Abstract
AIM:
To study which weight estimate calculation used in paediatric resuscitation results in optimal drug dosing; Advanced Paediatric and Life Support (APLS) or the UK Resuscitation Council age‐based formula.
Method
Commonly used drugs used in paediatric resuscitation were selected and a literature search conducted for each drug's pharmacokinetic properties, concentrating on the volume of distribution (Vd).
Hydrophobic drugs have a higher Vd than hydrophilic drugs as they distribute preferentially to fat mass (FM). The larger the Vd, the higher the initial dose required to achieve therapeutic plasma concentrations.
Actual body weight (ABW) estimates are a good indicator of Vd for hydrophobic drugs as they correlate well with FM. Ideal body weight (IBW) estimates may be a better indicator of Vd for hydrophilic drugs, as they correlate better with lean body mass. This highlights potential variation between ABW and IBW, which may result in toxic or sub‐therapeutic dosing.
Results
The new APLS formulae give higher estimates of expected weight for a wider age range. This may be a more accurate reflection of ABW due to increasing prevalence of obesity in children. The UK Resuscitation Council's formula appears to result in a lower estimate of weight, which may relate more closely to IBW.
Conclusion
The main drugs used in paediatric resuscitation are hydrophilic, thus the APLS formulae may result in too much being given. Therefore the UK Resuscitation Council's single formula may be preferred. In addition, a single formula may minimize error in the context of a child of unknown weight requiring administration of emergency resuscitation drugs.
Keywords: paediatric, pharmacokinetic, resuscitation, volume of distribution, weight
Introduction
Optimal therapeutic drug dosing is based on both the pharmacokinetic and pharmacodynamic properties of a drug and minimizes toxicity and avoids sub‐therapeutic levels 1. Optimal therapeutic drug dosing in children in a resuscitation setting is difficult for a number of reasons, one of the most significant being the ability to estimate the child's weight. An estimation of weight is essential as currently, a vast majority of the dosing guidelines in the BNF for Children (BNFC) are standardized by weight 2.
Both age and length have been used to estimate weight to calculate the most appropriate dosages of drugs required in an emergency setting. The exact methods of estimation have evolved over the years but at present there are three well‐recognized methods 3.
Estimation of actual body weight (ABW)
Age‐based formulae
The formulae commonly used are in accordance with the Advanced Paediatric Life Support (APLS) manual 4, where substituting the age of the child into a mathematical formula aids generation of an approximate weight for children aged 1–10 years. The formula below is still taught on the UK Resuscitation Council and European Resuscitation Council courses.
As of July 2011, the APLS guideline for weight estimation based on age has been altered to the following:
Measured 50th centile weight
Weight is referenced from growth chart data. It uses a table constructed using Child Growth Foundation data (their ‘Girls and Boys Four‐in‐One Growth Charts’). The BNFC contains an adapted version of this table 2 and the UK World Health Organisation (WHO) growth charts 2009 contain data for the 50th centile of weight split by gender 5 (see Table 1). An obvious difference is observed between the three sets of data, which can be attributed to how the data was collected and the ages of the subjects. Regarding the Child Growth Foundation data, for each age from ‘newborn’ to 11 years a weight is given. The girl‐boy weights in the table were calculated as an average of the 50th centile for a girl and the 50th centile for a boy. In contrast, the UK‐WHO growth charts combine data from UK‐90 6 based upon serial measurements of height and weight from 0 to 23 years and WHO data based upon breastfed infants from 0 to 4 years. It is unclear which set of weights is the most accurate, hence reducing the safety of its use in clinical practice.
Table 1.
