit during blood purification can be applied to overcome the urgent need for endotoxin elimination not only in treatment of sepsis, but also in liver failure.
Keywords: Polymyxin, lipopolysaccharide, sepsis, Inflammation, cytokines, endotoxin
Introduction
In recent years, endotoxins (LPS) have increasingly become the focus of interest for therapy of diseases that are treated with extracorporeal blood purification. LPS is a major constituent of the outer cell envelope of most Gram-negative bacteria and may strongly trigger inflammatory responses in humans, even at doses as low as 1 ng/kg body mass/h.1 LPS is released from the cell envelope of growing bacteria, as well as by lysis via antibiotics or complement.2,3 Endotoxins that enter the circulatory system bind to the soluble lipopolysaccharide binding protein (LBP). This complex initiates the inflammatory response by binding to the CD14 membrane protein of monocytes and macrophages, subsequently triggering the production of cytokines via TLR. LPS activation of TLR4 triggers the biosynthesis of diverse mediators of inflammation, such as TNF-α and IL-1β,4 and activates the production of co-stimulatory molecules required for the adaptive immune response.5 In mononuclear and endothelial cells, LPS also stimulates tissue factor production,6 and can therefore trigger the extrinsic coagulation pathway. As long as this process is limited to a local increase of pro- or anti-inflammatory cytokines, this is a normal response of the patient’s immune reaction against pathogens. However, in severe cases, the production of cytokines gets out of control leading to the more severe medical conditions such as systemic inflammatory response syndrome (SIRS) or sepsis. Thus, the effective removal of endotoxins is essential in order to reduce cytokine production in the case of Gram-negative sepsis.
Furthermore, endotoxins play an important role in liver failure. Endotoxins from patients’ intestines can pass the liver owing to reduced endotoxin removal via the reticuloendothelial system, which can lead to endotoxemia and, finally, to the symptoms described above.7,8
Different approaches have been followed to remove or inactivate endotoxins upon inflammation and sepsis. Antimicrobial peptides (AMPs) can block the endotoxin-initiated inflammatory cascade, which leads to a reduction of cytokine production. These AMPs are currently intensively investigated and provide, to some extent, promising results.9–11 While polymyxins are well known to inactivate the biological activity of LPS by shutting down the NF-κB pathway, owing to direct binding of LPS,12 the mechanisms of action for some other AMPs are not yet fully understood. There is some evidence that they enter another mode of action by inserting into CD14-positive cells and reducing the endotoxin activity by competitive inhibition due to their high affinity to LPS.13
Polymyxin B (PMB) is an antibiotic preferably applied to treat infections provoked by multidrug resistant Gram-negative bacteria. It is a cyclic, highly cationic decapeptide derived from Bacillus polymyxa.14 PMB has been applied for in vitro investigations, as well as intravenously in animal models, to demonstrate its capacity to inactivate endotoxins and to break down endotoxin aggregates in Gram-negative septicemia models.15–20 The bactericidal activity of the antibiotic PMB against Gram-negative bacteria relies on the ability to destabilize the outer bacterial wall by direct interaction with the lipid A moiety of LPS molecule. The model for action of PMB involves interaction of the positively charged diaminobutyric acid residues and the negatively charged phosphate groups of lipid A. This initial electrostatic interaction temporarily stabilizes the LPS–PMB complex and facilitates the interaction of the N-terminal fatty acyl chain of the PMB molecule into proximity with the lipid A fatty acyl chains.21 The LPS–PMB complex is very stable and has an association constant (Ka) according to the LPS type between 1.8 × 10−6 and 2.3 × 10−6 M.22 However, although these studies were rather focused on endotoxins than on bacteria, the applied PMB amounts mostly exceeded the minimal inhibitory concentration (MIC) of this antibiotic.
Currently, knowledge about pharmacokinetics and pharmacodynamics of polymyxins is very limited as intravenous administration was avoided within the last 50 yrs. Furthermore, nephro- and neurotoxicity restricted clinical application. Because of increasing numbers of multidrug-resistant Gram-negative pathogens and limited development of new antimicrobials, PMB experiences a revival as a therapeutic option for Gram-negative infections.23 In particular, the use of affinity chromatographic sorbents based on PMB ligands is reported as an appropriate method to remove endotoxins from protein solutions without denaturation and loss of products.24 Here we show that endotoxin inactivation by PMB or polymyxin E (PME; colistin) in patients with Gram-negative sepsis or endotoxemia could be an additional therapeutic option.
Materials and methods
Materials
PMB and endotoxins (LPS) from Pseudomonas aeruginosa and Escherichia coli were purchased from Sigma-Aldrich (Vienna, Austria). The PMB analyses were conducted using the Colistin & Polymyxin ELISA kit from Kwinbon Biotechnology Co. LTD (Beijing, China). The analyses of endotoxins were performed in pyrogen-free tubes and the kinetic chromogenic Limulus amebocyte lysate (LAL) test from Charles River Laboratories (Wilmington, MA, USA). Tubes (Vacuettes) for blood donation were obtained from Greiner (Kremsmünster, Austria). The Amicon Ultra-2 centrifugal filter devices with molecular cut-offs of 10, 30, 50 and 100 kDa were from Merck (Darmstadt, Germany). The ALBplus reagent set for albumin quantification and the TP set for measurement of total protein level was purchased from Roche (Mannheim, Germany).
