Abstract
Background
The Positive and Negative Affect Schedule (PANAS) is a widely used measure of affect, and a comprehensive psychometric evaluation has never been conducted among substance users.
Objective
To examine the psychometric properties of the PANAS in a sample of outpatient treatment substance users.
Methods
We used pooled data from four randomized clinical trials (N = 416; 34% female, 48% African American).
Results
A confirmatory factor analysis indicated adequate support for a two-factor correlated model comprised of Positive Affect and Negative Affect with correlated item errors (Comparative Fit Index = .93, Root Mean Square Error of Approximation = .07, χ2 = 478.93, df = 156). Cronbach’s α indicated excellent internal consistency for both factors (.90 and .91, respectively). The PANAS factors had good convergence and discriminability (Composite Reliability >.7; Maximum Shared Variance < Average Variance Extracted). A comparison from baseline to Week 1 indicated acceptable test-retest reliability (Positive Affect = .80, Negative Affect = .76). Concurrent and discriminant validity were demonstrated with correlations with the Brief Symptom Inventory and Addiction Severity Index. The PANAS scores were also significantly correlated with treatment outcomes (e.g., Positive Affect was associated with the maximum days of consecutive abstinence from primary substance of abuse, r = .16, p = .001).
Conclusion
Our data suggest that the psychometric properties of the PANAS are retained in substance using populations. Although several studies have focused on the role of Negative Affect, our findings suggest that Positive Affect may also be an important factor in substance use treatment outcomes.
Keywords: affect, cocaine, substance use, PANAS
Introduction
The use of drugs and alcohol to modify affective states is a central feature in the development and maintenance of substance use disorders, yet systematic assessment of affect has been infrequent in the addictions literature. This is likely due to the complex relationship between affective states and substance use (1–3). Substance use can alter affective states acutely during intoxication, during withdrawal, and also as a result of chronic use (4–6). Alternatively, changes in affect may precede substance use (7). The construct of affect has been linked to a variety of psychological treatment outcomes (8, 9). In order to address research questions regarding the relationship between affect and substance use, availability of a psychometrically sound measure of affect for substance-using populations is essential.
The Positive and Negative Affect Schedule (PANAS) is a widely used measure of Positive Affect and Negative Affect. Positive Affect is defined as the extent to which a person feels pleasantly alert. High levels of Positive Affect indicate a state of positive engagement and low levels of Positive Affect indicate a state of sadness and lethargy (10). Negative Affect is defined as a dimension of subjective distress, which encompasses states such as anger, guilt, and fear. High levels of Negative Affect are defined as a state of significant distress, while low levels of Negative Affect are defined as a state of calmness (10).
Although there are no previously published comprehensive psychometric evaluations of the PANAS among samples of illegal drug users, there is some evidence that the PANAS appears useful in understanding predictors of response to treatments for nicotine (11) and alcohol use disorders (12). Negative Affect has been associated with alcohol-related problems among college students (13), and drinking alcohol to cope with Negative Affect may be a risk factor for the development of alcohol dependence in some populations (14). With regards to treatment outcomes, there is some evidence that PANAS scores may change over time. For example, Magura and colleagues reported that Negative Affect significantly decreased from pre-treatment to post-treatment in the treatment condition (a neurobehavioral intervention) in a sample of cocaine users enrolled in a methadone treatment program (15). Similar outcomes were found in a sample of cocaine-dependent individuals participating in a randomized trial evaluating bromocriptine, with significant improvements in Negative Affect at post-treatment (16). Negative Affect has been associated with engagement in risky behaviors in a sample of veterans in substance use treatment (17). Finally, a formal review of the literature indicated support for targeting Negative Affect within substance use treatment to improve Negative Affect over the course of treatment, as well as to prevent relapse (18). However, within the substance use literature, the methodologies of the existing studies have been diverse, each targeting distinct outcome variables. Important variables such as the maximum duration of abstinence and treatment retention have not been fully explored; yet, they merit attention, as these variables are prominent indicators of treatment outcomes.
The psychometric properties of the PANAS were originally evaluated in the general non-clinical population, with the finding that Positive Affect and Negative Affect appeared to be independent constructs (10). After the original psychometric evaluation was conducted, other investigators found differences in Positive Affect and Negative Affect in subpopulations. For instance, several studies have found that females tend to report higher Negative Affect scores than men (19, 20), and data suggest African Americans report higher levels of Positive Affect and lower levels of Negative Affect (21). Other investigators have also replicated the independence of Positive Affect and Negative Affect (22, 23); however, there is also data to suggest that Positive Affect and Negative Affect are correlated, rather than independent, factors (19). The relative independence of Positive Affect and Negative Affect may depend on the population being evaluated, as well as other factors including self-reported intensity of affect (24). Still other studies have reported a more complex factor structure of the PANAS, with up to three factors accounting for the construct of affective experience (25–28). Thus, there are mixed findings in the literature regarding the factor structure of the PANAS. Crawford and Henry (2004) examined the PANAS scores by using competing models of factor structure and found that the model with the best fit to the data contained two correlated factors (Negative Affect and Positive Affect) with correlated item errors, providing support for the finding that Positive Affect and Negative Affect may not be independent constructs.