Comparison of weight estimates using Child Growth Foundation Data, BNFC and UK WHO Growth Chart Data
| Average UK weight (kg) | ||||
|---|---|---|---|---|
| Age | Child Growth Foundation data | BNFC | UK WHO growth chart 0–4 years | |
| Girls | Boys | |||
| Newborn | 3.5 | 3.5 | 3.5 | 3.75 |
| 3 months | 6 | 6.1 | 5.75 | 6.4 |
| 6 months | 7.8 | 7.6 | 7.25 | 7.9 |
| 9 months | 8.9 | — | 8.25 | 8.9 |
| 12 months | 9.8 | 9 | 9 | 9.7 |
| 18 months | 11.1 | — | 10.2 | 10.9 |
| 2 years | 12.2 | — | 11.5 | 12.1 |
| 3 years | 14.4 | 14 | 13.9 | 14.2 |
| 4 years | 16.4 | — | 16 | 16.2 |
| 5 years | 18.5 | 18.0 | — | — |
| 6 years | 20.6 | — | — | — |
| 7 years | 23.0 | 23.0 | — | — |
| 8 years | 25.8 | — | — | — |
| 9 years | 28.6 | — | — | — |
| 10 years | 31.8 | 32.0 | — | — |
| 11 years | 35.3 | — | — | — |
Tape‐based measurements for weight
A Broselow tape is a colour‐coded system that helps clinicians decide the dose of medication to administer; for each height an estimate of weight is given. The data is referenced from the National Health and Nutrition Examination Survey (NHANES) carried out by the Centers for Disease Control and Prevention in the United States. It includes height and weight measurements for children 0–18 years old 7. According to a recent prospective study, by Sandell and Charman, looking at the safety of using age‐based estimates when resuscitating children, they report that both Broselow tape length‐based and measured 50th centile age‐based weights outperformed the Resuscitation Council's mathematical formulae 3.
Due to issues of impracticality, such as trapped limbs, immobility and agitated patients 3, and ‘discontinuous pattern of change seen throughout infancy and early childhood’ 8, this method is not recommended for use in clinical practice.
Body composition
However, whichever method of weight estimation is used, and irrespective of the fact that correct weights may be ascertained, no indication is given of body composition. The body is composed of four compartments; fat, water, protein and mineral. Fat is often referred to as the fat mass (FM); and water, protein and mineral is often referred to as the fat free mass (FFM), which together form the two compartment model 9. FFM is sometimes referred to as lean body mass (LBM). It should be noted that LBM and FFM are not directly equivalent, but the difference as a percentage of ABW is usually less than 3–5% 10. Hence, for the purpose of this review, it will be assumed they are equal.
Body composition alters with age, and also within age groups. This is important because although two children may have the same weight estimation, they may have substantially different proportions of fat mass and lean body mass 11. A number of pharmacokinetic variables, based on body composition, can affect drug efficacy. These include the method of administration, rate of absorption, volume of distribution, metabolism and excretion 12.
Pharmacokinetic properties of the drugs used in paediatric resuscitation
Volume of distribution is deemed the most important pharmacokinetic factor affecting the ability to give a dose of drug within the correct therapeutic range. The other pharmacokinetic factors, including absorption, metabolism and excretion, do not alter the plasma levels to as great an extent, as discussed below.
In terms of absorption, the problems of varying gastric emptying rates, gastric pH and GI bacterial flora 13 are mainly overcome in resuscitation circumstances as the majority of drugs are given intravenously, thus bypassing these mechanisms. Hence the ‘bioavailability of parenterally administered drugs usually is considered to be 1’ 14. Other drug delivery methods include via the intramuscular (IM) or preterm neonates (PR) route. The IM route is not usually a problem, assuming it is given in the same location, and only in preterm neonates is it problematic as absorption is variable because they have a small percentage of body weight that is skeletal muscle or fat in comparison to older infants, children and adults. (Preterm neonates have been excluded from this discussion as neither of the age‐based estimations of weight account for babies born preterm.) Further, in neonates, blood flow in muscles varies in the first 2–3 weeks of life 15. This can either increase or decrease the absorption of the drug, but is not dependent on body composition. PR absorption in terms of rate and extent is dependent upon retention and rectal venous drainage. This has considerable inter‐individual variation, which is not dependent on age or weight 15.
The plasma protein binding of various drugs is less in the neonate compared to infants and children because of lower total plasma protein concentration and lower drug–protein binding capacities 16. As the pharmacological action of a drug is attributed to its unbound form of the drug, increased free concentrations can lead to toxicity 13. There is no definitive evidence to suggest that this is a factor relating to body composition or weight.
Drug metabolism occurs predominantly in the liver, although the kidneys, intestines, lungs and skin may be involved 17. Of the factors affecting hepatic metabolism, it is enzyme activity and blood flow that are relatively lower in children compared to adults. Elimination of drugs by the kidneys is dependent on glomerular filtration rate (GFR), tubular secretion and reabsorption. Both the hepatic and renal factors affecting metabolism and elimination depend on age rather than weight.
