Abstract

Three oligothiophenes were evaluated as PET ligands for the study of local and systemic amyloidosis ex vivo using tissue from patients with amyloid deposits and in vivo using healthy animals and PET-CT. The ex vivo binding studies revealed that all three labeled compounds bound specifically to human amyloid deposits. Specific binding was found in the heart, kidney, liver, and spleen. To verify the specificity of the oligothiophenes toward amyloid deposits, tissue sections with amyloid pathology were stained using the fluorescence exhibited by the compounds and evaluated with multiphoton microscopy. Furthermore, a in vivo monkey PET-CT study showed very low uptake in the brain, pancreas, and heart of the healthy animal indicating low nonspecific binding to healthy tissue. The biological evaluations indicated that this is a promising group of compounds for the visualization of systemic and localized amyloidosis.
Keywords: Positron emission tomography, amyloidosis, amyloid-β, transthyretin, AL amyloidosis, oligothiophenes
A number of pathological conditions are associated with aggregation of proteins forming insoluble amyloid deposits in tissue.1,2 These amyloid diseases are classified as local or systemic and the amyloid fibrils are characterized by a cross-β sheet structure where proteins self-aggregate into insoluble fibrillar amyloid deposits.3,4 The local aggregation of extracellular amyloid-β (Aβ) protein and intracellular neurofibrillary tangles (NFT) characterize the brain lesions in Alzheimer’s disease (AD), one of the most common forms of dementia.5 Another local amyloidosis affects the insulin producing β-cells of the pancreas by the polypeptide hormone islet amyloid polypeptide (IAPP), which forms localized amyloid deposits in islets of Langerhans in association with type II diabetes.3,6 The liver-produced plasma protein transthyretin (TTR) and immunoglobulin light chains (AL) from monoclonal plasma cells are examples of proteins that can aggregate into amyloid fibrils in systemic amyloidoses and often cause severe restrictive cardiomyopathy.4,7−9 Especially AL amyloidosis is a disease with very poor prognosis, although new treatment methods have been introduced prolonging the life span of the patients. A sensitive noninvasive diagnostic imaging method not requiring invasive sampling such as heart biopsy, which could detect the amyloid deposits in an early stage of the disease before irreversible damage to tissue have occurred, would be of significant clinical value.
Positron emission tomography (PET) is a powerful molecular imaging technique that uses radiolabeled compounds, PET ligands, labeled with short-lived positron emitting radionuclides. The distribution of these ligands can be followed in vivo using a PET camera thus creating a dynamic 3D distribution pattern of the tracer in tissue.10,11 Two of the most frequently used short-lived cyclotron-produced radionuclides are fluorine-18 (t1/2 = 109.8 min) and carbon-11 (t1/2 = 20.3 min).12 Several PET ligands have been developed for the visualization and quantification of Aβ-deposits in the human brain as potential noninvasive methods for the diagnosis of AD (Figure 1A).13−17
Figure 1.
Structures of [11C]PIB, Congo Red, Thioflavin T, q-FTAA/p-FTAA, and the 11C/18F LCO’s investigated scaffold in the present study.
Up to recently, little attention has been on PET ligands for amyloid deposits in organs other than the brain. However, the most used PET ligand for the study of senile plaque in AD patients, [11C]PIB,13 was recently shown to also visualize amyloid deposits of type ATTR and AL in the heart.18 Since [11C]PIB is excreted through liver and kidney high tracer accumulation in healthy organs preclude the use of this tracer for the study of amyloid in these organs. Thiophene-based ligands, abbreviated LCOs (luminescent conjugated oligothiophenes), display a striking specificity for protein aggregates associated with prion diseases, AD and systemic amyloidoses.19−22 In addition, LCOs bind to Aβ congophilic amyloid deposits as well as immunopositive disease-associated protein aggregates that go undetected by conventional amyloid ligands, such as Congo Red and Thioflavin T (ThT) (Figure 1A).23−25 These findings encouraged us to develop suitable syntheses strategies to radiolabel analogues of these oligothiophene derivatives with 11C and 18F and to investigate the potential of this class of compounds as PET ligands for the study of the different types of amyloid deposits found in systemic amyloidoses by in vitro binding assays and in vivo PET-CT (Figure 1B).
