Abstract
Diffraction at hard work: Modern heterogeneous catalysis would benefit from a multiscale science approach bridging the molecular world with the macroscopic world. Because of recent breakthroughs in X‐ray diffraction methods, including the surface X‐ray diffraction of atomically flat model catalysts, X‐ray diffraction tomography of catalyst bodies, and X‐ray profiling of an active catalyst in a chemical reactor, such an approach is now within reach.
Keywords: heterogeneous catalysis, pair distribution function, surface analysis, synchrotron radiation, X‐ray diffraction
The scientific community has recently celebrated the centennial of X‐ray diffraction. Ever since the first diffraction pattern was recorded by Max von Laue, X‐ray diffraction (XRD) has been developed into a widely used, powerful, and indispensable characterization method for structural analysis.1 A real revolution in using X‐rays for diffraction analysis happened with the advent of synchrotron radiation. Nowadays, numerous synchrotron facilities around the world provide highly brilliant, focused X‐rays with tunable energies. The energies of hard X‐rays (typically 5–100 keV) provide deep penetration into matter, which enables studies under elevated temperatures and pressures. Another benefit of hard X‐rays is that beam damage is significantly reduced. These features of hard X‐rays provide researchers with the ideal method to study catalysts under working conditions at different length scales, that is, at the level of the active surface of a catalyst material, of a catalyst body placed in a reactor, and of the chemical reactor itself. In this manner, it is possible to span a wide range of length scales, enabling a full multiscale science analysis of the dynamics of structurally complex catalytic materials. This highlight discusses the potential of modern hard X‐ray techniques to provide such a multiscale science approach for investigating heterogeneous catalysts at work by presenting some recent experiments that were the first of their kind (Figure 1).
Figure 1.
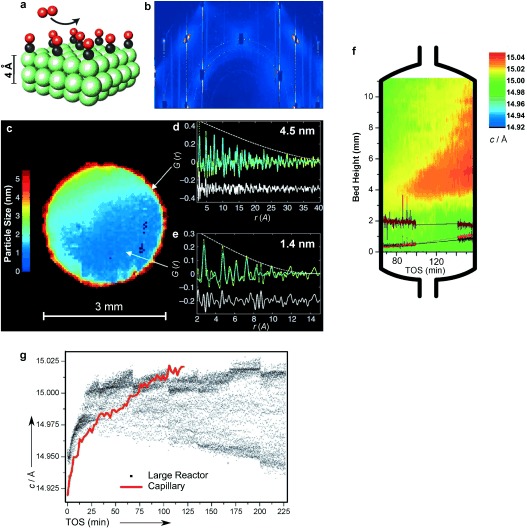
Three recent reports are highlighted to show the potential of a multiscale science approach for studying heterogeneous catalysts at work with advanced X‐ray diffraction methods. a) An atomic model of a Pd surface during CO oxidation.2 b) A single diffraction image representing all of the images recorded during a rotational scan. Individual CTRs and superlattice rods are visible.2 c) Pd/Al2O3 catalyst body showing the particle size distribution measured by PDF‐CT.3 d, e) Experimental and fitted PDF data for single pixels at the edge (d) and in the interior (e) of the particle.3 f) Schematic representation of a capillary reactor used for the MTO reaction, showing the crystallographic changes as a function of the catalyst bed height. The color scale (from blue to red) indicates an expansion of the crystallographic c axis with increasing time on stream.4 g) Scatter plot of a z‐scan of the MTO reaction in a large reactor, compared to a line plot from capillary reactor data. Images are adapted from Ref. 2–4.
XRD is essentially a bulk technique and may provide information on a surface structure only for model catalysts, that is, adsorbates on atomically flat surfaces of single‐crystal quality (Figure 1 a). By using a novel surface scattering geometry, Gustafson et al.2 took advantage of high‐energy X‐rays (85 keV) by grazing incidence geometry. Surface scattering results in streaking of the Bragg reflections, a phenomenon that is due to the termination of the surface. These streaks are commonly known as crystal truncation rods (CTRs). Most of the state‐of‐the‐art surface X‐ray diffraction (SXRD) measurements typically had to rely on the slow detection and scanning of particular diffraction features by a small two‐dimensional (2D) X‐ray detector. In the reported experiment, the high energies of the X‐rays increase the size of Ewald’s sphere and lead to a contraction of the reciprocal lattice space. This enables a much larger section of the CTRs to be sampled at once. Furthermore, by using a suitably large detector, one may record multiple reflections at the same time (Figure 1 b). This speeds up acquisition times by several orders of magnitude, which in turn allows the monitoring of surface reconstruction processes in situ with remarkable sub‐second time resolution. This was illustrated for CO oxidation on a highly active palladium surface. The newly developed technique provided structural information on lattice parameters and atom positions, together with information on surface termination and strain. This is essential not only for heterogeneous catalysis, but also for a wide range of scientific disciplines where the structure of an interface, such as the solid–liquid interface in an electrochemical cell, for example, is of great interest. With the large amount of information that can be collected in one experiment, one should not forget the potential of computational methods to facilitate the interpretation and understanding of experimental results. Many experiments require support by well‐established ab initio models, and combining these theoretical and experimental insights will be essential for understanding structural changes of catalytic solids.
