Abstract
Background
Information regarding the long-term outcome of enzyme replacement therapy (ERT) with recombinant human N-acetylgalactosamine 4-sulfatase (rhASB, galsulfase, Naglazyme®, BioMarin Pharmaceutical Inc.) for Taiwanese patients with mucopolysaccharidosis (MPS) VI is limited.
Methods
Nine Taiwanese patients with MPS VI (4 males and 5 females; age range, 1.4 to 21.1 years) treated with weekly intravenous infusions of galsulfase (1.0 mg/kg) in 5 medical centers in Taiwan were reviewed. A set of biochemical and clinical assessments were evaluated annually.
Results
After 6.2 to 11.2 years of galsulfase treatment, 6 patients experienced improvement over baseline in the 6-minute walk test by a mean of 150 m (59% change over time), and 3 patients also increased the 3-minute stair climb test by a mean of 60 steps (46%). In a manual dexterity test, 3 patients decreased the time required to pick up 10 coins and put the coins into a cup by 15 s (33%). Shoulder range of motion in all 9 patients improved, and Joint Pain and Stiffness Questionnaire scores improved by 0.42 points (21%). Four patients showed improved pulmonary function. Five patients had positive effects on cardiac-wall diameters. Four patients had improved cardiac diastolic function. Liver and spleen sizes as measured by abdominal ultrasonography remained the same or decreased in all 9 patients. However, the severity degree of valvular stenosis or regurgitation did not show improvement despite ERT. A mean overall 69% decrease in urinary glycosaminoglycan (GAG) excretion indicated a satisfactory biomarker response.
Conclusions
Long-term ERT was beneficial and safe for Taiwanese patients with MPS VI. This treatment reduced urinary GAG and had positive effects on a wide range of clinical functional assessments including endurance, mobility, joint function, pulmonary function, liver and spleen size, cardiac hypertrophy and diastolic dysfunction.
Abbreviations: MPS, mucopolysaccharidosis; ASB, N-acetylgalactosamine 4-sulfatase; GAG, glycosaminoglycan; ERT, enzyme replacement therapy; Galsulfase, recombinant human N-acetylgalactosamine 4-sulfatase; Z score, standard deviation score; 6MWT, 6-minute walk test; 3MSCT, 3-minute stair climb test; FVC, Forced vital capacity; FEV1, forced expiratory volume in 1 s; E/A, ratio between early and late (atrial) ventricular filling velocity; LVMI, left ventricular mass index; IVSd, interventricular septum thickness in diastole; LVPWd, left ventricular posterior wall thickness in diastole; LVM, left ventricular mass; BMD, bone mineral density; HAZ, height-for-age; DXA, dual energy x-ray absorptiometry; PTA, pure-tone audiometry; AC, air conduction; BC, bone conduction; HAQ, Health Assessment Questionnaire; CHAQ, Childhood Health Assessment Questionnaire
Keywords: Cardiac hypertrophy, Diastolic dysfunction, Enzyme replacement therapy, Glycosaminoglycans, Mucopolysaccharidosis VI, Pulmonary function
Highlights
-
•
Nine Taiwanese MPS VI patients treated with weekly intravenous infusions of galsulfase for 6.2 to 11.2 years were reviewed.
-
•
Long-term ERT was beneficial and safe for Taiwanese patients with MPS VI.
-
•
Long-term ERT reduced urinary GAG and had positive effects on a wide range of clinical functional assessments.
1. Introduction
Mucopolysaccharidosis VI (MPS VI; OMIM #253200, Maroteaux-Lamy syndrome) is an autosomal recessive disorder caused by lysosomal enzyme N-acetylgalactosamine 4-sulfatase (arylsulfatase B, or ASB) deficiency with a clinical spectrum of mild to severe phenotypes. Decreased ASB enzyme activity leads to impaired degradation of the glycosaminoglycan (GAG) dermatan sulfate. The resulting GAG accumulation in the lysosomes of connective tissue causes a chronic progressive disorder characterized by significant functional impairment and shortened lifespan. Patients with rapidly progressing disease often have coarse face, short stature, joint and skeletal deformities, corneal clouding, recurrent respiratory and ear infections, compromised cardiovascular and pulmonary function, spinal cord compression, and ultimately become wheelchair-bound or bedridden with early mortality in the late teens to early twenties. Although patients with MPS VI typically do not have neurocognitive impairment, their learning and development could be affected by physical limitations, particularly with vision deficit and hearing loss [1], [2]. MPS VI is an ultra-rare genetic disorder with a reported incidence of 0.14–0.38/100,000 live births [3]. Previous human clinical trials have demonstrated that enzyme replacement therapy (ERT) for MPS VI with recombinant human N-acetylgalactosamine 4-sulfatase (rhASB; galsulfase; Naglazyme®) significantly improves physical endurance, reduces urinary GAG, and has an acceptable safety profile [4], [5], [6], [7], [8]. Giugliani et al. [9] conducted a Resurvey Study of MPS VI patients to obtain 10-year follow-up data that included medical histories and clinical assessments (n = 59), and survival status over 12 years (n = 117). They reported that long-term ERT resulted in improvements in pulmonary function and endurance, stabilized cardiac function and increased survival. However, information on the long-term effects of ERT in Asian MPS VI patients is limited. In this study, we report our findings on the long-term effects of galsulfase treatment in 9 Taiwanese MPS VI patients.
2. Patients and methods
2.1. Selection of subjects
Data from 9 patients with MPS VI (4 males and 5 females) who received, or are currently receiving, ERT with galsulfase (1.0 mg/kg/week intravenously) between March 2004 and December 2015 in 5 medical centers in Taiwan, were retrospectively reviewed. Medical institutions included Mackay Memorial Hospital, China Medical University Hospital, National Cheng Kung University Hospital, Changhua Christian Hospital, and National Taiwan University Hospital. The patients' ages at which treatment was begun ranged widely from 1.4 to 21.1 years, and the duration of therapy ranged from 6.2 to 11.2 years. We routinely assessed a set of biochemical and clinical responses each year during treatment. Written informed consent for ERT was obtained from a parent for children and from patients over 18 years. The study was approved by the ethics committee of Mackay Memorial Hospital, Taipei, Taiwan.