LPS inactivation as a function of PMB concentration
To elucidate the dependency of LPS inactivation on the PMB concentration, fresh human heparinized plasma containing 5 ng/ml or 0.5 ng/ml LPS from either P. aeruginosa or E. coli were incubated with increasing amounts of PMB (0, 10, 100, 250, 500 and 1000 ng/ml) for 60 min at 37℃. The tests were performed in pyrogen-free 3-ml glass vials and LPS activity was determined using the LAL test. In order to compare the endotoxin neutralizing capability of PMB, the endotoxin neutralizing concentration (ENC50) was calculated. This is the PMB concentration that is capable of reducing the endotoxin activity compared with the LPS-spiked plasma control without PMB, by 50%.
Influence of PMB–LPS complex on cytokine induction
The PMB-dependent reduction of the LPS activity, as indicated by the LAL test, is not necessarily associated with a reduced inflammatory effect of LPS, namely the induction of the cytokine production. Therefore, further experiments were conducted in order to check if cytokine induction can be modulated by PMB-dependent inactivation of LPS. For these experiments, freshly drawn human heparinized blood was spiked with PMB to yield 0, 250, 500 and 1000 ng/ml PMB. Then, 0.5 ng/ml LPS from E. coli was added. The negative control was blood without LPS, spiked with 1000 ng/ml PMB to assess the influence of PMB alone on cytokine release. The samples were incubated in sterile polypropylene tubes (Greiner) for 4 h at 37℃ on a roller mixer and stored at −80℃ until the cytokines were quantified by ELISA. All assays were conducted at least in triplicates.
Combined use of PMB and cytokine adsorbent
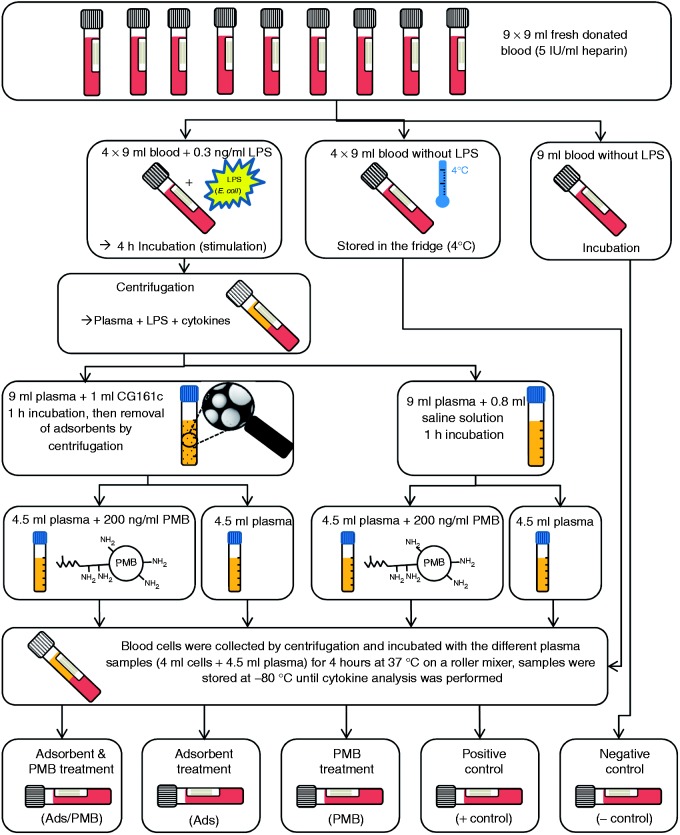
To verify whether the combination of adsorptive cytokine removal and PMB infusion is more effective than one of these treatments, an experiment as schematically displayed in Figure 1 was conducted. This experiment was carried out with blood from three different volunteers.
Figure 1.
Scheme of the experiment where three different treatment options: (i) adsorbent combined with PMB; (ii) adsorbent treatment only; (iii) PMB treatment only. The influence on inflammation was tested by determination of the cytokine levels after incubation of differently pre-treated plasma samples with native blood cells.
Binding of PMB to plasma proteins
Depending on the affinity to plasma proteins, which is a specific characteristic of a drug, one part of the drug is transported by plasma proteins and another part is freely distributed throughout the circulation. If protein binding is reversible, an equilibrium will exist between the bound and unbound proportion. To evaluate plasma protein binding, fresh citrate anticoagulated plasma from three donors was spiked with 1 µg/ml PMB. Five hundred µl PMB containing plasma was centrifuged (10,000 g, 20 min) through different ultrafiltration membranes with a molecular mass cut-off (MMCO) of 10, 30, 50 or 100 kDa. The filtrates were stored at −20℃ until quantification of PMB by ELISA and total protein content by an automated analyser (Hitachi 902; Hitachi, Tokyo, Japan). The positive control was unfiltered plasma spiked with 1 µg/ml PMB and the negative control was plasma without PMB. To detect any PMB-binding to the filter material, the same experiment was performed in 0.9% sodium chloride solution.
Clearance measurement of PMB
Since sepsis patients with acute kidney injury are treated with renal replacement therapy, a dialysis experiment was performed to estimate the clearance of PMB during a treatment. As intensive care treatment sessions usually take several hours, it can be assumed that most of the non-protein-bound fraction of PMB is removed during treatment. In order to avoid that the inflammatory activity of LPS is restored by PMB removal, PMB monitoring would be useful. To check the PMB clearance by a conventional dialysis filter, 1500 ml plasma spiked with 5 µg/ml PMB was circulated through a dialyzer (AV1000S, FMC, Bad Homburg, Germany) using the multiFiltrate device from Fresenius Medical Care (Bad Homburg, Germany). The schematic setup of this experiment is shown in Figure 2. The plasma flow rate (Qb) was 100 ml/min and dialysate (QD) flow rates of 2000 ml/h and 4800 ml/h were chosen. Samples for PMB quantification were collected pre- and postfilter after 5, 10, 15, 20, 25 and 30 min. The experiment was performed three times with different plasma and dialyzers and the PMB clearance was calculated according to the following formula:
Figure 2.