All the drugs selected for this study are used in paediatric resuscitation including CPR, hypertensive emergencies, anaphylaxis, seizures, infection, acute asthma and cerebral oedema.
Aim
The aim of this study is to determine which weight estimate calculation used in paediatric resuscitation results in optimal drug dosing: the calculations comprising the three new Advanced Paediatric and Life Support (APLS) age‐based formulae or the UK Resuscitation Council age‐based formula. Further, the study compares estimates of actual body weight and ideal body weight and their use in accurate drug dosing in children in a paediatric resuscitation setting.
Method
Drugs commonly used in paediatric resuscitation were selected and a literature search conducted for each drug's pharmacokinetic properties, concentrating on the volume of distribution derived from its hydrophilic and hydrophobic properties and their relationship to body composition (see Table 2). The primary medical search engines used were the Ovid MEDLINE (1948–December 2012) and EMBASE (1974–December 2012) databases. Phrases including ‘volume of distribution’, ‘pharmacokinetic’, ‘pharmacodynamics’, ‘hydrophilic’, ‘hydrophobic’, ‘paediatric’, ‘APLS’, ‘resuscitation’, ‘age’ and ‘weight’ were used in combination to achieve variation in search results. Estimates of actual body weight (ABW) and ideal body weight (IBW) were compared, and ABW estimates were calculated using both UK Resuscitation Council and APLS formulae.
Table 2.
Comparison of the pharmacokinetic properties of drugs used in paediatric resuscitation 13, 14, 15, 19, 21
| Hydrophilic | Ampiphilic | Hydrophobic |
|---|---|---|
| Adrenaline | Amiodarone | Atropine |
| Sodium Bicarbonate | Verapamil | |
| Calcium Gluconate | Lidocaine | |
| Isoprenaline | Diazepam | |
| Flecanide | Midazolam | |
| Adenosine | Lorazepam | |
| Salbutamol | Phenytoin | |
| Magnesium Sulphate | Thiopentone | |
| Aminophylline | Benzyl Penicillin | |
| Ceftriaxone | Paracetamol | |
| Sodium Nitroprusside | Prednisolone | |
| Morphine | Hydrocortisone | |
| Suxamethonium | Dexamethasone | |
| Chlorpheniramine | Ketamine | |
| Mannitol | Furosemide | |
| Labetalol | Epoprostenol | |
| Digoxin |
Results
The new APLS formulae give higher estimates of expected weight for a wider age range. This may be a more accurate reflection of ABW than the UK Resuscitation Council estimate, owing to an increasing prevalence of obesity in children. The UK Resuscitation Council's formula appears to result in a lower estimate of weight, which may relate more closely to IBW than ABW (see Table 3).
Table 3.
Comparison of weight estimates using the UK Resuscitation Council formula and the APLS formulae
| Age |
UK Resuscitation Council
Weight (kg) = 2 × (age in years + 4) (For ages 1–10 years) |
APLS formulae
1–12 months weight (kg) = (0.5 × age in months) + 4 1–5 years weight (kg) = (2 × age in years) + 8 6–12 years weight (kg) = (3 × age in years) + 7 (For ages 1 month–12 year) |
|---|---|---|
| 1 months | — | 4.5 |
| 6 months | — | 7 |
| 11 months | — | 9.5 |
| 1 years | 10 | 10 |
| 2 years | 12 | 12 |
| 3 years | 14 | 14 |
| 4 years | 16 | 16 |
| 5 years | 18 | 18 |
| 6 years | 20 | 25 |
| 7 years | 22 | 28 |
| 8 years | 24 | 31 |
| 9 years | 26 | 34 |
| 10 years | 28 | 37 |
| 11 years | — | 40 |
| 12 years | — | 43 |
The APLS formulae therefore would be most appropriate for use with hydrophobic drugs whilst the UK Resuscitation Council formula would be most appropriate for use with hydrophilic drugs.
Discussion
Volume of distribution of drugs, a major factor in drug efficacy, is dependent on the pharmacokinetic relationship of the proportion of body water and proportion of body fat 13. The body, as described in the two compartment model is comprised of fat mass and lean body mass 9 as described previously. An age‐based estimate of weight does not give any indication of either of these.
