Abstract
Biodegradable poly(d,l-lactide) (PDLLA), Poly(trimethylene carbonate) (PTMC), polycaprolactone (PCL), poly(caprolactone-co-d,l-lactide) (PCDLLA) and poly(trimethylene carbonate-co-caprolactone) (PTCL) are recently used for clinical drug delivery system such as subcutaneous contraceptive implant capsule due to their biodegradable properties that they could possess long-term stable performance in vivo without removal, however their permeation rate is unknown. In the work, biodegradable material membranes were prepared by solvent evaporation using chloroform, and commercial silicone rubber membrane served as a control. Gestodene was used as a model drug. Gestodene has high biologic progestational activity which allows for high contraceptive reliability at very low-dose levels. The permeation rate of gestodene for several biodegradable materials was evaluated. In vitro diffusion studies were done using Franz diffusion cells with a diffusion area of 1.33 cm2. Phosphate buffer solution (PBS, pH 7.4), 10% methanol solution and distilled water were taken in donor and receiver chambers at temperature of 37 °C respectively. The in vitro experiments were conducted over a period of 24 h during which samples were collected at regular intervals. The withdrawn samples were appropriately diluted and measured on UV–vis spectrophotometer at 247 nm. Conclusion data from our study showed that permeation rate of PCDLLA with CL ratio more than 70% could be more excellent than commercial silicone rubber membrane. They may be suitable as a candidate carrier for gestodene subcutaneous contraceptive implants in contraceptive fields.
Keywords: Permeation rate, Franz diffusion cell, Gestodene, Biodegradable materials, Membrane, Contraceptive implant capsule
1. Introduction
During the past decades, women had been looking forward to alternatives to the short-acting contraceptives and there were many studies with focus on long-acting contraceptives (Baldwin and Edelman, 2013, Neukom et al., 2011, Ferreira et al., 2014, Chen and Chen, 2007). Consistent use of short-acting methods such as injectables and pills is under constant threat from difficulty of the ‘surprise’ in case of a forgotten pill, inconvenience of daily intake, onset of side effects and other factors (Harel and Cromer, 1999, Lara-Torre and Schroeder, 2002, Likis, 2002, Brache and Faundes, 2010). Long-acting contraceptive methods such as intrauterine contraceptive device (Thonneau and Almont, 2008, Jacques et al., 1986), implants (Bhatia et al., 2011) and injections (Thurman et al., 2013, Rahimy et al., 1999) which could offer a long-period effect with good compliance (Urdl et al., 2005) and tolerance are more popular (Archer et al., 2004). Subcutaneous (SC) implantation (Zhang et al., 2011, Ma et al., 2006) is currently the most common route of self-administering biopharmaceuticals.
Gestodene (Stanczyk and Archer, 2014, Gao et al., 2009, Matějíček and Kubáň, 2007) – a progestin in the 19-nortestosterone series – today is widely used in recent years. Gestodene differs from levonorgestrel in chemical structure results in a shift in the conformational location of the 18-ethyl group and accounts for differences in the pharmacokinetics of the two steroids. Gestodene has high biologic progestational activity with respect to ovulation inhibition, endometrial morphology, and binding affinity to the progesterone receptor. This potent progestational activity allows for high contraceptive reliability at very low-dose levels (Shoupe, 1994). Gestodene is considered an effective, well-tolerated contraceptive option.
Controlled release drug delivery system (Bresolin et al., 2014, Campinez et al., 2013) is one of the most active fields of research and development, because of its advantages such as high-efficiency and low side effects. Coupled with this, methods for evaluation of drug carriers are in the center of attention. According to their degradation properties, carriers for implants can be further classified into biodegradable and non-biodegradable biopolymers such as silicone rubber. Degradable biomaterials do not need subsequent surgical removal after being implanted in bodies, a feature superior to non-degradable biomaterials. Long-term reversible contraceptives have been promoted as highly effective contraceptives that could lower rates of unintended pregnancy and are often viewed as particularly suitable methods for young women (Hoggart et al., 2013). Biodegradable polymers have been widely used and have greatly promoted the development of drug release system because of their biocompatibility and biodegradability. The development of biotechnology and medical technology has set higher requirements for biomedical materials.
Franz diffusion cell experiments (Kshirsagar et al., 2012, Rauma and Johanson, 2009) are emerging as a generally accepted method in the field of drug delivery. Although a new subcutaneous product is only of value if the clinical pharmacokinetic profile appears the appropriate pharmacodynamic response needed for the treatment of the patient, preclinical assessments strongly guide the product development. These include in vitro experiments for evaluating the penetration of a drug molecule through the biodegradable materials membranes. Moreover, measurement of the release profiles of the active pharmaceutical ingredient from the formulation is not only an important parameter for characterizing its release behavior, but it can also be considered as a significant quality attribute which is valuable in the development of a suitable formulation or in the evaluation of possible changes in formulation composition, production parameters and shelf-life stability (Vithlani et al., 2012, Boateng et al., 2012, Gao et al., 2014, Zare et al., 2008). Therefore, the regulatory health authorities are generally requesting these diffusion-release tests in the pharmaceutical dossier submitted to obtain the marketing authorization (Baert et al., 2011). In vitro permeation experiments are a valuable adjunct to in vivo absorption studies, and provide a convenient means for evaluating the permeation characteristics of drugs (Jung et al., 2012, Ng et al., 2012).