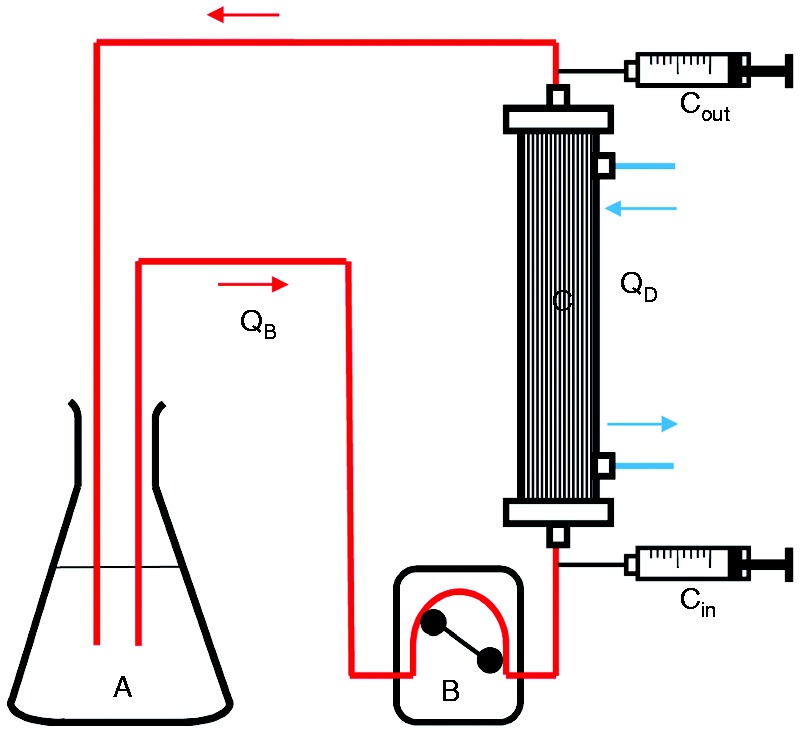
Setup for determination of PMB clearance during hemodialysis using the multiFiltrate. The experiment was performed with (A) 1500 ml plasma, (B) a blood pump with (QB) flow rates between 50 and 200 ml and (C) the dialyzer AV1000 S (Fresenius Medical Care). Samples were taken pre- (Cin) and post- (Cout) filter after 5, 10, 15, 20, 25 and 30 min.
PMB quantification
The blood and plasma levels of PMB were determined by use of a competitive enzyme immunoassay kit for analysis of PMB and PME with a detection limit of 1 ng/ml. If necessary, the samples were diluted with dilution reagent provided with the ELISA kit to reach the measuring range between 0 and 100 ng/ml PMB.
Cytokine quantification
The analysis of cytokines was conducted by ELISA with a Bio-Plex cytokine array system (Biorad, Vienna, Austria).
Statistics
Mean and SD were calculated with Excel 2010 (Microsoft, Redmond, WA, USA). The tests for normal distribution and the t-tests were conducted with SigmaStat for Windows 2.03.
Results
LPS inactivation as a function of PMB concentration and ENC50
To estimate the plasma level of PMB required to decrease the LPS activity to a certain level, the LPS inactivation in plasma was measured as a function of PMB concentration. At 0.5 ng/ml LPS, the ENC50 is 84 and 63 ng/ml for LPS from P. aeruginosa and E. coli, respectively. For 5 ng/ml LPS, the ENC50 is 219 and 381 ng/ml for LPS from P. aeruginosa and E. coli, respectively (Figures 3 and 4). The differences in the ENC50 for LPS from E. coli and P. aeruginosa, however, are not significant (t-test, P = 0.88 and P = 0.26, respectively) at both concentration levels.
Figure 3.
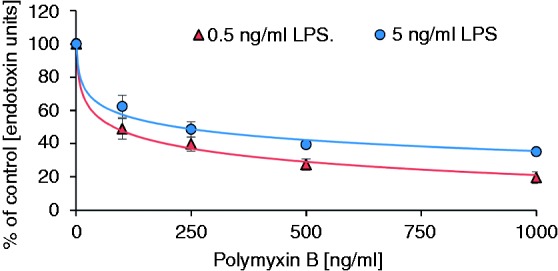
PMB-dependent inactivation of endotoxins from P. aeruginosa in human plasma.
Figure 4.
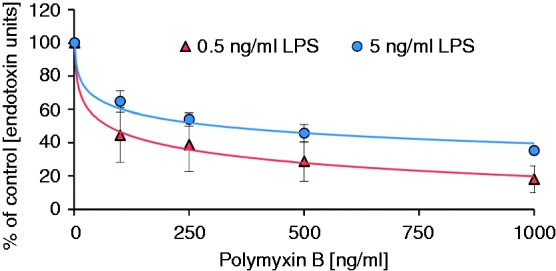
PMB-dependent inactivation of endotoxins from E. coli in human plasma.