Hydrophilic drugs preferentially distribute to body water; the extracellular fluid which consists of plasma and the interstitial fluid. Hydrophilic drugs, therefore, have a smaller volume of distribution than hydrophobic drugs, which distribute preferentially to fat tissue or freely between lean and fat tissue 18. The smaller the volume of distribution, the lower the dose of drug required to achieve adequate plasma concentrations.
Hydrophilic
The actual volume of distribution is dependent upon the amount of body water. Total body water in a term neonate is approximately 75%, decreasing to 60% (25% extracellular and 35% intracellular) by one year of age and only by 12–13 years of age does it reach adult values of around 60% (20% extracellular, 40% intracellular) 12. Thus neonates will have a larger volume of distribution in comparison to older infants and children and will require higher doses of hydrophilic drugs.
Take two children aged 6 years old: their ABW may be different, but the proportion of body water is likely to be similar (Figure 1). Hence the volume of distribution should be the same for both children, indicating that they require the same loading dose of hydrophilic drug. Using the UK Resuscitation Council formula the age‐based weight estimate would be 20 kg, whereas using the APLS formulae it would be 25 kg. This would mean the child given the hydrophilic drug dosed according to the APLS formulae would receive a higher dose, which may result in potential toxicity.
Figure 1.
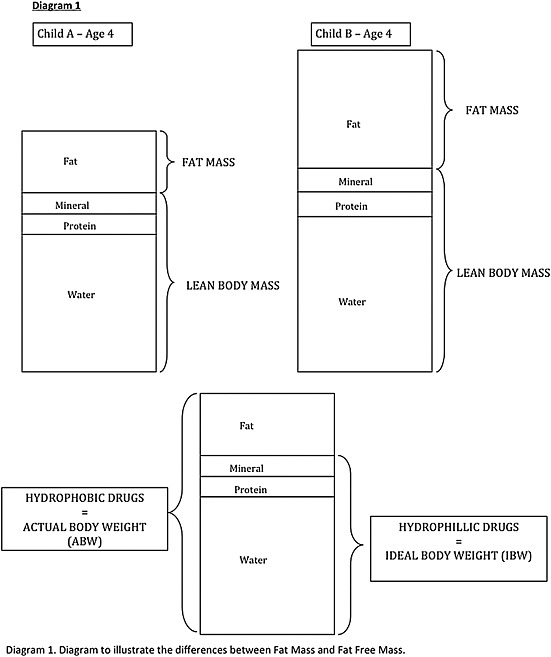
Differences between fat mass and fat free mass.
For drugs with a narrow therapeutic range (e.g. Digoxin, Aminophylline and Flecanide), there is only a small difference in dosage between sub‐therapeutic levels and toxicity 15.
Hydrophobic
Hydrophobic (lipophilic) drugs have a larger volume of distribution than hydrophilic drugs and thus require higher dosage levels to achieve therapeutic plasma concentrations 12. The volume of distribution is dependent on the amount of body fat. On average, a full‐term neonate has 12–16% body fat which increases to 20–25% by one year of age. This age range, however, is not accounted for in the age‐based estimates of weight. In adults, body fat accounts for approximately 18% of body weight 19, 20. Thus, due to higher proportion of fat, the volume of distribution for hydrophobic drugs will be higher in children than neonates.
Take two children aged 6 years old: their age‐based weight estimate according to the UK Resuscitation Council formula would be 20 kg and according to the APLS formulae would be 25 kg. Fat mass in children may vary significantly. If one child had a higher proportion of fat, the drug given would have a higher volume of distribution. The higher the volume of distribution, the higher the loading dose required to achieve therapeutic plasma concentrations. Thus the child given a dose of hydrophobic drug based on the UK Resuscitation Council formula may potentially receive a sub‐therapeutic dose.
For example, Phenytoin, used in status epilepticus, has a loading dose of 18 mg/kg for all ages. A child with a higher than average proportion of body fat and hence a high volume of distribution could receive a sub‐therapeutic dose, whilst a child with much lower than average proportion of body fat could potentially receive a toxic dose despite their age‐based weight estimate being the same, calculated by either formula. The possibility of toxicity is further exacerbated by the fact that Phenytoin displays zero‐order kinetics within the therapeutic range of plasma concentrations 21. Zero‐order kinetics is a ‘state at which the rate of an enzyme reaction is independent of the concentration of the substrate’ 22. Hence a small increase in the dose may lead to a large change in plasma concentrations if the metabolism is saturated. Knowledge of the volume of distribution can aid dosing to achieve appropriate plasma concentrations and minimize toxicity. Further difficulty is encountered as the time taken for hydrophobic drugs to distribute from water to fat compartments differs between drugs depending on the degree of lipophilicity 23. Hence it is not always possible to increase the dose without causing toxicity, and titration in small increments may be required.
