Abstract
The menopausal transition, or perimenopause, is associated with profound reproductive and hormonal changes. These changes have been well chronicled and matched with concomitant symptoms. The pattern of appearance of menopausal symptoms and their natural history have become increasingly clear thanks to the conduct of several long-term, longitudinal cohort studies that have examined many aspects of women's biology and psychology through this time of life. Menopausal symptoms are highly prevalent; they are sufficiently bothersome to drive almost 90% of women to seek out their healthcare provider for advice on how to cope.1 The classic symptom of menopause is the hot flash, which is experienced by most women, and is moderately to severely problematic for about 1/3 of women. While most women will have an experience of hot flashes limited to just a year or two, others will experience them for a decade or more, and a small proportion of women will never be free of them. Poor sleep becomes more common in perimenopausal women not only in association with the menopausal transition but also in relation to aging. Depressed mood and increased anxiety also increase during the transition, with an abrupt rise in prevalence as women approach the later stages of the menopausal transition and have longer bouts of amenorrhea. These common symptoms often interact with one another such that depressed women tend to experience worse hot flashes along with worse sleep. As women enter the latter stages of the transition, vaginal dryness and dyspareunia also become more likely, affecting about 1/3 of the population. Unlike hot flashes, mood issues, and sleep, vaginal symptoms will not go away without treatment. Clinical approaches to these problems often involve hormone therapy, which can be safely given to most perimenopausal women on a short-term basis. Therapeutic strategies that are nonhormonal and behavioral can also be deployed.
Introduction
The perimenopause is an ill-defined time period that surrounds the final years of a woman's reproductive life. It begins with the first onset of menstrual irregularity and ends after 1 year of amenorrhea has occurred, thereby defining the final menstrual period (FMP). There are two stages to the perimenopause or menopausal transition: the early transition, where cycles are mostly regular, with relatively few interruptions, and the late transition, where amenorrhea becomes more prolonged and lasts for at least 60 days, up to the FMP. Several worldwide cohorts have defined the natural history of the menopausal transition in sufficient detail such that these stages have been broken down and linked to specific hormonal events, which in turn are linked to symptoms. The purpose of this review is to integrate the research findings with the clinical presentations of symptomatic perimenopausal women, followed by a brief review of treatment options.
The Menopausal Transition Contains Some Speed Bumps
The stages of reproductive aging have been well described in two workshops, with the acronym STRAW.2,3 STRAW stages relevant to this topic include the late reproductive stages (−3b and −3a), the early and late menopausal transition stages (−2 and −1), and the early postmenopause (+1a and +1b). These stages are shown in Figure 1. It is important to note that the loss of ovarian reserve that accompanies the menopausal transition occurs before there is follicle failure, that is, an inability for granulosa cells to respond to a follicle-stimulating hormone (FSH) signal with estradiol production. Thus, the process is not simply a loss of estrogen accompanied by elevated FSH, at least not in its earlier stages.
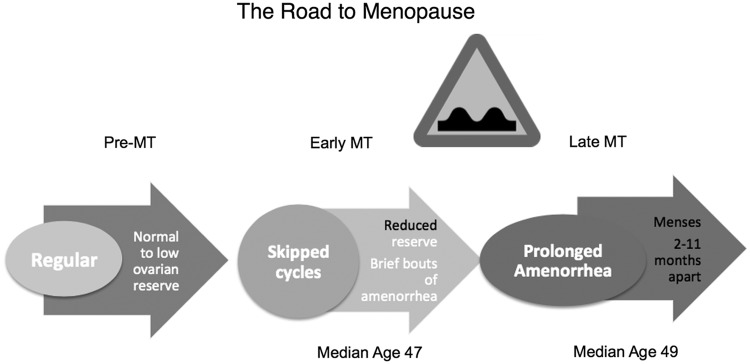
FIG. 1.
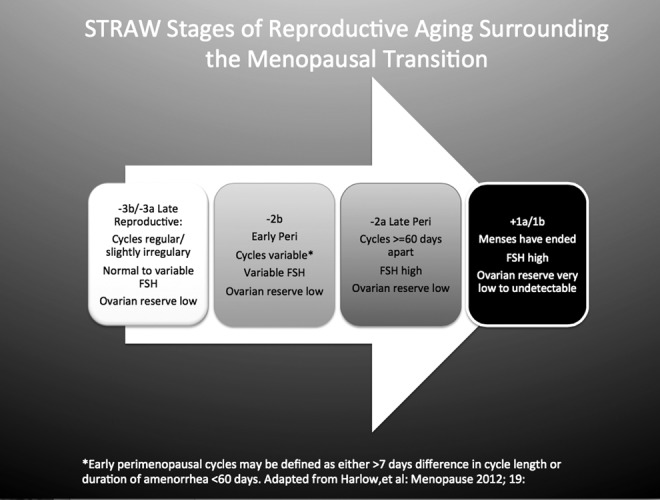
Diagram of the relevant changes in menstrual cycles and hormones associated with the transition to menopause. Ovarian reserve is defined above as a combination of measurements: inhibin B, estradiol, and ultrasound-assessed antral follicle counts.
