Abstract
The first human gene therapy trials using recombinant adeno-associated virus (rAAV) vectors were performed in cystic fibrosis (CF) patients. Over 100 CF patients were enrolled in 5 separate trials of rAAV2-CFTR administration via nasal, endobronchial, maxillary sinus, and aerosol delivery. Recombinant AAV vectors were designed to deliver the CF transmembrane regulator (CFTR) gene and correct the basic CFTR defect by restoring chloride transport and reverting the upregulation of proinflammatory cytokines. However, vector DNA expression was limited in duration because of the low incidence of integration and natural airway epithelium turnover. In addition, repeated administration of AAV-CFTR vector resulted in a humoral immune response that prevented effective gene transfer from subsequent doses of vector. AAV serotype 2 was used in human trials before the comparison with other serotypes and determination that serotypes 1 and 5 not only possess higher tropism for the airway epithelium, but also are capable of bypassing the binding and trafficking processes—both were important hindrances to the effectiveness of rAAV2. Although rAAV-CFTR gene therapy does not appear likely to supplant newer small-molecule CFTR modulators in the near future, early work with rAAV-CFTR provided an important foundation for later use of rAAV in humans.
Introduction
Cystic fibrosis (CF) is an autosomal recessive life-limiting disease caused by mutations in the gene encoding the CF transmembrane conductance regulator protein (CFTR). CF is characterized clinically by chronic airway obstruction and infection, exocrine pancreatic insufficiency, and abnormally high sweat electrolyte concentrations.1 Increased mucus viscosity, a prominent aspect of the presentation of CF, inhibits exocrine gland secretion and impedes clearance of bacteria from pulmonary airways via impaired mucociliary clearance, resulting in inflammation and polymorphonuclear neutrophilic leukocyte infiltration.2 The inflammatory characteristic of the disease is thought to be caused by an upregulation of interleukin-8 and tumor necrosis factor-α production and downregulation of the anti-inflammatory cytokine, interleukin-10.3 As a result of the increased mucus and aberrant inflammatory processes, CF patients experience chronic airway infections with specific bacteria, including Pseudomonas aeruginosa and Staphylococcus aureus.
When CF was first distinguished pathologically from celiac disease in 1938, the life expectancy was limited to 6 months as a result of malabsorption.4 The discovery of elevated ionic strength of sweat gland secretions in CF allowed for the implementation of a convenient diagnostic test based on sweat electrolyte concentrations and the generation of the hypothesis that chloride transport abnormalities represented the unifying defect in the organs affected in CF patients.1
Positional cloning allowed for the identification in 1989 of the CFTR gene as the primary defect in CF.5–7 The CFTR gene was identified as a member of the ATP-binding cassette (ABC) protein superfamily, functioning as a low-conductance, adenosine 3′,5′-cyclic monophosphate (cAMP)-dependent chloride channel in the affected exocrine organ systems and a regulator of other transmembrane channels.6,8,9 Five domains compose CFTR: two transmembrane domains, two nucleotide binding domains, and one regulatory domain.10 Internal sites in the transmembrane domain of CFTR can function as initiation codons for translation. Removal of more than the first four sections of transmembrane domain 1 results in loss of functional chloride channels, whereas removal of only the first four sections of the domain yield ion selectivity comparable to wild type though with diminished open probability and channel conductance.
Two thousand mutations have been defined with severe mutations either affecting chloride channel function (e.g., the G551D mutation) or its localization to the membrane (e.g., the F508-del mutation).11 There are six different classes of CFTR mutations. F508-del is the most common mutation affecting about 90% of patients with CF in the United States. This mutation involves the deletion of a phenylalanine and is characterized as a class II mutation—that is, the F508-del mutation sequesters the mutant CFTR in the endoplasmic reticulum, preventing the formation of CFTR chloride channels on the apical membrane.12 The other classes of CFTR mutations are less common in the United States and include the following: class I mutations result from premature truncation or nonsense alleles; class III mutations encode processed full-length CFTR proteins that reach the cell surface but possess little, or no, ion channel activity; class IV mutations exhibit only partial CFTR activity, whereas class V mutations have decreased CFTR transcripts; and class VI mutations have defective CFTR stability at the cell surface.13 The different classes of CFTR mutations have become particularly important over the last few years with the success of targeted small molecular therapy (see below).
CFTR activation requires protein kinase A phosphorylation and ATP binding to the nucleotide binding domains. In the absence of functional CFTR, protein kinase A is incapable of activating chloride channels.14 The CFTR anion channel is crucial to absorption and secretion, and is implicated in exocrine organ dysfunction. Functional CFTR is also responsible for normal immune regulation.15 Deficiency of CFTR in lymphocytes results in an exaggerated IgE response to pathogens with increased levels of proinflammatory cytokines, indicating a direct link between CFTR function in T-cells and immune function.
The median age of survival for patients with CF is now approximately 40 years. This is because of the treatment of malabsorption with enzyme replacement therapy, rigorous chest physical therapy and mucolytics to relieve airway obstruction, and treatment of airway infections with antibiotics.16–19 However, despite these interventions, many patients with CF still develop progressive airway obstruction, bronchiectasis, and some eventually undergo lung transplantation. This is in part because of the effects of an abnormal CFTR on the immune system. For example, CF causes abnormal ionic composition and disruption of normal polymorphonuclear neutrophilic leukocyte phagocytosis, which act as primary defenses against major bacterial invaders, such as S. aureus, P. aeruginosa, and Aspergillus fumigatus.2 The exact mechanism of polymorphonuclear neutrophilic leukocyte phagocytosis is unknown but most likely involves defective apoptosis and impaired degranulation in CF neutrophils.20,21 In addition, chronic P. aeruginosa infection results in a defective acid—sphingomyelinase—an enzyme important to the innate immune system, resulting in a 40% decrease in N-glycosylation, interleukin-8 augmentation, and the inability to cure the infection.22,23 Finally, as life expectancy lengthens without a cure for the genetic defect, the incidence of CF-related diabetes increases.24
The last few years have been promising for CF care because of the discovery of new small-molecule therapies. In particular, dramatic benefits have been observed from a CFTR potentiator, VX770 (or Ivacaftor), which is a potentiator of CFTR and allows the channel to open for more effective chloride transport. This therapy is useful for class III (e.g., G551D) and class IV mutations, which are CFTR channel gating and channel conductance defects, respectively.25 However, as mentioned above, the majority of patients with CF have the F508-del mutation, which is a class II, or CFTR protein processing mutation that prevents the protein from reaching the cell membrane. Orkambi, a therapy geared toward these class II mutations, is now FDA approved. Orkambi consists of a combination of ivacaftor and lumacaftor (a medication that improves CFTR processing and chloride secretion—i.e., a CFTR corrector).26 Unfortunately, this therapy has not been as effective as the CFTR potentiator and only results in a slight improvement in pulmonary function (4% improvement in FEV1), which is of questionable clinical significance.26 The limited success of these small-molecule therapies for the majority of CF patients who carry the type II mutations underscores the need for a treatment that offers mutation-independent correction of CFTR mutations. Gene therapy to correct the deficient mutation would ideally target all CFTR mutations. However, the focus of this review is to outline the historical significance of rAAV2-CFTR vector development, which provided important foundational safety data regarding the safety of recombinant adeno-associated virus (rAAV) in humans.