For many drugs differences in the volume of distribution will affect the plasma concentration. However, as the therapeutic range is large, this will not lead to toxic or sub‐therapeutic doses. Similar to hydrophilic drugs, those with a narrow therapeutic range are more likely to be affected by differences in fat mass and lean body mass.
Actual body weight vs ideal body weight
All age‐based estimates of weight used currently estimate actual body weight. Actual body weight represents fat mass plus lean body mass. An alternative is to estimate ideal body weight which represents lean body mass (Figure 1).
The attention paid to body composition is of increasing importance, as, in accordance with the Health Survey for England in 2006, the proportion of obese boys and girls aged 2–15 years was 17% and 15%, respectively 24. It has been found that obese children have excesses in whole body fat mass, lean body mass and bone mineral content. The excess in fat mass is the greatest and tends to account for 30–50% of weight 9.
Hence obesity in any age group will increase the volume of distribution of hydrophobic drugs, owing to increases in fat mass 13. It has been suggested that, for hydrophobic drugs, there is a good correlation between volume of distribution and total body weight (TBW). Hence ABW can be used as it reflects this 25, 26.
The volume of distribution of hydrophilic drugs is dependent on lean body mass as it is a close reflection of body water. Estimation of lean body mass in an emergency setting is near impossible owing to the complexity of the formula and variables involved.
Cheymol suggests that for use of relatively hydrophilic drugs, doses should be calculated based on IBW as it reflects lean body mass 25. In a paediatric population, the most common method of calculating IBW is to use body mass index (BMI) 27, the formula for which is:
Hence, by knowing the average BMI associated with a given age and sex, IBW could be calculated by measuring height. However, this has its own pitfalls as mentioned previously, such as immobility and agitated patients, as well as variation in respect to age and sex 28, 29. Despite impracticality, IBW would be useful if a simple method of estimation could be developed, as it more accurately represents body composition and therefore volume of distribution for hydrophilic drugs. Hence a more accurate drug dose could be given to minimize the likelihood of toxicity or sub‐therapeutic doses.
Overall, ABW estimates are a good indicator of volume of distribution for hydrophobic drugs, as they correlate well with FM. IBW estimates may be a better indicator of volume of distribution than ABW for hydrophilic drugs, as they correlate better with lean body mass. According to the European Resuscitation Council, the main drugs used in paediatric CPR are Adrenaline, Amiodarone, Atropine, Magnesium, Calcium and Sodium Bicarbonate 30, 31. All, with the exception of Atropine, are hydrophilic. However, it is important to note that all of the above formulae are estimates, hence it is vital that one looks at the child to ensure the estimated weight from the calculation is a rough match prior to drug dosage calculations. This practical point will help avoid mistakes when using the formula for children who are very over‐ or underweight for their age.
Limitations
Although the effect of body composition on volume of distribution was explored, we recognize that other pharmacokinetic properties must also be addressed. Some evidence shows an allometric correlation between weight and metabolism and clearance of drugs. However, other evidence shows this does not take into account changes in enzyme and organ function that occur with age 32, 33. Thus further work is required to see the effect of these pharmacokinetic factors, whether age or weight has more of an effect, and whether as a result the formulae need to be adjusted accordingly.
It must also be noted that pharmacodynamics properties can also affect drug concentration and effect. This was identified as an area of limited evidence and hence a potential source for further research.
Lastly, neither the APLS nor the UK Resuscitation Council formulae take into account variation in weight according to sex. This is likely because there is limited data looking at whether there is a significant weight difference according to sex and whether this weight difference is a result of differences in fat mass. This is a further area that could be explored in the future.
Conclusion
The gold standard in current practice, if time and circumstance permits, is to weigh the child in order to calculate the doses of resuscitation drugs. Often this is not the case and age‐based formulae are used to calculate actual body weight, despite the fact that simple and practical difficulty arises as a result of varying pharmacokinetic properties of drugs and varying body compositions of patients for a given weight. The two factors lead to difficulty in ensuring that the therapeutic dose of the drug is achieved.