Stages −3b and −3a encompass the late reproductive years when fertility may still be possible, but less likely than in earlier years. Ovarian reserve fluctuates over this time period, a reflection of a dwindling follicle pool that is variable over time. The cohort of ovarian follicles available for recruitment for the purpose of ovulation is ever diminishing. The hormones produced by small growing follicles in the ovary include antimullerian hormone (AMH) and inhibin B. These hormones have been used as peripheral serum markers of ovarian reserve, and AMH in particular is very effective in predicting the probability of a poor outcome with fertility therapy when it is low.4
Inhibin B, like AMH, is a TGF-beta superfamily peptide that is secreted by granulosa cells of growing follicles and directly suppresses pituitary secretion of FSH. As the follicle cohort shrinks, less inhibin B is produced, leading to the well-characterized monotropic rise in FSH that is a cardinal feature of the menopausal transition.5 Because the follicle cohort is relatively preserved at this point in the process, FSH is often normal and only occasionally elevated. Women usually do not observe any changes in their menstrual cycles or at most a slight increase in variability of menstrual cycle length.
By the time Stage −2, the early transition, is attained, the ovarian follicle cohort has shrunk to a critical level and, usually, a woman will note her first missed menstrual period. Some women may also note that the variation in their menstrual cycle length exceeds 7 days. Either menstrual-based definition heralds progress to the early transition. By this time, FSH is more consistently elevated and ovarian reserve measures, such as inhibin B, AMH, or an ultrasound-measured antral follicle count, are now critically low. Because the follicle cohort is still relatively preserved at these early stages of the transition, the rise in FSH causes folliculogenesis to occur more rapidly,6 and the follicular phase of the menstrual cycle becomes shorter. Follicles grow more quickly, but appear to ovulate at a smaller size.6 An increase in luteal phase follicle growth has also been observed, which means that the subsequent cycle's dominant follicle has already started to grow well before the onset of menses.7 These types of cycles, in which ovulations follow rapidly upon one another, with minimal follicular phase length, have been named luteal out-of-phase or LOOP events.8 These types of cycles contribute further to the menstrual irregularity of the perimenopause and are associated with hormone secretory patterns that deviate from midreproductive-aged women's hormone patterns. Specifically, lower luteal progesterone and higher FSH have been observed.8,9 We have also reported erratic estrogen secretory patterns in association with the transition.10 Thus, there may be some hormone changes associated with altering menstrual patterns and increasing cycle irregularity that can be quite profound and may contribute to symptomatology.
By Stage −1, the late menopausal transition, menstrual cyclicity becomes highly irregular and menstrual periods are scarce. Circulating estrogen is more likely to be low during anovulatory cycles, and the long periods of amenorrhea are accompanied by a sharp increase in the prevalence of common menopausal symptoms. However, when a woman does have a menstrual cycle, it may be ovulatory, anovulatory with relatively high estrogen levels, or anovulatory with low estrogen levels. This stage is the speed bump of the menopausal transition (Fig. 2). For the average woman, the menstrual milestone of the early transition (Stage −2) is age 47, the late transition (Stage −1) occurs at age 49, and the FMP occurs at age 51. However, there is substantial variability in the onset of these milestones. Entry into the transition at an earlier age is not necessarily associated with an earlier FMP; in fact, there is evidence that earlier onset of the transition may herald a longer and therefore more bothersome one.
FIG. 2.
Stages of reproductive aging workshop (STRAW) simplified to show the relevant stages and ages that precede the final menstrual period (FMP). MT, menopausal transition. The speed bump sign indicates the transition period from early to late MT, which is typically associated with an increase of the commonly observed menopausal symptoms: hot flashes, poor sleep, adverse mood, and vaginal dryness.
For the year that defines the FMP and the year after that (Stage +1), progesterone is no longer produced, but fluctuations in estrogen appear to occur. After that time period, hormones restabilize at their permanently postmenopausal levels. Although there are some further changes in hormones after the FMP has been defined, they are not nearly of the magnitude of the profound changes that occur as a woman traverses the menopause.
What Is the Natural History of Menopausal Symptoms?
Longitudinal studies of the menopausal transition have taught us a great deal about the changes that occur in populations of women from cohorts around the world.11–15 Earlier studies from the 1980s and 1990s included cohorts of 400–500 community-based women living in Massachusetts14 and western Pennsylvania.15 These studies were geared toward determining the menstrual milestones that accompanied the menopause transition and the cardiovascular risk factors that change during this time of life, respectively. The second generation of studies that followed women through the menopausal transition specifically sought to include hormonal markers of menopause and to cover a broader range of symptoms and behaviors to build a more comprehensive model of the menopausal experience of women.
These studies include the Melbourne Healthy Women's Study,16 SWAN, the Study of Women's Health Across the Nation,17 and the Penn Ovarian Aging Study.18 These studies have allowed us to define the transition process into menopause more fully and uncovered the concurrence of specific symptoms linked to specific stages of the transition. These longer-term, longitudinal cohort studies have included longer periods of follow-up (9 and 20 years, respectively) and collected much additional information on quality of life, sexual function, bone density, and reproductive hormones. In particular, SWAN included large numbers of women from multiple ethnic backgrounds.19 The Penn Ovarian Aging Study focused on reproductive hormones and collected twice annual specimens from each participant, as opposed to annual specimens in the other two studies.20 All of these studies have been either population based or community based, which is an important consideration in attempting to define the normal menopausal experience for women. However, none of these studies have tracked women with irregular menstrual cycles before the onset of the menopausal transition. This is because most of our definitions of menopausal milestones are still menstrual based.