The accurate assessment of affect among samples of substance users is important, given the centrality of affect in multiple conceptual models of addiction (1–7). However to study these processes in detail, the PANAS first needs to be assessed in a substance-using population. To achieve this goal, we evaluated the psychometric properties of the PANAS in a heterogeneous sample of substance users. We pooled data from four completed randomized clinical trials, each of which included the PANAS as part of the core set of assessments. We then evaluated factor structure, internal consistency, test-retest reliability, concurrent and discriminant validity, and predictive validity, with the expectation that the relatively strong psychometric properties of the PANAS (10, 19) would be retained in substance-using samples. We hypothesized that a confirmatory factor analysis would replicate the two-factor correlated structure with correlated item errors reported previously by Crawford & Henry (2004; see Table 2) as the best fit to the data through the use of competing models (19). In addition to evaluating the factor structure of the PANAS, a comprehensive evaluation of its validity (concurrent, discriminant, and predictive) is warranted. Because Negative Affect has been found to correlate with many symptoms of psychological disorders (29), we hypothesized that pre-treatment Negative Affect would be positively correlated with symptoms of psychopathology (i.e., depression, anxiety, paranoia), whereas pre-treatment Positive Affect would be negatively correlated in this population. Additionally, we hypothesized that pre-treatment Negative Affect would be positively correlated with poorer treatment outcome measures of drug and alcohol use, consistent with previous investigations (11–14), and that pre-treatment Positive Affect would be a protective factor, being negatively correlated with poor treatment outcome.
Table 2.
Summary of competing models1 of the Positive and Negative Affect Schedule (PANAS) scores. N = 416
| Model | X2 | df | CFI2 | RMSEA3 | X2/df | |
|---|---|---|---|---|---|---|
| 1a | 1 factor, uncorrelated | 2599.448 | 170 | .455 | .186 | 15.29 |
| 1b | 1 factor, correlated errors permitted (CE)4 | 1377.652 | 157 | .724 | .137 | 8.77 |
| 2a | 2 factor, uncorrelated | 844.415 | 170 | .847 | .098 | 4.97 |
| 2b | 2 factor, correlated | 824.732 | 169 | .853 | .097 | 4.88 |
| 2c | 2 factor, independent, CE permitted | 501.071 | 157 | .923 | .073 | 3.19 |
| 2d | 2 factor, correlated, CE permitted | 478.927 | 156 | .928 | .071 | 3.07 |
| 2e | 2 factor, correlated, CE permitted, includes “Excited” on both Positive Affect and Negative Affect | 478.884 | 155 | .928 | .071 | 3.09 |
Note. Minimal standards for acceptable model fit. CFI = >.90, RMSEA <.08, and relative chi-square < = 3.00.
Error correlational structure derived from Crawford and Henry (2004).
CFI = Comparative Fit Index.
RMSEA = Root Mean Square Error of Approximation.
Correlated Item Errors.
Method
The four clinical trials that contributed data for these analyses evaluated a range of types of substances (cocaine, marijuana, opioid, and alcohol). The protocols had similar exclusion and inclusion criteria as well as parallel assessment batteries, which facilitated pooling of the data for psychometric analyses. The first study assessed computer-delivered Cognitive Behavioral Therapy (CBT4CBT) as an adjunct to standard outpatient substance abuse treatment for 77 participants who met criteria for any current substance use disorder (30). The second study assessed combinations of CBT and contingency management among 127 individuals with marijuana use disorders (31). The third study evaluated the efficacy of CBT4CBT as an adjunct treatment in a sample of 101 cocaine-dependent individuals enrolled in a methadone program (32). The fourth study evaluated disulfiram and contingency management (N = 99) to enhance CBT outcomes in a sample of cocaine-dependent individuals (33). The four trials varied between 8 and 12 weeks of treatment duration.
All participants met current DSM-IV criteria for cocaine, opioid, marijuana, or alcohol dependence, assessed using the Structured Clinical Interview for DSM-IV (SCID), and were seeking treatment at an outpatient treatment facility (34). The inclusion criteria for all four studies required primary substance use within the previous 28 days, age greater than 18, and fluency in English. Participants were excluded from the studies if they were not sufficiently medically or psychiatrically stable for outpatient care, or if they were expecting imminent incarceration or changing residence out of the study locale.
Measures
Affect
Participants completed the 20-item PANAS at pre-treatment, weekly during treatment, and at each follow-up interview. The PANAS consists of 20 emotion words, with 10 loading on the Positive Affect factor and 10 on the Negative Affect factor (10). Sample words for Positive Affect include “alert”, “inspired”, and “enthusiastic”. Sample words for Negative Affect include “distressed”, “upset”, and “guilty”. Participants rate the degree to which they endorse each item on a rating scale (1 = very slightly or not at all; 5 = extremely). Items are then totaled to create a score for each factor: Positive Affect and Negative Affect. Higher scores represent greater endorsement of the construct.
Psychological symptoms
The Brief Symptom Inventory (BSI; 35) is a widely used self-report inventory that measures a broad domain of psychological symptoms (36): Somatization, Obsessive Compulsive, Depression, Interpersonal Sensitivity, Anxiety, Hostility, Psychoticism, Paranoid Ideation, and Phobic Anxiety. Items are rated on a five-point Likert scale from 1 (Not at All) to 5 (Extremely). The BSI was administered at pre-treatment. The Structured Clinical Interview for DSM-IV (SCID-IV) was used to assess the occurrence of lifetime psychological disorders (34).