The main drugs used in paediatric resuscitation are hydrophilic, thus the APLS formulae may result in too high a dose being given. Therefore the UK Resuscitation Council's single formula may be preferred. In addition, the single formula is simple and easy to remember so may minimize error in the context of a child of unknown weight that requires administration of emergency resuscitation drugs.
Suggestions for improvement include hydrophobic drugs dosed dependent on actual body weight, and hydrophilic drugs dosed according to ideal body weight. This indicates that in the resuscitation setting, the drug dose should be found using the most accurate method of calculation given the pharmacokinetic properties of the drug. Hence, the method of calculation should be drug‐dependent rather than a ‘one fits all’ approach, although it is recognized that this can be difficult in practice. More detailed research is required into methods and accuracy of IBW calculation in children.
Although the effect of body composition on volume of distribution was explored, more work is required into the effect on other pharmacokinetic properties. Furthermore, there is no substantial evidence yet as to the effects of pharmacodynamics parameters. These are possible areas for further research.
Carasco, C. F. , Fletcher, P. , and Maconochie, I. (2016) Review of commonly used age‐based weight estimates for paediatric drug dosing in relation to the pharmacokinetic properties of resuscitation drugs. Br J Clin Pharmacol, 81: 849–856. doi: 10.1111/bcp.12876.
References
- 1. Mulla H, Johnson TN. Dosing dilemmas in obese children. Arch Dis Childh Educ Pract Ed 2010; 95: 112–7. [DOI] [PubMed] [Google Scholar]
- 2. BNF . BNF for Children 2011–2012. London: Pharmaceutical Press, 2012. [Google Scholar]
- 3. Sandell JM, Charman SC. Can age‐based estimates of weight be safely used when resuscitating children? Emerg Med J 2009; 26: 43–7. [DOI] [PubMed] [Google Scholar]
- 4. Advanced Life Support Group . Advanced Paediatric Life Support: The Practical Approach, 5th edn., eds Samuels M, Wieteska S. Chichester: Wiley‐Blackwell, 2010. [Google Scholar]
- 5. Royal College of Paediatrics and Child Health . UK WHO Growth Charts, 0–4 years [online]. Available at http://www.rcpch.ac.uk/child‐health/research‐projects/uk‐who‐growth‐charts/uk‐who‐growth‐chart‐resources‐0‐4‐years/uk‐who‐0#0‐4 (last accessed 3 February 2016).
- 6. Cole TJ. Growth monitoring with the British 1990 growth reference. Arch Dis Child 1997; 76: 47–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. DuBois D, Baldwin S, King WD. Accuracy of weight estimation methods for children. Pediatr Emerg Care 2007; 23: 227–30. [DOI] [PubMed] [Google Scholar]
- 8. Cole TJ. A critique of the NCHS weight for height standard. Hum Biol 1985; 57: 183–96. [PubMed] [Google Scholar]
- 9. Wells JC, Fewtrell MS, Williams JE, Haroun D, Lawson MS, Cole TJ. Body composition in normal weight, overweight and obese children: matched case‐control analyses of total and regional tissue masses, and body composition trends in relation to relative weight. Int J Obes (Lond) 2006; 30: 1506–13. [DOI] [PubMed] [Google Scholar]
- 10. Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. Quantification of lean bodyweight. Clin Pharmacokinet 2005; 44: 1051–65. [DOI] [PubMed] [Google Scholar]
- 11. Reilly JJ, Dorosty AR. Epidemic of obesity in UK children. Lancet 1999; 354: 1874–5. [DOI] [PubMed] [Google Scholar]
- 12. Fernandez E. Factors and mechanisms for pharmacokinetic differences between pediatric population and adults. Pharmaceutics 2011; 3: 53–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Murphy JE, American Society of Health‐System Pharmacists . Clinical Pharmacokinetics, 4th edn. Bethesda, MD: American Society of Health‐System Pharmacists, 2008. [Google Scholar]
