Abstract
β-Thalassemia is a genetic disease characterized by reduced or non-functionality of β-globin gene expression, which is caused due to a number of variations and indels (insertions and deletions). In this case study, we have reported a rare occurrence of compound heterozygosity of two different variants, namely, HBBc.92G > C and HBBc.92 + 5G > C in maternal amniotic fluid sample. Prenatal β-thalassemia mutation detection in fetal DNA was carried out using nucleotide sequencing method. After analysis, the father was found to be heterozygous for HBBc.92G > C (Codon 30 (G > C)) mutation which is β0 type and the mother was heterozygous for HBBc.92 + 5G > C (IVS I-5 (G > C)) mutation which is β+ type. When amniotic fluid sample was analyzed for β-globin gene (HBB), we found the occurrence of heterozygous allelic pattern for aforesaid mutations. This compound heterozygous state of fetus sample was considered as β+/β0 category of β thalassemia which was clinically and genotypically interpreted as β-thalassemia major. Regular blood transfusions are required for the survival of thalassemia major patients hence prenatal diagnosis is imperative for timely patient management. Prenatal diagnosis helps the parents to know the thalassemic status of the fetus and enables an early decision on the pregnancy. In the present study, we have identified compound heterozygosity for β-thalassemia in the fetus which portrays the importance of prenatal screening.
Keywords: β-Thalassemia, Compound heterozygous, β+/β0 mutation, Amniotic fluid, Nucleotide sequencing
1. Introduction
Thalassemias, α- and β-thalassemias, are different forms of blood borne genetic disorders, which are inherited in an autosomal recessive pattern. β-Thalassemia is highly prevalent in Middle East, Mediterranean, Transcaucasus, Central Asia, Indian subcontinent, Africa and Far East [1]. β-Thalassemia is characterized by the defect in the synthesis of β-globin chains of hemoglobin tetramer. The mutations of HBB gene causes the abnormal formation of hemoglobin resulting in improper oxygen transportation and destruction of red blood cells. β-Thalassemia is caused by 21 important mutations out of 200 point mutations and indels published worldwide [2], [3]. These mutations either partially or completely terminate the synthesis of β-globin chain which is classified as β+ and β0 type mutations respectively. Depending on severity of hematological and clinical conditions, β-thalassemia is classified into three types, namely, β-thalassemia minor (also called as carrier), β-thalassemia intermedia and β-thalassemia major. People with β-thalassemia major require medical treatment including multiple blood transfusion regimens. Blood transfusion along with chelation therapy has resulted in improved life expectancy in thalassemia major patients. The clinical severity of β-thalassemia intermedia has ranged from asymptomatic carrier state to severe transfusion-dependent type. β-Thalassemia minor is clinically asymptomatic but can be characterized by specific hematological features.
Around 60,000 new births are recorded to be affected by β-thalassemia per year in the world [4]. Therefore, prenatal diagnosis of β-thalassemia is important for making an early decision about pregnancy. There are three possible genotypes leading to β-thalassemia major namely, β+ β+, β0β0 and β+ β0 [5]. β+ β+ type of thalassemia is classified as thalassemia major but it is comparatively less severe than other two types, because functionality of β-globin chain synthesis is not completely terminated. In β0β0 type of thalassemia, there will be no production of hemoglobin, because β-globin chain synthesis is stopped completely [6]. Patients with β0β0 type of β-thalassemia need RBC transfusion within two years of age. β+ β0 type of thalassemia is more severe than β+ β+ type but less than that of β0β0. Patients with β+ β0 type of thalassemia possess two β-globin chains one with reduced and another with absence of functionality. Severity of β+ β0 type of thalassemia varies from medium to high depending upon the mutations of the HBB gene [7]. The present case highlights the importance of voluntary premarital screening to detect carriers of β-thalassemia and marriage between both carriers should be strongly discouraged. In the present study, both the parents were heterozygous for two different mutations, the father was having a β0 type mutation and the mother was having β+ type mutation whereas the amniotic fluid sample was detected heterozygous for both mutations leading to β+ β0 type β-thalassemia major.