2.2. Baseline and follow-up biochemical and clinical evaluation
All patients had clinical manifestations of MPS VI, and diagnosis was confirmed by two-dimensional electrophoresis of urinary GAGs and ASB enzyme assay in serum, leukocytes and/or fibroblasts [10]. Each patient had a mutational analysis performed [11]. Height and weight were transformed to standard deviation scores (z scores) on the basis of a standard growth table for the Taiwanese population [12]. Prior to each intravenous infusion, patients were pre-medicated with diphenhydramine (0.5 mg/kg body weight). Galsulfase was diluted in 0.9% saline and administered at 1.0 mg/kg over 4 h once weekly according to product label administration instructions. Urinary GAGs and white blood cell ASB levels were measured at baseline and every year during treatment. Evaluation of mobility and physical function was performed at baseline and every year subsequently. Clinical functional assessments varied somewhat by institution and may have included a 6-minute walk test (6MWT), 3-minute stair climb test (3MSCT), joint range of motion, coin picking-up test, spirometry, echocardiography, abdominal ultrasonography, bone mineral density, and hearing test. Range of motion of the shoulders was measured with a goniometer by physical therapists. The goniometer assessments included both active and passive shoulder flexion, extension, and lateral rotation, and were consistent at all centers. Forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1) were evaluated by standard spirometry techniques according to American Thoracic Society guidelines [13], [14]. The cardiac ultrasound systems used were Philips Sonos 5500/7500 System (Andover, MA, USA), equipped with electronic transducers from 2 to 8 MHz. Diastolic and systolic diameters were measured using the M-mode, and the systolic function of the left ventricle was evaluated through the ejection fraction obtained by the Teichholz method [V = 7D3 / (2.4 + D), where V = LV volume and D = LV diameter] [15]. Diastolic filling was established using the E/A ratio by measuring mitral-inflow as determined by pattern-peak early filling (E) and late filling (A) velocities [16]. A reversed E/A ratio (E/A ratio < 1) was considered diastolic dysfunction. Severity of valvular stenosis and regurgitation were estimated and graded on the following scores: 0 (none), 1 (mild), 2 (moderate), and 3 (severe) based on the European Society of Cardiology/American Society of Echocardiography guidelines [17], [18]. The data of left ventricular mass index (LVMI), the thicknesses of the interventricular septum diameter in diastole (IVSd), and the thicknesses of the left ventricular posterior wall diameter in diastole (LVPWd) obtained by serial echocardiographic assessments [19] were recorded. These values were compared with normal values according to the study of Kampmann et al. [20]. Left ventricular mass (LVM) was calculated according to the American Society of Echocardiography simplified cubed equation. LVM was indexed (LVMI) by height2.7 to normalize heart size to body size. The LVMI was also calculated using the Devereux formula and indexed by body surface area with normal values according to the report of Poutanen et al. [21]. All above echocardiographic values were transformed into a z score derived by subtracting the mean reference value from an individual observed value, and then dividing the difference by the standard deviation from the reference value. Z score > 2 was considered abnormal. Abdominal ultrasonographic examinations were performed using high-resolution B-mode ultrasonography (SA-700A, Toshiba, Tokyo, Japan) with a 3.5-MHz curved array transducer. Liver size and spleen size were measured in comparison with the normal reference values for different body height of children [22]. The absolute value of bone mineral density (BMD) and height-for-age (HAZ) adjusted BMD z scores were evaluated by dual energy x-ray absorptiometry (DXA) as previously described [23]. The assessment of hearing loss by pure-tone audiometry (PTA) was performed as previously described [24] with the collection of the values of air conduction (AC), bone conduction (BC), and air-bone gap.
Height was used as a covariate for normal values. Joint Pain and Stiffness Questionnaire scores (Disability Index) were assessed by an analog scale based on the Health Assessment Questionnaire (HAQ) [25], [26] for patients who were > 18 years of age or Childhood Health Assessment Questionnaire (CHAQ) [27] completed by a parent or caregiver for younger patients. In 3 cases a manual dexterity test was performed where the patient was asked to pick up 10 coins and place them in a cup while their elbow was allowed to rest on the table; the time required to complete the task was recorded. All 9 patients were assessed using the Disability Index, with an overall score that addresses eight categories of activity, including dressing and grooming, arising, eating, walking, hygiene, reach, grip, and common daily activities. Adverse events were recorded as previously described [28].
2.3. Data analysis