Rationale for Studies of rAAV2-CFTR
When the CFTR gene was discovered in 1989, much hope was placed in gene replacement therapy to cure the disease. Human cell culture studies supported the potential for correction of the disease pathology with the expression of a normal copy of the CFTR gene.27 Successful in vitro complementation with retroviral delivery to CF pancreatic adenocarcinoma cells sparked investigation into viral transgene delivery to the airway epithelium to correct the loss of function mutation.28 Although Moloney murine leukemia-based virus vectors, vaccinia virus-based vectors, and baculovirus vectors had previously been used for CFTR gene expression in cell culture, none of these were well-suited for in vivo gene therapy of CF.27–29 Retroviral introduction to the airway epithelium was not ideal because of the requirement for ex vivo manipulation and actively dividing cells for transduction.9,28 In this case, an ideal gene therapy vector would allow for long-term persistence of CFTR in the absence of a host inflammatory response within cells that are not actively dividing.
AAV serotype 2 (AAV2), a member of Parvovirus family, is a small single-stranded DNA virus that was explored as a potential eukaryotic gene transfer vector because of its tropism for the respiratory epithelium with no associated pathology.18,30 (Table 1). Although over 100 natural variants of AAV are now known, most were discovered after 2002. Therefore, the “type-strain,” serotype 2, was the most thoroughly investigated for CF gene therapy. While retroviruses necessitate active cell division for integration and expression, AAV2 does not have this constraint. AAV enters a stable form of latency in the absence of adenovirus, herpesvirus, or vaccinia.3 As a small single-stranded DNA virus, AAV consists of only two genes, rep and cap,31 and so is relatively easy to manipulate in proviral plasmids.
Table 1.
Anticipated advantages of rAAV2-CFTR as understood during the preclinical and early phase clinical development of the rAAV2-CFTR vector and inherent limitations of AAV-mediated CF gene therapy as identified after preclinical and clinical trials of rAAV2-CFTR
| Advantages | Limitations |
|---|---|
| Nonpathogenic | Small packaging requirement of AAV virion |
| Lower risk of insertional mutagenesis | AAV persistence is largely episomal/low efficiency of integration |
| Higher viral titers | CF byproducts cause inactivation of transgene |
| Capable of transducing fully differentiated cells | Generation of neutralizing antibodies with repeat administration |
| Prolonged persistence | Optimal serotype not applied |
| Low activity of endogenous promoter |
AAV, adeno-associated virus; CF, cystic fibrosis; CFTR, CF transmembrane regulator.
Ind Enabling Studies: Preclinical Development, Proof-Of-Concept
The initial hurdle to be overcome in the development of rAAV2-CFTR was packaging this very large transgene (coding sequence of >4.4 kb) along with a promoter, polyadenylation signal, and the mandatory 145 bp inverted terminal repeats (ITRs) into a virus that naturally is only 4.7 kb in length. A series of constructs were created in which the CFTR gene was packaged in rAAV vectors, replacing the viral genes between the two ITRs. When packaged with an endogenous promoter, p5, the vector was slightly larger than the wild-type virus, but was still capable of correcting the cAMP-regulated chloride efflux.14,31 However, in the course of these studies, it was discovered that the ITR has intrinsic promoter activity allowing for expression of CFTR with a very compact promoter element.32 Truncated versions of the CFTR protein with the deletion of the amino-terminus were also capable of correcting the CF defect, as demonstrated by restoration of forskolin-stimulated chloride efflux.14 Forskolin increases intracellular cAMP, which facilitates chloride efflux when functioning CFTR is present. CFTR-deficient bronchial epithelial cells treated with forskolin showed an increase in cAMP with no corresponding increase in chloride efflux; however, the same cells transduced with AAV-CFTR resulted in an marked increase in chloride efflux.33 Immunofluorescence assay stained cells with anti-CFTR antibodies demonstrated the correlation between expression of CFTR and cAMP-regulated chloride conductance, as only cells with functional CFTR demonstrated an increase in chloride conductance. Western blot analysis demonstrated increased CFTR protein and Southern blots of Hirt extracts demonstrated replication of integrated rAAV vector genomes in complemented IB3 cells, validating the feasibility of using rAAV2-mediated CFTR gene transfer.32
Complementation with CFTR delivered by rAAV2 corrected the defective cAMP regulation of outwardly rectifying chloride channels characteristic of CF, allowing for normal chloride secretion.8 Correction of the defect through CFTR transgene delivery also resulted in the activation of outwardly rectifying chloride channels by protein kinase A, thus resolving a long-time discrepancy between the concept of CFTR as a chloride channel and that of CFTR as a regulator of other channels. This pivotal 1992 Nature article indicated that both concepts were valid. Patch-clamp experiments in human epithelial cells verified the roles of both CFTR chloride channels and outwardly rectifying chloride channels in cAMP-activated cell currents.8,10 It was presumed that complementation of only 6% of the bronchial epithelial cells would suffice in correction of the defect.34
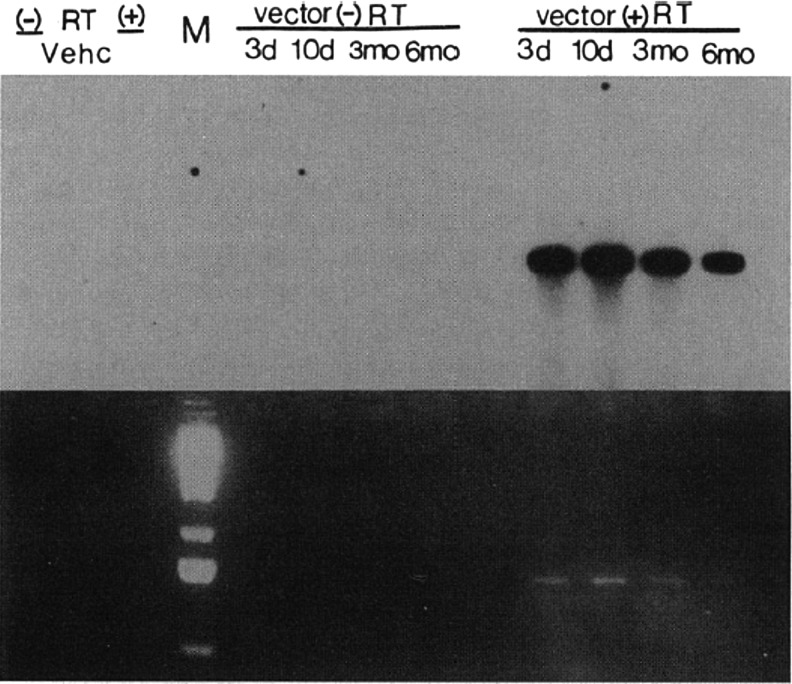
Preliminary attempts at in vivo recombinant AAV transgene delivery in Sprague-Dawley rats were performed intratracheally, resulting in low but detectable levels of gene transfer three to seven days postintroduction.18,31 In a subsequent milestone 1993 study, direct endobronchial instillation of rAAV2-CFTR was accomplished through a fiberoptic bronchoscope in New Zealand white rabbits to assess efficacy in vivo. Transgene delivery to the right lower lobe of the rabbit lung resulted in expression of CFTR protein and mRNA in a fashion that was sustained for 6 months35 (Fig. 1). CFTR expression was generally limited to the lobe to which the rAAV was directed. The stability of CFTR expression six months after in vivo delivery to the rabbit lung demonstrated the possibilities for applying gene therapy to correct the pathophysiologic defect and prevent progressive CF lung disease in humans.