Influence of PMB–LPS complex on cytokine induction
Although the LAL test revealed inactivation of LPS by PMB in a concentration-dependent manner, the influence on inflammation was tested by stimulating leukocytes from human blood with increasing amounts of PMB. The results of cytokine release show clearly that the formed LPS–PMB complex exerts a by far lower stimulating effect on blood cells than the native LPS molecule (Table 1; Figure 5). Among all cytokines under investigation, the highest impact was observed on TNF-α release. Only 50 ng PMB per ml blood reduced TNF-α secretion from leukocytes by 87.4 ± 5.9%. Increasing the PMB 2.5-fold to 125 ng/ml decreased the TNF-α level by about 94.8 ± 2.3%. A further increase of PMB caused no considerable decrease of cytokine concentrations (Table 1). The lowest impact of PMB was observed on IL-8 secretion. However, even the negative control without LPS showed high levels of IL-8. In general, the IL-10 level was very low (23 ± 8 pg/ml) because IL-10 is a late-related cytokine and a high level can only be reached if stimulation of the blood cells is prolonged to 12 h. The secretion of IL-1β was reduced by 75.1 ± 10.8% and that of IL-6 by 78.0 ± 12.0% in the presence of 125 ng PMB per ml blood.
Table 1.
The cytokine release in LPS-stimulated (E. coli) blood in presence of increasing amounts of PMB.
| TNF-α | IL-1β | IL-6 | IL-8 | IL-10 | |
|---|---|---|---|---|---|
| − Control | 0.3 ± 0.2 | 1.6 ± 1.8 | 0.2 ± 0.1 | 20.1 ± 13.2 | 18.9 ± 10.9 |
| + Control | 100 ± 17.6 | 100 ± 73.3 | 100 ± 19.1 | 100 ± 54.3 | 100 ± 32.8 |
| 50 ng/ml PMB | 12.6 ± 5.9 | 31.1 ± 9.6 | 40.0 ± 18.6 | 59.1 ± 19.4 | 53.1 ± 16.8 |
| 125 ng/ml PMB | 5.2 ± 2.3 | 24.9 ± 10.8 | 22.0 ± 12.0 | 53.6 ± 14.5 | 38.5 ± 16.2 |
| 250 ng/ml PMB | 4.2 ± 2.3 | 18.4 ± 11.2 | 19.0 ± 13.8 | 49.1 ± 11.0 | 34.9 ± 16.7 |
| 500 ng/ml PMB | 2.1 ± 0.7 | 10.2 ± 5.5 | 8.4 ± 4.5 | 40.6 ± 10.5 | 32.5 ± 15.8 |
The results are % SD of the positive control without PMB.
Figure 5.
The effect of LPS inactivation by PMB on cytokine release in whole blood from three different donors (mean ± SD; n = 3).
Combined use of PMB and cytokine adsorbent
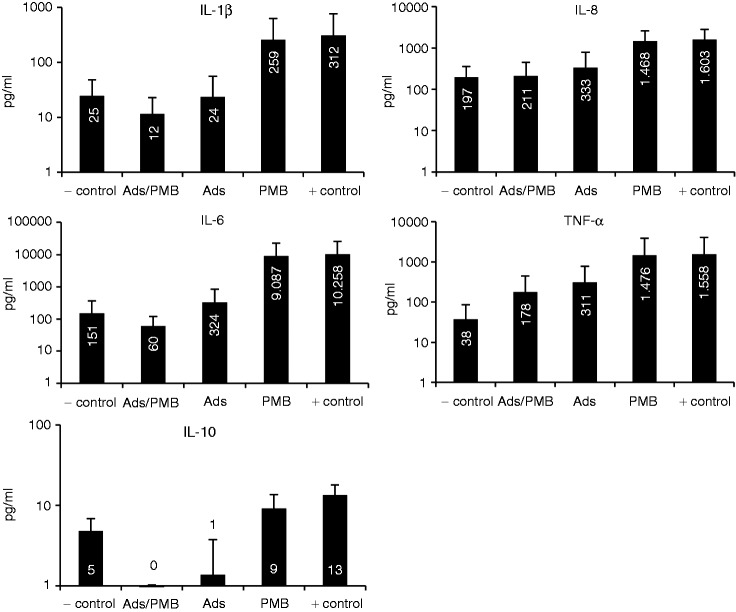
As pre-existing cytokines cannot be reduced by PMB infusion and the inflammatory acting LPS cannot be removed from blood by adsorption techniques sufficiently, these two types of treatments were simulated in an in vitro model (see Figure 1). Plasma that contained inflammatory mediators like LPS and secreted cytokines was pre-treated with adsorbent or PMB or both. Then, the treated plasma samples were incubated with blood cells from the same donor for 4 h and the cytokine levels were determined. Untreated plasma containing the inflammatory mediators served as a positive control and native blood as a negative control. According to the results, the addition of PMB after LPS stimulation scarcely reduced the cytokine secretion compared with the untreated plasma. The cytokine levels, however, were still high because of the presence of cytokines like TNF-α that still act as stimulating agents. Interestingly, the adsorbent CG161c decreased the cytokine level in plasma considerably to 30 ± 9% (TNF-α), 6 ± 5% (IL-1β), 2 ± 2% (IL-6), 1 ± 1% (IL-8) and 9 ± 13% (IL-10) compared with the untreated positive control. The styrene-divinylbenzene based CG161c adsorbent has a particle size of 120 µm and pores with an average diameter of 15 nm. In earlier studies, we have shown that CG161c is very effective in removing cytokines from plasma.25,26 After incubation of the different plasma samples with blood cells only the combination of adsorbent with PMB exhibited cytokine levels similar to the negative control where no LPS was added (see Table 2 and Figure 6). Obviously, PMB inactivates LPS and reduces leukocyte stimulation. The results show high SDs of the cytokine level due to the fact that blood from different donors was used.