Substance use
Information on severity of substance use and related problems was assessed via the Addiction Severity Index at pre-treatment, monthly during treatment, and at all post-treatment interviews (ASI; 37). The psychometric properties of the ASI have been well established (38). The ASI yields composite scores in 7 areas (medical, employment, legal, family/social, psychiatric, alcohol and drug use) that range from 0 to 1, with higher scores indicating greater severity of problems in that area. Participants reported on the frequency of their substance use by completing a Substance Use Calendar. Additionally, urine screens were administered weekly throughout treatment. Across studies, the urine screens measured five drug types (benzodiazepines, marijuana, cocaine, methamphetamine, and opiates). Only the urine screens collected during active treatment were included in these analyses in order to evaluate treatment outcomes and predictive validity. We used the percentage of urine screens positive for the primary substance as an outcome variable. Self-reported length of abstinence was defined as the maximum days of consecutive abstinence within the treatment period.
Data Analysis
Data were analyzed using SPSS version 21 and AMOS version 19. We tested competing models of the latent factor structure of the PANAS scores using confirmatory factor analysis (CFA) with a full information maximum likelihood model. Crawford and Henry (2004) outlined five competing models regarding the structure of the PANAS scores, which we sought to replicate. We evaluated the fit of the models using the Comparative Fit Index (CFI; 39) and the Root Mean Square Error of Approximation (RMSEA; 40). CFI values greater than .90 may indicate a reasonable model fit (41). RMSEA values that are less than .05 indicate an approximate fit, levels less than .08 suggest a reasonable error of approximation, and levels higher than .1 represent a poor fit (42).
Construct validity was assessed in several domains (43). The structural aspect of validity was assessed using Confirmatory Factor Analysis. Convergent and discriminant validity were evaluated using Pearson correlations with measures of psychopathological symptoms (measured with the BSI) as well as substance use (measured with the ASI). We expected that higher BSI scores on most dimensions would have positive Pearson correlations with Negative Affect and negative correlations with Positive Affect, and that ASI composite scores (legal, medical, family/social, and employment) would have fairly weak correlations with PANAS scores (greater than p = .05), as they do not directly focus on dimensions of affect. Predictive validity was evaluated using Pearson correlations with treatment outcome indicators demonstrated to be psychometrically strong and of clinical significance: positive urine toxicology screens, duration of self-reported abstinence, and completing treatment and reporting abstinence at the end of treatment (32, 44). Bonferroni-corrections were used for multiple comparisons. Internal consistency was evaluated using Cronbach’s alpha (standardized), as well as Composite Reliability (CR) values, with scores above .7 indicating adequate reliability. Test-retest reliability was assessed using intraclass correlation coefficients comparing PANAS scores at pre-treatment to scores during the first week of treatment and the end of treatment. Convergent and discriminant validity were further evaluated with the Average Variance Extracted value (AVE; values higher than .5 indicate support convergent validity), Maximum Shared Variance value (MSV), and Average Shared Variance value (ASV) from the Confirmatory Factor Analysis (39–42).
Results
For the full sample of 416 participants, the mean Negative Affect score was 20.25 (SD = 8.37) and the mean Positive Affect score was 30.21 (SD = 8.77). Table 1 presents Positive Affect and Negative Affect scores by baseline demographic and clinical measures, along with statistical comparisons. The Bonferroni-correction was calculated by dividing the number of tests by the level of significance (.05/22). With regard to Positive Affect, employed participants had significantly higher scores than unemployed participants, and those referred through the criminal justice system had higher Positive Affect scores than those without criminal justice involvement. There were no other significant differences in Positive Affect scores by baseline variables. Negative Affect scores, however, differed with higher scores for women, White non-Hispanics, those without current criminal justice involvement, and those diagnosed with a lifetime alcohol use disorder, major depression, or anxiety. There were significant differences between participants’ primary substance of choice and Positive and Negative affect. Using Tukey post-hoc comparisons, we found that participants who endorsed marijuana as their primary substance of choice scored higher on Positive Affect (M = 32.81, SD = 8.52) in comparison to those who reported alcohol (M = 30.79, SD = 6.74), cocaine (M = 28.72, SD = 8.65), and opiates as their substance of choice (M = 30.25, SD = 8.77) (F = 6.84, p < .001). Participants who endorsed marijuana as their primary substance of choice also scored lower on Negative Affect (M = 15.83, SD = 6.02) in comparison to alcohol (M = 22.71, SD = 7.41), cocaine (M = 22.49, SD = 8.54), and opiate users (M = 23.83, SD = 10.02) (F = 23.42, p < .001). All other comparisons between primary substances of choice were non-significant.
Table 1.