Figure 1.
The first demonstration of in vivo gene expression from an rAAV vector in a living animal was this RT-PCR from rabbit bronchial epithelial cells harvested at intervals from 3 days to 6 months after instillation of rAAV2-CFTR through a fiberoptic bronchoscope in a 1993 publication. A Southern blot (above) and an ethidium bromide-stained gel (below) depict the products of RT-PCR on total cellular RNA extracted from lung tissue homogenates collected 3 days, 10 days, 3 months, and 6 months after vector administration. The lung homogenate harvested from the vehicle-treated animal (vehc), along with the reactions without reverse transcriptase (− RT), act as control. rAAV, recombinant adeno-associated virus; CFTR, cystic fibrosis transmembrane regulator; RT, reverse transcriptase. [Reproduced with permission from Flotte et al.31]
Pivotal preclinical nonhuman primate data were then generated as endobronchial administration of AAV-CFTR to rhesus macaques resulted in vector gene transfer and expression six months postdelivery without inflammation or other adverse consequences.36,37 Vector DNA was detected in 50% of the bronchial epithelial cells in the highest dosage group.36 The safety and efficacy of single-dose vector delivery to the airway epithelium of macaques resulted in the approval of a phase I clinical trial by the National Institutes of Health Recombinant DNA Advisory Committee (RAC) and subsequent granting of an FDA Investigational New Drug (IND) application with Targeted Genetics Corporation as the sponsor and producer of the cGMP vector material.
Discovery of Episomal Persistence
Understanding the mechanism of persistence of rAAV vectors ultimately proved to be very important for the entire field of rAAV gene therapy. While site-specific integration of wild-type AAV2 into the AAVS1 site on human chromosome 19 had been detected at a frequency of 50–70% within immortalized cell lines,38 our laboratory's studies demonstrated that rep-deleted rAAV2 vectors persisted episomally.28,35,36 In nondividing cell cultures, both Southern blots and fluorescence in situ hybridization (FISH) indicated that recombinant rAAV2-CFTR persisted episomally rather than by site-specific integration, although wild-type AAV integration was predominantly site-specific in cell culture as had been reported earlier.39
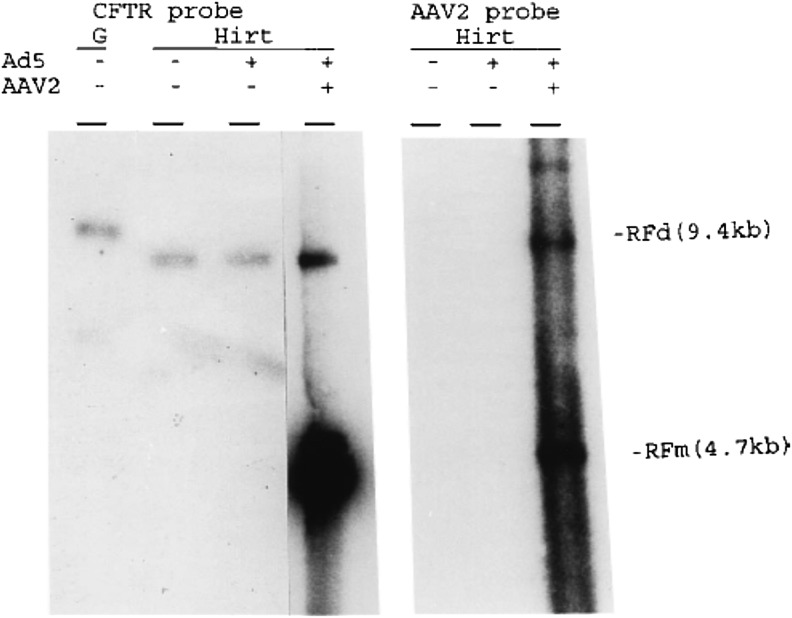
In order to confirm the significance of these findings, we modeled rAAV2-CFTR, wt-AAV2, and adenovirus replication and persistence in rhesus macaques. We used a host-range mutant adenovirus Ad2HR405, capable of replicating in rhesus and providing AAV helper function. In these studies, double-stranded rAAV2 vector DNA persisted episomally in bronchial epithelial cells, as judged by Southern blot and FISH, and was rescued from cells in vitro with the addition of wild-type AAV and adenovirus37 (Fig. 2). As natural hosts for AAV, these organisms were presumed to function as a better animal model for human gene therapy because most CF patients are likely AAV-seropositive.14 Because bronchial epithelium is more mitotically active in CF lungs compared with normal lungs, AAV's episomal persistence would ultimately prove to be a major limitation of the vector system.40
Figure 2.
Discovery of episomal persistence in vivo in the rhesus lung. [Reproduced with permission from Afione et al.37] Primary rhesus bronchial epithelial cells harvested from animals after in vivo AAV-CFTR delivery were cultured and evaluated by Southern blot with (+) and without (−) Ad5 and AAV2 supernatants. Lane G shows the genomic DNA after BamH1 digestion. The left Southern blot was analyzed by an internal specific CFTR cDNA probe and the right by an AAV2 internal specific probe. AAV-CFTR was determined to persist episomally, as Hirt supernatants without AAV2 resulted in episomal bands with sizes of AAV-CFTR and wild-type AAV2 replicating-form dimers.