- 14. Winter ME. Basic Clinical Pharmacokinetics, 4th edn Philadelphia, PA: Lippincott Williams & Wilkins, 2003. [Google Scholar]
- 15. Speight TM, Holford NHG. Avery's Drug Treatment: A Guide to the Properties, Choice, Therapeutic Use and Economic Value of Drugs in Disease Management, 4th edn Auckland: Adis International, 1997. [Google Scholar]
- 16. Strolin Benedetti M, Baltes EL. Drug metabolism and disposition in children. Fundam Clin Pharmacol 2003; 17: 281–99. [DOI] [PubMed] [Google Scholar]
- 17. Litterst CL, Mimnaugh EG, Reagan RL, Gram TE. Comparison of in vitro drug metabolism by lung, liver, and kidney of several common laboratory species. Drug Metab Dispos 1975; 3: 259–65. [PubMed] [Google Scholar]
- 18. Shock NW, Watkin DM, Yiengst MJ, Norris AH, Gaffney GW, Gregerman RI, Falzone JA. Age differences in the water content of the body as related to basal oxygen consumption in males. J Gerontol 1963; 18: 1–8. [DOI] [PubMed] [Google Scholar]
- 19. Evans WE, Schentag JJ, Jusko WJ. Applied Pharmacokinetics: Principles of Therapeutic Drug Monitoring, 3rd edn Vancouver, WA: Applied Therapeutics, 1992. [Google Scholar]
- 20. Friis‐Hansen B Body water compartments in children: changes during growth and related changes in body composition. Pediatrics 1961; 28: 169–81. [PubMed] [Google Scholar]
- 21. Grahame‐Smith DG, Aronson JK. Oxford Textbook of Clinical Pharmacology and Drug Therapy, 3rd edn Oxford: Oxford University Press, 2002. [Google Scholar]
- 22. Mosby's Medical Dictionary , 8th edn Edinburgh: Mosby, 2009. [Google Scholar]
- 23. Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science 2004; 303: 1818–22. [DOI] [PubMed] [Google Scholar]
- 24. Wake M, Canterford L, Patton GC, Hesketh K, Hardy P, Williams J, Waters E, Carlin JB. Comorbidities of overweight/obesity experienced in adolescence: longitudinal study. Arch Dis Child 2010; 95: 162–8. [DOI] [PubMed] [Google Scholar]
- 25. Cheymol G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet 2000; 39: 215–31. [DOI] [PubMed] [Google Scholar]
- 26. Morgan DJ, Bray KM. Lean body mass as a predictor of drug dosage: implications for drug therapy. Clin Pharmacokinet 1994; 26: 292–307. [DOI] [PubMed] [Google Scholar]
- 27. Phillips S, Edlbeck A, Kirby M, Goday P. Ideal body weight in children. Nutr Clin Pract 2007; 22: 240–5. [DOI] [PubMed] [Google Scholar]
- 28. Cameron N, Jones LL, Griffiths PL, Norris SA, Pettifor JM. How well do waist circumference and body mass index reflect body composition in pre‐pubertal children? Eur J Clin Nutr 2009; 63: 1065–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Demerath EW, Schubert CM, Maynard LM, Sun SS, Chumlea WC, Pickoff A, Czerwinski SA, Towne B, Siervogel RM. Do changes in body mass index percentile reflect changes in body composition in children? Data from the Fels Longitudinal Study. Pediatrics 2006; 117: e487–95. [DOI] [PubMed] [Google Scholar]
- 30. Biarent D, Bingham R, Eich C, López‐Herce J, Maconochie I, Rodríguez‐Núñez A, Rajka T, Zideman D. European Resuscitation Council Guidelines for Resuscitation 2010 Section 6. Paediatric life support. Resuscitation 2010; 81: 1364–88. [DOI] [PubMed] [Google Scholar]
- 31. Nolan JP, Soar J, Zideman DA, Biarent D, Bossaert LL, Deakin C, Koster RW, Wyllie J, Böttiger B. European Resuscitation Council Guidelines for Resuscitation 2010 Section 1. Executive Summary. Resuscitation 2010; 81: 1219–76. [DOI] [PubMed] [Google Scholar]
- 32. Edginton AN, Willmann S. Physiology‐based versus allometric scaling of clearance in children; an eliminating process based comparison. Paediatr Perinat Drug Ther 2006; 7: 146–53. [Google Scholar]
- 33. Shi R, Derendorf H. Pediatric dosing and body size in biotherapeutics. Pharmaceutics 2010; 2: 389–418. [DOI] [PMC free article] [PubMed] [Google Scholar]


