Abstract
Background and aims
N-terminal pro B-type natriuretic peptide (NT-proBNP) is inversely associated with diabetes mellitus, obesity and metabolic syndrome. We aim to characterize the association between NT-proBNP and nonalcoholic fatty liver disease (NAFLD), a condition strongly associated with metabolic syndrome.
Methods
4529 participants from the Multi-Ethnic Study of Atherosclerosis (MESA) free of cardiovascular disease, without self-reported liver disease and not diabetic at their baseline visit in 2000- 2002 were included in this analysis. NAFLD was defined by a liver attenuation <40 HU. Relative prevalence (RP) for NAFLD was assessed adjusted for age, race, and sex, percent of dietary calories derived from fat, total intentional exercise, alcoholic drinks per week, and interleukin-6 by quintiles of NT-proBNP. Adjusted linear spline model was used to characterize a non-linear association between NT-proBNP and liver fat. The inflection point (IP) was the NT-proBNP concentration where there was a change in slope in the association between liver attenuation and NT-proBNP.
Results
RP for NAFLD decreased by 30% from the lowest to the highest quintile of NT-proBNP, p = 0.01. We observed an inverse linear association between NT-proBNP and liver fat, which plateaued (IP) at an NT-proBNP concentration of 45 pg/mL. Linear regression coefficient (SE) per unit of NT-proBNP < and ≥ IP was of 0.05 (0.02), p = 0.001 and 0.0006 (0.0008), p = 0.5, respectively, differences between slopes p < 0.0001.
Conclusions
In this cross-sectional study of a community based multiethnic sample of non-diabetic adults, low levels of NT-proBNP are associated with greater prevalence of NAFLD.
Keywords: Natriuretic peptides, Non-alcohol fatty liver disease, NT-proBNP, Hounsfield Units
1. Introduction
Non-alcoholic fatty liver disease (NAFLD), defined as the accumulation of fat in the hepatic parenchyma (steatosis) with or without inflammation [1], is considered the hepatic component of the metabolic syndrome [2]. Not surprisingly, obesity, insulin resistance, metabolic syndrome and type 2 diabetes are associated cross-sectionally and prospectively with NAFLD [3, 4]. Therefore, the accumulation of fat in the liver, the development of insulin resistance, and type 2 diabetes may share common risk factors.
Natriuretic peptides are inversely associated with percent body fat, fasting blood glucose and triglycerides [5-7]. Furthermore, natriuretic peptides have been shown to be inversely associated with incident diabetes [8, 9]. These observations may stem from the metabolic effects of natriuretic peptides on lipoproteins [10], lipolysis, mitochondrial density and fat oxidation [11]. Given the common pathophysiological mechanisms of NAFLD with diabetes and other metabolic disorders, it is possible that natriuretic peptides have an effect on liver fat. In support of this assumption, Lazo M. et al. using liver enzymes as surrogate markers of liver fat demonstrated a U-shaped association between gamma-glutamyl transferase (GGT), and N-terminal pro B-type natriuretic peptide (NT-proBNP) [12].
The Multi-Ethnic Study of Atherosclerosis (MESA) measured baseline levels of NT-proBNP and estimated liver fat using computed tomographic imaging in a group of individuals free of cardiovascular disease. This allows for a quantitative assessment of the association between circulatory levels of NT-proBNP and the amount of liver fat.
The objective of this study is therefore to characterize the association between NT-proBNP and liver fat, as assessed by computed tomography, in a racially diverse group, without existing cardiovascular disease. We hypothesize that higher levels of NT-proBNP, but still within the “physiological range” is associated with less liver fat.
2. Methods
2.1. Study Subjects
This study included 6814 men and women MESA study participants of diverse ethnic and racial background (white, Africa-American, Chinese and Hispanics). They were recruited between July 2000 and August 2002, and were between 45 – 85 years of age and free of overt cardiovascular disease. Detailed description on the aims of the MESA study and the characteristics of this cohort is described in [13, 14]. The institutional review boards at all participating centers approved the study and written informed consent was obtained from every participant prior to data collection. For this cross sectional analysis data were restricted to 4529 MESA participants without self-reported liver disease, without diabetes at baseline and in whom NT-proBNP, gamma-glutamyl transpeptidase (GGT) and liver attenuation in Hounsfield units (HU) were measured at their baseline visit in 2000-2002.
2.2. Baseline demographic, anthropometric and metabolic characteristics
Among the anthropometric, cardiovascular and metabolic parameters that were included are: body mass index (BMI) (computed as weight (kg)/height2 (meters)), systolic and diastolic blood pressures; blood lipids, insulin, and glucose. Dietary energy and fat are derived by assigning tabulated weights to each food item in the food frequency questionnaire and summing [15]. Serum NT-proBNP was measured at the VA San Diego Healthcare System using an ElecSys 2010 analyzer (Roche Diagnostics, Indianapolis, Indiana, USA) with intra-assay and interassay coefficients of variation of 1.3 and 4.8%, respectively [16]. We assessed insulin resistance using the homeostasis model assessment of insulin resistance (HOMA-IR), calculated as insulin (mU/l) × (glucose [mg/dl] × 0.055)/22.5 [17]. In addition, IL-6 concentration was used to adjust for inflammatory status and was measured by ultrasensitive enzyme-linked immunosorbent assay (Quantikine HS human IL-6 immuno-assay; R&D Systems, Minneapolis, MN).