Animal Studies Performed Subsequent to Initiating Phase 1 Clinical Trials
In later studies, intratracheal AAV-CFTR delivery to CFTR knockout mice attenuated the hyper-IgE expression present in allergic bronchopulmonary aspergillosis, a syndrome seen in 15% of CF patients.15 Complementation with CFTR vector delivery or administration of a hypermannose water diet dissipates the inflammatory response and reverts the bacterial stress on the trachea and oropharynx of mice that act as in vivo models for P. aeruginosa.23
Investigation into the transduction of functional CFTR to the pulmonary epithelium during neonatal development was performed in newborn New Zealand white rabbits to test the hypothesis that the administration of the transgene early in life would allow for long-term persistence and prevent pulmonary inflammation and damage before the onset of the disease.41 Intratracheal instillation delivery of AAV-CFTR to the respiratory epithelium was found to be safe and efficacious through the alveolar developmental phase. Vector DNA was not evident six months after administration, marking a divergence from previous studies in rhesus macaques that could be attributed to the different vector introduction mechanisms, indicating that endobronchial administration may sustain more favorable results than intratracheal instillation. In addition, the higher rate of epithelial cell turnover in the neonates compared with the adults may explain the inconsistency between the trials in rhesus macaques and New Zealand white rabbits. This higher turnover likely results in the diminution of the vector DNA over time. Repeat administration appeared to be necessary to address the waning vector persistence.
Further investigation into the feasibility of repeat recombinant AAV vector administration was performed bronchoscopically in rabbits in an attempt to attain sustainable CFTR expression in the airway epithelium.42 Two doses of vector resulted in high titers of serum anti-AAV neutralizing antibodies. The vector capsid was then switched from AAV2 to AAV3 (one of the first examples of pseudotyping of rAAV) in order to avoid the neutralizing antibodies. Administration of the third AAV dose as a rAAV3 vector expressing green fluorescent protein (GFP) resulted in GFP expression within the airway epithelial cells three weeks after delivery. This demonstrated the ability of serotype switching to overcome neutralizing antibodies.
Four lung deposition techniques, nebulized through a mouthpiece, laryngeal mask, or endotracheal tube, or bronchoscopically delivered by microsprayer, were compared in rhesus macaques to determine the optimal method for vector instillation.43 Bronchoscopic delivery by microsprayer resulted in the highest lung deposition fraction compared with the other delivery methods and easily allowed for AAV expression in the lungs three weeks later. Successive administration of AAV2-CFTR vector by bronchoscopic microspraying allowed for gene transfer and transgene expression while neutralizing antibodies were generated toward the AAV serotype in rhesus macaques.44 Optimizing the deposition technique, increasing titer production, and knowing the efficacy of recurrent doses were needed for more effective clinical applications for CF.
The activities generated by the cytomegalovirus (CMV) Rous sarcoma virus (RSV) promoter, CMV enhancer/β actin (CB) promoter, and the CMV enhancer/RSV promoter hybrid were compared in vitro to assess whether an exogenous promoter would yield better expression than the previously implemented endogenous ITR promoter. The CB promoter was most effective in driving AAV to express the CFTR transgene.33 This new version of AAV-CFTR vector corrected defective chloride transport in bronchial cell culture and reverted the hyperinflammatory lung phenotype evident in CF mice.
Previous research with AAV2 in animal models proved safe and efficacious despite low target gene expression.45 As the prototype serotype, AAV2 inefficiently targets the apical receptors on the respiratory epithelium compared with other serotypes, such as AAV5, that target these receptors directly. Low gene expression was also attributed to the application of the endogenous promoter. A truncated CFTR in AAV5 allows for the construct to have an exogenous promoter such as the CB promoter, which was presumed to increase expression. Aerosolized vector delivery to the lungs of nonhuman primates resulted in quantifiable gene and protein expression that surpassed endogenous levels. The powerful nature of the CB promoter combined with the truncated CFTR in the construct showed promise in therapeutic applications.33,45
Different AAV pseudotypes may have higher tropism for the lung than AAV2 and the ability to bypass the host's humoral immune response.46 In 2004, a comparison of activity with different AAV capsid serotypes and promoters demonstrated that AAV5 was significantly more efficacious than the vector implemented previously, AAV2.40 AAV5 not only possessed higher expression levels in the airway epithelium, but it (along with AAV1) also had the capacity to bypass AAV2's hindrances, the binding and trafficking processes, indicating both AAV5 and AAV1's value as next-generation vectors.46 The low gene transfer efficiency of AAV2 was tied to the vector's inability to bind to the apical surface of human airway epithelial cells efficiently unless heparan sulfate proteoglycans are present on the cell surface. In most cases, the human airway epithelial cells appeared to be resistant to AAV2 infection because of the absence of heparan sulfate proteoglycans. The binding and trafficking processes of AAV2 are inhibited by soluble heparin because of the degradation of heparan sulfate proteoglycans that are necessary for the vector to bind. On the other hand, AAV5's processes are unaffected, indicating that AAV5-mediated gene transfer occurs by an alternative mechanism that allows for higher transduction efficiency.47 The benefits of AAV pseudotypes other than AAV2 led for a comparison between AAV1 and AAV5 to determine which serotype would serve as a better vector for gene transfer.48 Ninety days after vector administration to the chimpanzee airway, AAV1 yielded a 20-fold higher transduction efficiency compared with AAV5, the vector that also generated a more significant immune response to its viral capsid.
rAAV Biology in Polarized Human Airway Epithelia
These preclinical and clinical studies elucidated significant information about AAV serotypes and their tropisms for different preclinical models, providing an important perspective for the future development of CF gene therapy. Through these studies, differences in the biology of infection and efficiency of transduction from the apical surface of the human airway epithelium between rAAV serotypes were revealed.