Table 2.
Cytokine release from blood cells after incubation with differently treated inflammatory plasma. The plasma treatments were (i) addition of PMB, (ii) adsorbent to remove cytokines or (iii) the combination of both (see also Figure 1).
| TNF-α | IL-1β | IL-6 | IL-8 | IL-10 | |
|---|---|---|---|---|---|
| Donators | A/B/C | A/B/C | A/B/C | A/B/C | A/B/C |
| Positive control | 100 | 100 | 100 | 100 | 100 |
| Negative control | 73/2/0 | 69/58/1 | 20/3/0 | 11/13/13 | 54/56/14 |
| PMB | 92/38/95 | 98/80/82 | 101/80/88 | 91/91/92 | 91/41/67 |
| Adsorbent | 62/11/19 | 7/23/7 | 2/2/3 | 8/8/29 | 0/0/22 |
| PMB/adsorbent | 36/10/11 | 7/22/2 | 2/2/0 | 8/8/16 | 0/0/6 |
The results of each donator (A, B and C) are given in % as referred to the positive control without any treatment set at 100%.
Figure 6.
Comparison of the cytokine release pattern from blood cells after different ways of plasma pre-treatments: adsorbent combined with PMB (Ads/PMB); only adsorbent (Ads); only PMB (PMB); untreated plasma (+ control); native blood (− control).
Binding of PMB to plasma proteins
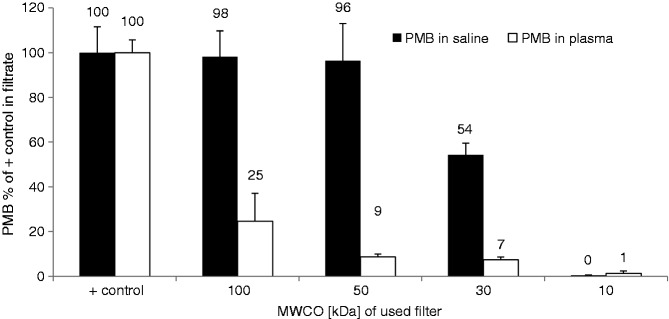
Most drugs bind to plasma proteins such as albumin, α1-acid glycoprotein, lipoproteins, α-, β- and γ-globulins, and to erythrocytes. Ultracentrifugation of PMB-spiked plasma through membranes with different MMCOs for separation of protein-bound PMB from free PMB revealed that this drug with a molecular weight of 1.3 kDa is mostly bound to plasma contents. Only the membrane with 100 kDa MMCO was permeable for higher amounts of PMB (25 ± 13%). The PMB-content of filtrates obtained from centrifugation through membranes with smaller pores was <10% compared with a PMB-solution without plasma (Figure 7). Thus, >90% of PMB is bound to plasma proteins and not even one-tenth exists in its free form in circulation.
Figure 7.
Binding of PMB to plasma proteins determined by ultrafiltration through membranes with different MMCO. The filtration rate of PMB in plasma was compared with that in 0.9% NaCl solution. The data represent the percentage of PMB in the filtrate as referred to the positive control (mean ± SD; n = 3).
Clearance measurement of PMB
The PMB clearance (ClPMB) for the AV1000S filter was determined using the multiFiltrate device (FMC) at a blood flow rate of 100 ml/min. The PMB clearance was 12.9 ± 5.1 ml/min (n = 18) at a dialysate flow rate of 2000 ml/h, and at a dialysate flow rate of 4800 ml/h the PMB clearance increased to 17.2 ± 8.5 ml/min (n = 13).
Discussion
In sepsis therapy, adsorption-based removal of LPS is still a challenge. The LPS molecule consists of a conserved hydrophobic domain known as lipid A, a non-repeating ‘core’ oligosaccharide and a highly variable distal polysaccharide. Until now, specific adsorption based on immobilized Abs did not offer promising results.27–30 In the case of Abs against the lipid A region, this was attributed to the low affinity of anti-lipid A Abs to lipid A and to cross-reactivity to plasma proteins.31,32 Adsorption based on Abs against the polysaccharide chain (O-antigen) is not feasible as the polysaccharide domain is highly variable. Although anion-exchanger resins efficiently remove endotoxins from aqueous solutions, as well as from protein solutions, they cannot be used in blood purification because of insufficient biocompatibility.33 Since 1994, a PMB-based extracorporeal hemoperfusion device, called Toraymyxin, has been commercially available and is now approved as a therapeutic device by the health insurance system in Japan.34 It is recommended for selective blood purification from endotoxins via direct hemoperfusion. Toraymyxin consists of polystyrene-derivative fibres with covalently immobilized PMB at a mass ratio of 0.5%.35 Direct hemoperfusion using such a PMB-immobilized fiber column (PMX-F) has been used for about 15 yrs for the treatment of septic shock.36 Direct hemoperfusion with PMX-F can be applied in patients with endotoxemia or suspected Gram-negative infection, who fulfill the conditions of SIRS and suffer from a septic shock requiring administration of vasoactive agents. Several studies claim that endotoxins are efficiently removed by PMX-F,35,37–39 and, concurrently, Staphylococcus aureus lipoteichoic acid-induced TNF-α production is suppressed.40 Even in clinical practice, adsorption techniques using Toraymyxin cartridges have been successfully applied in Japan since 1994 in more than 60,000 patients with severe sepsis.35 The fact that polymyxins bind endotoxins in blood very effectively and that PMB or