Taken together, these longitudinal cohort studies have provided a chronology to the menopausal transition that had been previously inaccessible. It has allowed investigators to link key symptoms that were previously believed to occur randomly to the reproductive aging milestones as defined by STRAW. In this process, we have learned that although menopausal symptoms tend to ramp up overall as women enter the late reproductive years and progress through the early menopausal transition, the biggest increment in symptomatology is clearly associated with the late transition—the time when amenorrhea becomes prolonged (60–364 days). In 2005, an NIH workshop associated three cardinal symptoms with the menopausal transition: hot flashes, poor sleep, and vaginal dryness/dyspareunia.21 Several years later, adverse mood also became appreciated as being linked to the menopausal transition.20,22,23 These core four symptoms will be addressed in turn.
Hot Flashes
Hot flashes, or vasomotor symptoms, are a cardinal feature of menopause that is almost universally experienced by women.13 Recent epidemiological evidence indicates that hot flashes are experienced by 30%–70% of premenopausal women,24 but they are likely to be mild in nature at these earlier stages of a woman's reproductive life. They begin to increase in prevalence as women attain the early transition, to about 39%, and nearly doubled for a 67% rate of cumulative reporting among the women in SWAN.25 Others report a cumulative prevalence of 85% for vasomotor symptoms.26 Vasomotor symptoms cause a substantial amount of distress and reduction in health-related quality of life (HRQOL).27 Despite their high prevalence, surprisingly little is understood about their exact pathophysiology.
Hot flash prevalence, and perhaps effectiveness of treatment, varies by race/ethnicity. African-American and Native American women appear to have the highest reporting of hot flashes.24 In the SWAN Study, Chinese and Japanese women had lower rates of hot flash reporting and a shorter overall duration of hot flashes.13 Body size also affects the severity and frequency of hot flash reporting in a complex way. Women of high body–mass index (BMI) report worse hot flashes when they are perimenopausal, but fewer and milder hot flashes once they are postmenopausal.28 Although hot flashes are believed to be related to the withdrawal of estradiol, epidemiological studies, which examine estradiol annually, do not demonstrate a relationship to estradiol, but elevated FSH is predictive of hot flashes.25 Cigarette smoking, anxiety, and depressed mood have all been associated with increased hot flash reporting in a number of studies.14,18,28–30
The duration of hot flashes can be much longer than previously believed.13 While earlier studies indicated that hot flashes were most pronounced in the years immediately preceding and following the FMP,14 more recent work has shown that duration of hot flashes for up to 10 years is not unusual.31 Women at particularly high risk for long duration of hot flashes include African-American women and women of high BMI.31 Moreover, women who begin to experience hot flashes earlier in their menopausal transition are more likely to have a prolonged duration.13,31 Thus, the clinician can help assess a patient for the likelihood that her hot flashes are going to go away quickly or are more likely to persist. This forecasting may help inform a woman's decision to try pharmacologic treatments.
About 20% of women in their late 50s, 10% of women in their 60s, and 5% of women in their 70s experience persistent hot flashes.32 Thus, although they thankfully diminish in prevalence over time for most women, a minority remains highly symptomatic and requires lifelong treatment to maintain quality of life.
Apart from their bother, hot flashes may have implications for a woman's health. Hot flashes have been shown to be linked to reduced heart rate variability (HRV), a marker of vagal control, the loss of which is associated with increased cardiovascular disease risk.33 Women who experience more hot flashes during sleeping hours have greater white matter hyperintensities on magnetic resonance imaging (MRI).34
Sleep Changes
Women begin to experience changes in their sleep patterns in their 40s, and these tend to worsen with entry into the menopausal transition. In addition, there appears to be a component of sleep that is adversely affected by the menopausal transition, although this has recently become controversial. A recent report by the Penn Ovarian Aging Study, which includes 16 years of follow-up, did not find an increasing incidence of self-reported poor sleep with progress through the menopausal transition.35 The latter cohort included 255 women and did not examine sleep architecture in detail, however. In the Melbourne Midlife Women's Health Project, an increase in the complaint of poor sleep was observed as women progressed through menopause.36 Significantly, more older women complain of insomnia compared with other demographic groups.37 However, it is not always clear whether such observations are directly related to menopause, estrogen withdrawal, or other hormone changes, or whether they are simply related to aging.