Although it was previously believed to exhibit low gene transfer efficiency, rAAV2 actually has high gene transfer efficiency on the apical surfaces despite the known absence of heparin sulfate proteoglycans.49 Instead, the marked low expression of apically internalized virions stems from differences in the endosomal processing and nuclear trafficking compared with virions internalized basolaterally.49 Because the barrier to effective transgene expression is at the level of endosomal processing and ubiquination, modulation of the ubiquitin–proteasome system could enhance gene expression lessening the distinction between AAV2 internalized apically and basolaterally, increasing the overall gene transfer efficiency of the AAV2 serotype.49
Selection of an appropriate preclinical model is as important as the selection of the AAV serotype. rAAV's tropisms for specific hosts could hinder the relevance or transferability of the findings to human clinical trials. Though nonhuman primates such as rhesus monkeys were widely applied as preclinical models for human CF gene therapy, they were later determined to poorly represent rAAV transduction of human airway epithelia.50 The efficiency of the apical and basolateral transduction of rAAV1, rAAV2, and rAAV5 was significantly higher in the rhesus monkey airway epithelium compared with human airway epithelium, indicating that these serotypes show a higher tropism for the rhesus monkey airway epithelium.50 rAAV5 also has a higher transduction efficiency in mouse airway epithelium compared with human airway epithelium.51 Cross-species variation in vector tropism has made it difficult to apply animal models to inform human trials; however, intratracheal delivery to chimpanzees has yielded promising results with AAV1 and AAV5 transduction efficiencies comparable to that in human airway epithelium.48
Clinical Development
Phase I study: nasal epithelium and endobronchial AAV-CFTR vector administration to adult CF patients with mild lung disease
Based on the positive findings in rhesus macaques, 12 adult CF patients presenting with mild lung disease were recruited to participate in a single-center, randomized, double-blind, placebo-controlled, dose escalation phase I clinical study, in which AAV was administered to humans for the first time.52 A single dose of either AAV-CFTR or placebo was administered to the nasal epithelium and all patients received a single, open-label AAV-CFTR dose to superior segment of the right lower lobe of the lung. Gene transfer efficiency to the nasal and bronchial epithelium was low, consistent with later findings that AAV2 transduction subsequent to apical internalization proceeds inefficiently.49,53 Reasons for the low gene transfer efficiency at the time were thought to be the competition for AAV binding by an excess of mucus glycoproteins, low cell proliferation, or inhibitory effects of host's immune defenses.54 A comparison of bronchoalveolar fluid from healthy and CF patients in vitro indicated that bronchoalveolar fluid from patients with the CF defect possessed inhibitory activity that resulted in a 5–20-fold decrease in AAV's transduction efficiency. The inherent AAV inhibitory activity of bronchoalveolar fluid from CF patients was attributed to neutrophil alpha defensins, which were inversely correlated with AAV's transduction efficiency and elevated 10,000-fold in samples with reduced efficiency. CF presents with elevated levels of neutrophil alpha defensins corresponding with bronchoalveolar fluid inhibitory activity because it is an inflammatory disease and the neutrophil alpha defensins reduce AAV transduction efficiency. As an acute-phase reactant protein, alpha-1 antitrypsin (AAT) serum concentrations elevate in response to inflammation in order to downregulate the inflammatory response. AAT levels were investigated in CF patients to determine if the AAV inhibitory properties of the bronochoalveolar fluid were affected by AAT. The inhibitory activity was mitigated and in some cases even completed reversed by AAT, indicating that the accumulation of neutrophil alpha defensins at inflammatory sites was disrupted by AAT.53 These results indicated the potential for improved gene transfer efficiency when AAT pretreatment was administered before gene therapy. This allowed for a reversal of the bronchoalveolar fluid inhibitory activity and increased AAV-CFTR transduction to the bronchial epithelial cells. These preliminary studies demonstrated the safety of CF gene therapy despite the low levels of expression.44
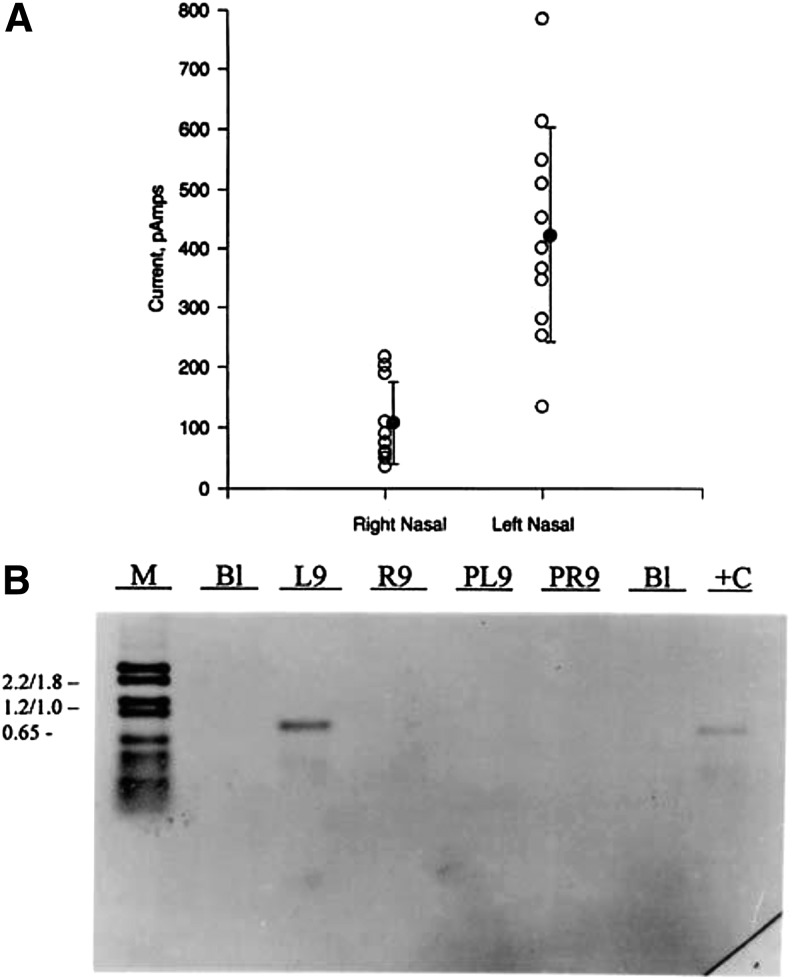
Analysis of primary nasal cells harvested after the administration of CF gene therapy demonstrated the correlation between AAV2-CFTR vector genomes, CFTR mRNA expression, and chloride channel activity in the cells33 (Fig. 3). Even low levels of mRNA resulted in physiological correction of CF pathology, demonstrating the possibility for complementation of the CFTR defect despite the low vector penetration.33
Figure 3.