PME in the form of colistin is a clinically approved drug for intravenous application reveals the idea of using them not as an antibiotic, but as an LPS neutralizing agent. We showed that PMB concentrations <100 ng/ml in plasma for 0.5 ng/ml LPS and <400 ng/ml for 5 ng/ml LPS from P. aeruginosa and E. coli are required to reduce the LAL activity to 50% of the control serum without PMB. These ENC50 are in the range of AMPs with the highest endotoxin inactivation capabilities that are currently known, namely peptides derived from the recombinant factor c of the LAL cascade.41 While LPS from P. aeruginosa is usually an order of magnitude less active than LPS from enterobacterial strains such as Escherichia, the inactivation ratio is similar. A potential explanation for this finding is that PMB directly interacts with the conserved lipid A region of the LPS molecule. In vitro, the formed LPS–PMB complex in blood exhibits lower inflammatory activity than the free LPS. Moreover, in presence of LPS the cytokine secretion of blood cells is clearly reduced when the PMB level in blood is ≥50 ng/ml (Figure 5). Although polymyxins are shown to reduce significantly the cytokine storm in endotoxemia, it has to be considered that patients developing or even suffering from sepsis already show high cytokine levels before starting any therapy. As polymyxins cannot reduce pre-existing cytokine levels, the administration of polymyxins should be supported by effective approaches for cytokine removal, such as adsorption or hemofiltration.42,43 Combining adsorptive cytokine removal with PMB administration (Figure 1) is proposed to be an effective approach to suppress inflammatory effects of Gram-negative infections (Figures 1 and 6; Table 2). The concentration of PMB necessary for effective inactivation of LPS is in a very low range but still close to the MIC.44 To minimize the risk of generating resistant germs, low-dose PMB for LPS inactivation should be applied only together with conventional antibiotic treatment. This procedure offers the advantage that endotoxins, which are released by antibiotic-induced bacterial lysis, are effectively inactivated by PMB.13,45 Furthermore, the half-lives of the different polymyxins have to be considered upon application for inactivation of LPS. However, the data available from the literature are inconsistent, and depend on the type of polymyxin and on the renal function of the patients, especially for the PME prodrug colistin methanonsulfonate.46–49 As polymyxins are removed by hemodialysis and adsorption,50,51 the clearance of the dialyzer and/or the adsorption unit has to be taken into account when PMB is applied concomitantly with extracorporeal blood purification. An in vitro simulation with a commercially available dialyser at dialysate flow rates between 2000 and 4800 ml/h yielded a PMB plasma clearance between 12% and 17%. This clearance rate agrees with data from the literature.52,53 Considering the small molecular mass of the PMB molecule (1.3 kDa), the calculated clearance rate is relatively low, which can be explained by binding of PMB molecules to plasma proteins. Plasma protein binding of PMB was assumed to be up to 90%,54 which is confirmed by our experiments (Figure 7). Titration calorimetry experiments show that in aqueous solution with 1–3 molecules of PMB are necessary to inactivate one LPS molecule.22 The association constant (Ka) of this interaction is between 1.8 × 10−6 and 2.3 × 10−6 M, which explains why very low amounts of free PMB inactivate LPS.
Conclusions
As endotoxins induce a strong host immune response, there is an urgent therapeutic need to reduce their activity. As a promising alternative or add-on to endotoxin adsorbents, endotoxin inactivation by low-dose PMB intravenous infusion or infusion into the extracorporeal circuit during blood purification is proposed to reduce considerably endotoxin activity not only in treatment of sepsis, but also in liver failure. However, the findings presented here are based on in vitro experiments. For an optimal and safe endotoxin inactivation therapy by polymyxin administration, further systematic investigations regarding drug monitoring and pharmacokinetic studies, especially in vivo studies, should be conducted.
Acknowledgements
The excellent technical support of Ute Fichtinger and Claudia Schildböck in the laboratories is gratefully acknowledged.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the government of Lower Austria and the European Commission (Project ID: WST3-T-91/036-2014).