The latest results of examinations for these 9 patients receiving ERT were compared with baseline data. Descriptive statistics were calculated, including means, standard deviations, and percentage change over time. Further statistical analysis was not performed due to the small sample size.
3. Results
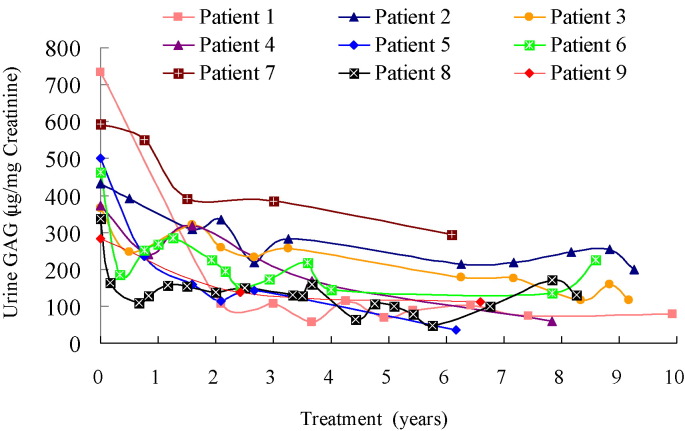
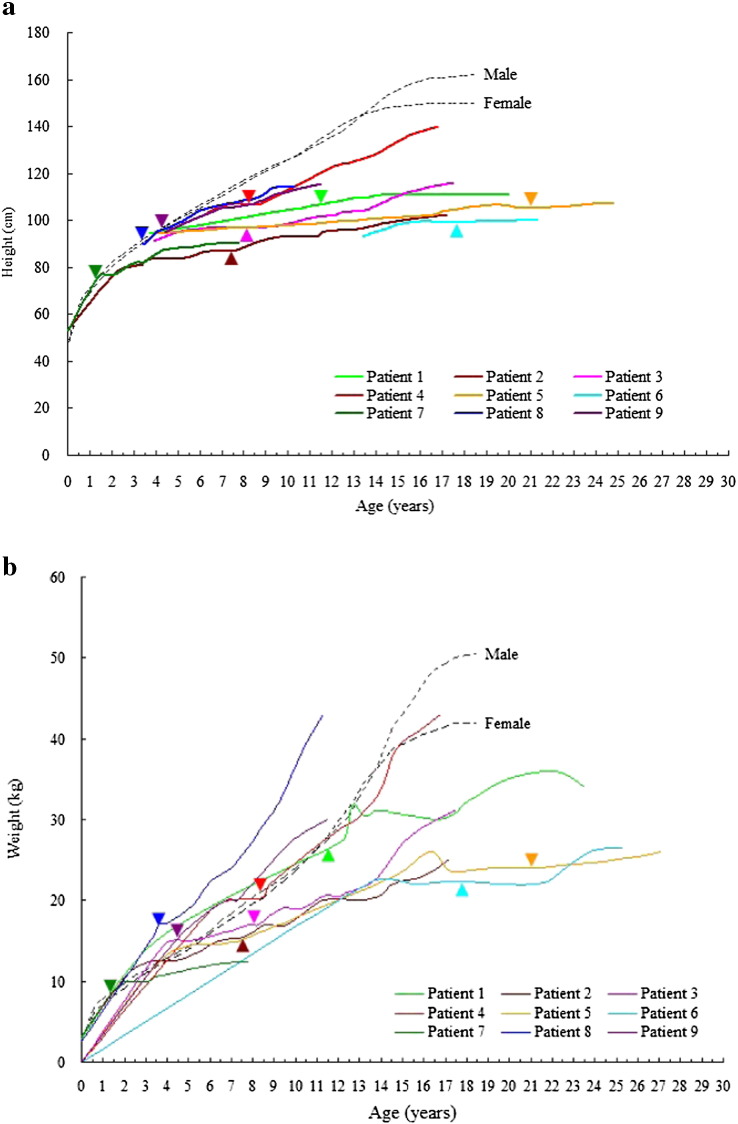
The diagnosis of MPS VI was confirmed in all 9 patients according to urinary GAG profiles, ASB enzyme activity level, and DNA mutation analysis. The z scores for height and weight at baseline were − 5.4 ± 3.4 and − 1.7 ± 1.3, respectively (Table 1). Seven patients had spinal cord compression symptoms. The baseline clinical assessment data was described as our previous study for these 9 Taiwanese patients with MPS VI in 2010 [28]. After 6.2 to 11.2 years of treatment, there was a 69% decrease in urinary excretion of GAG, indicating biomarker improvement (Table 1, Table 2, Fig. 1). Measures of function also improved, including 6MWT [in 6 patients, mean 150-meter (59%) increase over baseline] and 3MSCT [in 3 patients, mean 60-step (46%) increase]. The time requirement of picking up 10 coins and putting them into a cup in 3 patients decreased 15 s (33%). Shoulder range of motion improved in all 9 patients, as did the Disability Index (0.42 points, 21%). The 4 patients who underwent pulmonary function testing had improved FEV1 by 0.29 L (59%) and FVC by 0.42 L (80%) (Table 1, Table 2). The 5 patients who underwent echocardiographic evaluations had positive effects on cardiac-wall diameters. After ERT, the mean LVMI z score decreased from 3.18 to 1.88, the mean IVSd z score decreased from 6.86 to 2.33, and the mean LVPWd z score decreased from 1.70 to 0.30 (Fig. S1). Four of these 5 patients had improved cardiac diastolic function according to the improvement of E/A ratio (Fig. S2). However, the severity of valvular stenosis or regurgitation remained stable and did not show improvement despite ERT (Fig. S3). Liver and spleen size decreased or remained unchanged after ERT in all 9 patients by abdominal ultrasonographic assessments (Table 3). Three patients who underwent baseline and follow-up DXA screening after receiving ERT for 3.8 to 7.4 years showed an increase in absolute BMD values, as well as an improvement in HAZ adjusted BMD z score (Fig. S4). Three patients who had follow-up PTA after undergoing ventilation tube insertion while receiving ERT for 5.2 to 8.5 years showed improvements in AC and BC of the better ear, as well as a decrease in the air-bone gap (Fig. S5). Growth was not appreciably affected by ERT in all 9 patients assessed. Patient 4 did grow somewhat in height, but none of the patients achieved the third percentile for height (Fig. 2a).
Table 1.
Baseline and follow-up clinical assessment results of 9 Taiwanese patients with mucopolysaccharidosis VI receiving enzyme replacement therapy for 6.2 to 11.2 years.
| Patient | Gender | Mutation | ASB, nmol/h/mg proteina | Age at start of ERT (years) | Height (z score) | Weight (z score) | ERT durationb (years) | Urinary GAG, μg/mg creatininec |
6-min walk (m) |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | ||||||||
| 1 | F | p.L132P ND | 14.3 | 11.7 | − 5.9 | − 1.6 | 11.2 | 734.0 | 77.6 | 141 | 288 |
| 2 | M | p.G303E (homozygous) | 16.9 | 7.6 | − 6.6 | − 1.9 | 8.2 | 430.9 | 199.6 | 356.9 | 407 |
| 3 | M | p.R315X p.F399L | 7.5 | 8.3 | − 5.2 | − 1.7 | 8.2 | 366.4 | 116.0 | 367.2 | 512 |
| 4 | M | p.L132P (homozygous) | 9.6 | 8.5 | − 3.8 | − 1.3 | 8.2 | 372.1 | 60.1 | 392 | 570 |
| 5 | F | p.Q239R p.F399L | 12.6 | 21.1 | − 9.7 | − 3.4 | 6.3 | 501.5 | 36.5 | NA | NA |
| 6 | F | p.F399L p.S465X | 4.9 | 17.7 | − 11.2 | − 3.4 | 7.7 | 463.7 | 226.1 | 78 | NA |
| 7 | F | exon 4 deletions (homozygous) | 5.5 | 1.4 | − 0.9 | − 1.9 | 6.2 | 593.5 | 293.3 | NA | 378 |
| 8 | F | p.F399L (homozygous) | 2.3 | 3.6 | − 1.9 | 1 | 8.3 | 336.4 | 130.7 | 243 | 120 |
| 9 | M | p.H430N ND | 7.1 | 4.4 | − 3.2 | − 0.8 | 7.1 | 281.8 | 111.1 | 200 | 550 |
| Mean | 9 | 9.4 | − 5.4 | − 1.7 | 7.9 | 453.4 | 139.0 | 254.0 | 403.6 | ||
| SD | 4.8 | 6.5 | 3.4 | 1.3 | 1.5 | 140.8 | 84.4 | 121.9 | 160.9 | ||
| Patient | 3-min stair climb (stairs) | Coins picking up (sec) | FEV1 (L) | FVC (L) | Disability Indexd | Adverse events | |||||
| Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | ||
| 1 | 104 | 154 | 44 | NA | 0.61 | 0.65 | 0.66 | 0.76 | 1.75 | 0.375 | Y |
| 2 | 143 | 177 | 61 | 24 | 0.42 | 0.53 | 0.44 | 0.59 | 2.125 | 1.5 | Y |
| 3 | 154 | 243 | 35 | 38 | 0.67 | 0.8 | 0.75 | 0.92 | 2 | 1.625 | N |
| 4 | 198 | NA | 35 | 12 | NA | NA | NA | NA | 0.875 | 1.25 | N |
| 5 | NA | NA | NA | NA | NA | NA | NA | NA | 3 | 3 | N |
| 6 | 78 | NA | NA | NA | 0.28 | 1.48 | 0.29 | 1.94 | 1.75 | 3 | N |
| 7 | NA | 191 | NA | NA | NA | NA | NA | NA | 2.5 | 0.75 | N |
| 8 | 97 | NA | NA | 28 | NA | 0.48 | NA | 0.6 | 2.125 | 1.625 | Y |
| 9 | 142 | NA | NA | 44 | NA | NA | NA | NA | 1.5 | 0.75 | N |
| Mean | 130.9 | 191.3 | 43.8 | 29.2 | 0.50 | 0.79 | 0.54 | 0.96 | 1.958 | 1.542 | |
| SD | 40.8 | 37.7 | 12.3 | 12.5 | 0.18 | 0.41 | 0.21 | 0.56 | 0.603 | 0.933 | |
ND, not determined; ASB, arysulphatase B; ERT, enzyme replacement therapy; GAG, glycosaminoglycan; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; NA, not available; SD, standard deviation.