Sleep disturbances have been associated with hot flashes. Ohayon surveyed a sample of 3243 adults in California and observed a variable prevalence of hot flashes of 12.5% in premenopausal women, 79% in perimenopausal women, and 39.3% of postmenopausal women in association with insomnia rates of 36.5%, 56.6%, and 50.7%, respectively.38 The more severe the hot flashes, the more likely was a woman to report insomnia. A relationship between hot flashes and poor sleep is certainly intuitive, and some explanatory models consider that the anticipation of hot flashes may influence a woman's ability to fall asleep as well as her ability to stay asleep. This is consistent with the observation that self-reported sleep problems seem to become much more prevalent in women than in men as they age beyond 45 years—just about the timing of the onset of the menopausal transition.39
The SWAN Study has provided a number of observations that help clarify the relationships between menopause and sleep. At baseline, 9.8% of women in the SWAN cohort reported insomnia and 37.7% reported difficulty sleeping.39 Sleep difficulty varied with race/ethnicity and was the lowest (28%) in Japanese and highest (40%) in Caucasian women. Sleep difficulty was clearly seen to increase as women traversed the menopause, with late menopausal transition and surgically menopausal women reporting the greatest difficulty.
In addition to menopausal stage, the timing of sleep difficulty within the menstrual cycle was also explored in SWAN by performing daily diary estimates of trouble sleeping along with daily urinary hormone collection. In this study, difficulty sleeping was clearly associated with the perimenstrual phases of the cycle, with early perimenopausal women overall experiencing more poor sleep than women who had not yet experienced a break in their cycles.40 Hot flashes, adverse mood, poor self-perceived health and quality of life, and arthritis contributed to the complaint of poor sleep.39 Physical factors also influence sleep quality. Women with metabolic syndrome experienced substantially less sleep efficiency by polysomnography.41 Hormone therapy attenuated negative effects on sleep,39 particularly in surgically menopausal women.
Taken together, the data seem to indicate that at least some sleep difficulty that occurs concurrent with the menopausal transition can be attributed to the underlying hormonal changes and/or hot flashes.
Vaginal Dryness
Vaginal symptoms are common in postmenopausal women, and vaginal dryness, in particular, is reported to occur in about one-quarter to one-third42–44 women. The constellation of symptoms of vaginal dryness, irritation, and dysuria has been named genitourinary syndrome of menopause (GSM).45 The latter may be a truer reflection of the collective morbidity to the female genital tract caused by a lack of estrogen.
While it is easy to understand the relationship between low estrogen and GSM in postmenopause, the relationship between GSM and early and late perimenopause, when estrogen is not consistently low, is harder to explain. Nonetheless, vaginal symptoms appear relatively early in the transition.46 There is substantial ethnic variation in the reporting of vaginal dryness/dyspareunia, with up to almost 60% of Central American women in the SWAN Study reporting vaginal dryness at baseline compared with 21% of non-Hispanic Caucasian women.46 Unlike hot flashes and adverse mood, which tend to improve over time, vaginal dryness, similar to sleep difficulties, does not get better without specific ongoing treatment.
Adverse Mood
Depressive symptoms are more likely to be reported by women who are perimenopausal.22 Major depression, diagnosed using a Structured Clinical Interview for Diagnosis (SCID), was found to be more likely to occur in women during the late menopausal transition.47–49 Similarly, anxiety symptoms also appear to be more likely to be reported as women traverse the menopause50 and may be linked to the onset of major depression (odds ratio 1.47, p = 0.01).51 Interestingly, women with high anxiety at baseline continued to have high anxiety over the course of the menopausal transition, but those with low scores at study enrollment were more likely to become highly anxious as they progressed through menopause.50
It is important to distinguish depressive symptoms, which occur in a large proportion of women, from major depression, which is a far more serious psychiatric diagnosis. Depressive symptoms indicate a response score to the Center for Epidemiologic Studies-Depression scale (CES-D), that is, >16.52 The prevalence of depressive symptoms in the women in SWAN was 20.9% at baseline, 27.8% in the early menopausal transition, stabilized at 25.2% by the late menopausal transition, and decreased to 22% by postmenopause.49 Chinese and Japanese women in SWAN had the lowest prevalence of depressive symptoms at baseline. However, Japanese women as well as Hispanic women experienced a sharp upturn in depressive symptoms over time that was greater than the rest of the cohort, with a more than doubling among the Hispanic women (OR = 2.45).53 Vasomotor symptoms, stressful life events, smoking, poor social support, financial strain, low educational attainment, and higher BMI were all associated with greater reporting of depressive symptoms. Interestingly, among all the hormones examined, only a higher testosterone level was linked to reporting of depressive symptoms.53
Major depression is a far more severe disorder compared with depressive symptoms. It is diagnosed by five or more of the cardinal symptoms outlined in Table 1 being present for at least 2 weeks. The symptoms must not meet criteria for a mixed episode (manic and depressive symptoms together), they must cause clinically significant distress or impairment, must not be due to direct physiological effects of a substance, and cannot be accounted for by bereavement. Major depression is a life-threatening condition that is far less prevalent than depressive symptoms and was observed in 3% of women at the SWAN baseline assessment.53 As opposed to depressive symptoms, major depression did not vary by race/ethnicity. The risk for new onset of major depression increased as women became perimenopausal (OR = 2.27) and postmenopausal (OR = 3.57).53 Factors associated with major depression included stressful life events, history of an anxiety disorder, and psychotropic medication use—all of which approximately doubled the odds. BMI and hot flashes were associated with major depression in univariate analyses, but the association no longer remained after multivariable analysis.53
Table 1.