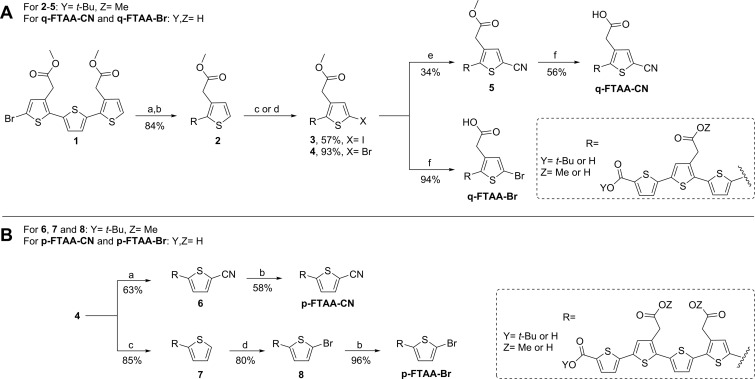
The synthesis of precursors and references was performed according to Scheme 1 and Scheme 2. The synthesis of compound q-FTAA-Br, precursor for labeling of [11C]q-FTAA, and the corresponding reference, q-FTAA-CN (quarternary formyl thiophene acetic acid cyanide), are shown in Scheme 1A. The synthesis of compound 1 has been described in the literature.26 Compound 1 was subjected to a Suzuki coupling with 5-(dihydroxyboryl)-2-thiophenecarboxylic acid using potassium carbonate and the palladium-catalyst PEPPSI-IPr, and subsequent tert-butyl ester protection employing tert-butyl 2,2,2-trichloroacetimidate and boron trifluoride diethyl etherate to afford compound 2 in 84% yield over two steps. The thiophene tetramer 2 was either iodinated using N-iodosuccinimide (NIS) to yield 3 in 57% or brominated using N-bromosuccinimide (NBS) to yield 4 in 93%. Compound 3 was subjected to a cyanation using CuCN forming 5 in 34% yield. Acid hydrolysis of 5 using trifluoroacetic acid (TFA) followed by basic hydrolysis using sodium hydroxide provided reference compound q-FTAA-CN in 56% yield over two steps. Compound 4 was treated according to the same hydrolysis protocol as q-FTAA-CN to give precursor q-FTAA-Br in 94% yield. A Suzuki coupling of compound 4 with 5-cyano-2-thienylboronic acid according to the same protocol as for the synthesis of compound 2 afforded compound 6 in 63% yield as shown in Scheme 1B. The reference compound p-FTAA-CN (pentameric formyl thiophene acetic acid cyanide) was obtained in 58% yield from compound 6 according to the two-step hydrolysis procedure vide supra. Compound 7 was synthesized in 85% yield from compound 4 using 2-thienylboronic acid according to the Suzuki coupling protocol using PEPPSI-IPr. Bromination of compound 7 with NBS provided compound 8 in 80% yield. Hydrolysis of compound 8 gave the precursor p-FTAA-Br in 96% yield.
Scheme 1. Synthesis of Precursors (q-FTAA-Br and p-FTAA-Br) and Reference Compounds (q-FTAA-CN and p-FTAA-CN).
Reagents and conditions: A. (a) 5-Carboxythiophene-2-boronic acid, PEPPSI-IPr, K2CO3, toluene, MeOH, 75 °C, 30 min; (b) tert-butyl 2,2,2-trichloroacetimidate, BF3•Et2O, dioxane, rt, 2 h; (c) NIS, TFA, DMF, 0 °C, 4 h; (d) NBS, DMF, -15°C, 2 h; (e) CuCN, DMF, microwave 175 °C, 15 min; (f) TFA, DCM, rt, 2 h then NaOH, dioxane, H2O, rt, 4 h. B. (a) 5-cyanothiophene-2-boronic acid pinacol ester, PEPPSI-IPr, K2CO3, toluene, MeOH, 75 °C, 30 min; (b) TFA, DCM, rt, 2 h then NaOH, dioxane, H2O; (c) 2-thienylboronic acid, PEPPSI-IPr, K2CO3, toluene, MeOH, 75 °C, 30 min; (d) NBS, DMF, −5 °C, 2 h.