Normal value: > 121.0.
ERT duration up until the time of this study.
Normal values are age dependent: 1–3 years, 20.0–110.5; 3–5 years, 10.7–112.0; > 5 years, 10.8–77.5.
Based on the Health Assessment Questionnaire (HAQ) for subjects > 18 years of age, or Child Health Assessment Questionnaire (CHAQ) for subjects ≤ 18 years of age. Disability Index ranged from 0 to 3, with 0 = no disability and 3 = severe disability.
Table 2.
The comparison of mean baseline and follow-up clinical assessment results of 9 Taiwanese patients with mucopolysaccharidosis VI underwent enzyme replacement therapy for 6.2–11.2 years.
| Clinical assessments | N | Baseline | Follow-up | Improvement (%) |
|---|---|---|---|---|
| Urinary GAG (μg/mg creatinine) | 9 | 453.4 | 139 | 69% |
| 6-min walk (m) | 6 | 254 | 404 | 59% |
| 3-min stair climb (stairs) | 3 | 131 | 191 | 46% |
| Coins picking up (sec) | 3 | 44 | 29 | 33% |
| Disability Index | 9 | 1.96 | 1.54 | 21% |
| FEV1 (L) | 4 | 0.50 | 0.79 | 59% |
| FVC (L) | 4 | 0.54 | 0.96 | 80% |
GAG, glycosaminoglycan; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
Fig. 1.
Urinary glycosaminoglycan (GAG) level vs. treatment year for 9 patients with mucopolysaccharidosis VI receiving enzyme replacement therapy for 6.2–11.2 years.
Table 3.
Abdominal ultrasonographic assessments of liver and spleen size at baseline and after 5.9–11.2 years of enzyme replacement therapy (ERT) for 9 patients with mucopolysaccharidosis VI.
| Patient | Age at start of ERT (years) | Treatment duration (years) | Baseline | After ERT | Liver and spleen size |
|---|---|---|---|---|---|
| 1 | 11.7 | 11.2 | Hepatospenomegaly | Mild hepatomegaly | Improvement |
| 2 | 7.6 | 9.2 | Hepatospenomegaly | No hepatospenomegaly | Improvement |
| 3 | 8.3 | 9.1 | Hepatospenomegaly | Borderline hepatosplenomegaly | Improvement |
| 4 | 8.5 | 9.2 | Hepatospenomegaly | No hepatospenomegaly | Improvement |
| 5 | 21.1 | 5.9 | Hepatomegaly, normal spleen | Hepatomegaly, normal spleen | Stationary |
| 6 | 17.7 | 7.3 | Hepatospenomegaly | No hepatospenomegaly | Improvement |
| 7 | 1.4 | 7.0 | No hepatomegaly, borderline enlarged spleen | No hepatosplenomegaly | Improvement |
| 8 | 3.6 | 9.4 | No hepatosplenomegaly | No hepatosplenomegaly | Stationary |
| 9 | 4.4 | 8.0 | No hepatosplenomegaly | No hepatosplenomegaly | Stationary |
Fig. 2.
Height (a) and weight (b) curves of 9 patients with mucopolysaccharidosis VI. Arrowheads represent start of enzyme replacement therapy; dotted lines represent third percentile growth and weight curves of the Taiwanese population.
3.1. Adverse events
Three patients had hypersensitivity reactions (skin itching/urticaria, dyspnea, fever) at some point during their treatment. However, following pre-medication with oral antihistamines, steroids and antipyretics, all were able to tolerate ERT despite mild reactions continuing for about 5 months in 2 of the 3 individuals. With continued pre-medication, all adverse events abated.
4. Discussion
This is the first report to demonstrate clinical benefits of long-term ERT in Taiwanese patients with MPS VI. As far as we are aware, the 9 cases presented here are the only MPS VI patients in Taiwan who are currently receiving ERT. Our review clearly demonstrates both biochemical and clinical functional improvement in all 9 patients after 6–11 years of ERT. Although several studies have shown that short-term ERT for MPS VI patients positively affects joint mobility, liver and spleen size, cardiac parameters, pulmonary function, and urinary GAGs levels [4], [5], [6], [7], [8], [28], [29], there is very limited literature reporting the long-term ERT effects for patients with MPS VI [9]. Swiedler et al. [30] carried out a cross-sectional survey of 121 patients with MPS VI, and reported an association between high urinary GAG values (> 200 μg/mg creatinine) and a worse clinical course of disease although the clinical manifestation varied widely among patients. In our study, all individuals had urinary GAG values above 200 μg/mg creatinine at baseline suggesting the severe phenotype may be more prevalent in the Taiwanese population (Table 1). The observed decrease in urinary GAG excretion seen with long-term ERT paralleled improvements in patients' functional status. Our results thus support the effectiveness of ERT for MPS VI, with functional improvement accompanying biochemical response. However, some aspects of disease phenotype are unaffected by long-term ERT. For example, no improvements in skeletal dysplasia or corneal clouding were observed in galsulfase treated Taiwanese patients, which were in accordance with those of McGill et al. [31].