2. Case report
In the present case, we have reported a β-thalassemia affected Gujarati family (Gujarat, India), wherein the husband is 35 years old and the wife is 32 years old. Clinician has shared the genetic history of the family along with a copy of β-thalassemia reports. Based on hemoglobin electrophoresis method, it was confirmed that the couple was β-thalassemia minor for different mutations. The father was carrying heterozygous HBBc.92G > C mutation and the mother was heterozygous for HBBc.92 + 5G > C mutation. The family consisted of two daughters, wherein the first daughter was thalassemia major and the second was thalassemia minor. Their treating clinician sent the maternal amniotic fluid (AF) sample (interchangeably referred as fetal sample) of couple's third pregnancy to Xcelris Labs Limited, Ahmedabad, India for genetic investigation of β-thalassemia. Clinician has taken couple's consent to carry out genetic testing on AF sample. We extracted DNA from trio samples, namely blood samples of parents and fetal sample, using Qiagen DNA mini kit and subjected them to Quality check. Fetal DNA was tested to be free from maternal DNA.
β-Thalassemia mutations were detected using nucleotide sequencing technology (ABI3730) on trio samples (parental blood and maternal amniotic fluid samples). DNA was subjected to quality check followed by target specific PCR amplification and Sanger sequencing. Our analysis confirmed that the father was heterozygous for HBBc.92G > C(Codon 30 (G > C)), which is β0 type of mutation and the mother was found to be heterozygous for HBBc.92 + 5G > C (IVS I-5 (G > C)), which is β+ type of mutation. When the chromogram of amniotic fluid sample was analyzed, we found the occurrence of HBBc.92G > C and HBBc.92 + 5G > C mutations in the compound heterozygous state (Fig. 1). This genotype pattern belonged to β+β0 category and causes clinically and phenotypically severe health conditions similar to β-thalassemia major. Our results corroborated the test results from hemoglobin electrophoresis method for parent samples.
Fig. 1.
Electropherogram of bidirectional sequencing analysis demonstrated heterozygous mutation [c.92 + 5G > C (IVS I-5 G > C)] and [c.92G > C (codon30 G > C)] in amniotic fluid sample.
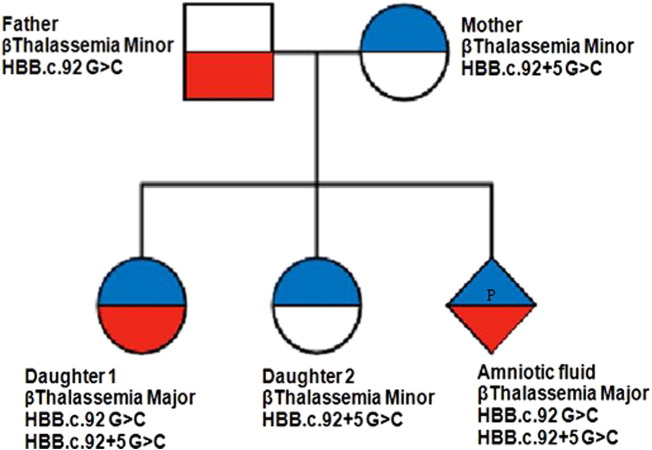
Our discussion with the clinician helped us to obtain β-thalassemia genetic results of the two daughters. Their first daughter was thalassemia major with the mutations HBBc.92G > C and HBBc.92 + 5G > C in the compound heterozygous state. This compound heterozygosity condition is similar to that of fetal DNA from current (third) pregnancy of couple. The second daughter was detected with thalassemia minor with the heterozygous HBBc.92 + 5G > C mutation. The data provided by the clinician for the first two daughters along with our findings in the present study is mentioned in Table 1. Pedigree chart was prepared to depict the autosomal recessive pattern of inheritance of β-thalassemia mutations (Fig. 2).
Table 1.