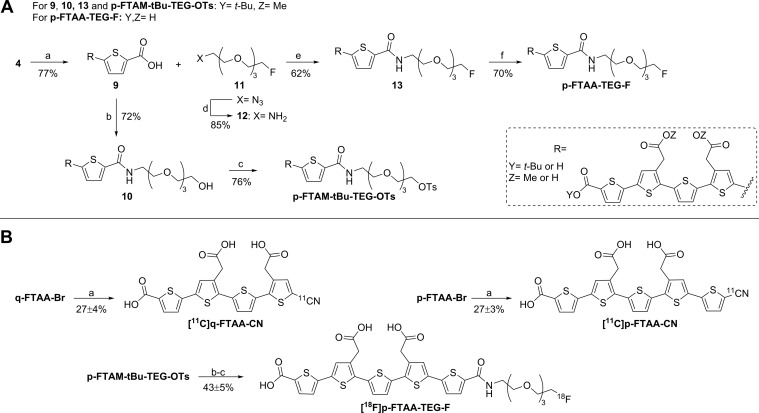
Scheme 2. Synthesis of Precursor p-FTAM-tBu-TEG-OTs and Reference p-FTAA-TEG-F and Radiosynthesis of [11C]q-FTAA-CN, [11C]p-FTAA-CN, and [18F]p-FTAA-TEG-F.
Reagents and conditions: A. (a) 5-Carboxythiophene-2-boronic acid, PEPPSI-IPr, K2CO3, toluene, MeOH, 75 °C, 30 min; (b) 2-(2-(2-(2-amino-ethoxy)ethoxy)ethoxy)-ethanol, HATU, DIPEA, DMF, rt, 2 h; (c) TsCl, pyridine, DCM, rt, 4 h; (d) PPh3, MeOH, H2O, 50 °C, 2 h; (e) 12, HATU, DIPEA, DMF, rt, 2 h; (f) TFA, DCM, rt, 2 h then NaOH, dioxane, H2O. B. (a) NH4[11C]CN, Pd(Xantphos)Cl2, DMF, 130 °C, 5 min. (b) K[18F]F,K2.2.2, MeCN, 80 °C, 10 min; (c) NaOH, 100 °C, 15 min.
Compound 9 was synthesized in 77% yield from compound 4 by the Suzuki procedure vide supra using 5-carboxythiophene-2-boronic acid (Scheme 2A). The resulting thiophenepentamer carboxylic acid was coupled to 2-(2-(2-(2-amino-ethoxy)ethoxy)ethoxy)-ethanol using the coupling reagent HATU, yielding alcohol 10 in 72% yield. Tosylation using tosyl chloride afforded precursor p-FTAM-tBu-TEG-OTs in 76% yield. The synthesis of compound 11 has been described in the literature.27 Compound 11 was subjected to a Staudinger reduction to afford 12 in 85% yield. Compound p-FTAA-TEG-F (pentameric formyl thiophene acetic acid tetra ethylene glycol fluoride) was synthesized by coupling compound 9 to compound 12 using the coupling reagent HATU, yielding the reference compound p-FTAA-TEG-F in 62% yield after hydrolysis of compound 13.