2.2.1. Diagnosis of hypertension, metabolic syndrome, diabetes, low estimated glomerular filtration rate (eGFR) and subclinical cardiovascular disease
Hypertension was defined as a systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg or the use of blood pressure lowering medications. Metabolic syndrome was defined according to the Adult Treatment Panel III report (ATPIII) [18]. For Chinese individuals the definition of metabolic syndrome included those with waist circumference > 90 cm and > 80 cm for males and females, respectively. Diabetes was defined as self-reported physician diagnosis, fasting glucose ≥126 mg/dL or the use of insulin or oral hypoglycemic medications each assessed at study clinic examinations. Low eGFR was defined as a eGFR < 60 mL/min/ 1.73m2 according to the EPI-CKD equation [19]. Subclinical cardiovascular disease was defined by the presence of at least two of the following: carotid plaque, left ventricular hypertrophy and a positive coronary artery calcium (CAC) score.
2.2.2. Measurement of liver fat and liver enzyme
Liver fat was estimated based on radiologic liver attenuation (Hounsfield units (HU)) using computerized tomographic imaging of the liver and the spleen. Liver attenuation is inversely associated with liver fat content, thus lower attenuation coefficients indicates greater fat content. A liver/spleen (L/S) attenuation ratio < 0.8 [20] and a liver attenuation < 42 HU are able to detect macrovesicular liver steatosis > 30% with 100% specificity and sensitivity ranging from 73% and 82% [20]. Previous reports using data from the MESA study have used liver attenuation < 40 HU and L/S ratio < 1 to define NAFLD [4, 21, 22]. An L/S ratio < 1 corresponds to mild steatosis (<9.9% fatty liver content) and a liver attenuation of < 40 HU corresponds to a moderate to severe steatosis (10% to > 25% fatty liver content) [23, 24]. The methods used to acquire this information from the liver and spleen CT has been described in detail previously [4, 25]. The inter-reader and intrareader intra-class correlation coefficient (ICC) for liver attenuation measurement was 0.96 and 0.99, respectively and the inter-reader and intrareader ICC for L/S was 0.99, for both measures [4]. GGT, another potential surrogate of fatty liver, was measured using methods previously described [26].
2.3. Statistical analysis
2.3.1. Descriptive statistics
Gender, race/ethnicity, sex, years of education, anthropometric, and metabolic parameters were used to characterize the sample at baseline by categories of liver attenuation < and ≥ 40 HU. Continuous variables with normal distributions are presented as mean ± SD and categorical variables as percentages. Variables with skewed distributions are log transformed and the mean log transformed value was exponentiated to obtain the geometric mean.
2.3.2. Assessing the relative prevalence of NAFLD
Due to the cross sectional nature of this analysis, the ratio of the prevalence of NAFLD in those exposed/prevalence of NAFLD in those not exposed is expressed as relative prevalence (RP) instead of relative risk. The RP of NAFLD (defined as an attenuation coefficient < 40 HU or liver to spleen ratio < 1) were assessed by quintiles of NT-proBNP adjusted for model 1 = age, race and sex; and model 2 = model 1 + percent of dietary calories derived from fat, number of alcoholic drinks per week, total intentional exercise and IL-6. Model 3 included model 2 + metabolic syndrome, which may be on the causal pathway between NT-proBNP and the development of NAFLD. Further adjusting model 2 for BMI and waist circumference was performed to assess if the association between NAFLD and NT-proBNP was attenuated by BMI. NT-proBNP values differ according to sex [27] and presence of subclinical cardiovascular disease (CVD) [5, 28]. For these reasons, a sex × NT-proBNP and also a subclinical CVD × NT-proBNP interaction term was assessed in model 2.
Sensitivity analyses was conducted excluding: 1) 422 heavier drinkers (i.e., males consuming >14 drinks/week and women consuming >7 drinks/week) [29], 2) 55 individuals with liver attenuations ≥ 80 HU (99th percentile) and 3) presence or absence of subclinical CVD.
2.3.3. Associations between liver attenuation and NT-proBNP
The association between categories of baseline NT-proBNP as an independent variable and liver attenuation (HU) as a dependent variable was assessed using linear regression procedures adjusting for the models 1, 2 and 3 as described above (figure 2). The NT-proBNP values at which the slopes of the dependent variables had a substantial change, i.e., the inflection point, was determined using linear splines adjusted for model 2 with serial knots at NT-proBNP values every 5 pg/mL for intervals between 20 – 300 pg/mL. The NT-proBNP concentration at which the linear spline model had the highest R2 was chosen as the inflection point [5]. Significance was set at p < 0.05. Statistical analysis was performed using SAS v 9.3 by SAS Institute Inc., Cary, NC.
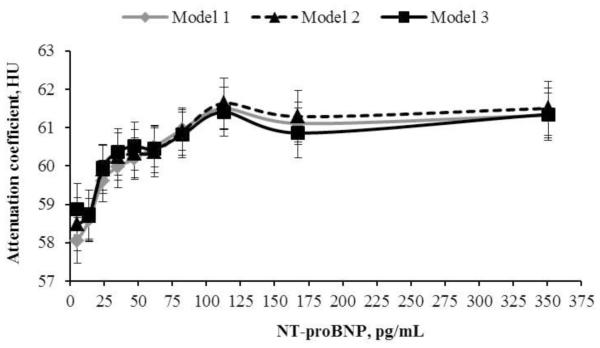
Figure 2.
The association between liver attenuation coefficient (HU) and deciles of baseline NT-proBNP in non-diabetic individuals without self-reported liver disease.
Legend. Model 1 adjusted for age, race and sex. Model 2 = model 1 + percent of calories derived from fat, total intentional exercise per week, number of alcoholic drinks per week and, IL-6. Model 3 = model 2 + metabolic syndrome at MESA baseline. HU = Hounsfield units.