β-Thalassemia mutation details in the family data.
| Sample | Mutations | Genotype | Inference |
|---|---|---|---|
| Father | HBBc.92G > C | β0β type | β-Thalassemia minor |
| Mother | HBBc.92 + 5G > C | β+ β type | β-Thalassemia minor |
| Daughter 1a | HBBc.92G > C | β+ β0 type | β-Thalassemia major |
| HBBc.92 + 5G > C | |||
| Daughter 2a | HBBc.92 + 5G > C | β+ β type | β-Thalassemia minor |
| Amniotic fluid | HBBc.92G > C | β+ β0 type | β-Thalassemia major |
| HBBc.92 + 5G > C |
Information given by the clinician.
Fig. 2.
Pedigree chart depicting the autosomal recessive inheritance pattern of β-thalassemia traits in the family.
3. Discussion
We have presented a case of a family, affected with two different mutations of β thalassemia. The mother is β thalassemia carrier for HBBc.92 + 5G > C mutation, which has the highest frequency of occurrence in Gujarat region of India [8]. Although, we have analyzed more than 15 families (trio samples) for β-thalassemia mutations, we have never found compound heterozygous β+ β0 type mutations in native Gujarati families. We have observed co-inheritance of β-thalassemia mutation (HBBc.92 + 5G > C) and sickle cell anemia disease causing mutation (HbE) in the amniotic fluid of parents, who were carriers [9]. Ambekar et al. has reported similar case of compound heterozygous state of β-thalassemia mutations in the Maharashtrian population of India [10].
The phenotype of compound heterozygous β-thalassemia can be intermedia or major. Individuals with β-thalassemia intermedia require RBC transfusion within few years after their birth, whereas β-thalassemia major would require medical treatment within two years [11]. In the present case, the compound heterozygosity has lead to β+ β0 type causing β-thalassemia major. Usually in β+ β0 type β-thalassemia, the Hb pattern shows HbA between 10 and 30%, HbF of 70–90% and HbA2 of 2–5% [12].
HBBc.92 + 5G > C mutation is the most prevalent variation worldwide and many documented cases of compound heterozygous mutations like deletion-inversion Gγ(Aγδβ)0, HBB: c. 93-2A > C and -42C > G with HBBc.92 + 5G > C are available in literature [13], [14], [15]. HBB gene is having two intronic and three exonic regions (Fig. 3). The variants detected in parents are located in the first intron and first exon of HBB gene. In HBBc.92 + 5G > C variant, 5th base of intron 1 changes from G to C which disrupts the normal splicing leading to the first exon being continued past the 5′ splice site and instead splice at a cryptic splice site that is few nucleotides past 5′ end causing β+ type mutation. The HBBc.92 G > C variant is located in the coding region of HBB gene. These two mutations HBBc.92G > C and HBBc.92 + 5G > C are located at the distance of 5 base pairs on HBB gene and probability of occurrence of both mutations in the same couple is very low. Segregation of compound heterozygosity has occurred twice in this family.
Fig. 3.
HBB gene structure denoting both the studied mutation.
4. Conclusion
Many advanced molecular diagnostic technologies have been used in healthcare. Despite, emerging technologies, β-thalassemia is considered as a major genetic disorder in the world [4]. The only survival strategy for β-thalassemia patients is lifelong blood transfusion to supply hemoglobin followed by iron chelation therapy to remove excess iron from the blood. This is not the permanent treatment regimen for the thalassemia diseases. Therefore, it is very important to counsel and recommend couples about the importance of prenatal genetic testing of β-thalassemia and other genetic diseases. It is imperative to recommend premarital counseling of couples before marriage in the β‐thalassemia rampant population of Asian Indians. Further, the nucleotide sequencing method has added advantages over RT-PCR method as it can detect novel mutations which may also be specific to ethnicity.
Conflict of interest
All the authors declare that they have no conflict of interest.