The two precursors q-FTAA-Br and p-FTAA-Br were subjected to a palladium-mediated cyanation reaction using dichloro[9,9-dimethyl-4,5-bis(diphenylphosphino)xanthene]palladium(II).The two radiolabeled oligothiophenes [11C]q-FTAA-CN and [11C]p-FTAA-CN were isolated in 27 ± 4% and 26 ± 3% decay corrected radiochemical yield based on the starting amount of [11C]cyanide (Scheme 2B). The reaction mixture was heated to 130 °C for 5 min and the labeled compounds were then obtained by semipreparative HPLC purification. The specific radioactivity for both [11C]q-FTAA-CN and [11C]p-FTAA-CN was in the order of 220–280 GBq/μmol at EOS and radiochemical purity higher than 99%. A normal production gave 0.5–1.5 GBq of [11C]q-FTAA-CN and [11C]p-FTAA-CN with a total synthesis time of approximately 50 min. The chemical purity was high with no UV traces of reagents. It was, however, not possible to achieve baseline separation of the corresponding precursors and products, and the concentration of precursor in the final product solution was approximately 10 μg/mL, about seven times the concentration of the product. It cannot be ruled out that the precursors also have affinity to the amyloid deposits and thus could lower the effective specific radioactivity. Assuming same binding affinity and pharmacokinetics of labeled product and precursor would give an effective specific radioactivity of about 25–40 GBq/μmol. However, considering the expected high number of binding sites in amyloid deposits and the high percentage of specific binding in the in vitro studies the pseudocarrier effect can probably be neglected (vide infra).
[18F]Fluoride was reacted with p-FTAM-tBu-TEG-OTs in a substitution reaction at 80 °C for 10 min (Scheme 2B). Hydrolysis of the esters with aqueous sodium hydroxide at 100 °C for 15 min gave crude [18F]p-FTAA-TEG-F, which was isolated using semipreparative HPLC. The decay corrected radiochemical yield was 43 ± 5% based on the initial activity of [18F]fluoride. A normal production gave 1–2 GBq of [18F]p-FTAA-TEG-F with a radiochemical purity of >99% and a specific radioactivity of 300–500 GBq/μmol, chemical purity was high with no UV traces of reagents or precursor. The total synthesis time was approximately 90 min.
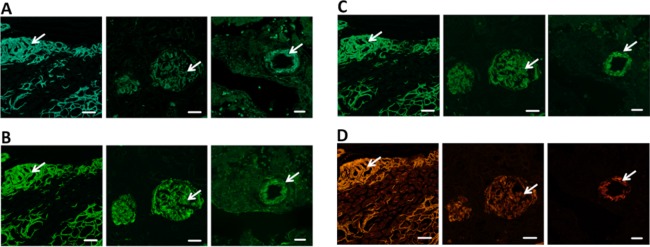
To verify the amyloid binding properties of the cyano and the TEG-fluoride modified LCOs, tissue sections from patients diagnosed with systemic amyloidoses were stained with the fluorescent reference compounds (Figure 2). When excited at 458 nm, all three oligothiophenes showed specific binding to Congo Red positive amyloid deposits in verified clinical samples from systemic amyloidosis patients with AA, AL, and ATTR. Hence, the three novel oligothiophenes showed a similar specificity toward protein aggregates as previously reported LCOs, verifying that the minor chemical modifications, as compared to q-FTAA (quarterneric thiophene) and p-FTAA (pentameric thiophene, see Figure 1), necessarily introduced for radiolabeling did not interfere with the amyloid binding potency of the dyes. Further, Kd values were obtained for the three ligands using fluorescence data. The values were determined to be 434 nM (q-FTAA-CN), 39 nM (p-FTAA-CN), and 149 nM (p-FTAA-TEG-F).
Figure 2.
Fluorescence images of tissues sections with ATTR (heart, first column), AL (kidney (second column), and AA (liver, third column) pathology stained by reference compound q-FTAA-CN (A), p-FTAA-CN (B), p-FTAA-TEG-F (C), and Congo Red (D). Congo Red positive amyloid deposits (white arrows) are clearly labeled by all reference compounds. Scale bars represent 20 μm (A,B) and 50 μm (C). All tissue sections were fixed with ethanol for 10 min and stained with 3 μM ligand in PBS after rehydration of the tissue.
The results from the fluorescence microscopy were confirmed in homogenate binding studies with the radiolabeled compounds [11C]q-FTAA-CN, [11C]p-FTAA-CN, and [18F]p-FTAA-TEG-F. PIB and an amyloid binding LCO, p-FTAA,22 were used as blocking substances.