Harmatz et al. [8] reported that FEV1 and FVC increased over baseline by 11% and 17%, respectively, after 96 weeks of ERT for 56 patients with MPS VI. This improvement in pulmonary function is one factor underlying the increased physical endurance documented in the 6-minute and 12-minute walk tests. Our spirometry results in 4 patients were consistent with theirs. ERT appeared to be effective in reducing cardiac hypertrophy in our 5 patients who had detailed echocardiographic examinations. Four of these 5 patients had improved cardiac diastolic function according to the improvement of E/A ratio. These results suggest that ERT may have some effect on GAG accumulation in cardiac tissue, and thus was effective in reducing cardiac hypertrophy and diastolic dysfunction. However, ERT seemed to have little or no effect on valvular heart disease. Our results are consistent with other studies describing the cardiac effects of ERT for patients with MPS VI [32], [33], [34].
The older patients in our study had hepatosplenomegaly, while the younger patients showed relatively normal liver and spleen size (Table 3), reflecting the progressive nature of the disease. Brands et al. [29] reported that after ERT for 1.3 to 5.4 years, liver and spleen size decreased in all eleven MPS VI patients when assessed manually by physicians. Similarly, our results showed that liver and spleen size decreased or remained unchanged after ERT for all 9 patients as measured by abdominal ultrasonography. There is a paucity of literature describing the effects of ERT on BMD in MPS [23], [35]. In our study, 3 MPS VI patients who underwent follow-up DXA after receiving ERT for 3.8 to 7.4 years showed an increase in absolute BMD values, as well as an improvement in their HAZ adjusted BMD z scores. Improvements in BMD in MPS VI patients receiving ERT may occur through multiple mechanisms including reduced GAG storage in the bones, increased muscle strength and endurance, and improved pulmonary function and mobility [14], [23], [28], [36]. There is also limited literature describing the effects of ERT on hearing loss. Brands et al. [29] described that 2 of 3 patients with MPS VI improved conductive hearing loss after ERT, possibly due to ERT-related decrease in occurrence of upper airway infections. In our study, 3 MPS VI patients improved or stabilized their BC values [sensorineural hearing loss component] after receiving ERT for 5.2 to 8.5 years. Improvement of conductive hearing loss in MPS patients receiving ERT may be due to fewer upper airway infections, as well as reducing middle ear effusions causing pressure on the Eustachian tube. However, it remains unclear whether sensorineural hearing loss has a congenital basis or is acquired secondarily to deposition of GAGs in the inner ear or central nervous system [37]. The issue concerning the effect of ERT for MPS on sensorineural hearing loss may be resolved after longer follow-up. Overall, our experience with treating Taiwanese patients is thus consistent with the results of ERT elsewhere in the world.
In our series, none of the patients reached the third percentile of the height curve, suggesting a limited effect of ERT on growth (Fig. 2a). Reports from Scarpa et al. [34] in Italy are similar. In cats treated from birth, ERT prevented or slowed skeletal dysplasia, however, no parallel studies of early therapy in humans are available as yet [38], [39]. Whether it is possible in humans to identify the disease early enough to begin ERT before irreversible skeletal pathology has occurred remains to be seen. Spinal cord compression is a significant issue in many MPS VI patients. Our results showed that 7 out of 9 patients had spinal cord compression symptoms, which were consistent with those of Scarpa et al. [34].
Adverse events, such as skin rash, dyspnea, and pyrexia, were reported in clinical trials of galsulfase for MPS VI [4], [5], [6], [7]. A third of our patients had similar symptoms, but the reactions were easily managed. None of the 3 had serious sequelae, and all 3 were able to continue with treatment.
4.1. Limitations
This retrospective and multicenter study did not compare the effects of ERT in these patients with untreated control subjects. Meanwhile, clinical assessments varied by institutions and treating physicians. Besides, the range of age at which treatment started was quite wide, as was the degree of disease severity. However, our experience reflects what clinicians probably come across in daily practice with unselected patients.
5. Conclusion
In our case series of 9 Taiwanese patients with MPS VI, long-term ERT with galsulfase improved endurance, mobility, joint function, pulmonary function, cardiac hypertrophy and diastolic dysfunction, liver size and spleen size, while reducing urinary GAG excretion. ERT was well-tolerated, with mild hypersensitivity reactions in 3 patients easily managed with routine pre-medication therapy. ERT for treatment of MPS VI has been endorsed by the National Health Insurance program in Taiwan since February 2006. Our clinical experience confirms that long-term ERT is clearly beneficial for Taiwanese patients with MPS VI, just as it is for other global MPS VI populations.
Conflict of interest statement
The authors declare that they have no competing interests.
Authors' contributions
HYL performed acquisition, statistical analysis and interpretation of data, and drafting of the manuscript. SPL participated in design of the study, interpretation of the data and helped to draft the manuscript. CKC performed biochemical analyses and revised the manuscript. CHW, YHC, YMW, FJT, YYC, SJL, HPP, DMN, WLH and YYK were responsible for patient screening. All authors read and accepted the manuscript.
Acknowledgements
The investigators acknowledge the participation of study patients and their families. We also wish to thank Ms. Virginia Tsai, the founder of the Taiwan MPS Society, for her devotion in the family care and collection of some of the important data. This study was supported by the research grants from the National Science Council, Taiwan (NSC-104-2314-B-195-019, NSC-102-2314-B-195-006, and NSC-102-2314-B-195-017-MY3), and Mackay Memorial Hospital (MMH-103-092, MMH-101-111 and MMH-I-S-600). We would like to express our sincere thanks to Ms. Tsai-Feng Ho for her professional assistance in biostatistics.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ymgmr.2016.04.003.