Demographic and relevant treatment variables. N = 416
| Positive Affect | Negative Affect | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| M | SD | F | df | p | M | SD | F | df | p | n | |
|
|
|
||||||||||
| Gender | |||||||||||
| Female | 28.73 | 8.67 | 6.44 | 1,414 | .01 | 23.15 | 9.18 | 28.25 | 1,414 | .00* | 144 |
| Male | 31.00 | 8.74 | 18.71 | 7.49 | 272 | ||||||
| Race/Ethnicity † | |||||||||||
| White, Non-Hispanic | 28.74 | 8.77 | 3.07 | 2,396 | .05 | 22.75 | 8.87 | 12.16 | 2,396 | .00* | 155 |
| African American | 30.81 | 8.64 | 18.43 | 7.76 | 200 | ||||||
| Hispanic | 31.45 | 9.14 | 20.02 | 4.62 | 44 | ||||||
| Education | |||||||||||
| No High School or GED | 29.73 | 8.60 | 0.48 | 1,414 | .14 | 19.84 | 8.30 | 0.39 | 1,414 | .54 | 115 |
| High School or GED | 30.40 | 8.84 | 20.41 | 8.41 | 301 | ||||||
| Marital Status | |||||||||||
| Never Married/Living Alone | 30.27 | 8.85 | 0.09 | 1,414 | .77 | 20.12 | 8.36 | 0.47 | 1,414 | .50 | 346 |
| Married/Long Relationship | 29.93 | 8.40 | 20.87 | 8.47 | 70 | ||||||
| Employment Status | |||||||||||
| Employed | 31.91 | 8.60 | 11.10 | 1,413 | .00* | 19.40 | 8.58 | 3.07 | 1,411 | .08 | 174 |
| Not Employed | 29.04 | 8.70 | 20.85 | 8.06 | 241 | ||||||
| Referred by Criminal Justice | |||||||||||
| Yes | 32.69 | 8.54 | 20.75 | 1,411 | .00* | 16.70 | 7.03 | 51.13 | 1,413 | .00* | 155 |
| No | 28.74 | 8.52 | 22.45 | 8.40 | 258 | ||||||
| On Public Assistance | |||||||||||
| Yes | 29.22 | 8.91 | 3.32 | 1,411 | .07 | 21.73 | 8.72 | 7.45 | 1,411 | .01 | 155 |
| No | 30.83 | 8.58 | 19.42 | 8.06 | 258 | ||||||
| Lifetime Alcohol Use Disorder | |||||||||||
| Yes | 29.88 | 8.49 | 1.02 | 1,410 | .31 | 21.92 | 8.54 | 24.18 | 1,410 | .00* | 243 |
| No | 30.76 | 9.16 | 17.92 | 7.55 | 169 | ||||||
| Lifetime Major Depression | |||||||||||
| Yes | 29.15 | 9.07 | 1.37 | 1,409 | .24 | 23.67 | 8.07 | 15.13 | 1,409 | .00* | 73 |
| No | 30.48 | 8.70 | 19.54 | 8.27 | 339 | ||||||
| Lifetime Any Anxiety Disorder | |||||||||||
| Yes | 28.51 | 9.29 | 3.52 | 1,410 | .06 | 26.26 | 9.84 | 51.79 | 1,410 | .00* | 74 |
| No | 30.62 | 8.62 | 18.96 | 7.41 | 338 | ||||||
| Antisocial Personality Disorder | |||||||||||
| Yes | 32.47 | 9.20 | 5.13 | 1,410 | .02 | 19.82 | 8.19 | 0.23 | 1,410 | .63 | 66 |
| No | 29.82 | 8.63 | 20.36 | 8.42 | 346 | ||||||
Note.
p < .002 (Bonferroni-Corrected). The psychological diagnoses were derived from the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders. Scores from the Positive and Negative Affect Schedule (PANAS) range from 10–50, with higher scores representing higher affect. GED = General Education Diploma.
Excludes Asian, Native American, Multiracial, and Other (n = 17) due to small sample sizes.
Following methods outlined by Crawford and Henry (2004), the simple single factor model with no correlated item errors was tested first and indicated poor fit (CFI=.45, RMSEA=.15, Table 2). Another single factor model, proposed by Zevon and Tellegen (1982), was evaluated with correlated item errors: interested, alert, and attentive; excited and enthusiastic and inspired; proud and determined; and strong and active; distressed and upset; guilty and ashamed; hostile and irritable; nervous and jittery; scared and afraid (46). This model was also found to have a poor fit (CFI =.73, RMSEA =.14). The next model, 2a, tests two uncorrelated factors (Positive Affect and Negative Affect) with no correlated item errors and was also found to be inadequate (CFI = .85, RMSEA .10). Model 2b, which also had a poor fit, tested the 2 factors and allowed for 13 correlations tested in 1b.
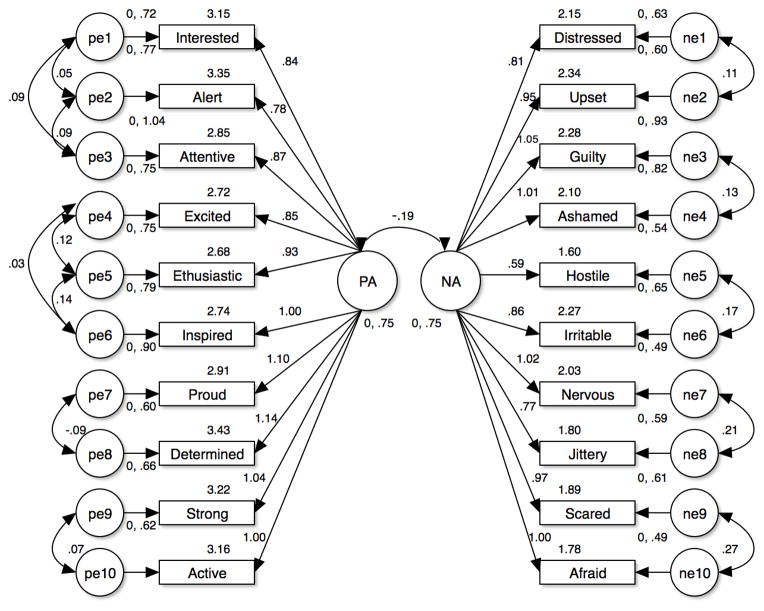
Model 2c tested the fit of 2 independent factors, Positive Affect and Negative Affect, with the correlated item errors used in Models 1b and 2b. This model was adequate with a CFI of .92 and an RMSEA of .07. To look for improved fit, the next model, 2d, allowed for both correlation between the 2 factors and the 13 correlated item errors in Models 1b and 2b. This model was also found to have good fit (CFI = .93, RMSEA = .07). Finally, in order to replicate Crawford and Henry, Model 2e, which added the correlation of one item from the Positive Affect factor to Negative Affect, was analyzed. This model also had good fit, but was not improved relative to Model 2d. The six models of factor structure are summarized in Table 2. As shown in Figure 1, the two factor correlated model (Positive and Negative Affect as correlated) with correlated item errors indicated the best fit (CFI = .93, RMSEA = .07, χ2 = 478.93, df = 156). Because of the heterogeneity of types of substance use, we also tested for invariance of the models with a multi-group confirmatory factor analysis. We compared the model fit for primary cocaine and primary marijuana users, but did not include primary alcohol (n=14) or opiate users (n=12) because of sample size restrictions. The two factor correlated model (Positive and Negative Affect as correlated) with correlated item errors demonstrated invariance with regard to cocaine and marijuana as primary substance.