3. Results
3.1. General characteristics
The mean (SD) liver attenuation of non-diabetic individuals without self-reported liver disease was 60.2 (11.6) HU with an interquartile range between 55.5 and 67.0 HU. As shown in table 1, compared to those without fatty liver, individuals with fatty liver were on average 3 years younger had greater BMI, HOMA-IR, systolic and diastolic blood pressure; greater levels of blood triglycerides, fasting blood glucose, IL-6 values, a higher percent of dietary calories derived from fat, a greater percentage of them were heavier alcohol drinkers and had higher circulatory GGT levels. The prevalence of NAFLD was greater among white/Caucasians and Hispanics than among Chinese and African Americans. In addition, individuals with fatty liver were also more likely to have metabolic syndrome, have lower plasma NT-proBNP concentration, total intentional exercise per week and lower L/S ratio. There was no difference in the prevalence low eGFR (eGFR < 60) between those with HU < or ≥ 40.
Table 1.
Demographic and metabolic characteristics and presence of subclinical disease by categories of liver attenuation coefficient < or ≥ 40 HU at baseline in individuals without diabetes and without self-reported liver disease.
| ≥40 HU | < 40 HU | ||
|---|---|---|---|
| N (%) | 4281 (94.5) | 248 (5.5) | p value |
| Sex, % females | 52.9 | 49.6 | 0.3 |
| Age, years | 62.7 (0.2) | 59.1 (0.7) | <0.0001 |
| Race | <0.0001 | ||
| White | 42.7 | 37.5 | |
| Chinese | 12.2 | 10.9 | |
| African American | 24.2 | 12.5 | |
| Hispanics | 20.9 | 39.1 | |
| Education, % | 83.2 | 77.8 | 0.02 |
| Current Smoker, % | 12.2 | 12.1 | 1.0 |
| HTN, % | 44.38 | 48.79 | 0.2 |
| Low eGFR, % | 9.7 | 7.3 | 0.2 |
| BMI, kg/m2 | 27.8 (0.1) | 31.9 (0.3) | <0.0001 |
| Systolic blood pressure, mmHg |
131.3 (0.3) | 136.9 (1.4) | <0.0001 |
| Diastolic blood pressure, mmHg |
71.7 (0.1) | 74.2 (0.6) | <0.0001 |
| HDL-C, mg/dL | 51.8 (0.2) | 46.2 (0.9) | <0.0001 |
| Triglycerides, mg/dL | 126.5 (1.2) | 170.3 (4.9) | <0.0001 |
| Glucose, mg/dL | 89.3 (0.2) | 96.3 (0.6) | <0.0001 |
| HOMA-IR, (mU/L*mmol/dL/22.5) |
2.06 (0.02) | 3.72 (0.08) | <0.0001 |
| Interleukin-6, pg/mL | 1.49 (0.02) | 2.01 (0.08) | <0.0001 |
| Percent calories from saturated fat, % |
10.1 (0.05) | 10.6 (0.2) | 0.02 |
| NT-proBNP, pg/mL | 51.3 (49.9 - 52.8) | 39.8 (35.4 - 44.9) | <0.0001 |
| NT-proBNO > 100 pg/mL, % | 29.1 | 17.3 | <0.0001 |
| Heavy drinkers, % | 9.2 | 11.3 | 0.3 |
| Total intentional exercise, MET/min/week |
1228 (1186 - 1272) | 1031 (884 - 1202) | 0.03 |
| GGT, U/L | 39.2 (0.5) | 54.4 (1.9) | <0.0001 |
| Liver/spleen | 1.23 (0.004) | 0.66 (0.018) | <0.0001 |
| Metabolic syndrome, % | 29.2 | 64.0 | <0.0001 |
| Subclinical CVD, % | 26.8 | 28.8 | 0.6 |
Categorical variables are expressed as % and continuous variables are expressed as means (SE), except for NT-proBNP, which is the geometric mean (95% CI). Continuous variables were adjusted for age, race and gender, except when age was the dependent variable. HU = Hounsfield units. Education refers to the percent of individuals who completed at least high school. HTN = individuals with hypertension. Low estimated glomerular filtration rate (eGFR) = eGFR , 60 mL/kg/min based on the CKD-EPI equation. BMI = body mass index kg/m2. HDL-C = high density lipoprotein-cholesterol. GGT = gamma-glutamyl transpeptidase. Heavy drinkers were defined as males consuming > 14 drinks/week and women consuming > 7 drinks/week. Total intentional exercise = geometric mean (95% CI). Subclinical cardiovascular disease is defined as those individuals who had at least two of the following conditions: left ventricular hypertrophy, carotid plaque and coronary artery calcium > 0 Agatston units.
3.2. Relative prevalence of non-alcoholic fatty liver disease (NAFLD) by quintiles of baseline NT-proBNP
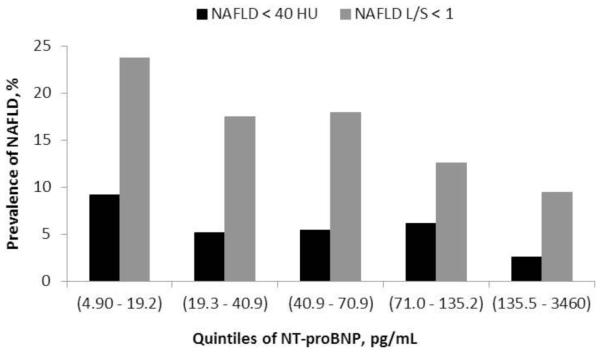
Non-alcoholic fatty liver disease defined as a liver attenuation coefficient < 40 HU represented 5.5% of the sample and was 3.6-fold (9.2% vs. 2.6%) more prevalent in the lowest quintile of NT-proBNP (range: 4.9 - 19.2 pg/mL) than at the highest quintile of NT-proBNP (≥ 135.5 pg/mL), Figure 1. Relative prevalence adjusted for model 1 decreased across categories of NT-proBNP with a linear trend for each log unit of NT-proBNP of 0.81 (0.72 – 0.92), p = 0.001. Further adjusting for percent of dietary calories derived from fat, total intentional exercise, alcoholic drinks per week and interleukin-6 did not substantially change the association between NAFLD and NT-proBNP. However, adding metabolic syndrome or BMI to model 2, the association between NAFLD and NT-proBNP was significantly weakened, p = 0.07 and = 0.08 for adjustment for metabolic syndrome and BMI, respectively.