Correlation between rAAV2-CFTR gene transfer and restoration of cAMP-activated chloride flux (A). Cells were harvested from rAAV2-CFTR trial patients after in vivo gene delivery and then grown in primary culture for analysis. Primary cultures taken by bronchial or nasal brushings (B1, L9, R9) or from nasal polypectomy (PL9, PR9) were analyzed by DNA PCR for rAAV2-CFTR sequences (B). The primary culture from L9 (left nasal sample of patient 9), which had shown strong positive DNA signal, was then analyzed by whole cell current analysis after stimulation with a cAMP activation cocktail. [Reproduced with permission from Flotte et al.33]
Phase I/II study: maxillary sinus delivery of AAV-CFTR for the treatment of chronic sinusitis in patients with CF
This clinical study served the purpose of investigating the safety of AAV-CFTR in humans and determining the dosage that results in the transduction of either 25% of maxillary sinus epithelial cells or the maximum tolerated dose. In addition, the study examined the differences between placebo and AAV-CFTR administration to patients with CF who had recurrent sinusitis. Maxillary sinus analysis was conducted because of the similarity of their ion transport systems and microbiology with the lower respiratory epithelium.55
Before enrollment in the study, patients' nasal mucosa was screened for adenovirus and AAV to ensure that they did not have prior exposure.55 Patients were assigned to participate in either the dose escalation or the double-blind nasal exposure to AAV-CFTR vector with exposure to a placebo in the other sinus. The purpose of the dose escalation study was to determine the optimal or maximum allowable AAV-CFTR dose. The randomized nasal exposure aimed to study the effects of the AAV-CFTR on the presentation of sinusitis. Vector-mediated complementation and induced cAMP chloride conductance was assessed through analysis of the in vivo transepithelial potential difference—the difference in voltage across the maxillary sinus epithelium. Transepithelial potential difference is applied in the diagnosis of CF because these individuals have a more negative nasoepithelium than those without CF—this is a result of reduced chloride secretion and increased sodium absorption. As reverse transcriptase PCR was limited in its ability to detect vector transcripts, transepithelial potential difference measurements were performed after vector administration and compared with a baseline voltage. Hyperpolarization corresponding with CFTR expression and improved chloride secretion elucidated the dose-dependent relationship between AAV-CFTR treatment and expression in the maxillary sinus. Vector administration resulted in safe, dose-dependent gene transfer that persisted for up to 10 weeks with no significant immune response or contralateral exposure to the untreated sinus, demonstrating the potential for targeted exposure.55,56 AAV-CFTR treatment also decreased the concentrations of the inflammatory cytokine, interleukin-8, in sinus fluid.3
Phase II study: maxillary sinus delivery of AAV-CFTR to CF patients with antrostomies
Based on the positive results of the phase I/II clinical trial demonstrating the safety and dose-dependent nature of CFTR gene transfer in the maxillary sinuses, a phase II trial was conducted.57 Patients who had previously received endoscopic antrostomies were administered AAV-CFTR in a double-blind, placebo-controlled manner with one sinus receiving the vector and the other a placebo. The rate of relapse, defined by the clinical manifestation of sinusitis, in the treated and untreated maxillary sinuses did not differ significantly. The secondary endpoints, sinus transepithelial potential difference, histopathology, and sinus fluid interleukin-8, were not significantly different between the treated maxillary sinus and the untreated contralateral sinus. One secondary endpoint, measurement of anti-inflammatory cytokine interleukin-10, was significantly increased in the fluid from the treated sinus compared with the control 90 days after vector delivery. Increased levels of IL-10 with CFTR transgene administration reduced inflammation, indicating improved CFTR function and demonstrating that there may be a significant treatment effect 90 days postadministration. Though the safety of vector delivery was demonstrated with this phase II clinical trial, its efficacy was put into question because of the absence of statistically significant differences between the treated and untreated maxillary sinuses.57
Phase II/IIB study: repeat administration of aerosolized AAV-CFTR to lungs of patients with CF
A phase II multicenter double-blind placebo-controlled study was performed to assess the safety of repeated vector administration to the airways of patients with CF.58 Patients with mild lung disease received three doses of either aerosolized DNase-resistant vector or placebo at 30-day intervals. Evidence of a significant improvement in induced sputum interleukin-8 and forced expiratory volume in the first second (FEV1) was seen between the treatment groups.58 All patients administered the transgene generated serum-neutralizing antibodies to the AAV capsid. In a subset of patients enrolled in the study, gene transfer, but not gene expression, was evident, as a result of effective infection and inefficient intracellular processing.49
As a follow-up to this study, a phase IIB study was executed to further investigate the safety and benefit of repeated aerosolized vector delivery.59 Over 100 subjects with mild to moderate CF received two doses of either aerosolized DNase-resistant AAV-CFTR or placebo. Even though the vector was tolerated well, the FEV1 was not statistically significant between the treatment groups, contradicting the results from the previous study.58,59 The absence of significant improvements in FEV1, changes in induced sputum biologic markers, or duration of antibiotic use indicated that AAV-CFTR did not sustainably improve lung function in CF patients.
Current Status of Gene Therapy for CF
Despite the strides made toward an effective gene therapy treatment for CF over the past 25 years, obstacles remain limiting its feasibility. Correction of CF pathophysiology was achieved with CFTR gene transfer; however, the low expression of the endogenous promoter and low efficiency of integration coupled with the initiation of an immune response with subsequent administrations presented hurdles for effective therapy. Other obstacles associated with gene therapy for CF are inherent to the disease pathophysiology, such as the location of the defect on the conducting airway epithelium requiring the vector to transport from the airway to the nuclei within airway epithelium.60
In recent years, less emphasis has been made on developing gene therapy for CF because of the improvement of treatments for the disease and the creation of pharmaceuticals targeted toward the manifestation of the disease.19 The success with small-molecule studies has demonstrated the efficacy of treating CF with these molecules; thus, the role of gene therapy is quite uncertain.
Genome editing with CRISPR/Cas9, TALENs, or Zn-finger nucleases (ZFNs) could be used for more specific and definitive correction of the CF defect. The remarkable efficiency of the CRISPR/Cas9 system has attracted particular attention. Initial genome editing efforts with CRISPR/Cas9 have focused on ex vivo manipulation of stem cells or organoid cultures.61 If AAV were to have a role in CF gene therapy, it would most likely be in the context of delivering the necessary components for genome editing. The promise of such gene therapy approaches will rely on the development of a greater understanding of stem cell and regenerative biology and a greater technical ability to manipulate and reintroduce cells into the airways of the lungs. Such approaches will ultimately need to be objectively evaluated in order to determine if they do, in fact, offer any advantage over small-molecule CFTR modulators and conventional CF therapies.
Acknowledgments
The National Institute of Diabetes and Digestive and Kidney Diseases (R01DK098252) and the National Institute of Child Health and Human Development (1 K08 HD077040-01A1) funded the work described in this review.
Author Disclosure
T.R.F. is a paid consultant for Dimension Therapeutics and Editas Medicine. H.S.L. and M.K.E. have no competing financial interests.