The purpose of this paper was to evaluate the permeation properties of gestodene on several biodegradable materials such as PTMC (Zhang et al., 2006, Kluin et al., 2009), PCL (Shen et al., 2013, Yen et al., 2009), PDLLA (Guo et al., 2007, Kumar et al., 2014), PCDLLA (Zhang et al., 2013b) and PTCL (Campos et al., 2013), which are proved to be used in human body by FDA (Tian et al., 2012, Ishaug-Riley et al., 1999, San Miguel et al., 2008, Kowalczuk et al., 2014). Although these biomaterials had been intensively studied, the permeation profiles of these materials can be different, especially when considering certain drug delivery system and were never reported. Gestodene was used as a model drug. The permeation studies, particularly for drug permeation, involved the use of Franz-type diffusion cells. These consisted of two compartments with a membrane clamped between the donor and receiver chambers. Such diffusion or permeation cells had a fixed volume of agitated donor and receptor solutions and can then be used to evaluate the time course for permeation of gestodene through these membranes. The concentration of gestodene was measured by UV–vis spectrophotometry. The method was previously validated and verified for accuracy, precision and linearity. A comprehensive profile of their properties comparing to commercial medical silicone rubber membrane was also provided. The results obtaining from UV–vis spectrophotometer clearly indicated that the diffusion cells provide a simple, precise and reliable system to monitor in vitro experiment.
2. Materials and methods
2.1. Materials
1,3-Trimethylene carbonate (TMC, Jinan Daigang Biomaterial Co., Ltd, China) and d,l-lactide (DLLA, Jinan Daigang Biomaterial Co., Ltd, China) were used without further purification. ε-caprolactone (CL, Alfa Aesar, USA) was purified by drying over CaH2 (Sinopharm Chemical Reagent Co., Ltd, China) and distilled under reduced nitrogen pressure. Stannous octoate (SnOct2, Sigma–Aldrich, USA) was used as received. Gestodene was obtained from Beijing (China) Zizhu Pharmaceutical Co., Ltd. Medical silicone rubber membrane was received from Jinan (China) Chensheng Medical Silicone Rubber Product CO., Ltd. Solvents used for the sample preparation and analytical procedures were of HPLC or analytical grade.
2.2. Polymer synthesis
PTMC, PCL, PDLLA, PCDLLA and PTCL were synthesized as previously described (Yang et al., 2010, Zhang et al., 2012, Zhang et al., 2013a). Briefly, the polymerizations were conducted by ring opening polymerization in evacuated and sealed glass ampules using SnOct2 as catalyst (Feng et al., 2009). Polymerization was carried out for 24 h at 130 ± 2 °C. The synthesized polymers were purified by dissolution in chloroform and precipitation into methanol. After being washed in methanol several times, the polymers were dried under reduced pressure until constant weight.
2.3. Gel permeation chromatography (GPC) analysis
The molecular weight of gels and their distribution (Polydispersity index, PDI) were measured by GPC using a Waters model 1515 isocratic HPLC pump with a Waters model 2414 refractive index detector, at a flow rate of 1.0 ml/min (eluent: THF; 35 °C). Polystyrene standards (Waters) were used for calibration.
2.4. Preparation of membranes
Homogeneous membranes were prepared from PDLLA, PTMC, PCL, PCDLLA and PTCL by solvent evaporation at room temperature as described (Bormashenko et al., 2006, Tsuji and Miyauchi, 2001). Briefly, polymers were dissolved in chloroform. A total of 50 ml of the polymer solution were cast into glass Petri dishes. The solvent was allowed to evaporate slowly in a fume hood at room temperature. Finally the membranes with a 15-cm diameter were weighed individually and kept in a desiccator containing calcium chloride at room temperature. The final weight was noted when there was no further change in the weight of individual membrane. Thickness of the membranes was measured using a vernier caliper at different places of the membrane and average thickness determined was 0.022 cm.
2.5. In vitro permeation studies – Franz diffusion cell
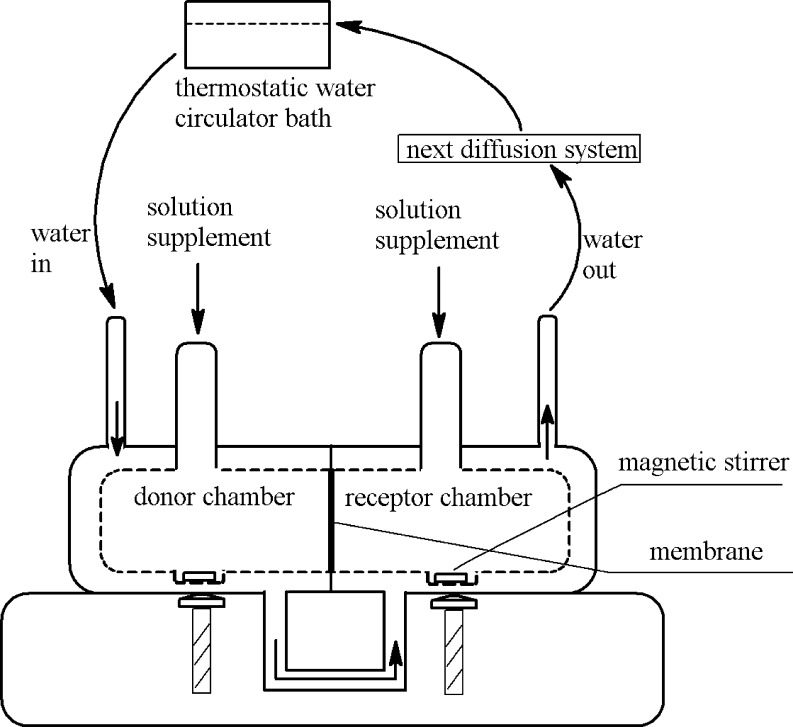
In vitro diffusion studies were done using Franz diffusion cell having a diffusion area of 1.33 cm2. This automation of such experiments had increased in the last few years (Lobo et al., 2014, Li et al., 2012). A protocol suitable to validate the automated diffusion equipment with several in-line flow-through cells was used. The diffusion system was machined with inlet and outlet where thermostatic water-circulator bath can be attached. The temperature was precisely controlled in a thermostatic cabinet to minimize variations in experimental conditions (△T ⩽ 1 °C). The Franz diffusion cell system designed, constructed and operated in this study is shown schematically in Fig. 1. PBS, 10% methanol solution and distilled water were taken in donor and receiver chambers at 37 °C for 24 h respectively. The prepared membranes separated the two chambers. Saturated solution of Gestodene was placed in the donor chamber, and corresponding solvent was placed in receiver chamber. The contents of the both chambers were stirred continuously at about 150 rpm using a magnetic bead. 6 ml of the solution from the receiver medium was removed at regular intervals, 0, 0.5, 1, 2, 3, 6, 8, 12, and 24 h and was replaced with equal volume of fresh solvent to maintain sink conditions. All the determinations were made in triplicate for each cell.