Figure 1.
Prevalence of non-alcoholic fatty liver disease defined as a liver attenuation < 40 HU or a liver to spleen ratio < 1 by quintiles of NT-proBNP.
Legend. HU = Hounsfield units. NAFLD= non-alcoholic fatty liver disease. Individuals with self-reported liver disease and diabetes were excluded.
NAFLD defined as a liver/spleen ratio (L/S) < 1 represented 16% of the sample and was 2.5-fold higher (23.7% vs. 9.5%) in the lowest quintile of NT-proBNP than in the highest quintile, Table 2. There was an inverse association in relative prevalence for NAFLD across quintiles of NT-proBNP following adjustment for model 1 and 2 covariates, p < 0.001 and p = 0.001, respectively. Further adjusting for metabolic syndrome or BMI (p = 0.01) did not substantially change the association between NAFLD and NT-proBNP, model 3 in Table 2.
Table 2.
Cross sectional adjusted relative prevalence for non-alcoholic fatty liver disease by quintiles of baseline NT-proBNP in non-diabetic individuals without self-reported liver disease.
| Quintiles of NT-proBNP, pg/mL |
||||||||
|---|---|---|---|---|---|---|---|---|
| (rang e) |
(4.9 - 19.2) |
(19.3 - 40.9) |
(41.0 - 70.9) |
(71.0 - 135.2) |
(135.5 - 3460) |
|||
| NAF LD |
n | 888 | 902 | 929 | 919 | 891 | Linear trend |
p value |
| < 40 HU |
Mode l 1 |
1 | 0.63 (0.44 - 0.89) |
0.70 (0.49 - 1.01) |
0.67 (0.46 - 0.99) |
0.40 (0.24 - 0.68) |
0.81 (0.72 - 0.92) |
0.001 |
| Mode l 2 |
1 | 0.64 (0.41 - 1.00) |
0.78 (0.51 - 1.21) |
0.72 (0.45 - 1.15) |
0.48 (0.26 - 0.88) |
0.82 (0.71 - 0.96) |
0.01 | |
| Mode l 3 |
1 | 0.66 (0.43 - 1.02) |
0.78 (0.51 - 1.2) |
0.74 (0.47 - 1.18) |
0.54 (0.29 - 1.00) |
0.88 (0.76 - 1.01) |
0.07 | |
| < 1 | Mode l 1 |
1 | 0.77 (0.61 - 0.98) |
0.81 (0.63 - 1.03) |
0.58 (0.44 - 0.77) |
0.46 (0.33 - 0.63) |
0.82 (0.75 - 0.89) |
<0.00 01 |
| Mode l 2 |
1 | 0.83 (0.62 - 1.12) |
0.86 (0.63 - 1.17) |
0.6 (0.42 - 0.85) |
0.49 (0.33 - 0.74) |
0.87 (0.78 - 0.97) |
0.001 | |
| Mode l 3 |
1 | 0.87 (0.65 - 1.15) |
0.86 (0.64 - 1.16) |
0.63 (0.45 - 0.89) |
0.54 (0.37 - 0.81) |
0.87 (0.78 - 0.97) |
0.01 | |
HU = Hounsfield units. Relative risk adjusted by model 1 = age, race, sex. Model 2 = model 1 + percent of dietary calories derived from fat, total intentional exercise, alcoholic drinks per week and interleukin-6. Model 3 = model 2 + metabolic syndrome. NAFLD = non-alcohol fatty liver disease defined as an average liver attenuation coefficient < 40 HU or a liver to spleen ration < 1. Linear trend is per unit of log (NT-proBNP).
Using either definition for NAFLD (< 40 HU or L/S ratio < 1), the association between relative prevalence of NAFLD and NT-proBNP was not different between sex, as reflected by the lack of a sex × NAFLD interaction, p = 0.09 and p = 0.7, respectively. Excluding individuals with alcohol consumption > 14 drinks per week if males and > 7 drinks per week if females, did not substantially change the association between NT-proBNP and relative prevalence of NAFLD for model 2, RP per unit increase in ln(NT-proBNP) = 0.83 (0.71 - 0.97), p = 0.02 and 0.83 (0.74 - 0.93), p = 0.002, respectively. There was no race × NAFLD interaction whether defining NAFLD as HU < 40 or by an L/S ratio < 1, p = 1.0 and p 0.3, respectively. Similarly, there was no subclinical CVD × NAFLD interaction, p > 0.2. Excluding individuals with liver attenuation > 80 HU did not substantially change the association between RP of NAFLD and NT-proBNP, RP per unit increase in ln(NT-proBNP) = 0.85 (0.74 - 0.98) and 0.84 (0.76 - 0.93), p < 0.03 for NAFLD and L/S < 1, respectively. In addition, there was not a BMI category × NAFLD interaction, p = 0.6 and p = 0.8 when defining NAFLD as HU < 40 or an L/S ratio < 1, respectively.