Symptoms of Major Depression
| Depressed, sad mood |
| Decreased interest/pleasure in activities |
| Significant weight loss/gain or decreased/increased appetite |
| Insomnia or hypersomnia |
| Psychomotor agitation or retardation |
| Fatigue |
| Feelings of worthlessness or excessive guilt |
| Decreased ability to think or concentrate, or indecisiveness |
| Recurrent thoughts of death, suicidal ideation with or without a plan, or suicide attempt |
Symptoms must be present for at least 2 weeks. Taken from American Psychiatric Association (2000). Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington, DC.
Approaches to Treatment
The above list is by no means comprehensive. It represents a first pass at a series of longitudinal data sets that have examined symptoms over time and, as such, can detect only the most prominent symptoms that are universally experienced by many women. It is quite possible that there are subsets of women who are particularly prone to certain types of symptoms, and it will take additional research time and effort to isolate those groups and make the appropriate linkages between precipitating factors and individual vulnerabilities. Therefore, the key principle in clinical management of women suffering from menopausal symptoms is to distinguish what is typical from what is not and address each woman's individual symptom experience with an eye toward providing maximal relief.
Typical symptoms
Women who present with any of the four symptoms above have a strong evidence base supporting the likelihood that their symptom(s) are related to their menopausal transition, and hormonal therapy is therefore likely to be of benefit. For most women without contraindications to their use, hormones are the most effective way to improve quality of life. Commonly used hormone preparations are listed in Table 2. All of the preparations used for menopausal hormone therapy (MHT) are low dose compared with a woman's premenopausal hormonal fluctuations. They are about ¼ the equivalent estrogen dose of an oral contraceptive pill. Women who experience breakthrough bleeding on lower-dose hormones or who require contraception may benefit from oral contraceptives instead of these lower-dose preparations.
Table 2.
Preparations Used for Menopausal Hormone Therapy
| Trade name | Estrogen | Progestin | FDA approved | Dose |
|---|---|---|---|---|
| Premarin | CEE | — | Yes | 0.3–1.25 mg PO daily |
| Cenestin | Synthetic CE | — | Yes | 0.3–1.25 mg PO daily |
| Menest | Esterified Estrogen | — | Yes | 0.3–1.25 mg PO daily |
| Estrace | 17 β-estradiol | — | Yes | 1–2 mg PO daily |
| Estinyl | Ethinyl estradiol | — | Yes | 0.02–0.05 mg PO 1–3× daily |
| Evamist | 17 β-estradiol | — | Yes | 1–3 sprays daily |
| Alora, Climara, Esclim, Menostar, Vivelle, Vivelle Dot, Estraderm | 17 β-estradiol | — | Yes | 1 patch weekly-twice weekly, 0.014–0.1 mg |
| Estrogel | 17 β-estradiol | — | Yes | 1.25 g daily transdermal gel (equivalent 0.75 mg estradiol) |
| Estrasorb | 17 β-estradiol | — | Yes | 1–2 foil pouches daily of transdermal topical emulsion |
| Activella | Estradiol 1 mg | NETA 0.5 mg | Yes | 1 tab PO daily |
| FemHRT | Ethinyl Estradiol 5 mcg | NETA 1 mg | Yes | 1 tab PO daily |
| Ortho-Prefest | 17 β-estradiol 1 mg | Norgesti-mate 0.09 mg | Yes | First 3 tablets contain estrogen, next 3 contain both hormones; alternate pills every 3 days |
| Premphase | CEE 0.625 mg | MPA 5 mg | Yes | First 14 tablets contain estrogen only and remaining 14 tablets contain both hormones. |
| 1 tab PO daily | ||||
| Prempro | CEE 0.3–0.625 mg | MPA 1.5–5 mg | Yes | 1 tab PO daily |
| Combipatch | 17 β-estradiol 0.05 mg | NETA 0.14 or 0.25 mg | Yes | 1 patch transdermal twice weekly |
| Climara-Pro | 17 β-estradiol 0.045 mg | LNG 0.015 mg | Yes | 1 patch weekly |
| Estrace | 17 β-estradiol vaginal cream | — | Yes | 2–4 g daily ×1 week, then 1 g three times weekly |
| Femring | Estradiol vaginal ring | 0.05 or 0.1 mg | Yes | 1 ring inserted vaginally every 3 months |
| Duavee | CEE 0.45 mg/bazedoxefine 20 mg | Yes | 1 tablet daily |
It is important to remember that a woman in the late menopausal transition is likely to become symptomatic if she takes oral contraceptive pills or other forms of hormonal contraception such as a ring (the patch is not recommended for perimenopausal women) in the usual 3 weeks on/1 week off regimen. Continuous oral contraceptive hormone use is preferred and has been shown to be effective in symptom control.54 Another option that can be considered is the concurrent use of estrogen in combination with a levonorgestrel intrauterine system, which provides endometrial protection and contraception.55
Because observational studies indicate that transdermal estradiol does not increase a woman's risk of venous thromboembolism,56 several clinical guidelines recommend the use of nonoral estrogens when treating postmenopausal women.57 However, the need for concern in perimenopausal women is less clear, and the clinician should aim for the preparation that is best tolerated by the patient.