Figure 1.
Schematic drawing of the diffusion cell with thermostatic water bath.
2.6. UV–vis spectroscopy
Solutions removed from receptor chamber were taken for drug content estimation. The solution samples were filtered through 0.45 μm filter membranes and analyzed for the determination of gestodene permeated. UV–vis absorption measurement was carried out at room temperature on a PerkinElmer lambda 20 UV–vis spectrometer using a quartz cell. A dilute solution was used for this analysis. The UV–vis spectrum of gestodene in the range of 190–400 nm was obtained. Finally, the filtrate was examined for the drug content by measuring absorbance at λmax of 247 nm. The cumulative gestodene release was investigated. The unknown concentrations of the remained solutions were determined using the equation obtained from the standard curve, A = 0.0528C (μg/ml) – 0.0078, with a correlation coefficient of R2 = 0.99999.
3. Results and discussion
3.1. Macroscopic observations
Membrane-forming capabilities of biodegradable polymers were different (Table 1). The membranes morphology was found to be homogeneous for most of materials, except for small molecular weight PDLLA. However, it was also difficult to remove the membrane from Petri dish for PCDLLA (40:60 mol%) due to its viscosity. The permeation experiments could not be carried out for small molecular weight PDLLA and PCDLLA (40:60 mol%) membranes.
Table 1.
Comparison of membrane-forming capabilities for different materials.
| Polymer | Molar ratio | Mn × 10−5 | Membrane-forming capabilities |
|---|---|---|---|
| PTMC17 | – | 17 | Fine; easy to be removed from Petri dishes; flexible; transparent; adhesive |
| PTMC22 | – | 22 | |
| PTMC29 | – | 29 | |
| PCL6 | – | 6 | Fine; easy to be removed from Petri dishes; slightly hard; a white appearance |
| PCL10 | – | 10 | |
| PCL16 | – | 16 | |
| PDLLA4 | – | 4 | Difficult to separate them from Petri dishes |
| PDLLA5 | – | 5 | |
| PDLLA7 | – | 7 | Fine; easy to be removed from Petri dishes; slightly hard; transparent |
| PDLLA12 | – | 12 | |
| PDLLA17 | – | 17 | |
| PCDLLA1:9 | 10:90 | 13 | Fine; easy to be removed from Petri dishes; moderate hard; anti-adhesive |
| PCDLLA2:8 | 20:80 | 15 | |
| PCDLLA3:7 | 30:70 | 10 | |
| PCDLLA4:6 | 40:60 | 15 | Fine; flexible; adhesive |
| PCDLLA5:5 | 50:50 | 17 | |
| PCDLLA6:4 | 60:40 | 15 | Very poor; difficult to separate them from Petri dishes |
| PCDLLA7:3 | 70:30 | 20 | Fine; easy to be removed from Petri dishes; moderate hard; anti-adhesive |
| PCDLLA8:2 | 80:20 | 16 | |
| PCDLLA9:1 | 90:10 | 15 | |
| PTCL1:9 | 10:90 | 25 | |
| PTCL2:8 | 20:80 | 24 | |
| PTCL3:7 | 30:70 | 23 | |
| PTCL4:6 | 40:60 | 23 | Fine; easy to be removed from Petri dishes; anti-adhesive |
| PTCL5:5 | 50:50 | 25 | An increase in hardness and opaqueness was correlated with increased caprolactone molar ratio |
| PTCL6:4 | 60:40 | 25 | |
| PTCL7:3 | 70:30 | 25 | |
| PTCL8:2 | 80:20 | 26 | |
| PTCL9:1 | 90:10 | 26 |
3.2. Molecular weight and distribution analysis by GPC
Table 2 shows number molecular weight of different materials before and after membrane-forming. In drug delivery systems, a moderate molecular weight was desired, which can affect the polymer properties such as morphology, membrane-forming, and permeation rate, directly. We aimed at searching the optimal molecular weight of different biodegradable materials in the experiment for permeation studies. As a little change in the molecular weight of materials before and after membrane-forming, it was supposed that the solvent evaporation method did not affect the molecular weight values of these materials.
Table 2.