References
- 1.Vichinsky Elliott P., MacKlin Eric A., Waye John S., Lorey Fred, Olivieri Nancy F. Changes in the epidemiology of thalassemia in North America: a new minority disease. Pediatrics. 2005;116(6):e818–e825. doi: 10.1542/peds.2005-0843. [DOI] [PubMed] [Google Scholar]
- 2.Weatherall David J., Clegg John B. John Wiley & Sons; 2008. The Thalassaemia Syndromes. [Google Scholar]
- 3.Huisman, HJ Titus, Carver Marianne F.H., Baysal Erol. Sickle Cell Anemia Foundation. 1997. A syllabus of thalassemia mutations. [Google Scholar]
- 4.Higgs D., Engel J., Stamatoyannopoulos G. The Lancet. HTML & CSS. 2011. Thalassemia. (2012, 07 04) [Google Scholar]
- 5.Cunningham Melody J., Macklin Eric A., Neufeld Ellis J., Cohen Alan R., Thalassemia Clinical Research Network Complications of β-thalassemia major in North America. Blood. 2004;104(1):34–39. doi: 10.1182/blood-2003-09-3167. [DOI] [PubMed] [Google Scholar]
- 6.Gaeke Vanessa. 2012. A Database-driven Website of β-Thalassemia Mutations. [Google Scholar]
- 7.Taher Ali, Isma'eel Hussain, Cappellini Maria D. Thalassemia intermedia: revisited. Blood Cell Mol. Dis. 2006;37(1):12–20. doi: 10.1016/j.bcmd.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Shrivastava, Naina, N. P. Sharma, P. Parmar, R. Patel. Real-time PCR for the Rapid Detection of Common β-Thalassemia Mutation in Gujarat.
- 9.Dhawan D., Chaudhary S., Chandratre K., Ghosh A., Sojitra N. Prenatal screening for co-inheritance of sickle cell anemia and β-thalassemia traits. Clin. Med. Biochem. Open Access. 2016;1(108):2. [Google Scholar]
- 10.Ambekar S.S., Phadke M.A., Balpande D.N., Mokashi G.D., Khedkar V.A., Bankar M.P., Gambhir P.S., Bulakh P.M., Basutkar D.G. The prevalence and heterogeneity of beta-thalassemia mutations in the Western Maharashtra population: a hospital based study. IJHG. 2001;1(3):219–223. [Google Scholar]
- 11.Cao Antonio, Galanello Renzo. Beta-thalassemia. Genitourin. Med. 2010;12(2):61–76. doi: 10.1097/GIM.0b013e3181cd68ed. [DOI] [PubMed] [Google Scholar]
- 12.Dehury Snehadhini, Purohit Prasanta, Meher Satyabrata, Das Kishalaya, Patel Siris. Compound heterozygous state of β-thalassemia with IVS1-5 (G → C) mutation and Indian deletion-inversion Gγ (Aγδβ) 0-thalassemia in eastern India. Rev. Bras. Hematol. Hemoter. 2015;37(3):202–206. doi: 10.1016/j.bjhh.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agarwal S., Tamhankar P.M., Kumar R., Dalal A. Clinical and haematological features in a compound heterozygote (HBB: c. 92 + 5G > C/HBB: c. 93-2A > C) case of thalassaemia major. Int. J. Lab. Hematol. 2010;32(3):369–372. doi: 10.1111/j.1751-553X.2009.01157.x. [DOI] [PubMed] [Google Scholar]
- 14.Fedorov A.N., Nasyrov F. Yu, Smirnova E.A., Bocharova T.N., Limborska S.A. IVS-1-1 (G → C) in combination with-42 (C → G) in the promoter region of the β-globin gene in patients from Tajikistan. Hemoglobin. 1993;17(3):275–278. doi: 10.3109/03630269308998904. [DOI] [PubMed] [Google Scholar]
- 15.Galanello Renzo, Origa Raffaella. Beta-thalassemia. Orphan. J. Rare Dis. 2010;5(1):1. doi: 10.1186/1750-1172-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]