Figure 1.
Structural validity of the Positive and Negative Affect Schedule (PANAS) scores. PA = Positive Affect. NA = Negative Affect. The values listed above each item name are the intercept estimates.
Next, we evaluated the internal consistency of the PANAS scores. For the full scale, Cronbach’s alpha was .80; values were higher for the subscales (Positive Affect = .90, Negative Affect = .91). PANAS scores appeared relatively stable over time, with an intraclass correlation coefficient for the PANAS scores from baseline to the first week of treatment of .80 for Positive Affect and .76 for Negative Affect. For the comparison of baseline to end of treatment, ICCs were .70 for Positive Affect and .67 for Negative Affect.
We then evaluated convergent and discriminant validity. We evaluated convergent validity with composite reliability (CR) and average variance extracted (AVE) (Positive Affect Factor: CR = .90, AVE of .47; Negative Affect Factor: CR =.91, AVE = .49). Composite reliability scores above .7 are indicative of adequate model reliability, while AVE scores higher than .5 indicate high convergent validity. In our sample, the scores were very near the .5 threshold. In order to evaluate discriminant validity, we evaluated the maximum shared variance (MSV) and average shared variance (ASV) (Positive Affect Factor: MSV of .07, and ASV of .02; Negative Affect Factor: MSV =.18, and ASV =.06). For strong discriminant validity, MSV and ASV values should be lower than AVE values. The Positive and Negative Affect scores in our sample were below the cutoff, demonstrating discriminant validity.
Concurrent and discriminant validity were further evaluated by correlating Positive Affect and Negative Affect scores with psychiatric distress scores from the Brief Symptom Inventory (BSI) and the Addiction Severity Index (ASI) scores, as summarized in Table 3. The Bonferroni correction was calculated by dividing the number of tests by the level of significance (.05/32) and was rounded to three decimal places. Overall, as expected, Positive Affect was negatively correlated with several of the subscales from the BSI, and Negative Affect was positively correlated with all of the subscales from the BSI. We hypothesized that Positive Affect and Negative Affect would not be correlated with the legal, employment, medical, or family/social composite scores of the ASI. The Positive Affect score was significantly correlated with ASI drug composite score, but no other composites. As expected, there were no significant correlations of Negative Affect with the ASI legal and employment composite scores. Negative Affect, however, was significantly correlated with the medical and family/social composite, as well as the alcohol, and psychiatric composite scores of the ASI.
Table 3.
Concurrent and discriminant validity of the Positive and Negative Affect Schedule (PANAS) scores: Correlations with the BSI and the ASI at pre-treatment. N = 410
| Positive Affect | Negative Affect | |
|---|---|---|
| BSI
| ||
| Somatization | −0.15 | 0.48* |
| Obsessive Compulsive | −0.23* | 0.58* |
| Depression | −0.37* | 0.70* |
| Interpersonal Sensitivity | −0.19* | 0.58* |
| Anxiety | −0.25* | 0.69* |
| Hostility | −0.12 | 0.50* |
| Phobic Anxiety | −0.17* | 0.50* |
| Psychoticism | −0.22* | 0.55* |
| Paranoid Ideation | −0.08 | 0.44* |
|
| ||
| ASI
| ||
| Legal | −0.08 | 0.04 |
| Medical | −0.09 | 0.18* |
| Family/Social | −0.04 | 0.25* |
| Employment | −0.07 | 0.00 |
| Alcohol | 0.01 | 0.17* |
| Drug | −0.22* | 0.37* |
| Psychiatric | −0.16 | 0.47* |
Note.
p < .002 (Bonferroni-Corrected). BSI = Brief Symptom Inventory. ASI = Addiction Severity Index.
Finally, in terms of relationships with treatment outcome, baseline Positive Affect was significantly positively associated with maximum days of consecutive abstinence from primary substance of abuse during active treatment (r = .16, p = .001) and treatment completion with abstinence in the last week of treatment (r = .17, p = .001), while Negative Affect was negatively associated with both of these outcomes (consecutive abstinence r = −.17, p = .001, treatment completion r = −.18, p = .001), as well as percentage of treatment days abstinent from primary substance (r =−.16, p = .002).