All three oligothiophene derivatives showed high degree of specific binding to ATTR, AL, and AA amyloid in human samples from heart, spleen, and liver (Figure 3A). The specific binding was determined by competition binding using excess (10 μM) of p-FTAA. Interestingly, it was shown that [11C]q-FTAA-CN, [11C]p-FTAA-CN, and [18F]p-FTAA-TEG-F binding to amyloid was blocked to a much lower extent by coincubation with excess of PIB (10 μM) than the degree of blocking obtained by coincubation with an excess of p-FTAA (10 μM). The mean values for the three ligands are summarized in Figure 3B. This is a clear indication that PIB and oligothiophenes do not compete for the same binding site. Moreover, in a recent study28 it was shown that [3H]PIB was able to bind to the same tissue samples that were used in the present study but to a lower extent. For the binding of [3H]PIB to heart amyloid of ATTR type, the Bmax was 10.4 pmol/mg tissue, as compared to 270, 210, and 21 pmol/mg tissue at 10 nM concentrations of [11C]q-FTAA-CN, [11C]p-FTAA-CN, and [18F]p-FTAA-TEG-F, respectively (Figure 3C). The potentially very high number of oligothiophene binding sites, especially in the heart, holds promises for the new PET ligands to be very sensitive and specific ligands for amyloid deposits in several organs. The high degree of specific binding also suggests that the precursor either lacks affinity for the amyloid deposits or that the effective specific radioactivity was sufficient.
Figure 3.
(A) Specific binding (%) of the three ligands using amyloid tissue from human and mice with corresponding controls lacking amyloid pathology. The experiments were performed through competition binding with p-FTAA (10 μM). (B) Percentage blocking (mean value of the three ligands) using either p-FTAA (10 μM) or PIB (10 μM) as blocking compound. (C) Quantification of Bmax (pmol/mg tissue) using the three ligands to amyloid tissues (ATTR, AL λ, AL κ, and control).
To further characterize the radiolabeled oligothiophenes, a PET-CT study was performed in one healthy cynomolgus monkey. The images of the brain, summarized over time, clearly showed that the uptake is very low, in the brain (not shown). This is consistent with the studies using rats. The uptake in the liver, and especially in the gall bladder, is apparent. [11C]q-FTAA-CN, but not the other compounds, is also excreted through the kidneys, as some activity is found in the urinary bladder (Figure 4). The heart is also visualized by the high blood radioactivity. The radioactivity was determined in blood samples collected throughout the studies, and the time course of the blood activity. The blood radioactivity was high throughout the study with [11C]q-FTAA-CN being slightly more rapidly excreted.29
Figure 4.
Fused PET-CT whole body scans of a cynomolgus monkey with [11C]p-FTAA-CN (left), [11C]q-FTAA-CN (middle), and [18F]p-FTAA-TEG-F (right) 60–75 min (3 × 5 min scans). Arrows points to LI (liver), HE (heart), GB (gall bladder), and BL (bladder). Injected dose: 184, 201, and 52 MBq, respectively.
In conclusion, the three new oligothiophene derivatives showed binding to amyloid deposit in heart, kidney, and liver tissue slices as shown by fluorescence binding. Homogenate binding studies with [11C]q-FTAA-CN, [11C]p-FTAA-CN, and [18F]p-FTAA-TEG-F showed specific binding for all three tracers to human amyloid deposits of type AL, TTR, and AA from heart, spleen, and liver. The oligothiophene could not be displaced with the current golden standard, PIB, suggesting another binding site. The cynomolgus PET-CT studies in a nonhuman primate indicate the potential usefulness of [11C]q-FTAA-CN as PET biomarkers for amyloid deposits in the pancreas and the heart in humans. The very high amount of binding in amyloid containing heart myocardium is another important finding suggesting that the sensitivity of the new tentative PET ligand could be superior to [11C]PIB for studies of amyloid deposits in the heart. However, the high blood radioactivity may present some problems, and therefore, PET studies in animals with induced amyloid deposits are thus needed to verify the potential of these new amyloid PET ligands. Such studies are being conducted in our laboratory and will be reported in due course.