3.3. Association between liver attenuation and NT-proBNP
Linear regression coefficients between liver attenuation and log of NT-proBNP were 0.86 (0.18), p < 0.0001 when adjusted for model 1 covariates and 0.81 (0.21) when adjusted for model 2 covariates, p = 0.0002. Adjusting for metabolic syndrome (model 3) slightly weakened the association, 0.68 (0.21), p = 0.001. Further adjusting for BMI or waist circumference did not substantially change the association between liver attenuation and NT-proBNP. There was no low eGFR × NT-proBNP interaction for model 1 or 2, p = 0.9 and p = 0.5, respectively. Figure 2 shows that the association between liver attenuation and NT-proBNP did not follow a linear pattern. Linear spline analysis adjusted for model 2 covariates showed that the inflection point (highest R2 = 0.173) occurred at an NT-proBNP concentration of 45 pg/mL, corresponding to a liver attenuation of 60 HU. Linear regression coefficient below the inflection point was 0.05 (0.02) for every pg/mL increase in NT-proBNP, p = 0.001 and above the inflection point was 0.0006 (0.0008), p = 0.5. Linear slopes at NT-proBNP concentrations < and ≥ 45 pg/mL were different, p < 0.001.
4. Discussion
The results of this cross-sectional analysis show a positive linear association between liver attenuation in HU (less attenuation more fat) and baseline values of NT-proBNP. However, the association between NT-proBNP and liver attenuation is not linear and plateaus at NT-proBNP value ≥ 45 pg/mL. This study also shows an inverse association between baseline NT-proBNP and relative prevalence of fatty liver disease. Furthermore, the inverse association between relative risk for NAFLD and NT-proBNP persists after adjusting for age, race, sex, and percent of calories derived from fat, total intentional exercise, number of alcoholic drinks per week and IL-6.
4.1. Association between liver fat and NT-proBNP
Although plasma levels of NT-proBNP differ by race, race does not modify the association between NT-proBNP and NAFLD. This is similar to the result report by Al Rifai et al. [21] that found that the association between metabolic syndrome and NAFLD was not dependent on race. The greater prevalence of NAFLD when defining it as an L/S ratio < 1 is related to the inclusion of individuals with mild liver disease. This is likely the explanation for the weakening of the association between NT-proBNP and risk of NAFLD when including metabolic syndrome in the model and defining NAFLD as a liver attenuation <40 HU (a more restrictive definition of NAFLD), but not when defining it as an L/S < 1. The weakening of the association between NT-proBNP and NAFLD when adding metabolic syndrome to the model depends on the defining criteria for NAFLD and may suggest that metabolic syndrome is on the causal pathway for the development of NAFLD. However, further research is required to assess the dose response effect of natriuretic peptides on the metabolism and accumulation of fat in the liver and the development of NAFLD.
The plateauing of the positive association between NT-proBNP and liver attenuation at levels of NT-proBNP ≥ 45 pg/mL is most likely related to the reported upper limit in liver attenuation of individuals without clinical signs of liver disease [30, 31]. In this study, the upper interquartile value of liver attenuation for those non-diabetics and without self-reported liver disease was of 67 HU, which is near to the corresponding liver attenuation value (60 HU) at the NT-proBNP inflection point. The association between NT-proBNP and liver fat follows a linear pattern along the range of values that describes healthy and fatty liver tissue. Liver attenuation values greater than 67 HU in liver CT scans may reflect areas of abnormal liver tissue where the association between NT-proBNP and liver attenuation is no longer significant.
4.2. Potential biological mechanism
The biologically active B-type natriuretic peptide (BNP) and the inactive NT-proBNP are released on an equimolar basis by the heart due to myocyte stretching and cleavage of the precursor peptide proBNP by the enzymes corin and/or furin [32]. Low plasma NT-proBNP has been associated in cross sectional studies with greater BMI [5, 33], increased visceral adipose tissue [6], presence of metabolic syndrome [34, 35] and in longitudinal studies low levels of NT-proBNP have shown to be predictive of incident diabetes [8, 36-38]. The epidemiological associations between natriuretic peptides with obesity and incident diabetes could result from reverse causality. However, a Mendelian randomization study found that individuals with a genetic variant BNP, which was associated with elevated plasma levels of BNP, were less likely to develop incident diabetes [37]. In addition to epidemiological evidence, animal and human models have shown that atrial natriuretic peptide (ANP) and BNP have metabolic actions that can explain the associations observed in epidemiological studies. Cellular actions of natriuretic peptides occur by binding to natriuretic peptide (NP) receptors -A and -B (NPR-A and NPR-B, respectively). Binding of NP to its receptors leads to activation of the gene that codes for the peroxisome proliferator-activated receptor γ coactivator-1 α (PGC1A) [39] and stimulates an increase in mitochondrial density, oxygen consumption and an increase in insulin sensitivity [11, 40] and increased lipolysis in adipose tissue independently from catecholamine induced lipolysis [41, 42]. These metabolic actions may provide a biological explanation for the accumulation of fat and the development of NAFLD associated with low levels of NT-proBNP.
NPs are cleared from the circulation by natriuretic peptide receptor-C (NPR-C) [43, 44]. NP receptors have been detected in adipose tissue, cardiac and skeletal muscle, kidneys and liver [45, 46]. It was further shown that insulin concentration decreased NPR-A and NPR-B in adipose tissue and increased NPR-C, whereas fasting induced the opposite effect [45]. Therefore, any event that increases insulin concentration can lower natriuretic peptide levels (due to increased clearance) and decrease the ability of natriuretic peptides to exert their cellular actions due to lower numbers of NPR –A and –B.