Discussion
Data from this study suggest that the PANAS Positive and Negative Affect scores are valid and reliable for substance users. The PANAS scores demonstrated good reliability (both internal consistency and test-retest). Convergent and discriminant validity were supported in that Negative Affect scores were strongly correlated with multiple symptoms of psychopathology (as measured by the BSI), while Positive Affect showed strong inverse correlations. Convergent and discriminant validity were further supported with analyses conducted using Confirmatory Factor Analysis (e.g., composite reliability, average variance extracted, maximum shared variance, average shared variance). Predictive validity for both scales was indicated with correlations with primary outcome measures including the maximum duration of abstinence achieved within treatment, and whether the participant completed treatment and was abstinent in the final week of treatment. These variables have been found to be strong indicators of long-term treatment outcomes (32, 44).
We found, through a series of Confirmatory Factor Analyses, that the best-fitting model to our data was a two-factor correlated model with correlated item errors, demonstrating that Positive Affect and Negative Affect are separate, yet correlated, constructs. The two-factor model has been supported in several other studies that have examined the psychometric properties of the PANAS in other populations (10, 19, 23, 46). The finding that Positive Affect and Negative Affect were correlated was also found in previous investigations in other samples (19, 21). The correlated item errors in our model suggest that there may be underlying constructs or content categories, as described in the literature (19, 45). Thus, while there is empirical support and utility in considering Positive Affect and Negative as separate constructs, our results indicate that they are not entirely independent.
The mean scores for Positive Affect and Negative Affect in our sample differ from several other published studies, both in non-clinical samples and within the substance use literature. Crawford and Henry (2004) found, in their non-clinical sample, a mean Negative Affect score of 16.00 (SD = 5.90) and a mean Positive Affect score of 31.31 (SD = 7.65). Our substance-using sample had a higher level of Negative Affect and a similar level of Positive Affect. Negative Affect and Positive Affect scores were also higher in this sample than previous reports from samples of methadone-maintained patients (16) and heavy drinkers (47), indicating that there may be differences in PANAS scores in treatment subpopulations. However, in our sample, we found that the factor structure of the PANAS scores held even after running multi-group analyses by self-reported primary substance type.
Despite Negative Affect receiving more attention in the substance use literature with regards to substance use and treatment outcomes (13–16), our findings support previous research that has found both levels of Positive and Negative Affect at pre-treatment are associated with substance use treatment outcomes (48–50). Of particular interest was the finding that Positive Affect was associated with substance use treatment outcomes, as many investigations have examined the role of Negative Affect, but comparatively few have focused on Positive Affect (13,17, 48). This finding is congruent with a separate study that found that higher Positive Affect was associated with approach-oriented coping, abstinence-related action tendencies, and abstinence-specific social support (48). Thus, Positive Affect may be an important construct that warrants further attention in the substance abuse treatment research.
Although this pooled sample was of moderate size and included individuals who reported multiple types of substances used, several limitations should be noted. First, the sample was comprised of treatment-seeking outpatients enrolled in randomized clinical trials; therefore, these results may not generalize to other samples or treatment settings. In addition, the evaluation of convergent and discriminant validity was constrained to measures that were already included in the assessment batteries of the four randomized clinical trials. Further, all participants were nested within trials. Additionally, there were more males than females in this sample. Despite these limitations, this was the first comprehensive psychometric evaluation of the PANAS in a substance-using population. Our results provide relatively strong support for administering the PANAS in this population, and point to its potential for evaluating the role of affect in the treatment of substance use.
The results of this study may support a deeper understanding of the complex relationship between substance use and affect. Future studies may examine the temporal relationship between substance use and affect in unique substance-using populations, with the goal of identifying the degree to which changes in one are implicated in changes in the other. Specific to treatment, there is clinical potential in investigating how Positive Affect and Negative Affect are influenced by treatment, and to what degree. It is unknown if specifically targeting Positive Affect and Negative Affect within treatment interventions will contribute to improved substance use treatment outcomes (e.g., abstinence, treatment retention). Additionally, future research may examine how Negative Affect and Positive Affect are related to treatment outcomes in other types of interventions. A psychometrically-validated measure of affect in substance-using populations will be critical in advancing this field of research.
Acknowledgments
Funding
This research was supported in part through a National Institute on Drug Abuse (NIDA) T-32 grant, 5T32DA007238-23 (Petrakis), by a supplement to NIDA grant R01 DA015969-09S1 (PI: Carroll), as well as NIDA grants P50-DA09241 (PI: Carroll), and U10 DA015831 (PI: Carroll).