When hormones are contraindicated or otherwise unacceptable to a patient, there are some other options that are now on-label for treatment. For vasomotor symptoms, paroxetine mesylate, a 7.5 mg long-acting salt of paroxetine, was recently FDA approved for this indication. For vaginal dryness, ospemifene, 60 mg, a new selective estrogen receptor modulator (SERM), has also been FDA approved. For adverse mood symptoms, the selective serotonin reuptake inhibitor (SSRI) class of drugs is a reasonable alternative for depression. Similarly, while there are no menopause-specific remedies for poor sleep, treatments ranging from behavioral modification for insomnia58 to melatonin receptor agonists may be tried.59 For women who have hot flashes that are bothersome only at night, gabapentin in a small nightly dose of 100–300 mg may be highly effective.
Other pharmacological agents that have demonstrated effectiveness against hot flashes in clinical trials include the SSRI/selective norepinephrine reuptake inhibitor (SNRI) class of drugs, gabapentin, clonidine, and progesterone/progestins.26 Nonpharmacologic or botanical remedies for menopausal symptoms have been largely ineffective in well-conducted clinical trials. These ineffective treatments include yoga,60 omega-3 fatty acid supplementation,61 and black cohosh.62
Approach to atypical symptoms
Since the full panoply of menopausal symptoms is not yet known with any great degree of certainty, it is important to seek an accurate history of all of the symptoms that a woman associates with her menopausal transition. Quite often, a commitment to a short course (3 months) of MHT will clarify whether or not a true relationship exists between symptoms and hormones. When in doubt, withdrawal of the hormones and a rechallenge will help to eliminate doubt.
Multiple symptom treatment with a single agent
Several menopausal symptoms may be intertwined. For example, hot flashes may exacerbate sleep disruption and contribute to depressive symptoms. It therefore makes sense to consider single agents that may be active against more than one symptom. Since midlife women also often have additional health problems such as hypertension, it also makes sense to try to introduce as few additional medications as possible and therefore finding an agent that is active against multiple concurrent conditions can be ideal. Women with hot flashes and depression can be treated with hormone therapy if the depression is mild to moderate in severity, but an SSRI/SNRI agent may be an excellent alternative. Women with concomitant hypertension and vasomotor symptoms may be treated with clonidine, a centrally active alpha-1-adrenergic blocker, and, if successful, the single medication may treat both problems. Sometimes, addressing a different health issue, such as impaired glucose tolerance, will lead to weight loss, which will have a positive impact on hot flashes. These approaches should be part of every clinician's armamentarium.
Summary
The menopausal transition is a challenging period of life for many women, yet these are women who are in the prime of their careers and are often caught in the sandwich generation situation, in which they are caring for both children and parents. They and society can ill afford to lose any of their human potential. It is therefore critical to continue the efforts outlined herein to understand how perimenopause fits into the context of womens' lives and to anticipate and address the common and not so common symptoms that may cause morbidity or at least loss of quality of life.
Author Disclosure Statement
Dr. Santoro has investigator-initiated grant support from Bayer and stock options in Menogenix, Inc.
References
- 1.Guthrie JR, Dennerstein L, Taffe JR, Donnelly V. Healthcare-seeking for menopausal problems. Climacteric 2003;6:112–117 [PubMed] [Google Scholar]
- 2.Harlow SD, Gass M, Hall JE, et al. Executive summary of the Stages of Reproductive Aging Workshop +10: Addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab 2012;97:1159–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soules MR, Sherman S, Parrott E, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW). Climacteric 2001;4:267–272 [PubMed] [Google Scholar]
- 4.Santoro N, Cedars M, Hansen K. AMH, FSH and AFC: The ABC's of ovarian reserve testing for the generalist. ACOG Update 2015;41 [Google Scholar]
- 5.Sherman BM, West JH, Korenman SG. The menopausal transition: Analysis of LH, FSH, estradiol, and progesterone concentrations during menstrual cycles of older women. J Clin Endocrinol Metab 1976;42:629–636 [DOI] [PubMed] [Google Scholar]
- 6.Santoro N, Isaac B, Neal-Perry G, et al. Impaired folliculogenesis and ovulation in older reproductive aged women. J Clin Endocrinol Metab 2003;88:5502–5509 [DOI] [PubMed] [Google Scholar]
- 7.Brink HV, Chizen D, Hale G, Baerwald A. Age-related changes in major ovarian follicular wave dynamics during the human menstrual cycle. Menopause 2013;20:1243–1254 [DOI] [PubMed] [Google Scholar]
- 8.Hale GE, Hughes CL, Burger HG, Robertson DM, Fraser IS. Atypical estradiol secretion and ovulation patterns caused by luteal out-of-phase (LOOP) events underlying irregular ovulatory menstrual cycles in the menopausal transition. Menopause 2009;16:50–59 [DOI] [PubMed] [Google Scholar]