Number molecular weight of different polymers before and after membrane-forming.
| Polymer | Molar ratio | Mn × 10−5 |
|
|---|---|---|---|
| Before membrane-forming | After membrane-forming | ||
| PTMC17 | – | 17 | 16 |
| PTMC22 | – | 22 | 22 |
| PTMC29 | – | 29 | 28 |
| PCL6 | – | 6 | 6 |
| PCL10 | – | 10 | 9 |
| PCL16 | – | 16 | 16 |
| PDLLA7 | – | 7 | 6 |
| PDLLA12 | – | 12 | 10 |
| PDLLA17 | – | 17 | 15 |
| PCDLLA1:9 | 10:90 | 9 | 10 |
| PCDLLA2:8 | 20:80 | 11 | 9 |
| PCDLLA3:7 | 30:70 | 10 | 9 |
| PCDLLA4:6 | 40:60 | 11 | 9 |
| PCDLLA5:5 | 50:50 | 11 | 10 |
| PCDLLA6:4 | 60:40 | 10 | 8 |
| PCDLLA7:3 | 70:30 | 13 | 10 |
| PCDLLA8:2 | 80:20 | 10 | 10 |
| PCDLLA9:1 | 90:10 | 10 | 8 |
| PTCL1:9 | 10:90 | 25 | 24 |
| PTCL2:8 | 20:80 | 24 | 22 |
| PTCL3:7 | 30:70 | 23 | 23 |
| PTCL4:6 | 40:60 | 23 | 22 |
| PTCL5:5 | 50:50 | 25 | 23 |
| PTCL6:4 | 60:40 | 25 | 24 |
| PTCL7:3 | 70:30 | 25 | 23 |
| PTCL8:2 | 80:20 | 26 | 25 |
| PTCL9:1 | 90:10 | 26 | 24 |
PDI was defined as the ratio of the weight average molecular weight, Mw, to the number average molecular weight, Mn. High PDI values correspond to a conglomerate of chains spanning a wide range of molecular weights. The lower values in PDI indicated that homogeneous polymers were synthesized.
3.3. Permeation studies
3.3.1. Constant homopolymer but varying molecular weight and release media
As shown in Table 3 and Fig. 2, total cumulative release within 24 h was lower than 15 μg. When the molecular weight of designated homopolymer was constant, the permeation rate was not similar to that observed in different media and lower than that obtained with the silicone rubber, suggesting that these homopolymers have lower permeation efficacy than that of commercial rubber silicone product.
Table 3.
Effect of molecular weight and release media on permeation efficacy for homopolymer.
| Polymer | Mn × 10−5 | Permeation efficacy |
||
|---|---|---|---|---|
| Water | PBS | Methanol | ||
| PTMC17 | 17 | +++ | +++ | +++++ |
| PTMC22 | 22 | ++ | ++ | +++++ |
| PTMC29 | 29 | ++ | + | +++ |
| PCL6 | 6 | +++ | +++ | ++++ |
| PCL10 | 10 | ++ | ++ | +++ |
| PCL16 | 16 | ++ | ++ | ++ |
| PDLLA7 | 7 | + | + | ++ |
| PDLLA12 | 12 | − | + | +++ |
| PDLLA17 | 17 | + | + | ++ |
Cumulative drug release after 24 h was used for the comparison. The number of signs represented different cumulative drug release values: −, <2 μg; +, 2–5 μg; ++, 5–10 μg; +++, 10–15 μg; ++++, 15–20 μg; +++++, >20 μg.
Figure 2.
Permeation profiles of gestodene through different type of homopolymers and silicone rubber after 24 h.
The summed results of the permeation rate, as shown in Fig. 2, indicated that permeation rate was affected by both molecular weight and release media, but no significant difference was observed in release profiles.
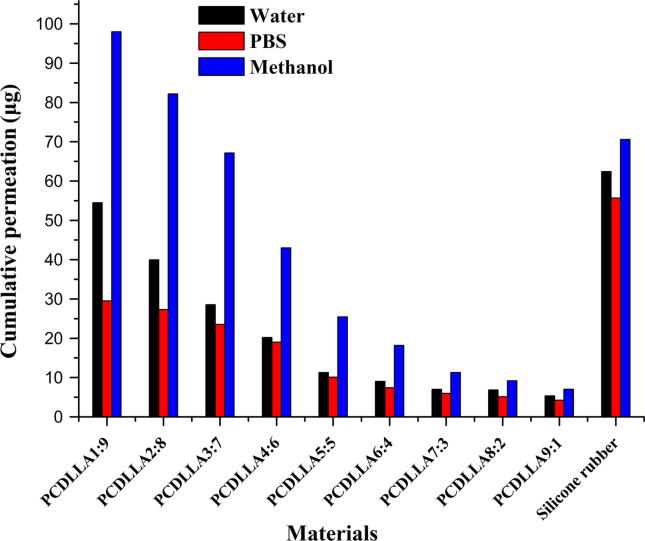
3.3.2. Constant molecular weight of copolymer PCDLLA but varying molar ratio and release media
The results obtained from Table 4 and Fig. 3 showed that the high molar ratio of caprolactone part in copolymer and the high permeation efficacy of copolymers were exhibited. Among these release media, the maximal permeation rate was occurred in 10% methanol solution. The permeation rate of copolymer in PBS or 10% methanol solution appeared the remarkable decrease with the decrease in the molar ratio of caprolactone. However, the permeation rate of copolymer in distilled water decreased slightly with the decrease in the molar ratio of caprolactone.
Table 4.
Effect of molar ratio and release media on permeation efficacy for copolymer PCDLLA.
| Copolymer | Molar ratio | Permeation efficacy |
||
|---|---|---|---|---|
| Water | PBS | Methanol | ||
| PCDLLA1:9 | 10:90 | +++++ | +++++ | +++++ |
| PCDLLA2:8 | 20:80 | +++++ | +++++ | +++++ |
| PCDLLA3:7 | 30:70 | +++++ | +++++ | +++++ |
| PCDLLA4:6 | 40:60 | +++++ | ++++ | +++++ |
| PCDLLA5:5 | 50:50 | +++ | +++ | +++++ |
| PCDLLA6:4 | 60:40 | ++ | ++ | +++ |
| PCDLLA7:3 | 70:30 | ++ | ++ | +++ |
| PCDLLA8:2 | 80:20 | ++ | ++ | ++ |
| PCDLLA9:1 | 90:10 | ++ | + | ++ |
Cumulative drug release after 24 h was used for the comparison. The number of signs represented different cumulative drug release values: −, <2 μg; +, 2–5 μg; ++, 5–10 μg; +++, 10–15 μg; ++++, 15–20 μg; +++++, >20 μg.