Acknowledgments
This work was supported from The Swedish Research Council (PH, KPRN), Swedish Foundation for Strategic Research (KPRN), LiU-Neuro (DS), the Ehrling-Persson Foundation (to K.P.R..N and L.B.G.J.), and the Göran-Gustafsson Foundation (P.H.). K.P.R.N. is further supported by an ERC Starting Independent Researcher grant (Project: MUMID). L.B.G.J. is enrolled in the doctoral program Forum Scientium.
Glossary
ABBREVIATIONS
- AA
amyloid of serum amyloid A protein
- AD
Alzheimer’s disease
- AL
amyloid lightchain
- ATTR
amyloid of transthyretin
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.5b00309.
Experimental details for all compounds synthesized; spectroscopic data for standards and precursors; protocols for animal imaging studies (PDF)
Author Contributions
The manuscript was written through contributions of all authors.
The authors declare no competing financial interest.
Supplementary Material
References
- Sipe J.; Benson M. D.; Buxbaum J. N.; Ikeda S.; Merlini G.; Saraiva M. J.; Westermark P. Nomeclature 2014: Amyloid Fibril Proteins and Clinical Classification of the Amyloidosis. Amyloid 2014, 21, 221–224. 10.3109/13506129.2014.964858. [DOI] [PubMed] [Google Scholar]
- Eisenberg D.; Jucker M. The Amyloid State of Proteins in Human Diseases. Cell 2012, 148, 1188–1203. 10.1016/j.cell.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald J.; Riek R. Biology of Amyloid: Structure, Function, and Regulation. Structure 2010, 18, 1244–1260. 10.1016/j.str.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Dobson C. M. Protein Folding and Misfolding. Nature 2003, 426, 884–890. 10.1038/nature02261. [DOI] [PubMed] [Google Scholar]
- DeToma A.; Salamekh S.; Ramamoorthy A.; Lim M. Misfolded Proteins in Alzheimer’s Disease and Type II Diabetes. Chem. Soc. Rev. 2012, 41, 608–621. 10.1039/C1CS15112F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark P.; Andersson A.; Westermark G. Islet Amyloid Polypeptide, Islet Amyloid, and Diabetes Mellitus. Physiol. Rev. 2011, 91, 795–826. 10.1152/physrev.00042.2009. [DOI] [PubMed] [Google Scholar]
- Merlini G.; Bellotti V. Molecular Mechanisms of Amyloidosis. N. Engl. J. Med. 2003, 349, 583–596. 10.1056/NEJMra023144. [DOI] [PubMed] [Google Scholar]
- Ihse E.; Rapezzi C.; Merlini G.; Benson M.; Ando Y.; Suhr O.; Ikeda S.; Lavatelli F.; Obici L.; Quarta C.; Leone O.; Jono H.; Ueda M.; Lorenzini M.; Liepnieks J.; Ohshima T.; Tasaki M.; Yamashita T.; Westermark P. Amyloid Fibrils Containing Fragmented ATTR may be the Standard Fibril Composition in ATTR Amyloidosis. Amyloid 2013, 20, 142–150. 10.3109/13506129.2013.797890. [DOI] [PubMed] [Google Scholar]
- Jacobson D.; Pastore R.; Yaghoubian R.; Kane I.; Gallo G.; Buck F.; Buxbaum J. Variant-sequence Transthyretin (isoleucine 122) in Late-onset Cardiac Amyloidosis in Black Americans. N. Engl. J. Med. 1997, 336, 466–473. 10.1056/NEJM199702133360703. [DOI] [PubMed] [Google Scholar]
- Herschman H. R. Molecular Imaging: Looking at Problems, Seeing Solutions. Science 2003, 302, 605–608. 10.1126/science.1090585. [DOI] [PubMed] [Google Scholar]