- 9.Santoro N, Crawford SL, Lasley WL, et al. Factors related to declining luteal function in women during the menopausal transition. J Clin Endocrinol Metab 2008;93:1711–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santoro N, Brown JR, Adel T, Skurnick JH. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab 1996;81:1495–1501 [DOI] [PubMed] [Google Scholar]
- 11.Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet Gynecol 2000;96:351–358 [DOI] [PubMed] [Google Scholar]
- 12.Freeman EW, Sammel MD, Sanders RJ. Risk of long-term hot flashes after natural menopause: Evidence from the Penn Ovarian Aging Study cohort. Menopause 2014;21:924–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med 2015;175:531–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas 1992;14:103–115 [DOI] [PubMed] [Google Scholar]
- 15.Matthews KA, Wing RR, Kuller LH, Meilahn EN, Plantinga P. Influence of the perimenopause on cardiovascular risk factors and symptoms of middle-aged healthy women. Arch Intern Med 1994;154:2349–2355 [PubMed] [Google Scholar]
- 16.Burger HG, Dudley E, Mamers P, Groome N, Robertson DM. Early follicular phase serum FSH as a function of age: The roles of inhibin B, inhibin A and estradiol. Climacteric 2000;3:17–24 [DOI] [PubMed] [Google Scholar]
- 17.Santoro N, Randolph JF., Jr Reproductive hormones and the menopause transition. Obstet Gynecol Clin North Am 2011;38:455–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeman EW, Sammel MD, Lin H. Temporal associations of hot flashes and depression in the transition to menopause. Menopause 2009;16:728–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sowers M, Crawford S, Sternfeld B. Design, survey sampling and recruitment methods of SWAN: A mutli-center, multi-ethnic, community-based cohort study of women and the menopausal transition. In: Kelsey J, Lobo RA, Marcus R, eds. Menopause: Biology and Pathobiology, Vol. 175. San Diego, CA: Academic Press, 2000;175–188. [Google Scholar]
- 20.Freeman EW, Sammel MD, Lin H, et al. Symptoms associated with menopausal transition and reproductive hormones in midlife women. Obstet Gynecol 2007;110:230–240 [DOI] [PubMed] [Google Scholar]
- 21.Multiple A. Management of menopausal symptoms. Am J Med 2005;118:1–171 [Google Scholar]
- 22.Bromberger JT, Matthews KA, Schott LL, et al. Depressive symptoms during the menopausal transition: The Study of Women's Health Across the Nation (SWAN). J Affect Disord 2007;103:267–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen LS, Soares CN, Vitonis AF, Otto MW, Harlow BL. Risk for new onset of depression during the menopausal transition: The Harvard study of moods and cycles. Arch Gen Psychiatry 2006;63:385–390 [DOI] [PubMed] [Google Scholar]
- 24.Reed SD, Lampe JW, Qu C, et al. Premenopausal vasomotor symptoms in an ethnically diverse population. Menopause 2014;21:153–158 [DOI] [PubMed] [Google Scholar]
- 25.Randolph JF, Jr, Sowers M, Bondarenko I, et al. The relationship of longitudinal change in reproductive hormones and vasomotor symptoms during the menopausal transition. J Clin Endocrinol Metab 2005;90:6106–6112 [DOI] [PubMed] [Google Scholar]
- 26.ACOG Practice Bulletin No. 141: Management of menopausal symptoms. Obstet Gynecol 2014;123:202–216 [DOI] [PubMed] [Google Scholar]
- 27.Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: A comprehensive review. Health Qual Life Outcomes 2005;3:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thurston RC, Sowers MR, Sternfeld B, et al. Gains in body fat and vasomotor symptom reporting over the menopausal transition: The study of women's health across the nation. Am J Epidemiol 2009;170:766–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gold EB, Block G, Crawford S, et al. Lifestyle and demographic factors in relation to vasomotor symptoms: Baseline results from the Study of Women's Health Across the Nation. Am J Epidemiol 2004;159:1189–1199 [DOI] [PubMed] [Google Scholar]
- 30.Stearns V, Ullmer L, Lopez JF, Smith Y, Isaacs C, Hayes D. Hot flushes. Lancet 2002;360:1851–1861 [DOI] [PubMed] [Google Scholar]
- 31.Freeman EW, Sammel MD, Lin H, Liu Z, Gracia CR. Duration of menopausal hot flushes and associated risk factors. Obstet Gynecol 2011;117:1095–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunner RL, Aragaki A, Barnabei V, et al. Menopausal symptom experience before and after stopping estrogen therapy in the Women's Health Initiative randomized, placebo-controlled trial. Menopause 2010;17:946–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thurston RC, Christie IC, Matthews KA. Hot flashes and cardiac vagal control during women's daily lives. Menopause 2012;19:406–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thurston RC, Aizenstein HJ, Derby CA, Sejdic E, Maki PM. Menopausal hot flashes and white matter hyperintensities. Menopause 2015. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 35.Freeman EW, Sammel MD, Gross SA, Pien GW. Poor sleep in relation to natural menopause: A population-based 14-year follow-up of midlife women. Menopause 2015;22:719–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dennerstein L, Lehert P, Guthrie JR, Burger HG. Modeling women's health during the menopausal transition: A longitudinal analysis. Menopause 2007;14:53–62 [DOI] [PubMed] [Google Scholar]