Footnotes
Declaration of Interest
Kathleen M. Carroll is a member of CBT4CBT, LLC, which makes CBT4CBT available to qualified clinical providers and organizations on a commercial basis. Dr. Carroll works with Yale University to manage any potential conflicts of interest. The other authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- 1.de Graaf R, Radovanovic M, Van Laar M, et al. Early cannabis use and estimated risk of later onset of depression spells: Epidemiologic evidence from the population-based World Health Organization World Mental Health Survey Initiative. Am J Epidemiol. 2010:1–11. doi: 10.1093/aje/kwq096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glantz MD, Anthony JC, Berglund PA, et al. Mental disorders as risk factors for later substance dependence: estimates of optimal prevention and treatment benefits. Psychol Med. 2009;39:1365–1377. doi: 10.1017/S0033291708004510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch Gen Psychiat. 1997;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- 4.Baker TB, Japuntich SJ, Hogle JM, McCarthy DE, Curtin JJ. Pharmacologic and behavioral withdrawal from addictive drugs. Curr Dir in Psychol Sci. 2006;15:232–236. [Google Scholar]
- 5.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 6.Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL, Handlesman L. Long-term frontal brain metabolic changes in cocaine abusers. Synapse. 1992;11:184–190. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- 7.Khantzian EJ. The self-medication hypothesis of addictive disorders: Focus on heroin and cocaine. Am J Psychiat. 1985;142:1259–1264. doi: 10.1176/ajp.142.11.1259. [DOI] [PubMed] [Google Scholar]
- 8.Stice E. A prospective test of the dual-pathway model of bulimic pathology: mediating effects of dieting and negative affect. J Abnorm Psychol. 2001;110:124–135. doi: 10.1037//0021-843x.110.1.124. [DOI] [PubMed] [Google Scholar]
- 9.Vrieze E, Demyttenaere K, Bruffaerts R, et al. Dimensions in major depressive disorder and their relevance for treatment outcome. J Affect Disorders. 2014;155:35–41. doi: 10.1016/j.jad.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 11.Lerman C, Roth D, Kaufmann V, et al. Mediating mechanisms for the impact of bupropion in smoking cessation treatment. Drug Alcohol Depen. 2002;67:219–223. doi: 10.1016/s0376-8716(02)00067-4. [DOI] [PubMed] [Google Scholar]
- 12.Uva MCDS, Timary PD, Cortesi M, Mikolajczak M, Blicquy PDRD, Luminet O. Moderating effect of emotional intelligence on the role of negative affect in the motivation to drink in alcohol-dependent subjects undergoing protracted withdrawal. Pers Indiv Differ. 2010;48:16–21. [Google Scholar]
- 13.Martens MP, Neighbors C, Lewis MA, Lee CM, Oster-Aaland L, Larimer ME. The roles of negative affect and coping motives in the relationship between alcohol use and alcohol-related problems among college students. J Stud Alcohol Drugs. 2008;69:412–419. doi: 10.15288/jsad.2008.69.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carpenter KM, Hasin DS. Drinking to cope with negative affect and DSM-IV alcohol use disorders: A test of three alternative explanations. J Stud Alcohol Drugs. 1999;60:694–704. doi: 10.15288/jsa.1999.60.694. [DOI] [PubMed] [Google Scholar]
- 15.Magura S, Rosenblum A, Lovejoy M, Handelsman L, Foote J, Stimmel B. Neurobehavioral treatment for cocaine-using methadone patients: A preliminary report. J Addict Dis. 1995;13:143–160. doi: 10.1300/j069v13n04_03. [DOI] [PubMed] [Google Scholar]
- 16.Handelsman L, Rosenblum A, Palij M, Magura S, Foote J, Lovejoy M, Stimmel B. Bromocriptine for cocaine dependence. Am J Addiction. 1997;6:54–64. [PubMed] [Google Scholar]
- 17.Weiss NH, Williams DC, Connolly KM. Preliminary examination of negative affect, emotion dysregulation, and risky behaviors among military veterans in residential substance abuse treatment. Military Behavioral Health. 2015 doi: 10.1080/21635781.2015.1038405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olson KL, Cooper RL, Nugent WR, Reid RC. Addressing negative affect in substance use relapse prevention. Journal of Human Behavior in the Social Environment. 2015 [Google Scholar]
- 19.Crawford JR, Henry JD. The Positive and Negative Affect Schedule (PANAS): Construct validity, measurement properties and normative data in a large non-clinical sample. Bri J Clin Psychol. 2004;43:245–265. doi: 10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- 20.Mackinnon A, Jorm AF, Christensen H, Korten AE, Jacomb PA, Rodgers B. A short form of the Positive and Negative Affect Schedule: Evaluation of factorial validity and invariance across demographic variables in a community sample. Pers Indiv Differ. 1999;27:405–416. [Google Scholar]
- 21.Merz EL, Malcarne VL, Roesch SC, et al. Psychometric properties of Positive and Negative Affect Schedule (PANAS) original and short forms in an African American community sample. J Affect Disorders. 2013;151:942–949. doi: 10.1016/j.jad.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmukle SC, Egloff B, Burns LR. The relationship between positive and negative affect in the Positive and Negative Affect Schedule. J Res Pers. 2002;36:463–475. [Google Scholar]
- 23.Tuccitto DE, Giacobbi PR, Leite WL. The internal structure of positive and negative affect: A confirmatory factor analysis of the PANAS. Educ Psychol Meas. 2009;1:1–17. [Google Scholar]
- 24.Diener E, Larsen RJ, Levine S, Emmons RA. Intensity and frequency: Dimensions underlying Positive and Negative Affect. J Pers Soc Psychol. 1985;48:1253–1265. doi: 10.1037//0022-3514.48.5.1253. [DOI] [PubMed] [Google Scholar]