Figure 3.
Permeation profiles of gestodene through various molar ratios of PCDLLA copolymers and silicone rubber after 24 h.
The release of gestodene from different membranes could be best described by cumulative release values from Fig. 3. Cumulative drug release of commercial silicone rubber membrane was found to be more than 30 μg/d which indicating that commercial silicone rubber membrane had good permeation performance in different media.
In the permeability experiments using the Franz diffusion cells, the permeation efficacy of copolymers was correlated with molar ratio of caprolactone. In general, drug permeation of copolymers was larger with a range of caprolactone molar ratios, from 70 to 90, especially in 10% methanol solution. The permeation rate of copolymers in methanol can reach a maximum of 60.12 μg/d, whereas the molar ratio value should be 80%. When the molar content of caprolactone part in PCDLLA was 90%, the permeation results of copolymer were similar to those of commercial silicone rubber membranes. It can be explained by the fact that copolymers with a range of caprolactone molar ratios from 70 to 90 have a significant lipophilic property. Copolymers showed more outstanding permeability data than those of homopolymers for gestodene, generally.
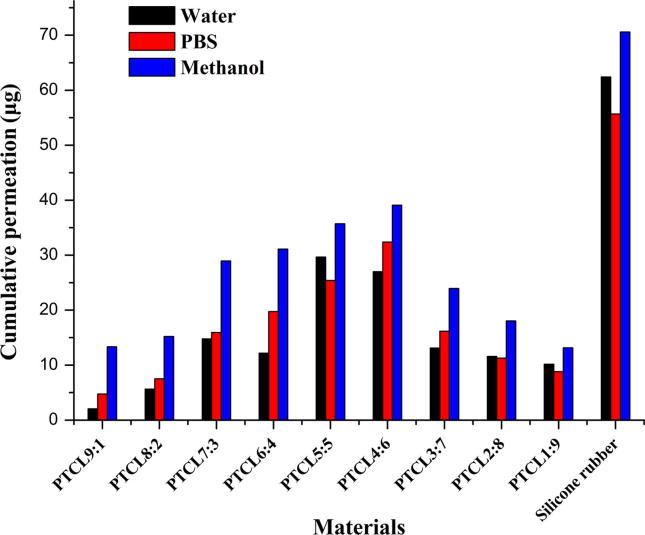
3.3.3. Constant molecular weight of copolymer PTCL but varying molar ratio and release
The permeation properties of the copolymers PTCL in different media were investigated and the results are shown in Fig. 4 and Table 5. The overall drug release of PTCL gradually increased with time in distilled water, methanol and PBS. However, the cumulative release was found to be low when the molar ratio was far away from 1:1 and close to homopolymer. The results observed were consistent with those of homopolymers we had been summarized. Gestodene release profiles of homopolymers were not excellent in the current study. The release results also demonstrated that the slightly high cumulative drug release in methanol medium is mainly due to the higher degree of swelling of the polymer matrix in methanol. This polymer chain–solution interaction decreases the physical cross-linking between PTCL chains and allowing a slightly higher degree of swelling of the polymer matrix and follows a more complete release of gestodene.
Figure 4.
Permeation profiles of gestodene through various molar ratios of PTCL copolymers and silicone rubber after 24 h.
Table 5.
Effect of molar ratio and release media on permeation efficacy for copolymer PTCL.
| Copolymer | Molar ratio | Permeation efficacy |
||
|---|---|---|---|---|
| Water | PBS | Methanol | ||
| PTCL1:9 | 10:90 | +++ | ++ | +++ |
| PTCL2:8 | 20:80 | +++ | +++ | +++ |
| PTCL3:7 | 30:70 | +++ | ++++ | +++++ |
| PTCL4:6 | 40:60 | +++++ | +++++ | +++++ |
| PTCL5:5 | 50:50 | +++++ | +++++ | +++++ |
| PTCL6:4 | 60:40 | +++ | ++++ | +++++ |
| PTCL7:3 | 70:30 | +++ | ++++ | +++++ |
| PTCL8:2 | 80:20 | +++ | +++++ | +++++ |
| PTCL9:1 | 90:10 | ++ | ++ | ++++ |
Cumulative drug release after 24 h was used for the comparison. The number of signs represented different cumulative drug release values: -, <2 μg; +, 2–5 μg; ++, 5–10 μg; +++, 10–15 μg; ++++, 15–20 μg; +++++, >20 μg.
4. Conclusion
Biodegradable materials based drug delivery formulations research has reached significant maturity in the last few years (Dash and Konkimalla, 2012). There are numerous compelling evidences for the potential of biodegradable materials for many novel challenging drug delivery and tissue engineering applications. It is worth mentioning that the outstanding permeation efficacy of these membranes obviously offers immense potential in drug delivery systems.
Various homopolymers and copolymers membranes were prepared using the solvent evaporation method for permeation studies and commercial silicone rubber membrane was selected as the control. The model drug gestodene was used in the experiment.