- 37.Bixler EO, Kales A, Soldatos CR, Kales JD, Healey S. Prevalence of sleep disorders in the Los Angeles metropolitan area. Am J Psychiatry 1979;136:1257–1262 [DOI] [PubMed] [Google Scholar]
- 38.Ohayon MM. Severe hot flashes are associated with chronic insomnia. Arch Intern Med 2006;166:1262–1268 [DOI] [PubMed] [Google Scholar]
- 39.Kravitz HM, Joffe H. Sleep during the perimenopause: A SWAN story. Obstet Gynecol Clin North Am 2011;38:567–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kravitz HM, Janssen I, Santoro N, et al. Relationship of day-to-day reproductive hormone levels to sleep in midlife women. Arch Intern Med 2005;165:2370–2376 [DOI] [PubMed] [Google Scholar]
- 41.Hall MH, Okun ML, Sowers M, et al. Sleep is associated with the metabolic syndrome in a multi-ethnic cohort of midlife women: The SWAN Sleep Study. Sleep 2012;35:783–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barnabei VM, Grady D, Stovall DW, et al. Menopausal symptoms in older women and the effects of treatment with hormone therapy. Obstet Gynecol 2002;100:1209–1218 [DOI] [PubMed] [Google Scholar]
- 43.Pastore LM, Carter RA, Hulka BS, Wells E. Self-reported urogenital symptoms in postmenopausal women: Women's Health Initiative. Maturitas 2004;49:292–303 [DOI] [PubMed] [Google Scholar]
- 44.Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med 2009;6:2133–2142 [DOI] [PubMed] [Google Scholar]
- 45.Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: New terminology for vulvovaginal atrophy from the International Society for the Study of Women's Sexual Health and the North American Menopause Society. Climacteric 2014;17:557–563 [DOI] [PubMed] [Google Scholar]
- 46.Green R, Polotsky AJ, Wildman RP, et al. Menopausal symptoms within a Hispanic cohort: SWAN, the Study of Women's Health Across the Nation. Climacteric 2010;13:376–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen L, Soares C, Vitonis A, Otto M, Harlow B. Risk for new onset of depression during the menopausal transition: The Harvard study of moods and cycles. Arch Gen Psychiatry 2006;63:386–390 [DOI] [PubMed] [Google Scholar]
- 48.Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, Hollander L. Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch Gen Psychiatry 2004;61:62–70 [DOI] [PubMed] [Google Scholar]
- 49.Bromberger JT, Kravitz HM, Chang YF, Cyranowski JM, Brown C, Matthews KA. Major depression during and after the menopausal transition: Study of Women's Health Across the Nation (SWAN). Psychol Med 2011;41:1879–1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bromberger JT, Kravitz HM, Chang Y, et al. Does risk for anxiety increase during the menopausal transition? Study of women's health across the nation. Menopause 2013;20:488–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kravitz HM, Schott LL, Joffe H, Cyranowski JM, Bromberger JT. Do anxiety symptoms predict major depressive disorder in midlife women? The Study of Women's Health Across the Nation (SWAN) Mental Health Study (MHS). Psychol Med 2014;44:2593–2602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401 [Google Scholar]
- 53.Bromberger JT, Kravitz HM. Mood and menopause: Findings from the Study of Women's Health Across the Nation (SWAN) over 10 years. Obstet Gynecol Clin North Am 2011;38:609–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sulak PJ. The perimenopause: A critical time in a woman's life. Int J Fertil Menopausal Stud 1996;41:85–89 [PubMed] [Google Scholar]
- 55.Somboonporn W, Panna S, Temtanakitpaisan T, Kaewrudee S, Soontrapa S. Effects of the levonorgestrel-releasing intrauterine system plus estrogen therapy in perimenopausal and postmenopausal women: Systematic review and meta-analysis. Menopause 2011;18:1060–1066 [DOI] [PubMed] [Google Scholar]
- 56.Canonico M, Oger E, Plu-Bureau G, et al. Hormone therapy and venous thromboembolism among postmenopausal women: Impact of the route of estrogen administration and progestogens: The ESTHER study. Circulation 2007;115:840–845 [DOI] [PubMed] [Google Scholar]
- 57.Santen RJ, Allred DC, Ardoin SP, et al. Postmenopausal hormone therapy: An Endocrine Society scientific statement. J Clin Endocrinol Metab 2010;95:s1–s66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buysse DJ. Insomnia. JAMA 2013;309:706–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bruyneel M. Sleep disturbances in menopausal women: Aetiology and practical aspects. Maturitas 2015;81:406–409 [DOI] [PubMed] [Google Scholar]
- 60.Newton KM, Reed SD, Guthrie KA, et al. Efficacy of yoga for vasomotor symptoms: A randomized controlled trial. Menopause 2014;21:339–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cohen LS, Joffe H, Guthrie KA, et al. Efficacy of omega-3 for vasomotor symptoms treatment: A randomized controlled trial. Menopause 2014;21:347–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nedrow A, Miller J, Walker M, Nygren P, Huffman LH, Nelson HD. Complementary and alternative therapies for the management of menopause-related symptoms: A systematic evidence review. Arch Intern Med 2006;166:1453–1465 [DOI] [PubMed] [Google Scholar]