- 25.Gaudreau P, Sanchez X, Blondin JP. Positive and negative affective states in a performance-related setting: Testing the factorial structure of the PANAS across two samples of French-Canadian participants. Eur J Psychol Assess. 2006;22:240–249. [Google Scholar]
- 26.Killgore WDS. Evidence for a third factor on the Positive and Negative Affect Schedule in a college student sample. Percept Motor Skill. 2000;90:147–152. doi: 10.2466/pms.2000.90.1.147. [DOI] [PubMed] [Google Scholar]
- 27.Leue A, Beauducel A. The PANAS structure revisited: on the validity of a bifactor model in community and forensic samples. Psychol Assessment. 2011;23:215–225. doi: 10.1037/a0021400. [DOI] [PubMed] [Google Scholar]
- 28.Mehrabian A. Comparison of the PAD and PANAS as models for describing emotions and for differentiating anxiety from depression. J Psychopathol Behav. 1997;19:331–357. [Google Scholar]
- 29.Bradley B, DeFife JA, Guarnaccia C, et al. Emotion dysregulation and negative affect: association with psychiatric symptoms. J Clin Psychiatry. 2011;72:685–691. doi: 10.4088/JCP.10m06409blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carroll KM, Ball SA, Martino S, Nich C, Gordon MA, Portnoy GA, Rounsaville BJ. Computer-assisted delivery of cognitive behavioral therapy for addiction: A randomized trial of CBT4CBT. Am J Psychiat. 2008;165:881–889. doi: 10.1176/appi.ajp.2008.07111835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carroll KM, Nich C, LaPaglia DM, Peters EN, Easton CJ, Petry NM. Combining cognitive behavioral therapy and contingency management to enhance their effects in treating cannabis dependence: Less can be more, more or less. Addiction. 2012;107:1650–1659. doi: 10.1111/j.1360-0443.2012.03877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carroll KM, Kiluk BD, Nich C, Gordon MA, Portnoy GA, Marino DR, Ball SA. Computer-assisted delivery of cognitive-behavioral therapy: efficacy and durability of CBT4CBT among cocaine-dependent individuals maintained on methadone. Am J Psychiat. 2014;171:436–444. doi: 10.1176/appi.ajp.2013.13070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carroll KM, Nich C, Petry NM, Eagan DA, Shi JM, Ball SA, Sofuoglu M. Disulfiram and contingency management to enhance CBT for cocaine dependence: Effects on cocaine use and preliminary evidence for interactions with dopamine B-hydroxylase polymorphism. under review. Manuscript submitted for publication. [Google Scholar]
- 34.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition, January 1995 FINAL: SCID-I/P Version 2.0. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; [Google Scholar]
- 35.Derogatis LR, Melisaratos N. The brief symptom inventory: an introductory report. Psychol Med. 1983;13:595–605. [PubMed] [Google Scholar]
- 36.Thomas ML. Rewards of bridging the divide between measurement and clinical theory: Demonstration of a bifactor model for the Brief Symptom Inventory. Psychol Assessment. 2012;24:101–113. doi: 10.1037/a0024712. [DOI] [PubMed] [Google Scholar]
- 37.McLellan AT, Kushner H, Metzger D, et al. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 38.McLellan AT, Cacciola JC, Alterman AI, Rikoon SH, Carise D. The Addiction Severity Index at 25: origins, contributions and transitions. American J Addiction. 2006;15:113–124. doi: 10.1080/10550490500528316. [DOI] [PubMed] [Google Scholar]
- 39.Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- 40.Steiger JS. Structural model evaluation and modification: An interval estimation approach. Multivar Behav Res. 1990;25:173–180. doi: 10.1207/s15327906mbr2502_4. [DOI] [PubMed] [Google Scholar]
- 41.Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1–55. [Google Scholar]
- 42.Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing Structural Equation Models. Newbury Park, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- 43.Messick S. Validity of psychological assessment: Validation of inferences from persons’ responses and performances as scientific inquiry into score meaning. Am Psychol. 1995;50:741–749. [Google Scholar]
- 44.Higgins ST, Wong CJ, Badger GJ, Ogden DEH, Dantona RL. Contingent reinforcement increases cocaine abstinence during outpatient treatment and 1 year of follow-up. J Consult Clin Psych. 2000;68:64–72. doi: 10.1037//0022-006x.68.1.64. [DOI] [PubMed] [Google Scholar]
- 45.Zevon MA, Tellegen A. The structure of mood change: An idiographic/nomothetic analysis. J Pers Soc Psychol. 1982;43:111–122. [Google Scholar]
- 46.Thompson ER. Development and validation of an internationally reliable short-form of the positive and negative affect schedule (PANAS) J Cross Cult Psychol. 2007;38:227–242. [Google Scholar]
- 47.Kambouropoulos N, Staiger PK. The influence of sensitivity to reward on reactivity to alcohol-related cues. Addiction. 2001;96:1175–1185. doi: 10.1046/j.1360-0443.2001.968117510.x. [DOI] [PubMed] [Google Scholar]
- 48.Carrico AW, Woods WJ, Siever MD, et al. Positive affect and processes of recovery among treatment-seeking methamphetamine users. Drug Alcohol Depen. 2013;132:624–629. doi: 10.1016/j.drugalcdep.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hall SM, Havassy BE, Wasserman DA. Effects of commitment to abstinence, positive moods, stress, and coping on relapse to cocaine use. J Consult Clin Psych. 1991;59:526–532. doi: 10.1037//0022-006x.59.4.526. [DOI] [PubMed] [Google Scholar]
- 50.McHugh RK, Kaufman JS, Frost KH, Fitzmaurice GM, Weiss RD. Positive affect and stress reactivity in alcohol-dependent outpatients. J Stud Alcohol Drugs. 2013;74:152–157. doi: 10.15288/jsad.2013.74.152. [DOI] [PMC free article] [PubMed] [Google Scholar]