References
- 1.Greisman SE, Hornick RB. Comparative pyrogenic reactivity of rabbit and man to bacterial endotoxin. Proc Soc Exp Biol Med Soc Exp Biol Med 1969; 131: 1154–1158. [DOI] [PubMed] [Google Scholar]
- 2.Buttenschoen K, Radermacher P, Bracht H. Endotoxin elimination in sepsis: physiology and therapeutic application. Langenbecks Arch Surg 2010; 395: 597–605. [DOI] [PubMed] [Google Scholar]
- 3.Morrison DC. Antibiotic-mediated release of endotoxin and the pathogenesis of Gram-negative sepsis. Prog Clin Biol Res 1998; 397: 199–207. [PubMed] [Google Scholar]
- 4.Dinarello CA. Interleukin-1 and interleukin-1 antagonism. Blood 1991; 77: 1627–1652. [PubMed] [Google Scholar]
- 5.Medzhitov R, Janeway C., Jr Innate immunity. N Engl J Med 2000; 343: 338–344. [DOI] [PubMed] [Google Scholar]
- 6.Li A, Chang AC, Peer GT, et al. Comparison of the capacity of rhtnf-alpha and Escherichia coli to induce procoagulant activity by baboon mononuclear cells in vivo and in vitro. Shock 1996; 5: 274–279. [DOI] [PubMed] [Google Scholar]
- 7.Kuratsune H, Koda T, Kurahori T. The relationship between endotoxin and the phagocytic activity of the reticuloendothelial system. Hepatogastroenterology 1983; 30: 79–82. [PubMed] [Google Scholar]
- 8.Nolan JP. The role of intestinal endotoxin in liver injury: a long and evolving history. Hepatology 2010; 52: 1829–1835. [DOI] [PubMed] [Google Scholar]
- 9.Brandenburg K, Andrä J, Garidel P, Gutsmann T. Peptide-based treatment of sepsis. Appl Microbiol Biotechnol 2011; 90: 799–808. [DOI] [PubMed] [Google Scholar]
- 10.Schuerholz T, Doemming S, Hornef M, et al. The anti-inflammatory effect of the synthetic antimicrobial peptide 19-2.5 in a murine sepsis model: A prospective randomized study. Crit Care 2013; 17: R3–R3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lohner K. Antimicrobial mechanisms: A sponge against fungal infections. Nat Chem Biol 2014; 10: 411–412. [DOI] [PubMed] [Google Scholar]
- 12.Tsuzuki H, Tani T, Ueyama H, Kodama M. Lipopolysaccharide: neutralization by polymyxin b shuts down the signaling pathway of nuclear factor kappab in peripheral blood mononuclear cells, even during activation. J Surg Res 2001; 100: 127–134. [DOI] [PubMed] [Google Scholar]
- 13.Schuerholz T, Brandenburg K, Marx G. Antimicrobial peptides and their potential application in inflammation and sepsis. Crit Care 2012; 16: 207–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mares J, Kumaran S, Gobbo M, Zerbe O. Interactions of lipopolysaccharide and polymyxin studied by nmr spectroscopy. J Biol Chem 2009; 284: 11498–11506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cardoso LS, Araujo MI, Goes AM, et al. Polymyxin B as inhibitor of LPS contamination of schistosoma mansoni recombinant proteins in human cytokine analysis. Microb Cell Fact 2007; 6: 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morresey PR, Mackay RJ. Endotoxin-neutralizing activity of polymyxin b in blood after iv administration in horses. Am J Vet Res 2006; 67: 642–647. [DOI] [PubMed] [Google Scholar]
- 17.Nanjo Y, Ishii Y, Kimura S, et al. Effects of slow-releasing colistin microspheres on endotoxin-induced sepsis. J Infect Chemother 2013; 19: 683–690. [DOI] [PubMed] [Google Scholar]
- 18.Petsch D, Anspach FB. Endotoxin removal from protein solutions. J Biotechnol 2000; 76: 97–119. [DOI] [PubMed] [Google Scholar]
- 19.Rifkind D. Prevention by polymyxin b of endotoxin lethality in mice. J Bacteriol 1967; 93: 1463–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharp CR, DeClue AE, Haak CE, et al. Evaluation of the anti-endotoxin effects of polymyxin B in a feline model of endotoxemia. J Feline Med Surg 2010; 12: 278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Velkov T, Thompson PE, Nation RL, Li J. Structure–activity relationships of polymyxin antibiotics. J Med Chem 2010; 53: 1898–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srimal S, Surolia N, Balasubramanian S, Surolia A. Titration calorimetric studies to elucidate the specificity of the interactions of polymyxin B with lipopolysaccharides and lipid A. Biochem J 1996; 315: 679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Falagas ME, Kasiakou SK. Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Crit Care 2006; 10: R27–R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anspach FB, Hilbeck O. Removal of endotoxins by affinity sorbents. J Chromatogr A 1995; 711: 81–92. [DOI] [PubMed] [Google Scholar]
- 25.Harm S, Falkenhagen D, Hartmann J. Pore size—a key property for selective toxin removal in blood purification. Int J Artif Organs 2014; 37: 668–678. [DOI] [PubMed] [Google Scholar]
- 26.Harm S, Gabor F, Hartmann J. Characterization of adsorbents for cytokine removal from blood in an in vitro model. J Immunol Res 2015; 2015: 484736–484736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atan R, Crosbie D, Bellomo R. Techniques of extracorporeal cytokine removal: a systematic review of the literature on animal experimental studies. Int J Artif Organs 2013; 36: 149–158. [DOI] [PubMed] [Google Scholar]
- 28.Atan R, Crosbie DC, Bellomo R. Techniques of extracorporeal cytokine removal: a systematic review of human studies. Ren Fail 2013; 35: 1061–1070. [DOI] [PubMed] [Google Scholar]
- 29.Hurley JC. Towards clinical applications of anti-endotoxin Abs; a re-appraisal of the disconnect. Toxins 2013; 5: 2589–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angus DC, Birmingham MC, Balk RA, et al. E5 murine monoclonal antiendotoxin Ab in Gram-negative sepsis: A randomized controlled trial. E5 study investigators. JAMA 2000; 283: 1723–1730. [DOI] [PubMed] [Google Scholar]