Appendix A. Supplementary data
Parameter changes of echocardiographic assessments at baseline and after 5.2–8.4 years of enzyme replacement therapy (ERT) for 5 patients with mucopolysaccharidosis VI (Patients No. 1 to 5). LVMI, left ventricular mass index; IVSd, the thicknesses of the interventricular septum diameter in diastole; LVPWd, the thicknesses of the left ventricular posterior wall diameter in diastole. Fig. S2 E/A ratio changes of echocardiographic assessments of diastolic dysfunction at baseline and after 5.9–10.8 years of enzyme replacement therapy (ERT) for 5 patients with mucopolysaccharidosis VI (Patients No. 1 to 5). E/A ratio, ratio of the early (E) to late (A) ventricular filling velocities. E/A ratio ≧ 1, normal diastolic function; E/A ratio < 1, impaired relaxation. Fig. S3 Parameter changes of echocardiographic assessments of valvular heart diseases at baseline and after 5.9–10.8 years of enzyme replacement therapy (ERT) for 5 patients with mucopolysaccharidosis VI (Patients No. 1 to 5). MS, mitral stenosis; MR, mitral regurgitation; AS, aortic stenosis; AR, aortic regurgitation. Severity of valvular stenosis and regurgitation were estimated and graded on the following scores: 0 (none), 1 (mild), 2 (moderate), and 3 (severe). Fig. S4 Baseline and follow-up absolute bone mineral density (BMD) values and height-for-age z score (HAZ) adjusted BMD z scores of 3 patients with mucopolysaccharidosis VI (Patients No. 1 to 3) underwent enzyme replacement therapy (ERT) for 3.8 to 7.4 years. Fig. S5 Baseline and follow-up pure-tone audiometry (PTA) parameters of 3 patients with mucopolysaccharidosis VI (Patients No. 1 to 3) who underwent ventilation tube insertion and received enzyme replacement therapy (ERT) for 5.2 to 8.5 years. AC, air conduction; BC, bone conduction.
References
- 1.Neufeld E.F., Muenzer J. The mucoplysaccharidoses. In: Scriver C.R., Beaudet A.L., Sly W.S., Valle D., Childs B., Kinzler K.W., Vogelstein B., editors. The Metabolic and Molecular Bases of Inherited Disease. eighth ed. McGraw-Hill; New York: 2001. pp. 3421–3452. assoc, eds. [Google Scholar]
- 2.Chuang C.K., Lin S.P. Neurochemical changes and therapeutical approaches in mucopolysaccharidoses. In: Surendran Sankar, Aschner Michael, Bhatnagar Maheep., editors. Neurochemistry of Metabolic Diseases-lysosomal Storage Diseases, Phenylketonuria and Canavan Disease. Transworld Research Network; Trivandrum, India: 2007. pp. 1–20. [Google Scholar]
- 3.Lin H.Y., Lin S.P., Chuang C.K., Niu D.M., Chen M.R., Tsai F.J., Chao M.C., Chiu P.C., Lin S.J., Tsai L.P., Hwu W.L., Lin J.L. Incidence of the mucopolysaccharidoses in Taiwan, 1984–2004. Am. J. Med. Genet. A. 2009;149A:960–964. doi: 10.1002/ajmg.a.32781. [DOI] [PubMed] [Google Scholar]
- 4.Harmatz P., Whitley C.B., Waber L., Pais R., Steiner R., Plecko B., Kaplan P., Simon J., Butensky E., Hopwood J.J. Enzyme replacement therapy in mucopolysaccharidosis VI (Maroteaux-Lamy syndrome) J. Pediatr. 2004;144:574–580. doi: 10.1016/j.jpeds.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Harmatz P., Ketteridge D., Giugliani R., Guffon N., Teles E.L., Miranda M.C., Yu Z.F., Swiedler S.J., Hopwood J.J., MPS VI Study Group Direct comparison of measures of endurance, mobility, and joint function during enzyme-replacement therapy of mucopolysaccharidosis VI (Maroteaux-Lamy syndrome): results after 48 weeks in a phase 2 open-label clinical study of recombinant human N-acetylgalactosamine 4-sulfatase. Pediatrics. 2005;115:e681–e689. doi: 10.1542/peds.2004-1023. [DOI] [PubMed] [Google Scholar]
- 6.Harmatz P., Giugliani R., Schwartz I., Guffon N., Teles E.L., Miranda M.C., Wraith J.E., Beck M., Arash L., Scarpa M., Yu Z.F., Wittes J., Berger K.I., Newman M.S., Lowe A.M., Kakkis E., Swiedler S.J., MPS VI Phase 3 Study Group Enzyme replacement therapy for mucopolysaccharidosis VI: a phase 3, randomized, double-blind, placebo-controlled, multinational study of recombinant human N-acetylgalactosamine 4-sulfatase (recombinant human arylsulfatase B or rhASB) and follow-on, open-label extension study. J. Pediatr. 2006;148:533–539. doi: 10.1016/j.jpeds.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Harmatz P., Giugliani R., Schwartz I.V., Guffon N., Teles E.L., Miranda M.C., Wraith J.E., Beck M., Arash L., Scarpa M., Ketteridge D., Hopwood J.J., Plecko B., Steiner R., Whitley C.B., Kaplan P., Yu Z.F., Swiedler S.J., Decker C., MPS VI Study Group Long-term follow-up of endurance and safety outcomes during enzyme replacement therapy for mucopolysaccharidosis VI: final results of three clinical studies of recombinant human N-acetylgalactosamine 4-sulfatase. Mol. Genet. Metab. 2008;94:469–475. doi: 10.1016/j.ymgme.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Harmatz P., Yu Z.F., Giugliani R., Schwartz I.V., Guffon N., Teles E.L., Miranda M.C., Wraith J.E., Beck M., Arash L., Scarpa M., Ketteridge D., Hopwood J.J., Plecko B., Steiner R., Whitley C.B., Kaplan P., Swiedler S.J., Hardy K., Berger K.I., Decker C. Enzyme replacement therapy for mucopolysaccharidosis VI: evaluation of long-term pulmonary function in patients treated with recombinant human N-acetylgalactosamine 4-sulfatase. J. Inherit. Metab. Dis. 2010;33:51–60. doi: 10.1007/s10545-009-9007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giugliani R., Lampe C., Guffon N., Ketteridge D., Leão-Teles E., Wraith J.E., Jones S.A., Piscia-Nichols C., Lin P., Quartel A., Harmatz P. Natural history and galsulfase treatment in mucopolysaccharidosis VI (MPS VI, Maroteaux-Lamy syndrome)–10-year follow-up of patients who previously participated in an MPS VI survey study. Am. J. Med. Genet. A. 2014;164A:1953–1964. doi: 10.1002/ajmg.a.36584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chuang C.K., Lin S.P., Chung S.F. Diagnostic screening for mucopolysaccharidoses by the dimethylmethylene blue method and two dimensional electrophoresis. Zhonghua Yi Xue Za Zhi (Taipei) 2001;64:15–22. [PubMed] [Google Scholar]