- Wester H.-J. Nuclear Imaging Probes: From Bench to Bedside. Clin. Cancer Res. 2007, 13, 3470–3481. 10.1158/1078-0432.CCR-07-0264. [DOI] [PubMed] [Google Scholar]
- Ametamey S. M.; Honer M.; Schubiger P. A. Molecular Imaging with PET. Chem. Rev. 2008, 108, 1501–1516. 10.1021/cr0782426. [DOI] [PubMed] [Google Scholar]
- Klunk W.; Engler H.; Nordberg A.; Wang Y.; Blomqvist G.; Holt D.; Bergström M.; Savitcheva I.; Huang G.; Estrada S.; Ausen B.; Debnath M.; Barletta J.; Price J.; Sandell J.; Lopresti B.; Wall A.; Koivisto P.; Antoni G.; Mathis C.; Långström B. Imaging Brain Amyloid in Alzheimer’s Disease with Pittsburgh Compound-B. Ann. Neurol. 2004, 55, 306–319. 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Leinonen V.; Rinne J.; Virtanen K.; Eskola O.; Rummukainen J.; Huttunen J.; Fraunberg M.; Nerg O.; Koivisto A.; Rinne J.; Jaaskelainen J.; Buckley C.; Smith A.; Jones P.; Sherwin P.; Farrar G.; McLain R.; Kailajarvi M.; Heurling K.; Grachev I. Positron Emission Tomography with [18F]Flutemetamol and [11C]PiB for in Vivo Detection of Cerebral Cortical Amyloid in Normal Pressure Hydrocephalus Patients. Eur. J. Neurol. 2013, 20, 1043–1052. 10.1111/ene.12102. [DOI] [PubMed] [Google Scholar]
- Johnson A.; Jeppsson F.; Sandell J.; Wensbo D.; Neelissen J.; Jureus A.; Ström P.; Norman H.; Farde L.; Svensson S. AZD2184: a Radioligand for Sensitive Detection of Beta-amyloid Deposits. J. Neurochem. 2009, 108, 1177–1186. 10.1111/j.1471-4159.2008.05861.x. [DOI] [PubMed] [Google Scholar]
- Wolk D.; Rinne J.; Wong D.; Leinonen V.; Arnold S.; Buckley C.; Smith A.; McLain R.; Sherwin P.; Farrar G.; Kailajarvi M.; Grachev I. [F-18]-Flutemetamol PET Amyloid Imaging and Cortical Biopsy Histopathology in Normal Pressure Hydrocephalus: Pooled Analysis of Four Studies. Acta Neuropathol. 2012, 124, 833–845. 10.1007/s00401-012-1051-z. [DOI] [PubMed] [Google Scholar]
- Clark C.; Schneider J.; Bedell B.; Beach T.; Bilker W.; Mintun M.; Pontecorvo M.; Hefti F.; Carpenter A.; Flitter M.; Krautkramer M.; Kung H.; Coleman R.; Doraiswamy P.; Fleisher A.; Sabbagh M.; Sadowsky C.; Reiman P.; Zehntner S.; Skovronsky D.; Grp A.-A. S. Use of Florbetapir-PET for Imaging β-Amyloid Pathology. JAMA-J. Am. Med. Assoc. 2011, 305, 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni G.; Lubberink M.; Estrada S.; Axelsson J.; Carlson K.; Lindsjo L.; Kero T.; Långström B.; Granstam S.; Rosengren S.; Vedin O.; Wassberg C.; Wikström G.; Westermark P.; Sörensen J. In Vivo Visualization of Amyloid Deposits in the Heart with 11C-PIB and PET. J. Nucl. Med. 2013, 54, 213–220. 10.2967/jnumed.111.102053. [DOI] [PubMed] [Google Scholar]
- Sigurdson C. J.; Nilsson K. P. R.; Hornemann S.; Manco G.; Polymenidou M.; Schwarz P.; Leclerc M.; Hammarström P.; Wuthrich K.; Aguzzi A. Prion Strain Discrimination Using Luminescent Conjugated Polymers. Nat. Methods 2007, 4, 1023–1030. 10.1038/nmeth1131. [DOI] [PubMed] [Google Scholar]
- Nilsson K. P. R.; Åslund A.; Berg I.; Nyström S.; Konradsson P.; Herland A.; Inganäs O.; Stabo-Eeg F.; Lindgren M.; Westermark G. T.; Lannfelt L.; Nilsson L. N. G.; Hammarström P. Imaging Distinct Conformational States of Amyloid-β Fibrils in Alzheimer’s Disease Using Novel Luminescent Probes. ACS Chem. Biol. 2007, 2, 553–560. 10.1021/cb700116u. [DOI] [PubMed] [Google Scholar]