References
- 1.Di Sant'Agnese PA, Darling RC, Perera GA, et al. Abnormal electrolyte composition of sweat in cystic fibrosis of the pancreas; clinical significance and relationship to the disease. Pediatrics 1953;12:549–563 [PubMed] [Google Scholar]
- 2.Flotte TR, Carter BJ. Adeno-associated virus vectors for gene therapy of cystic fibrosis. Methods Enzymol 1998;292:717–732 [DOI] [PubMed] [Google Scholar]
- 3.Flotte TR. Gene therapy for cystic fibrosis. Curr Opin Mol Ther 1999;1:510–516 [PubMed] [Google Scholar]
- 4.Davis PB. Cystic fibrosis since 1938. Am J Resp Crit Care Med 2006;173:475–482 [DOI] [PubMed] [Google Scholar]
- 5.Kerem B, Rommens JM, Buchanan JA, et al. Identification of the cystic fibrosis gene: Genetic analysis. Science 1989;245:1073–1080 [DOI] [PubMed] [Google Scholar]
- 6.Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science 1989;245:1066–1073 [DOI] [PubMed] [Google Scholar]
- 7.Rommens JM, Iannuzzi MC, Kerem B, et al. Identification of the cystic fibrosis gene: Chromosome walking and jumping. Science 1989;245:1059–1065 [DOI] [PubMed] [Google Scholar]
- 8.Egan M, Flotte T, Afione S, et al. Defective regulation of outwardly rectifying Cl- channels by protein kinase A corrected by insertion of CFTR. Nature 1992;358:581–584 [DOI] [PubMed] [Google Scholar]
- 9.Flotte TR, Carter BJ. In vivo gene therapy with adeno-associated virus vectors for cystic fibrosis. Adv Pharmacol 1997;40:85–101 [DOI] [PubMed] [Google Scholar]
- 10.Carroll TP, Morales MM, Fulmer SB, et al. Alternate translation initiation codons can create functional forms of cystic fibrosis transmembrane conductance regulator. J Biol Chem 1995;270:11941–11946 [DOI] [PubMed] [Google Scholar]
- 11.Cutting GR. Spectrum of mutations in cystic fibrosis. J Bioenerg Biomembr 1993;25:7–10 [DOI] [PubMed] [Google Scholar]
- 12.Swiatecka-Urban A, Brown A, Moreau-Marquis S, et al. The short apical membrane half-life of rescued {Delta}F508-cystic fibrosis transmembrane conductance regulator (CFTR) results from accelerated endocytosis of {Delta}F508-CFTR in polarized human airway epithelial cells. J Biol Chem 2005;280:36762–36772 [DOI] [PubMed] [Google Scholar]
- 13.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med 2005;352:1992–2001 [DOI] [PubMed] [Google Scholar]
- 14.Carter BJ, Flotte TR. Development of adeno-associated virus vectors for gene therapy of cystic fibrosis. Curr Top Microbiol Immunol 1996;218:119–144 [DOI] [PubMed] [Google Scholar]
- 15.Mueller C, Torrez D, Braag S, et al. Partial correction of the CFTR-dependent ABPA mouse model with recombinant adeno-associated virus gene transfer of truncated CFTR gene. J Gene Med 2008;10:51–60 [DOI] [PubMed] [Google Scholar]
- 16.MacLusky IB, Gold R, Corey M, et al. Long-term effects of inhaled tobramycin in patients with cystic fibrosis colonized with Pseudomonas aeruginosa. Pediatr Pulmonol 1989;7:42–48 [DOI] [PubMed] [Google Scholar]
- 17.FitzSimmons SC. The changing epidemiology of cystic fibrosis. Curr Probl Pediatr 1994;24:171–179 [DOI] [PubMed] [Google Scholar]
- 18.Flotte TR, Carter BJ. Adeno-associated virus vectors for gene therapy. Gene Ther 1995;2:357–362 [PubMed] [Google Scholar]
- 19.Cutting GR. Cystic fibrosis genetics: From molecular understanding to clinical application. Nat Rev Genet 2015;16:45–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pohl K, Hayes E, Keenan J, et al. A neutrophil intrinsic impairment affecting Rab27a and degranulation in cystic fibrosis is corrected by CFTR potentiator therapy. Blood 2014;124:999–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moriceau S, Lenoir G, Witko-Sarsat V. In cystic fibrosis homozygotes and heterozygotes, neutrophil apoptosis is delayed and modulated by diamide or roscovitine: Evidence for an innate neutrophil disturbance. J Innate Immun 2010;2:260–266 [DOI] [PubMed] [Google Scholar]
- 22.Yu H, Zeidan YH, Wu BX, et al. Defective acid sphingomyelinase pathway with Pseudomonas aeruginosa infection in cystic fibrosis. Am J Resp Cell Mol Biol 2009;41:367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martino AT, Mueller C, Braag S, et al. N-glycosylation augmentation of the cystic fibrosis epithelium improves Pseudomonas aeruginosa clearance. Am J Resp Cell Mol Biol 2011;44:824–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stalvey MS, Brusko TM, Mueller C, et al. CFTR mutations impart elevated immune reactivity in a murine model of cystic fibrosis related diabetes. Cytokine 2008;44:154–159 [DOI] [PubMed] [Google Scholar]
- 25.Ramsey BW, Davies J, McElvaney NG, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 2011;365:1663–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wainwright CE, Elborn JS, Ramsey BW, et al. Lumacaftor-Ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med 2015;373:220–231 [DOI] [PubMed] [Google Scholar]
- 27.Rich DP, Anderson MP, Gregory RJ, et al. Expression of cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells. Nature 1990;347:358–363 [DOI] [PubMed] [Google Scholar]
- 28.Drumm ML, Pope HA, Cliff WH, et al. Correction of the cystic fibrosis defect in vitro by retrovirus-mediated gene transfer. Cell 1990;62:1227–1233 [DOI] [PubMed] [Google Scholar]
- 29.Kartner N, Hanrahan JW, Jensen TJ, et al. Expression of the cystic fibrosis gene in non-epithelial invertebrate cells produces a regulated anion conductance. Cell 1991;64:681–691 [DOI] [PubMed] [Google Scholar]
- 30.Carter BJ, Antoni BA, Klessig DF. Adenovirus containing a deletion of the early region 2A gene allows growth of adeno-associated virus with decreased efficiency. Virology 1992;191:473–476 [DOI] [PubMed] [Google Scholar]
- 31.Flotte TR, Afione SA, Conrad C, et al. Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc Natl Acad Sci U S A 1993;90:10613–10617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flotte TR, Afione SA, Solow R, et al. Expression of the cystic fibrosis transmembrane conductance regulator from a novel adeno-associated virus promoter. J Biol Chem 1993;268:3781–3790 [PubMed] [Google Scholar]