- 11.Lin W.D., Lin S.P., Wang C.H., Hwu W.L., Chuang C.K., Lin S.J., Tsai Y., Chen C.P., Tsai F.J. Genetic analysis of mucopolysaccharidosis type VI in Taiwanese patients. Clin. Chim. Acta. 2008;394:89–93. doi: 10.1016/j.cca.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Chen W., Chang M.H. New growth charts for Taiwanese children and adolescents based on World Health Organization standards and health-related physical fitness. Pediatr. Neonatol. 2010;51:69–79. doi: 10.1016/S1875-9572(10)60014-9. [DOI] [PubMed] [Google Scholar]
- 13.American Thoracic Society Standardization of spirometry, 1994 update. Am. J. Respir. Crit. Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 14.Lin S.P., Shih S.C., Chuang C.K., Lee K.S., Chen M.R., Niu D.M., Chiu P.C., Lin S.J., Lin H.Y. Characterization of pulmonary function impairments in patients with Mucopolysaccharidoses—changes with age and treatment. Pediatr. Pulmonol. 2014;49:277–284. doi: 10.1002/ppul.22774. [DOI] [PubMed] [Google Scholar]
- 15.Teichholz L.E., Kreulen T., Herman M.V., Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am. J. Cardiol. 1976;37:7–11. doi: 10.1016/0002-9149(76)90491-4. [DOI] [PubMed] [Google Scholar]
- 16.Eidem B.W., McMahon C.J., Cohen R.R., Wu J., Finkelshteyn I., Kovalchin J.P., Ayres N.A., Bezold L.I., O'Brian Smith E., Pignatelli R.H. Impact of cardiac growth on Doppler tissue imaging velocities: a study in healthy children. J. Am. Soc. Echocardiogr. 2004;17:212–221. doi: 10.1016/j.echo.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Lin S.M., Lin H.Y., Chuang C.K., Lin S.P., Chen M.R. Cardiovascular abnormalities in Taiwanese patients with mucopolysaccharidosis. Mol. Genet. Metab. 2014;111:493–498. doi: 10.1016/j.ymgme.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Baumgartner H., Hung J., Bermejo J., Chambers J.B., Evangelista A., Griffin B.P., Iung B., Otto C.M., Pellikka P.A., Quiñones M., American Society of Echocardiography, European Association of Echocardiography Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J. Am. Soc. Echocardiogr. 2009;22:1–23. doi: 10.1016/j.echo.2008.11.029. quiz 101-102. [DOI] [PubMed] [Google Scholar]
- 19.Lang R.M., Bierig M., Devereux R.B., Flachskampf F.A., Foster E., Pellikka P.A., Picard M.H., Roman M.J., Seward J., Shanewise J.S., Solomon S.D., Spencer K.T., Sutton M.S., Stewart W.J., Chamber Quantification Writing Group, American Society of Echocardiography's Guidelines and Standards Committee, European Association of Echocardiography Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Kampmann C., Wiethoff C.M., Wenzel A., Stolz G., Betancor M., Wippermann C.F., Huth R.G., Habermehl P., Knuf M., Emschermann T., Stopfkuchen H. Normal values of M mode echocardiographic measurements of more than 2000 healthy infants and children in central Europe. Heart. 2000;83:667–672. doi: 10.1136/heart.83.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poutanen T., Jokinen E. Left ventricular mass in 169 healthy children and young adults assessed by three-dimensional echocardiography. Pediatr. Cardiol. 2007;28:201–207. doi: 10.1007/s00246-006-0101-5. [DOI] [PubMed] [Google Scholar]
- 22.Konuş O.L., Ozdemir A., Akkaya A., Erbaş G., Celik H., Işik S. Normal liver, spleen, and kidney dimensions in neonates, infants, and children: evaluation with sonography. AJR Am. J. Roentgenol. 1998;171:1693–1698. doi: 10.2214/ajr.171.6.9843315. [DOI] [PubMed] [Google Scholar]
- 23.Lin H.Y., Shih S.C., Chuang C.K., Chen M.R., Niu D.M., Lin S.P. Assessment of bone mineral density by dual energy X-ray absorptiometry in patients with mucopolysaccharidoses. Orphanet J. Rare Dis. 2013;8:71. doi: 10.1186/1750-1172-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin H.Y., Shih S.C., Chuang C.K., Lee K.S., Chen M.R., Lin H.C., Chiu P.C., Niu D.M., Lin S.P. Assessment of hearing loss by pure-tone audiometry in patients with mucopolysaccharidoses. Mol. Genet. Metab. 2014;111:533–538. doi: 10.1016/j.ymgme.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Fries J.F. The hierarchy of quality-of-life assessment, the health assessment questionnaire (HAQ), and issues mandating development of a toxicity index. Control. Clin. Trials. 1991;12(4 Suppl):106S–117S. doi: 10.1016/s0197-2456(05)80016-9. [DOI] [PubMed] [Google Scholar]
- 26.Ramey D.R., Raynauld J.P., Fries J.F. The health assessment questionnaire 1992: status and review. Arthritis Care Res. 1992;5:119–129. doi: 10.1002/art.1790050303. [DOI] [PubMed] [Google Scholar]
- 27.Singh G., Athreya B.H., Fries J.F., Goldsmith D.P. Measurement of health status in children with juvenile rheumatoid arthritis. Arthritis Rheum. 1994;37:1761–1769. doi: 10.1002/art.1780371209. [DOI] [PubMed] [Google Scholar]
- 28.Lin H.Y., Chen M.R., Chuang C.K., Chen C.P., Lin D.S., Chien Y.H., Ke Y.Y., Tsai F.J., Pan H.P., Lin S.J., Hwu W.L., Niu D.M., Lee N.C., Lin S.P. Enzyme replacement therapy for mucopolysaccharidosis VI-experience in Taiwan. J. Inherit. Metab. Dis. 2010;33(Suppl. 3):S421–S427. doi: 10.1007/s10545-010-9212-5. [DOI] [PubMed] [Google Scholar]