- Åslund A.; Herland A.; Hammarström P.; Nilsson K. P. R.; Jonsson B.-H.; Inganäs O.; Konradsson P. Studies of Luminescent Conjugated Polythiophene Derivatives: Enhanced Spectral Discrimination of Protein Conformational States. Bioconjugate Chem. 2007, 18, 1860–1868. 10.1021/bc700180g. [DOI] [PubMed] [Google Scholar]
- Åslund A.; Sigurdson C. J.; Klingstedt T. s.; Grathwohl S.; Bolmont T.; Dickstein D. L.; Glimsdal E.; Prokop S.; Lindgren M.; Konradsson P.; Holtzman D. M.; Hof P. R.; Heppner F. L.; Gandy S.; Jucker M.; Aguzzi A.; Hammarström P.; Nilsson K. P. R. Novel Pentameric Thiophene Derivatives for in Vitro and in Vivo Optical Imaging of a Plethora of Protein Aggregates in Cerebral Amyloidoses. ACS Chem. Biol. 2009, 4, 673–684. 10.1021/cb900112v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan V.; Klingstedt T.; Simon R.; Nilsson K. P. R.; Thueringer A.; Kashofer K.; Haybaeck J.; Denk H.; Abuja P. M.; Zatloukal K. Cross β-sheet Conformation of Keratin 8 is a Specific Feature of Mallory-Denk Bodies Compared With Other Hepatocyte Inclusions. Gastroenterology 2011, 141, 1080–1090. 10.1053/j.gastro.2011.05.039. [DOI] [PubMed] [Google Scholar]
- Klingstedt T.; Blechschmidt C.; Nogalska A.; Prokop S.; Häggqvist B.; Danielsson O.; Engel W. K.; Askanas V.; Heppner F. L.; Nilsson K. P. R. Luminescent Conjugated Oligothiophenes for Sensitive Fluorescent Assignment of Protein Inclusion Bodies. ChemBioChem 2013, 14, 607–616. 10.1002/cbic.201200731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingstedt T.; Shirani H.; Åslund K. O. A.; Cairns N. J.; Sigurdson C. J.; Goedert M.; Nilsson K. P. R. The Structural Basis for Optimal Performance of Oligothiophene-Based Fluorescent Amyloid Ligands: Conformational Flexibility is Essential for Spectral Assignment of a Diversity of Protein Aggregates. Chem. - Eur. J. 2013, 19, 10179–10192. 10.1002/chem.201301463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingstedt T.; Åslund A.; Simon R. A.; Johansson L. B. G.; Mason J. J.; Nyström S.; Hammarström P.; Nilsson K. P. R. Synthesis of a Library of Oligothiophenes and Their Utilization as Fluorescent Ligands for Spectral Assignment of Protein Aggregates. Org. Biomol. Chem. 2011, 9, 8356–8370. 10.1039/c1ob05637a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breyholz H.-J.; Wagner S.; Faust A.; Riemann B.; Höltke C.; Hermann S.; Schober O.; Schäfers M.; Kopka K. Radiofluorinated Pyrimidine-2,4,6-triones as Molecular Probes for Noninvasive MMP-Targeted Imaging. ChemMedChem 2010, 5, 777–789. 10.1002/cmdc.201000013. [DOI] [PubMed] [Google Scholar]
- Hellström-Lindahl E.; Westermark P.; Antoni G.; Estrada S. In Vitro Binding of [3H]PIB to Human Amyloid Deposits of Different Types. Amyloid 2014, 21, 21–27. 10.3109/13506129.2013.860895. [DOI] [PubMed] [Google Scholar]
- The average human plasma protein binding was 35 ± 7% as tested for 11 different LCOs at 10 μM LCO in 10% human blood plasma.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.