- 33.Flotte TR, Schwiebert EM, Zeitlin PL, et al. Correlation between DNA transfer and cystic fibrosis airway epithelial cell correction after recombinant adeno-associated virus serotype 2 gene therapy. Hum Gene Ther 2005;16:921–928 [DOI] [PubMed] [Google Scholar]
- 34.Johnson LG, Olsen JC, Sarkadi B, et al. Efficiency of gene transfer for restoration of normal airway epithelial function in cystic fibrosis. Nat Genet 1992;2:21–25 [DOI] [PubMed] [Google Scholar]
- 35.Flotte TR, Afione SA, Zeitlin PL. Adeno-associated virus vector gene expression occurs in nondividing cells in the absence of vector DNA integration. Am J Resp Cell Mol Biol 1994;11:517–521 [DOI] [PubMed] [Google Scholar]
- 36.Conrad CK, Allen SS, Afione SA, et al. Safety of single-dose administration of an adeno-associated virus (AAV)-CFTR vector in the primate lung. Gene Ther 1996;3:658–668 [PubMed] [Google Scholar]
- 37.Afione SA, Conrad CK, Kearns WG, et al. In vivo model of adeno-associated virus vector persistence and rescue. J Virol 1996;70:3235–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kotin RM, Siniscalco M, Samulski RJ, et al. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci U S A 1990;87:2211–2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kearns WG, Afione SA, Fulmer SB, et al. Recombinant adeno-associated virus (AAV-CFTR) vectors do not integrate in a site-specific fashion in an immortalized epithelial cell line. Gene Ther 1996;3:748–755 [PubMed] [Google Scholar]
- 40.Sirninger J, Muller C, Braag S, et al. Functional characterization of a recombinant adeno-associated virus 5-pseudotyped cystic fibrosis transmembrane conductance regulator vector. Hum Gene Ther 2004;15:832–841 [DOI] [PubMed] [Google Scholar]
- 41.Rubenstein RC, McVeigh U, Flotte TR, et al. CFTR gene transduction in neonatal rabbits using an adeno-associated virus (AAV) vector. Gene Ther 1997;4:384–392 [DOI] [PubMed] [Google Scholar]
- 42.Beck SE, Jones LA, Chesnut K, et al. Repeated delivery of adeno-associated virus vectors to the rabbit airway. J Virol 1999;73:9446–9455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beck SE, Laube BL, Barberena CI, et al. Deposition and expression of aerosolized rAAV vectors in the lungs of Rhesus macaques. Mol Ther 2002;6:546–554 [DOI] [PubMed] [Google Scholar]
- 44.Flotte TR, Zeitlin PL, Reynolds TC, et al. Phase I trial of intranasal and endobronchial administration of a recombinant adeno-associated virus serotype 2 (rAAV2)-CFTR vector in adult cystic fibrosis patients: A two-part clinical study. Hum Gene Ther 2003;14:1079–1088 [DOI] [PubMed] [Google Scholar]
- 45.Fischer AC, Smith CI, Cebotaru L, et al. Expression of a truncated cystic fibrosis transmembrane conductance regulator with an AAV5-pseudotyped vector in primates. Mol Ther 2007;15:756–763 [DOI] [PubMed] [Google Scholar]
- 46.Virella-Lowell I, Zusman B, Foust K, et al. Enhancing rAAV vector expression in the lung. J Gene Med 2005;7:842–850 [DOI] [PubMed] [Google Scholar]
- 47.Zabner J, Seiler M, Walters R, et al. Adeno-associated virus type 5 (AAV5) but not AAV2 binds to the apical surfaces of airway epithelia and facilitates gene transfer. J Virol 2000;74:3852–3858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flotte TR, Fischer AC, Goetzmann J, et al. Dual reporter comparative indexing of rAAV pseudotyped vectors in chimpanzee airway. Mol Ther 2010;18:594–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duan D, Yue Y, Yan Z, et al. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J Clin Invest 2000;105:1573–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu X, Luo M, Trygg C, et al. Biological differences in rAAV transduction of airway epithelia in humans and in Old World non-human primates. Mol Ther 2007;15:2114–2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu X, Yan Z, Luo M, et al. Species-specific differences in mouse and human airway epithelial biology of recombinant adeno-associated virus transduction. Am J Resp Cell Mol Biol 2006;34:56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flotte T, Carter B, Conrad C, et al. A phase I study of an adeno-associated virus-CFTR gene vector in adult CF patients with mild lung disease. Hum Gene Ther 1996;7:1145–1159 [DOI] [PubMed] [Google Scholar]
- 53.Virella-Lowell I, Poirier A, Chesnut KA, et al. Inhibition of recombinant adeno-associated virus (rAAV) transduction by bronchial secretions from cystic fibrosis patients. Gene Ther 2000;7:1783–1789 [DOI] [PubMed] [Google Scholar]
- 54.Fasbender A, Zabner J, Zeiher BG, et al. A low rate of cell proliferation and reduced DNA uptake limit cationic lipid-mediated gene transfer to primary cultures of ciliated human airway epithelia. Gene Ther 1997;4:1173–1180 [DOI] [PubMed] [Google Scholar]
- 55.Wagner JA, Reynolds T, Moran ML, et al. Efficient and persistent gene transfer of AAV-CFTR in maxillary sinus. Lancet 1998;351:1702–1703 [DOI] [PubMed] [Google Scholar]
- 56.Wagner JA, Messner AH, Moran ML, et al. Safety and biological efficacy of an adeno-associated virus vector-cystic fibrosis transmembrane regulator (AAV-CFTR) in the cystic fibrosis maxillary sinus. Laryngoscope 1999;109:266–274 [DOI] [PubMed] [Google Scholar]
- 57.Wagner JA, Nepomuceno IB, Messner AH, et al. A phase II, double-blind, randomized, placebo-controlled clinical trial of tgAAVCF using maxillary sinus delivery in patients with cystic fibrosis with antrostomies. Hum Gene Ther 2002;13:1349–1359 [DOI] [PubMed] [Google Scholar]
- 58.Moss RB, Rodman D, Spencer LT, et al. Repeated adeno-associated virus serotype 2 aerosol-mediated cystic fibrosis transmembrane regulator gene transfer to the lungs of patients with cystic fibrosis: A multicenter, double-blind, placebo-controlled trial. Chest 2004;125:509–521 [DOI] [PubMed] [Google Scholar]
- 59.Moss RB, Milla C, Colombo J, et al. Repeated aerosolized AAV-CFTR for treatment of cystic fibrosis: A randomized placebo-controlled phase 2B trial. Hum Gene Ther 2007;18:726–732 [DOI] [PubMed] [Google Scholar]
- 60.Armstrong DK, Cunningham S, Davies JC, et al. Gene therapy in cystic fibrosis. Arch Disease Child 2014;99:465–468 [DOI] [PubMed] [Google Scholar]
- 61.Schwank G, Koo BK, Sasselli V, et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 2013;13:653–658 [DOI] [PubMed] [Google Scholar]