When comparisons were made among these materials, it was found that there were some differences in the release of gestodene. It was reported that there are contraceptive implants, containing 42 mg gestodene. And effective release area was 2.26 cm2. Effective dose of gestodene of preventing pregnancy was reported to be in the range of 10–20 μg/d (Chen et al., 1996). However, Franz diffusion cells had a diffusion area of 1.33 cm2 in the present studies. After the corresponding conversion of diffusion area to silicone rubber membrane, permeation rate of PCL in different media was greater than or close to 10 μg/d; almost permeation rate of all the PDLLA copolymers in different media was less than 10 μg/d, except for the one whose molecular weight was 120,000 in 10% methanol solution; the PCDLLA copolymers, ranging in molar ratio of caprolactone from 70 to 90, exhibited excellent permeation performance similar to commercial silicone rubber membrane; the PTCL copolymers exhibited superior permeation characteristics, when the molar ratio of trimethylene carbonate to caprolactone was close to 1. Details of the overall permeation data can be put into evidence and analyzed in drug delivery system research in the future.
Acknowledgments
Authors would like to express their gratitude to the National Key Technology Research and Development Program of the Ministry of Science and Technology of China (2006BAI03B05) and the Liaoning Science and Technology Program Project (No. 2006225001-20) for their financial support for the development of the present work.
Footnotes
Peer review under responsibility of King Saud University.
References
- Archer D.F., Cullins V., Creasy G.W., Fisher A.C. The impact of improved compliance with a weekly contraceptive transdermal system (Ortho Evra®) on contraceptive efficacy. Contraception. 2004;69:189–195. doi: 10.1016/j.contraception.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Baert B., Vansteelandt S., De Spiegeleer B. Ion mobility spectrometry as a high-throughput technique for in vitro transdermal Franz diffusion cell experiments of ibuprofen. J. Pharm. Biomed. Anal. 2011;55:472–478. doi: 10.1016/j.jpba.2011.02.027. [DOI] [PubMed] [Google Scholar]
- Baldwin M.K., Edelman A.B. The effect of long-acting reversible contraception on rapid repeat pregnancy in adolescents: a review. J. Adolesc. Health. 2013;52:S47–S53. doi: 10.1016/j.jadohealth.2012.10.278. [DOI] [PubMed] [Google Scholar]
- Bhatia P., Nangia S., Aggarwal S., Tewari C. Implanon: subdermal single rod contraceptive implant. J. Obstet. Gynecol. India. 2011;61:422–425. doi: 10.1007/s13224-011-0066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boateng J.S., Matthews K.H., Auffret A.D., Humphrey M.J., Eccleston G.M., Stevens H.N. Comparison of the in vitro release characteristics of mucosal freeze-dried wafers and solvent-cast films containing an insoluble drug. Drug Dev. Ind. Pharm. 2012;38:47–54. doi: 10.3109/03639045.2011.590496. [DOI] [PubMed] [Google Scholar]
- Bormashenko E., Pogreb R., Musin A., Stanevsky O., Bormashenko Y., Whyman G., Barkay Z. Patterning in rapidly evaporated polymer solutions: formation of annular structures under evaporation of the poor solvent. J. Colloid Interface Sci. 2006;300:293–297. doi: 10.1016/j.jcis.2006.03.064. [DOI] [PubMed] [Google Scholar]
- Brache V., Faundes A. Contraceptive vaginal rings: a review. Contraception. 2010;82:418–427. doi: 10.1016/j.contraception.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Bresolin J.R., Largura M.C.T., Dalri C.C., Hoffer G., Rodrigues C.A., Lucinda-Silva R.M. Spray-dried O-carboxymethyl chitosan as potential hydrophilic matrix tablet for sustained release of drug. Drug Dev. Ind. Pharm. 2014;40:503–510. doi: 10.3109/03639045.2013.771644. [DOI] [PubMed] [Google Scholar]
- Campinez M.D., Aguilar-de-Leyva A., Ferris C., de Paz M.V., Galbis J.A., Caraballo I. Study of the properties of the new biodegradable polyurethane PU (TEG-HMDI) as matrix forming excipient for controlled drug delivery. Drug Dev. Ind. Pharm. 2013;39:1758–1764. doi: 10.3109/03639045.2012.736516. [DOI] [PubMed] [Google Scholar]
- Campos J.M., Ribeiro M.R., Ribeiro M.F., Deffieux A., Peruch F. Copolymerisation of ε-caprolactone and trimethylene carbonate catalysed by methanesulfonic acid. Eur. Polym. J. 2013;49:4025–4034. [Google Scholar]
- Chen H.-L., Chen J.-X. Research on the new one rod contraceptive implant containing gestodene. J. Reprod. Contracept. 2007;18:86–88. [Google Scholar]
- Chen, H., Ye, Zhihou, Chen, L., Chen, J., Liu, C., Bai, X., 1996. A Long-Term Contraceptive Implant Containing Gestodene. Chinese patent CN 1179942A.