An intrinsic problem of conventional powder XRD analysis is that it is essentially blind to amorphous and nano‐crystalline components. A promising way to overcome these limitations of XRD and to study small nanoparticles (<2 nm) with XRD is to use a pair distribution function (PDF) analysis. PDF analysis gives the probability distribution of finding pairs of atoms at a certain real‐space distance. As it utilizes all Bragg and diffuse‐scattering signals, it contains information on the local structure of both nano‐crystalline and amorphous materials. Recently, Jacques et al. revealed the strong potential of the PDF computed tomography (PDF‐CT) technique to provide more information on truly nano‐crystalline and amorphous materials.3 This method may provide information on the 3D distribution of multiple crystalline and amorphous phases in complex catalytic bodies. This was demonstrated for Pd/PdO nanoparticles dispersed within a millimeter‐sized γ‐Al2O3 catalyst body. With the PDF‐CT technique, it was possible to resolve the size and distribution of the supported nanoparticles in three dimensions (Figure 1 c), including those of diffraction‐visible 4.5 nm large, but also diffraction‐silent 1.4 nm large Pd nanoparticles (Figure 1 d and e, respectively).
Understanding catalytic processes at the level of a chemical reactor is equally important as it is challenging to study these processes under in situ conditions. Recent work by Wragg et al. has illustrated that the XRD method may be applied to the methanol‐to‐olefins (MTO) reaction on the industrially important microporous catalyst SAPO‐34.4 Instead of a common single‐point experiment, a fast scanning method was applied in a reactor‐like capillary to spatially map what is happening in the reactor. More specifically, the expansion of the crystal lattice was monitored along the catalytic reactor (Figure 1 f) and with time on stream (Figure 1 g). It was noted that the formation of the hydrocarbon pool species (HCP) was responsible for the expansion of the crystal lattice along the c axis. At a later stage of the reaction process, coke‐like aromatic species were formed, blocking the pores and deactivating the catalyst. It is also interesting that the HCP species formed first at the middle of the reactor and evolved toward its end. The developed method provided new insights into the induction period of the MTO reaction and the complex mechanisms of catalyst bed deactivation.
It is clear that XRD alone cannot provide all relevant information that is required for understanding the working principles of catalytic solids. However, XRD may be combined with other synchrotron techniques, such as extended X‐ray absorption fine structure (EXAFS) analysis or X‐ray fluorescence, to simultaneously make use of methods that are sensitive to other parameters, such as the local environment of specific elements. These systems may be further combined with infrared, UV/Vis, or fluorescence microscopy and spectroscopy. It is certain that existing problems will provide clear incentives for experimental improvements in the future. Surface diffraction is particularly sensitive to temperature changes, making sample alignment rather difficult. Furthermore, scattered intensity and detector sensitivity drop with an increase in photon energy, whereas the time resolution of tomographic experiments is still a serious drawback for monitoring fast reaction processes.
With the upgrades of the third‐generation synchrotrons, the performance of XRD has greatly improved over the past decade. These efforts should lead to even more significant improvements of the X‐ray beam brilliance and coherence, the speed and sensitivity of X‐ray detectors, and nano‐focusing optics. Considering also the potential of free electron lasers (FELs),5 one can only foresee a brilliant future for XRD in unravelling catalytic processes in detail and in bridging the molecular world with the macroscopic world.
This work was supported by the NWO Gravitation program, the Netherlands Center for Multiscale Catalytic Energy Conversion (MCEC), and an European Research Council (ERC) Advanced Grant (321140).
References
- 1. Sumner T., Science 2014, 343, 1092–1093; [DOI] [PubMed] [Google Scholar]; Jones N., Nature 2014, 505, 602–603. [DOI] [PubMed] [Google Scholar]
- 2. Gustafson J., Shipilin M., Zhang C., Stierle A., Hejral U., Ruett U., Gutowski O., Carlsson P.‐A., Skoglundh M., Lundgren E., Science 2014, 343, 758–761. [DOI] [PubMed] [Google Scholar]
- 3. Jacques S. D. M., Di Michiel M., Kimber S. A. J., Yang X., Cernik R. J., Beale A. M., Billinge S. J. L., Nat. Commun. 2013, 4, 2536. [DOI] [PubMed] [Google Scholar]
- 4. Wragg D. S., O’Brien M. G., Bleken F. L., Di Michiel M., Olsbye U., Fjellvåg H., Angew. Chem. 2012, 124, 8080–8083; [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2012, 51, 7956–7959. [DOI] [PubMed] [Google Scholar]
- 5. Miller R. J. D., Science 2014, 343, 1108–1116; [DOI] [PubMed] [Google Scholar]; Waldrop M. M., Nature 2014, 505, 604–606. [DOI] [PubMed] [Google Scholar]