4.3. Strengths and limitations
The strengths of this analysis is that it included a large sample of individuals of diverse ethnic and racial background free of CVD and a wide range of liver attenuation coefficients. Another strong point is the very high intra- and intereader correlation for the determination of HU and the low intra-assay and interassay coefficient of variation for NT-proBNP. A weakness is the cross-sectional and observational nature of this analysis, which prevents the ability to establish a causal effect for NT-proBNP in the development of NAFLD. However, a Mendelian randomization study showed an inverse association between NT-proBNP and incidence diabetes [37]. The result from that study provides further support for the hypothesis that natriuretic peptides may be an important factor to consider in the development of type 2 diabetes mellitus. Also, although the inverse association between HU by computed tomography and fat liver storage has been well documented, using magnetic resonance imaging to evaluate the difference in resonance frequency between water and fat may have a better biophysical basis to evaluate fatty liver [47]. A number of participants had conditions which could increase plasma levels of NT-proBNP such as chronic obstructive pulmonary disease (COPD) [48], subclinical CVD [49] and low eGFR [50] and confound the association between NT-proBNP and NAFLD. While the prevalence of COPD was not assessed at baseline, the prevalence of current or former smokers was similar in those with and without NAFLD. Similarly, the prevalence of those with low eGFR or subclinical CVD was not different between those with and without NAFLD and the lack of an interaction between NT-proBNP and subclinical CVD or with low eGFR supports the results that NT-proBNP is an independent risk factor associated with NAFLD.
5.0. Future research and potential clinical implications
There is increasing epidemiological and experimental evidence describing associations between low levels of natriuretic peptides and disorders of glucose and fat metabolism. Whether there is a cause and effect relationship between low levels of natriuretic peptides and the development of obesity, metabolic syndrome and overt type 2 diabetes has yet to be clarified. Furthermore, the mechanism(s) that are involved in lowering blood levels of natriuretic peptides have only been scarcely studied. Blood levels of NT-proBNP may have important clinical applications in order to identify, prevent and treat individuals at risk of metabolic disorders of glucose and fat.
5.1. Conclusions
This cross-sectional analysis has shown that low concentrations of NT-proBNP (<19.3 pg/mL) is an independent risk factor associated with the presence of NAFLD and that for NT-proBNP values < 45 pg/mL there is an inverse association between NT-proBNP and amount of liver fat.
Acknowledgments
The authors also thank the investigators and staff of the Multi-Ethnic Study of Atherosclerosis (MESA) for their valuable contributions. A full list of MESA investigators and institutions can be found at http://www.mesa-nhlbi.org/.
Funding
This research was supported by contracts N01-HC-95159 through N01-HC-95169 and grants grant R01 HL071739 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-TR-001079 from NCRR, and by Roche Diagnostics.
Footnotes abbreviations
- NT-proBNP
N-terminal pro B-type natriuretic peptide
- MESA
Multi-Ethnic Study of Atherosclerosis
- NAFLD
nonalcoholic fatty liver disease
- RP
relative prevalence
- IL-6
interleukin-6
- IP
inflection point
- HU
Hounsfield units
- GGT
gamma-glutamyl transferase
- BMI
body mass index
- HOMA-IR
homeostasis model assessment of insulin resistance
- eGFR
estimated glomerular filtration rate
- ATP III
the Adult Treatment Panel III
- CAC
coronary artery calcium
- ICC
intraclass correlation coefficient
- CVD
cardiovascular disease
- PGC1A
peroxisome proliferator-activated receptor γ coactivator-1 α;
- NPR-A
B and C, natriuretic peptide receptor-A, B and C
- COPD
chronic obstructive pulmonary disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
Otto A. Sanchez, no disclosures. Daniel Duprez, consultant to Novartis Astra Zeneca. Mariana Lazo, no disclosures. Irfan Zeb, no disclosures. Russell P Tracy, no disclosures. Ryan Bradley, no disclosures. Hossein Bahrami no disclosures. Lori B Daniels consultant to Alere, Inc; speaking fees from Critical Diagnostics and Roche. Joao A. Lima, consultant to Toshiba Medical Systems, Bracco. Alan Maisel, consultant to Alere, BG Medicine, Brahms, Critical Diagnostics, EFG diagnostics, Novartis, Abbott. Carmen A. Peralta, no disclosures. David R Jacobs, no disclosures. Mathew Budoff, consultant to General Electric.
Author contribution
Proposed the topic and wrote the manuscript and performed statistical analysis: Otto A. Sanchez. Analysis and writing the manuscript: Mariana Lazo, Lori B Daniels, Daniel Duprez, Hossein Bahrami, Joao A. Lima, Alan Maisel, Carmen A. Peralta, , Russell P Tracy and Ryan Bradley.
Expertise in liver disease and imaging: Irfan Zeb and Mathew Budoff.
Statistical analysis and writing manuscript: David R Jacobs.