- Dash T.K., Konkimalla V.B. Poly--caprolactone based formulations for drug delivery and tissue engineering: a review. J. Control. Release. 2012;158:15–33. doi: 10.1016/j.jconrel.2011.09.064. [DOI] [PubMed] [Google Scholar]
- Feng J., Wang H.-F., Zhang X.-Z., Zhuo R.-X. Investigation on lipase-catalyzed solution polymerization of cyclic carbonate. Eur. Polym. J. 2009;45:523–529. [Google Scholar]
- Ferreira J.M., Nunes F.R., Modesto W., Gonçalves M.P., Bahamondes L. Reasons for Brazilian women to switch from different contraceptives to long-acting reversible contraceptives. Contraception. 2014;89:17–21. doi: 10.1016/j.contraception.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Gao X., Chen L., Xie J., Yin Y., Chang T., Duan Y., Jiang N. In vitro controlled release of vitamin C from Ca/Al layered double hydroxide drug delivery system. Mater. Sci. Eng.: C. 2014;39:56–60. doi: 10.1016/j.msec.2014.02.028. [DOI] [PubMed] [Google Scholar]
- Gao Y., Liang J., Liu J., Xiao Y. Double-layer weekly sustained release transdermal patch containing gestodene and ethinylestradiol. Int. J. Pharm. 2009;377:128–134. doi: 10.1016/j.ijpharm.2009.05.017. [DOI] [PubMed] [Google Scholar]
- Guo X., Zhang L., Qian Y., Zhou J. Effect of composition on the formation of poly(dl-lactide) microspheres for drug delivery systems: mesoscale simulations. Chem. Eng. J. 2007;131:195–201. [Google Scholar]
- Harel Z., Cromer B. The use of long-acting contraceptives in adolescents. Pediatr. Clin. North Am. 1999;46:719–732. doi: 10.1016/s0031-3955(05)70148-6. [DOI] [PubMed] [Google Scholar]
- Hoggart L., Louise Newton V., Dickson J. “I think it depends on the body, with mine it didn’t work”: explaining young women’s contraceptive implant removal. Contraception. 2013;88:636–640. doi: 10.1016/j.contraception.2013.05.014. [DOI] [PubMed] [Google Scholar]
- Ishaug-Riley S.L., Okun L.E., Prado G., Applegate M.A., Ratcliffe A. Human articular chondrocyte adhesion and proliferation on synthetic biodegradable polymer films. Biomaterials. 1999;20:2245–2256. doi: 10.1016/s0142-9612(99)00155-6. [DOI] [PubMed] [Google Scholar]
- Jacques M., Marrie T., William Costerton J. In vitro quantitative adherence of microorganisms to intrauterine contraceptive devices. Curr. Microbiol. 1986;13:133–137. [Google Scholar]
- Jung Y., Yoon J.-H., Kang N., Park S., Jeong S. Diffusion properties of different compounds across various synthetic membranes using Franz-type diffusion cells. J. Pharmaceut. Invest. 2012;42:271–277. [Google Scholar]
- Kluin O.S., van der Mei H.C., Busscher H.J., Neut D. A surface-eroding antibiotic delivery system based on poly-(trimethylene carbonate) Biomaterials. 2009;30:4738–4742. doi: 10.1016/j.biomaterials.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Kowalczuk A., Trzcinska R., Trzebicka B., Müller A.H.E., Dworak A., Tsvetanov C.B. Loading of polymer nanocarriers: factors, mechanisms and applications. Prog. Polym. Sci. 2014;39:43–86. [Google Scholar]
- Kshirsagar S.J., Bhalekar M.R., Mohapatra S.K. Development and evaluation of carvedilol-loaded transdermal drug delivery system: in-vitro and in-vivo characterization study. Drug Dev. Ind. Pharm. 2012;38:1530–1537. doi: 10.3109/03639045.2012.656271. [DOI] [PubMed] [Google Scholar]
- Kumar V., Kumari A., Kumar D., Yadav S.K. Biosurfactant stabilized anticancer biomolecule-loaded poly (d,l-lactide) nanoparticles. Colloids Surf., B. 2014;117:505–511. doi: 10.1016/j.colsurfb.2014.01.057. [DOI] [PubMed] [Google Scholar]
- Lara-Torre E., Schroeder B. Adolescent compliance and side effects with quick start initiation of oral contraceptive pills. Contraception. 2002;66:81–85. doi: 10.1016/s0010-7824(02)00326-8. [DOI] [PubMed] [Google Scholar]
- Li S., Qiu Y.Q., Zhang S.H., Gao Y.H. Enhanced transdermal delivery of 18 beta-glycyrrhetic acid via elastic vesicles: in vitro and in vivo evaluation. Drug Dev. Ind. Pharm. 2012;38:855–865. doi: 10.3109/03639045.2011.630395. [DOI] [PubMed] [Google Scholar]
- Likis F.E. Contraceptive applications of estrogen. J. Midwifery Women’s Health. 2002;47:139–156. doi: 10.1016/S1526-9523(02)00234-9. [DOI] [PubMed] [Google Scholar]
- Lobo S., Li H.N., Farhan N., Yan G. Evaluation of diclofenac prodrugs for enhancing transdermal delivery. Drug Dev. Ind. Pharm. 2014;40:425–432. doi: 10.3109/03639045.2013.767828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma G., Song C., Sun H., Yang J., Leng X. A biodegradable levonorgestrel-releasing implant made of PCL/F68 compound as tested in rats and dogs. Contraception. 2006;74:141–147. doi: 10.1016/j.contraception.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Matějíček D., Kubáň V. High performance liquid chromatography/ion-trap mass spectrometry for separation and simultaneous determination of ethynylestradiol, gestodene, levonorgestrel, cyproterone acetate and desogestrel. Anal. Chim. Acta. 2007;588:304–315. doi: 10.1016/j.aca.2007.02.028. [DOI] [PubMed] [Google Scholar]
- Neukom J., Chilambwe J., Mkandawire J., Mbewe R.K., Hubacher D. Dedicated providers of long-acting reversible contraception: new approach in Zambia. Contraception. 2011;83:447–452. doi: 10.1016/j.contraception.2010.08.021. [DOI] [PubMed] [Google Scholar]