- 29.Brands M.M., Oussoren E., Ruijter G.J., Vollebregt A.A., van den Hout H.M., Joosten K.F., Hop W.C., Plug I., van der Ploeg A.T. Up to five years experience with 11 mucopolysaccharidosis type VI patients. Mol. Genet. Metab. 2013;109:70–76. doi: 10.1016/j.ymgme.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Swiedler S.J., Beck M., Bajbouj M., Giugliani R., Schwartz I., Harmatz P., Wraith J.E., Roberts J., Ketteridge D., Hopwood J.J., Guffon N., Sá Miranda M.C., Teles E.L., Berger K.I., Piscia-Nichols C. Threshold effect of urinary glycosaminoglycans and the walk test as indicators of disease progression in a survey of subjects with mucopolysaccharidosis VI (Maroteaux-Lamy syndrome) Am. J. Med. Genet. A. 2005;134A:144–150. doi: 10.1002/ajmg.a.30579. [DOI] [PubMed] [Google Scholar]
- 31.McGill J.J., Inwood A.C., Coman D.J., Lipke M.L., de Lore D., Swiedler S.J., Hopwood J.J. Enzyme replacement therapy for mucopolysaccharidosis VI from 8 weeks of age–a sibling control study. Clin. Genet. 2010;77:492–498. doi: 10.1111/j.1399-0004.2009.01324.x. [DOI] [PubMed] [Google Scholar]
- 32.Kampmann C., Lampe C., Whybra-Trümpler C., Wiethoff C.M., Mengel E., Arash L., Beck M., Miebach E. Mucopolysaccharidosis VI: cardiac involvement and the impact of enzyme replacement therapy. J. Inherit. Metab. Dis. 2014;37:269–276. doi: 10.1007/s10545-013-9649-4. [DOI] [PubMed] [Google Scholar]
- 33.Braunlin E., Rosenfeld H., Kampmann C., Johnson J., Beck M., Giugliani R., Guffon N., Ketteridge D., Sá Miranda C.M., Scarpa M., Schwartz I.V., Leão Teles E., Wraith J.E., Barrios P., Dias da Silva E., Kurio G., Richardson M., Gildengorin G., Hopwood J.J., Imperiale M., Schatz A., Decker C., Harmatz P., MPS VI Study Group Enzyme replacement therapy for mucopolysaccharidosis VI: long-term cardiac effects of galsulfase (Naglazyme®) therapy. J. Inherit. Metab. Dis. 2013;36:385–394. doi: 10.1007/s10545-012-9481-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scarpa M., Barone R., Fiumara A., Astarita L., Parenti G., Rampazzo A., Sala S., Sorge G., Parini R. Mucopolysaccharidosis VI: the Italian experience. Eur. J. Pediatr. 2009;168:1203–1206. doi: 10.1007/s00431-008-0910-z. [DOI] [PubMed] [Google Scholar]
- 35.Fung E.B., Johnson J.A., Madden J., Kim T., Harmatz P. Bone density assessment in patients with mucopolysaccharidosis: a preliminary report from patients with MPS II and VI. J. Pediatr. Rehabil. Med. 2010;3:13–23. [PMC free article] [PubMed] [Google Scholar]
- 36.Hendriksz C.J., Giugliani R., Harmatz P., Lampe C., Martins A.M., Pastores G.M., Steiner R.D., Leão Teles E., Valayannopoulos V., CSP Study Group Design, baseline characteristics, and early findings of the MPS VI (mucopolysaccharidosis VI) clinical surveillance program (CSP) J. Inherit. Metab. Dis. 2013;36:373–384. doi: 10.1007/s10545-011-9410-9. [DOI] [PubMed] [Google Scholar]
- 37.Simmons M.A., Bruce I.A., Penney S., Wraith E., Rothera M.P. Otorhinolaryngological manifestations of the mucopolysaccharidoses. Int. J. Pediatr. Otorhinolaryngol. 2005;69:589–595. doi: 10.1016/j.ijporl.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 38.Auclair D., Hopwood J.J., Brooks D.A., Lemontt J.F., Crawley A.C. Replacement therapy in Mucopolysaccharidosis type VI: advantages of early onset of therapy. Mol. Genet. Metab. 2003;78:163–174. doi: 10.1016/s1096-7192(03)00007-6. [DOI] [PubMed] [Google Scholar]
- 39.Crawley A.C., Niedzielski K.H., Isaac E.L., Davey R.C., Byers S., Hopwood J.J. Enzyme replacement therapy from birth in a feline model of mucopolysaccharidosis type VI. J. Clin. Invest. 1997;99:651–662. doi: 10.1172/JCI119208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Parameter changes of echocardiographic assessments at baseline and after 5.2–8.4 years of enzyme replacement therapy (ERT) for 5 patients with mucopolysaccharidosis VI (Patients No. 1 to 5). LVMI, left ventricular mass index; IVSd, the thicknesses of the interventricular septum diameter in diastole; LVPWd, the thicknesses of the left ventricular posterior wall diameter in diastole. Fig. S2 E/A ratio changes of echocardiographic assessments of diastolic dysfunction at baseline and after 5.9–10.8 years of enzyme replacement therapy (ERT) for 5 patients with mucopolysaccharidosis VI (Patients No. 1 to 5). E/A ratio, ratio of the early (E) to late (A) ventricular filling velocities. E/A ratio ≧ 1, normal diastolic function; E/A ratio < 1, impaired relaxation. Fig. S3 Parameter changes of echocardiographic assessments of valvular heart diseases at baseline and after 5.9–10.8 years of enzyme replacement therapy (ERT) for 5 patients with mucopolysaccharidosis VI (Patients No. 1 to 5). MS, mitral stenosis; MR, mitral regurgitation; AS, aortic stenosis; AR, aortic regurgitation. Severity of valvular stenosis and regurgitation were estimated and graded on the following scores: 0 (none), 1 (mild), 2 (moderate), and 3 (severe). Fig. S4 Baseline and follow-up absolute bone mineral density (BMD) values and height-for-age z score (HAZ) adjusted BMD z scores of 3 patients with mucopolysaccharidosis VI (Patients No. 1 to 3) underwent enzyme replacement therapy (ERT) for 3.8 to 7.4 years. Fig. S5 Baseline and follow-up pure-tone audiometry (PTA) parameters of 3 patients with mucopolysaccharidosis VI (Patients No. 1 to 3) who underwent ventilation tube insertion and received enzyme replacement therapy (ERT) for 5.2 to 8.5 years. AC, air conduction; BC, bone conduction.