References
- [1].Bi WR, Yang CQ, Shi Q, Xu Y, Cao CP, Ling J, et al. Large-scale analysis of factors influencing nonalcoholic fatty liver disease and its relationship with liver enzymes. Genetics and molecular research : GMR. 2014;13:5880–91. doi: 10.4238/2014.August.7.3. [DOI] [PubMed] [Google Scholar]
- [2].Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–23. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- [3].Chang E, Park C-Y, Park SW. Role of thiazolidinediones, insulin sensitizers, in non-alcoholic fatty liver disease. Journal of Diabetes Investigation. 2013;4:517–24. doi: 10.1111/jdi.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zeb I, Katz R, Nasir K, Ding J, Rezaeian P, Budoff MJ. Relation of nonalcoholic fatty liver disease to the metabolic syndrome: the Multi-Ethnic Study of Atherosclerosis. Journal of cardiovascular computed tomography. 2013;7:311–8. doi: 10.1016/j.jcct.2013.08.011. [DOI] [PubMed] [Google Scholar]
- [5].Sanchez OA, Duprez DA, Bahrami H, Daniels LB, Folsom AR, Lima JA, et al. The associations between metabolic variables and NT-proBNP are blunted at pathological ranges: the Multi-Ethnic Study of Atherosclerosis. Metabolism. 2014;63:475–83. doi: 10.1016/j.metabol.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Neeland IJ, Winders BR, Ayers CR, Das SR, Chang AY, Berry JD, et al. Higher natriuretic peptide levels associate with a favorable adipose tissue distribution profile. Journal of the American College of Cardiology. 2013;62:752–60. doi: 10.1016/j.jacc.2013.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Christensen HM, Kistorp C, Schou M, Keller N, Zerahn B, Frystyk J, et al. Cross-talk between the heart and adipose tissue in cachectic heart failure patients with respect to alterations in body composition: a prospective study. Metabolism. 2014;63:141–9. doi: 10.1016/j.metabol.2013.09.017. [DOI] [PubMed] [Google Scholar]
- [8].Lazo M, Young JH, Brancati FL, Coresh J, Whelton S, Ndumele CE, et al. NH2-terminal pro-brain natriuretic peptide and risk of diabetes. Diabetes. 2013;62:3189–93. doi: 10.2337/db13-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Magnusson M, Jujic A, Hedblad B, Engstrom G, Persson M, Struck J, et al. Low plasma level of atrial natriuretic peptide predicts development of diabetes: the prospective Malmo Diet and Cancer study. J Clin Endocrinol Metab. 2012;97:638–45. doi: 10.1210/jc.2011-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sanchez OA, Duprez DA, Daniels LB, Maisel A, Otvos JD, Peralta CA, et al. The association between N-terminal pro B-type natriuretic peptide and lipoprotein particle concentration plateaus at higher N-terminal pro B-type natriuretic peptide values: Multi-Ethnic Study on Atherosclerosis. Metabolism: Clinical & Experimental. 2015 doi: 10.1016/j.metabol.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Miyashita K, Itoh H, Tsujimoto H, Tamura N, Fukunaga Y, Sone M, et al. Natriuretic peptides/cGMP/cGMP-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes. 2009;58:2880–92. doi: 10.2337/db09-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lazo M, Rubin J, Clark JM, Coresh J, Schneider AL, Ndumele C, et al. The association of liver enzymes with biomarkers of subclinical myocardial damage and structural heart disease. J Hepatol. 2014 doi: 10.1016/j.jhep.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. American Journal of Epidemiology. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- [14].Bild DE, Detrano R, Peterson D, Guerci A, Liu K, Shahar E, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111:1313–20. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- [15].Nettleton JA, Rock CL, Wang Y, Jenny NS, Jacobs DR. Associations between dietary macronutrient intake and plasma lipids demonstrate criterion performance of the Multi-Ethnic Study of Atherosclerosis (MESA) food-frequency questionnaire. Br J Nutr. 2009;102:1220–7. doi: 10.1017/S0007114509382161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Patton KK, Heckbert SR, Alonso A, Bahrami H, Lima JA, Burke G, et al. N-terminal pro-B-type natriuretic peptide as a predictor of incident atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis: the effects of age, sex and ethnicity. Heart. 2013;99:1832–6. doi: 10.1136/heartjnl-2013-304724. [DOI] [PubMed] [Google Scholar]
- [17].Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- [18].Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C. Definition of Metabolic Syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on Scientific Issues Related to Definition. Circulation. 2004;109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. Participants ftC. [DOI] [PubMed] [Google Scholar]
- [19].Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Annals of Internal Medicine. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Park SH, Kim PN, Kim KW, Lee SW, Yoon SE, Park SW, et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology. 2006;239:105–12. doi: 10.1148/radiol.2391050361. [DOI] [PubMed] [Google Scholar]
- [21].Al Rifai M, Silverman MG, Nasir K, Budoff MJ, Blankstein R, Szklo M, et al. The association of nonalcoholic fatty liver disease, obesity, and metabolic syndrome, with systemic inflammation and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2015;239:629–33. doi: 10.1016/j.atherosclerosis.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hamirani YS, Katz R, Nasir K, Zeb I, Blaha MJ, Blumenthal RS, et al. Association between inflammatory markers and liver fat: The Multi-Ethnic Study of Atherosclerosis. Journal of clinical & experimental cardiology. 2014:5. doi: 10.4172/2155-9880.1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pamilo M, Sotaniemi EA, Suramo I, Lahde S, Arranto AJ. Evaluation of liver steatotic and fibrous content by computerized tomography and ultrasound. Scand J Gastroenterol. 1983;18:743–7. doi: 10.3109/00365528309182089. [DOI] [PubMed] [Google Scholar]