- Ng S.-F., Rouse J., Sanderson F., Eccleston G. The relevance of polymeric synthetic membranes in topical formulation assessment and drug diffusion study. Arch. Pharmacal Res. 2012;35:579–593. doi: 10.1007/s12272-012-0401-7. [DOI] [PubMed] [Google Scholar]
- Rahimy M.H., Cromie M.A., Hopkins N.K., Tong D.M. Lunelle™ monthly contraceptive injection (medroxyprogesterone acetate and estradiol cypionate injectable suspension): effects of body weight and injection sites on pharmacokinetics. Contraception. 1999;60:201–208. doi: 10.1016/s0010-7824(99)00085-2. [DOI] [PubMed] [Google Scholar]
- Rauma M., Johanson G. Comparison of the thermogravimetric analysis (TGA) and Franz cell methods to assess dermal diffusion of volatile chemicals. Toxicol. In Vitro. 2009;23:919–926. doi: 10.1016/j.tiv.2009.04.003. [DOI] [PubMed] [Google Scholar]
- San Miguel V., Limer A.J., Haddleton D.M., Catalina F., Peinado C. Biodegradable and thermoresponsive micelles of triblock copolymers based on 2-(N,N-dimethylamino)ethyl methacrylate and ε-caprolactone for controlled drug delivery. Eur. Polym. J. 2008;44:3853–3863. [Google Scholar]
- Shen Y.Q., Lu F., Hou J.W., Shen Y.Y., Guo S.R. Incorporation of paclitaxel solid dispersions with poloxamer188 or polyethylene glycol to tune drug release from poly(epsilon-caprolactone) films. Drug Dev. Ind. Pharm. 2013;39:1187–1196. doi: 10.3109/03639045.2012.704042. [DOI] [PubMed] [Google Scholar]
- Shoupe D. New progestins–clinical experiences: gestodene. Am. J. Obstet. Gynecol. 1994;170:1562–1568. doi: 10.1016/s0002-9378(94)05020-9. [DOI] [PubMed] [Google Scholar]
- Stanczyk F.Z., Archer D.F. Gestodene: a review of its pharmacology, potency and tolerability in combined contraceptive preparations. Contraception. 2014;89:242–252. doi: 10.1016/j.contraception.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Thonneau P.F., Almont T.E. Contraceptive efficacy of intrauterine devices. Am. J. Obstet. Gynecol. 2008;198:248–253. doi: 10.1016/j.ajog.2007.10.787. [DOI] [PubMed] [Google Scholar]
- Thurman A., Kimble T., Hall P., Schwartz J.L., Archer D.F. Medroxyprogesterone acetate and estradiol cypionate injectable suspension (Cyclofem) monthly contraceptive injection: steady-state pharmacokinetics. Contraception. 2013;87:738–743. doi: 10.1016/j.contraception.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Tian H., Tang Z., Zhuang X., Chen X., Jing X. Biodegradable synthetic polymers: preparation, functionalization and biomedical application. Prog. Polym. Sci. 2012;37:237–280. [Google Scholar]
- Tsuji H., Miyauchi S. Poly(l-lactide): VI Effects of crystallinity on enzymatic hydrolysis of poly(l-lactide) without free amorphous region. Polym. Degrad. Stab. 2001;71:415–424. [Google Scholar]
- Urdl W., Apter D., Alperstein A., Koll P., Schönian S., Bringer J., Fisher A.C., Preik M. Contraceptive efficacy, compliance and beyond: factors related to satisfaction with once-weekly transdermal compared with oral contraception. Eur. J. Obstetr. Gynecol. Reprod. Biol. 2005;121:202–210. doi: 10.1016/j.ejogrb.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Vithlani S., Sarraf S., Chaw C.S. Formulation and in vitro evaluation of self-emulsifying formulations of Cinnarizine. Drug Dev. Ind. Pharm. 2012;38:1188–1194. doi: 10.3109/03639045.2011.643895. [DOI] [PubMed] [Google Scholar]
- Yang D., Zhang C., Li M., Zhang W., Guo J., Guan Y., Li J. Synthesis and characterization of poly(trimethylene carbonate) Polym. Mater. Sci. Eng. 2010;26:24–26. [Google Scholar]
- Yen C., He H., Lee L.J., Ho W.S.W. Synthesis and characterization of nanoporous polycaprolactone membranes via thermally- and nonsolvent-induced phase separations for biomedical device application. J. Membr. Sci. 2009;343:180–188. [Google Scholar]
- Zare M., Mobedi H., Barzin J., Mivehchi H., Jamshidi A., Mashayekhi R. Effect of additives on release profile of leuprolide acetate in an in situ forming controlled-release system: in vitro study. J. Appl. Polym. Sci. 2008;107:3781–3787. [Google Scholar]
- Zhang C., Yang D., Li M. Preparation and characterization of poly (ε-caprolactone-co-d,l-lactide) Modern Chem. Ind. 2012:69–71. [Google Scholar]
- Zhang C., Yang D., Meng S., Guan Y. Application progress of biodegradable polymeric materials in contraceptive domain. Plastics. 2011 49–51+20. [Google Scholar]
- Zhang C., Zhang X., Yang D., Wang P. Biodegradation of in situ-forming gel of poly(DLLA-co-CL) in vivo. J. Appl. Polym. Sci. 2013;130:3800–3808. [Google Scholar]
- Zhang X., Zhang C., Zhang W., Meng S., Liu D., Wang P., Guo J., Li J., Guan Y., Yang D. Feasibility of poly (ε-caprolactone-co-DL-lactide) as a biodegradable material for in situ forming implants: evaluation of drug release and in vivo degradation. Drug Dev. Ind. Pharm. 2013 doi: 10.3109/03639045.2013.866140. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Grijpma D.W., Feijen J. Poly(trimethylene carbonate) and monomethoxy poly(ethylene glycol)-block-poly(trimethylene carbonate) nanoparticles for the controlled release of dexamethasone. J. Control. Release. 2006;111:263–270. doi: 10.1016/j.jconrel.2005.12.001. [DOI] [PubMed] [Google Scholar]