- 31.McCloskey RV, Straube RC, Sanders C, et al. Treatment of septic shock with human monoclonal Ab ha-1a. A randomized, double-blind, placebo-controlled trial. Chess trial study group. Ann Intern Med 1994; 121: 1–5. [DOI] [PubMed] [Google Scholar]
- 32.Helmerhorst EJ, Maaskant JJ, Appelmelk BJ. Anti-lipid a monoclonal Ab centoxin (ha-1a) binds to a wide variety of hydrophobic ligands. Infect Immun 1998; 66: 870–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harm S, Falkenhagen D, Hartmann J. Endotoxin adsorbents in extracorporeal blood purification: do they fulfill expectations? Int J Artif Organs 2014; 37: 222–232. [DOI] [PubMed] [Google Scholar]
- 34.Shoji H, Tani T, Hanasawa K, Kodama M. Extracorporeal endotoxin removal by polymyxin b immobilized fiber cartridge: designing and antiendotoxin efficacy in the clinical application. Ther Apher 1998; 2: 3–12. [DOI] [PubMed] [Google Scholar]
- 35.Cruz DN, Perazella MA, Bellomo R, et al. Effectiveness of polymyxin b-immobilized fiber column in sepsis: a systematic review. Crit Care 2007; 11: R47–R47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garidel P, Brandenburg K. Current understanding of polymyxin b applications in bacteraemia/sepsis therapy prevention: clinical, pharmaceutical, structural and mechanistic aspects. Antiinfect Agents Med Chem 2009; 8: 367–385. [Google Scholar]
- 37.Esteban E, Ferrer R, Alsina L, Artigas A. Immunomodulation in sepsis: the role of endotoxin removal by polymyxin b-immobilized cartridge. Mediators Inflamm 2013; 2013: 507539–507539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yaroustovsky M, Abramyan M, Krotenko N, et al. Combined extracorporeal therapy for severe sepsis in patients after cardiac surgery. Blood Purif 2014; 37: 39–46. [DOI] [PubMed] [Google Scholar]
- 39.Yaroustovsky M, Abramyan M, Popok Z, et al. Preliminary report regarding the use of selective sorbents in complex cardiac surgery patients with extensive sepsis and prolonged intensive care stay. Blood Purif 2009; 28: 227–233. [DOI] [PubMed] [Google Scholar]
- 40.Jaber BL, Barrett TW, Cendoroglo Neto M, et al. Removal of cytokine inducing substances by polymyxin-b immobilized polystyrene-derivative fibers during in vitro hemoperfusion of 10% human plasma containing Staphylococcus aureus challenge. ASAIO J 1998; 44: 48–53. [DOI] [PubMed] [Google Scholar]
- 41.Tan NS, Ho B, Ding JL. High-affinity lps binding domain(s) in recombinant factor C of a horseshoe crab neutralizes LPS-induced lethality. FASEB J 2000; 14: 859–870. [DOI] [PubMed] [Google Scholar]
- 42.Falkenhagen D, Harm S and Hartmann J. Dosierungsanleitung für endotoxinbindende lipopeptide. European Patent Application, EP 2679236 A1 2014.
- 43.Falkenhagen D, Hartmann J and Harm S. Extracorporeal perfusion device. European Patent Application, EP2679301 A1 2014.
- 44.Urban C, Tiruvury H, Mariano N, et al. Polymyxin-resistant clinical isolates of Escherichia coli. Antimicrob Agents Chemother 2011; 55: 388–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cohen J, McConnell JS. Release of endotoxin from bacteria exposed to ciprofloxacin and its prevention with polymyxin B. Eur J Clin Microbiol 1986; 5: 13–17. [DOI] [PubMed] [Google Scholar]
- 46.Bergen PJ, Landersdorfer CB, Zhang J, et al. Pharmacokinetics and pharmacodynamics of ‘old' polymyxins: what is new? Diagn Microbiol Infect Dis 2012; 74: 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Markou N, Markantonis SL, Dimitrakis E, et al. Colistin serum concentrations after intravenous administration in critically ill patients with serious multidrug-resistant, Gram-negative bacilli infections: a prospective, open-label, uncontrolled study. Clin Ther 2008; 30: 143–151. [DOI] [PubMed] [Google Scholar]
- 48.Michalopoulos AS, Falagas ME. Colistin: recent data on pharmacodynamics properties and clinical efficacy in critically ill patients. Ann Intensive Care 2011; 1: 30–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohamed AF, Karaiskos I, Plachouras D, et al. Application of a loading dose of colistin methanesulfonate in critically ill patients: population pharmacokinetics, protein binding, and prediction of bacterial kill. Antimicrob Agents Chemother 2012; 56: 4241–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li J, Rayner CR, Nation RL, et al. Pharmacokinetics of colistin methanesulfonate and colistin in a critically ill patient receiving continuous venovenous hemodiafiltration. Antimicrob Agents Chemother 2005; 49: 4814–4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harm S, Gruber A, Gabor F, Hartmann J. Adsorption of selected antibiotics to resins in extracorporeal blood purification. Blood Purif 2015; 41: 55–63. [DOI] [PubMed] [Google Scholar]
- 52.Koomanachai P, Landersdorfer CB, Chen G, et al. Pharmacokinetics of colistin methanesulfonate and formed colistin in end-stage renal disease patients receiving continuous ambulatory peritoneal dialysis. Antimicrob Agents Chemother 2014; 58: 440–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jitmuang A, Nation RL, Koomanachai P, et al. Extracorporeal clearance of colistin methanesulphonate and formed colistin in end-stage renal disease patients receiving intermittent haemodialysis: implications for dosing. J Antimicrob Chemother 2015; 70: 1804–1811. [DOI] [PubMed] [Google Scholar]
- 54.Zavascki AP, Goldani LZ, Cao G, et al. Pharmacokinetics of intravenous polymyxin b in critically ill patients. Clin Infect Dis 2008; 47: 1298–1304. [DOI] [PubMed] [Google Scholar]