- [24].Zeb I, Li D, Nasir K, Katz R, Larijani VN, Budoff MJ. Computed tomography scans in the evaluation of fatty liver disease in a population based study: the multi-ethnic study of atherosclerosis. Acad Radiol. 2012;19:811–8. doi: 10.1016/j.acra.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr., et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- [26].Bradley R, Fitzpatrick AL, Jenny NS, Lee DH, Jacobs DR., Jr Associations between total serum GGT activity and metabolic risk: MESA. Biomarkers in medicine. 2013;7:709–21. doi: 10.2217/bmm.13.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Manson JE, Bassuk SS. Biomarkers of cardiovascular disease risk in women. Metabolism. 2015;64:S33–9. doi: 10.1016/j.metabol.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sanchez OA, Jacobs DR, Jr., Bahrami H, Peralta CA, Daniels LB, Lima JA, et al. Increasing aminoterminal-pro-B-type natriuretic peptide precedes the development of arterial hypertension: the multiethnic study of atherosclerosis. J Hypertens. 2015;33:966–74. doi: 10.1097/HJH.0000000000000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- [30].Phatak MG. Computed tomography of the liver. J Comput Tomogr. 1984;8:157–69. doi: 10.1016/0149-936x(84)90102-4. [DOI] [PubMed] [Google Scholar]
- [31].Piekarski J, Goldberg HI, Royal SA, Axel L, Moss AA. Difference between liver and spleen CT numbers in the normal adult: its usefulness in predicting the presence of diffuse liver disease. Radiology. 1980;137:727–9. doi: 10.1148/radiology.137.3.6934563. [DOI] [PubMed] [Google Scholar]
- [32].Mair J. Biochemistry of B-type natriuretic peptide--where are we now? Clin Chem Lab Med. 2008;46:1507–14. doi: 10.1515/CCLM.2008.295. [DOI] [PubMed] [Google Scholar]
- [33].Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Wilson PW, et al. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109:594–600. doi: 10.1161/01.CIR.0000112582.16683.EA. [DOI] [PubMed] [Google Scholar]
- [34].Feng SQ, Ye P, Luo LM, Xiao WK, Xu RY, Wu HM. [Relationship between serum N-terminal pro-brain natriuretic peptide and metabolic syndrome: a cross-sectional study] Zhonghua xin xue guan bing za zhi. 2013;41:130–4. [PubMed] [Google Scholar]
- [35].Chang HR, Hsieh JC, Hsu BG, Wang LY, Yu-Chih Chen M, Wang JH. N-terminal pro-B-type natriuretic peptide is inversely associated with metabolic syndrome in hypertensive patients. American Journal of the Medical Sciences. 2014;348:210–4. doi: 10.1097/MAJ.0000000000000234. [DOI] [PubMed] [Google Scholar]
- [36].Everett BM, Cook NR, Chasman DI, Magnone MC, Bobadilla M, Rifai N, et al. Prospective evaluation of B-type natriuretic peptide concentrations and the risk of type 2 diabetes in women. Clinical Chemistry. 2013;59:557–65. doi: 10.1373/clinchem.2012.194167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pfister R, Sharp S, Luben R, Welsh P, Barroso I, Salomaa V, et al. Mendelian randomization study of B-type natriuretic peptide and type 2 diabetes: evidence of causal association from population studies. PLoS Med. 2011;8:e1001112. doi: 10.1371/journal.pmed.1001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sanchez OA, Duprez DA, Bahrami H, Peralta CA, Daniels LB, Lima JA, et al. Changes in N-terminal pro-B-type natriuretic peptide and incidence of diabetes: The Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Metab. 2015 doi: 10.1016/j.diabet.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Engeli S, Birkenfeld AL, Badin PM, Bourlier V, Louche K, Viguerie N, et al. Natriuretic peptides enhance the oxidative capacity of human skeletal muscle. Journal of Clinical Investigation. 2012;122:4675–9. doi: 10.1172/JCI64526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Souza SC, Chau MD, Yang Q, Gauthier MS, Clairmont KB, Wu Z, et al. Atrial natriuretic peptide regulates lipid mobilization and oxygen consumption in human adipocytes by activating AMPK. Biochem Biophys Res Commun. 2011;410:398–403. doi: 10.1016/j.bbrc.2011.05.143. [DOI] [PubMed] [Google Scholar]
- [41].Lafontan M, Moro C, Berlan M, Crampes F, Sengenes C, Galitzky J. Control of lipolysis by natriuretic peptides and cyclic GMP. Trends in endocrinology and metabolism: TEM. 2008;19:130–7. doi: 10.1016/j.tem.2007.11.006. [DOI] [PubMed] [Google Scholar]
- [42].Sengenes C, Berlan M, De Glisezinski I, Lafontan M, Galitzky J. Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB Journal. 2000;14:1345–51. [PubMed] [Google Scholar]
- [43].Mangiafico S, Costello-Boerrigter LC, Andersen IA, Cataliotti A, Burnett JC., Jr Neutral endopeptidase inhibition and the natriuretic peptide system: an evolving strategy in cardiovascular therapeutics. Eur Heart J. 2013;34:886–93. doi: 10.1093/eurheartj/ehs262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Woodard GE, Rosado JA. Natriuretic peptides in vascular physiology and pathology. Int Rev Cell Mol Biol. 2008;268:59–93. doi: 10.1016/S1937-6448(08)00803-4. [DOI] [PubMed] [Google Scholar]
- [45].Nakatsuji H, Maeda N, Hibuse T, Hiuge A, Hirata A, Kuroda Y, et al. Reciprocal regulation of natriuretic peptide receptors by insulin in adipose cells. Biochem Biophys Res Commun. 2010;392:100–5. doi: 10.1016/j.bbrc.2010.01.008. [DOI] [PubMed] [Google Scholar]
- [46].Vollmar AM, Paumgartner G, Gerbes AL. Differential gene expression of the three natriuretic peptides and natriuretic peptide receptor subtypes in human liver. Gut. 1997;40:145–50. doi: 10.1136/gut.40.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bohte AE, van Werven JR, Bipat S, Stoker J. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol. 2011;21:87–97. doi: 10.1007/s00330-010-1905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].van Gestel YR, Sin DD, Poldermans D. Elevated N-terminal pro-B-type natriuretic peptide levels: the effect of chronic obstructive pulmonary disease. J Am Coll Cardiol. 2009;53:458. doi: 10.1016/j.jacc.2008.10.026. author reply −9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sanchez OA, Jacob DR, Jr., Bahrami H, Peralta CA, Daniels LB, Lima JA, et al. Increasing aminoterminal-pro-B-type natriuretic peptide precedes the development of arterial hypertension: themultiethnic study of atherosclerosis. J Hypertens. 2015;33 doi: 10.1097/HJH.0000000000000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].DeFilippi C, van Kimmenade RR, Pinto YM. Amino-terminal pro-B-type natriuretic peptide testing in renal disease. Am J Cardiol. 2008;101:82–8. doi: 10.1016/j.amjcard.2007.11.029. [DOI] [PubMed] [Google Scholar]